Abstract
Effective entomological surveillance planning stresses a careful consideration of methodology, trapping technologies, and analysis techniques. Herein, the basic principles and technological components of arthropod surveillance plans are described, as promoted in the symposium “Advancements in arthropod monitoring technology, techniques, and analysis” presented at the 58th annual meeting of the Entomological Society of America in San Diego, CA. Interdisciplinary examples of arthropod monitoring for urban, medical, and veterinary applications are reviewed. Arthropod surveillance consists of the three components: 1) sampling method, 2) trap technology, and 3) analysis technique. A sampling method consists of selecting the best device or collection technique for a specific location and sampling at the proper spatial distribution, optimal duration, and frequency to achieve the surveillance objective. Optimized sampling methods are discussed for several mosquito species (Diptera: Culicidae) and ticks (Acari: Ixodidae). The advantages and limitations of novel terrestrial and aerial insect traps, artificial pheromones and kairomones are presented for the capture of red flour beetle (Coleoptera: Tenebrionidae), small hive beetle (Coleoptera: Nitidulidae), bed bugs (Hemiptera: Cimicidae), and Culicoides (Diptera: Ceratopogonidae) respectively. After sampling, extrapolating real world population numbers from trap capture data are possible with the appropriate analysis techniques. Examples of this extrapolation and action thresholds are given for termites (Isoptera: Rhinotermitidae) and red flour beetles.
Keywords: insect monitoring, collection technique, trapping technology, analysis technique, management plan
Arthropod surveillance is the use of sampled individuals to gather information about the dynamics of the larger population. The early detection of infestations or changes to population size by monitoring programs can initiate pre-emptive or preventative control measures to reduce damage to structures and crops, and morbidity and mortality in animals and humans.
Insects of structural, urban, and agricultural importance have significant economic impacts. Broadly, they damage structures, decrease product value through feeding damage and contamination, and produce allergens, stings, or bites. Better sampling, mass trapping, and baiting methods decrease costs and improve the quality of life and income for farmers, processors, sellers, homeowners, and the general public. Blood-feeding, or hematophagous, arthropods are the vectors of many of the world’s most significant human and animal pathogens. Hematophagous arthropods also may cause considerable nuisance to hosts being fed upon; and at high attack rates this nuisance can result in substantial economic costs to domestic animal agriculture industries through hide damage, reductions in animal weight gains, or reduced production of animal products such as milk or eggs (Reviewed by Lehane 2005). Even at low attack rates, hosts may suffer hypersensitivity reactions as a result of insect biting.
After detection, the surveillance data of urban, agricultural, veterinary, and medical pests must be evaluated routinely to chart the progress and efficacy of control measures. Careful attention to the surveillance program components will improve them by increasing efficiency, enhancing sensitivity, and strengthening inferences from trap capture data. The following is a review of the three arthropod surveillance components: sampling methods, trap technology, and analysis techniques.
Sampling Methods
Sampling methods are selected depending on the temporal and spatial requirements of the surveillance question. For example, sampling intervals (time between samplings) and spatial distribution, may change if the purpose is to detect seasonality, population expansion, or introduced organisms within an area. If the objective is to estimate the abundance and distribution of a single species, a specific collection technique or type of trapping may suffice. Alternatively, one method may not function optimally in all environments, just as one trap does not work for all arthropods. Therefore surveillance plans must include a variety of collection methods if the goal is to characterize species diversity in an area. After reviewing the advantages and disadvantages of a range of mosquito sampling methods, the suitability of each method will be discussed in relation to surveillance of Aedes aegypti (L.), a dengue virus vector. Similarly, tick sampling methods will be reviewed to evaluate the benefits and limitations of temporal and spatial sampling extents. One important consideration when designing a sampling plan is a cost-benefit analysis of effort versus sampling accuracy—this is discussed using tick sampling as an example.
The use of Multiple Trapping Techniques for each Life History Stage to Rapidly Evaluate Mosquito Populations (Peter Obenauer)
Mosquito sampling is necessary for assessing species diversity and the abundance and distribution of species within the sampled area. When using a well-planned sampling method, collection data also may be used to address population size fluctuations and seasonality, pathogen infection prevalence, and habitat invasions. Sampling methods often focus on collecting immature (larval or pupal) stages from aquatic habitats or adults seeking sugar, blood, and oviposition sites. Collection techniques for each life history stage optimize mosquito capture efficiency, but each trapping technique may be biased for specific species (Service 1993). Trapping from multiple mosquito life history stages and using a variety of sampling techniques will yield a more accurate understanding of the mosquito species diversity in an area. Furthermore, some sampling techniques require fewer material resources, less collection effort, and are less prone to human error. Regardless of sampling method or collection technique, the goal remains to ensure that each species of mosquito is sampled in proportion to its respective occurrence in the heterogeneous mosquito population (Huffaker and Rack 1943).
Larval or pupal sampling is efficient because the immature life-history stages are restricted to aquatic habitats and therefore are less mobile than adults. Sampling from pools or containers is easy, cost effective, and technologically simple; however, locating all larval and pupal habitats may be difficult (see Aedes aegypti surveillance in the following section). Although mosquito larvae are found in a diversity of aquatic habitats, each species has characteristic habitat preferences. Sampling immature stages is a very sensitive technique for presence or absence monitoring of local mosquito populations, and pupal numbers are closely correlated to adult populations. Lastly, larval and pupal sampling is not gender biased. The aquatic life history stages may be sampled with tools as simple as dippers and nets or as complicated as floating quadrats, submerged light traps, cylinder traps with screens, and vegetation sampling (uprooting, washing, chemical treatments). These tools can be used to sample habitats as diverse as rock pools, tree holes, ground containers, special plant associations (e.g., bromiliads and pitcher plants), and crab holes (Service 1993).
Sampling mobile adult mosquitoes captures a spectrum of species, ages, and physiological states by using either nonattractive sampling (such as collecting from resting sites) or attractive sampling (employing techniques that take advantage of a mosquito’s positive taxis for specific environmental cues). Adult capture may collect mosquitoes that migrate from more distant areas and that may not be present in local larval habitats. Furthermore, sampling adults simultaneously yields biting pressure and population age structure data for multiple species. The latter is an important aspect of disease surveillance because older mosquitoes are more likely to be infected with pathogens. Attractant sampling methods can be used to target specific physiological states of females, such as newly emerged, sugar and host seeking, blood-fed, gravid, or ovipositing.
Nonattractant sampling consists of collecting mosquitoes resting indoors or outdoors. This method collects both sexes, across a range of physiological states and ages. For example, Williams et al. (2006) determined that the RG Sentinel trap was the most effective at collecting Ae. aegypti, but that the Centers for Disease Control (CDC) backpack aspirator collected more blood-fed females. The diversity of mosquito species found in resting sites are proportional to the species present in an area, although resting site results may not account for all species that can disperse into that area. An additional problem is that collection from resting sites is time consuming and labor intensive in spite of the array of techniques available, including oral or mechanical aspirators, hand or truck-mounted nets, suction sweeps, rotary traps, insecticide spray collections, malaise or tent traps, sticky traps, or resting boxes.
Attractant sampling is less labor intensive because mosquitoes find and enter traps based on the traps’ attractant properties. Furthermore, attractant sampling is not dependent on collectors’ search abilities, which may vary by effort expended, habitat familiarity, knowledge of local landscape, and general experience. Traps baited with humans or animals are most effective for collection of host-seeking mosquitoes because they emit attractants such as carbon dioxide (CO2) and other olfactory cues that are highly attractive to target mosquito species. Although baited traps are good for evaluating disease risk (biting pressure), the use of people and animals is time-consuming and poses ethical problems. Therefore, traps may be baited with synthetic or natural compounds that approximate host attractive odors and reduce the variation between hosts such as surface area, body temperature, and skin emissions. Visual attractants such as specific shapes and colors also may be used alone or in combination with olfactory cues (Allan et al. 1987). One specific type of attractant sampling targets gravid female mosquitoes that are searching for oviposition sites. These ovitraps may use sticky paper, insecticides, or nets to capture mosquitoes that are attracted to oviposition cues mimicked by the trap. These traps are particularly good for collecting older mosquitoes that may be infected with pathogens. These traps are easy to use and manipulate, but they are very species specific depending on the water-holding container and water infusion (plants, mosquito larval water, organic discharge, etc.).
Surveillance of mosquito species that occupy more than one habitat or are capable of moving between habitat interfaces is especially challenging. Aedes al-bopictus (Skuse), the Asian tiger mosquito, is a species that is capable of occupying urban, suburban, and semiforested habitats, with larval habitats in both artificial and natural containers (Hawley 1988). Alternate surveillance techniques may be required when assessing the abundance of this species in different habitats (Obenauer et al. 2010). Furthermore, each mosquito species may respond best to a unique trap and bait configuration. For example, recent trapping studies in Kenya determined that whereas Culex quinquefasciatus Say responded in significantly greater numbers to CDC light traps baited with CO2, no significant differences in trapping abundance were observed with Anopheles arabiensis Patton (Muturi et al. 2007).
Aedes aegypti Surveillance Methods (Roberto Bar-rera)
Aedes aegypti surveillance methods were crucial to detect the presence or absence of this mosquito during the eradication campaign (Soper 1967). They consisted of human bait captures of biting females by using manual aspirators or hand nets, ovitraps, and surveys of immature stages in containers (Conner and Monroe 1923, Breteau 1954, Fay and Eliason 1966, Reiter and Gubler 1997). Indices of relative population abundance were developed from the number of biting females captured per unit time, percentage of positive ovitraps, number of positive containers (i.e., containers with at least one larva) per 100 houses (Breteau Index), and percentage of positive houses (House Index). Fay and Prince (1970) developed traps for adult Ae. aegypti, but the traps were bulky and heavy, precluding the deployment of traps in the field sufficient for a reliable estimation of population abundance. Techniques for indoor collections of the resting adult population were developed based on the en-dophagic or endophilic behavior of Ae. aegypti and consisted of hand nets or electro-mechanical aspirators (Clark et al. 1994). This sampling technique allowed for a nearly absolute estimate of population density by taking into account the number of houses and the average number of mosquitoes collected per house in a representative sample of the urban setting. Mark-release-recapture approaches were the first techniques used to estimate the absolute population size of adult Ae. aegypti, and they also allowed the determination of daily survival and longevity (Sheppard et al. 1969). However, these techniques are difficult to implement as operational tools in vector control programs.
More recently, Focks and Chadee (1997) applied the pupal survey method, based on the premise that pupal density is a better proxy for adult mosquito productivity than larval density—pupal mortality is relatively low compared with larval mortality. In addition, other researchers established that most pupae and adults are produced in a few types of containers (WHO 2006). They proposed that if control was applied in only the most productive containers, the number of mosquitoes can be reduced below a mosquito population threshold that prevents or interrupts dengue virus transmission (Focks et al. 2000, Focks 2003). However, the presence of cryptic, underground aquatic habitats of Ae. aegypti also have been documented. These can produce even more mosquitoes than the traditional surface containers and, therefore, limit the utility of pupal surveys to areas where those hidden habitats do not exist (Gonzalez and Suarez 1995, Kay et al. 2000, Barrera et al. 2008). The great difficulty is in determining whether a complex urban area has cryptic aquatic habitats of Ae. aegypti. Targeting the most productive containers also requires a nearly complete surveillance of households. In practice this is difficult to accomplish because residents may be absent or some may deny access to vector control personnel. The large variation in the number of Ae. aegypti pupae in space and time typically requires large sample sizes for accurate estimates of population densities. Simplified schemes to sample pupae more efficiently were developed only recently (Barrera 2009).
Ovitraps are rather sensitive tools to detect the presence of Ae. aegypti and have been used extensively for mosquito monitoring since the eradication era. Traps are monitored for either eggs or larvae on a routine basis to assess reproduction rates in an area; however, ovitraps may be too simplistic for mimicking aquatic habitats and do not compete adequately with the natural and artificial containers typical of urban areas (Focks 2003). Several attempts have been made to increase ovitrap attractiveness (Reiter et al. 1991), but the question of whether ovitraps actually reflect the population density of Ae. aegypti still persists. Ovitraps are inexpensive, allow wide area deployment and assessment, can be operated by personnel with minimal training, and some simple models can be implemented to eliminate the need to count every egg contained in ovitraps (Mogi et al. 1990). Recent data have suggested a good relationship between egg density per ovitrap and dengue incidence (Centers for Disease Control, unpublished data). Based on similar principles, gravid traps that capture container-ovipositing Aedes females have been developed, mostly using sticky surfaces (Ritchie et al. 2003). Limited available data suggest a nonlinear relationship between number of gravid females in gravid traps and number of eggs in ovitraps (Facchinelli et al. 2007). The BG-Sentinel, a portable trap for host-seeking adult Ae. aegypti, permit efficient monitoring of recently emerged as well as parous female adult mosquitoes (Geier et al. 2006); however, a major shortcoming is the elevated price and maintenance costs. The ever increasing transmission of dengue viruses in tropical and subtropical areas demands the development of inexpensive and reliable Ae. aegypti surveillance devices.
Ixodid Tick Sampling Techniques (Sandra Allan)
Tick sampling provides a basis for assessing population density and diversity, as well as prevalence and intensity of infection with tick-associated pathogens. Sampling methods focus on collecting host-seeking ticks from vegetation, attraction of ticks to odor sources, and sampling from host animals. Methods of off-host sampling provide a relative estimate of numbers present (abundance) or in a unit area (density). In contrast, sampling from hosts provides an expression of mean density per host, prevalence, or intensity of infestation (Wilson 1994).
Sampling methods such as flagging, dragging, and walking surveys rely on contact with primarily host-seeking ticks from vegetation or other substrate. Flagging, which is sometimes used interchangeably with dragging, consists of using a small fabric flag on a dowel to pass over vegetation, stir up leaf litter, or run through low vegetation to collect questing ticks. Collecting is done along transects (100 m) with ticks collected at set distances. In contrast, dragging consists of pulling a large piece of light-colored textured cloth (1 m2) along the ground behind the collector over transects with ticks removed at routine intervals. The latter method generally does not sample ticks on high or dense vegetation or under leaf litter. Walking surveys are conducted by a collector wearing white or light-colored clothing who walks along transects and collects ticks from clothing at routine intervals. Although labor intensive, these methods provide immediate results.
Attractant-based sampling most commonly involves the use of a CO2 trap that is often placed on a white cloth to enhance collections (Wilson et al. 1972, Sonenshine 1993). These traps may sample an area as large as 23 m2 for highly responsive species such as Amblyomma americanum L. (Wilson et al. 1972). Tick species, however, vary in their responsiveness to CO2 with some species responding strongly (Amblyomma hebraeum Koch, Amblyomma variegatum F.); moderately (Ixodes scapularis Say); or poorly (Dermacentor variabilis Say) (reviewed in Sonenshine 1993). Addition of a pheromone such as the attraction-aggregation-attachment pheromone of A. variegatum to a CO2 trap can enhance attraction (Maranga et al. 2003). In contrast to vertebrate-seeking hematophagous insects such as mosquitoes, the use of other host attractant volatiles in traps for ticks remains relatively unexplored (Allan 2010). The CO2 traps provide a measure of relative abundance, however, the area being sampled is unknown, unless a trap is tested using marked individuals for release-recapture. This sampling approach, although effective in sparse or dense vegetation and less dependent on dry conditions (Gray 1985), does require several hours for each collection.
Host-based sampling uses the tick’s ability to find suitable hosts for sampling and includes trapping wild animals, restraining livestock and household animals, and sampling from fresh carcasses (e.g., deer), and provides relative-abundance data. This method is advantageous because preferred hosts can be sensitive collectors of ticks at low densities. Sampling ticks from natural habitats include approaches such as aspiration from animal nests or borrows and from artificial nest boxes (Wilson et al. 1972) and provide an estimate of relative abundance. Disadvantages of this method are that it is labor intensive, may require worker protection measures (i.e., Peromyscus and hantavirus), difficulty in obtaining large sample sizes, inconsistency in collection between workers, and requirements for animal handling approval.
Numerous studies examining different sampling methods indicate that the efficacy of these methods varies with factors such as tick species, age and sex, height of vegetation sampled, type of vegetation, stages and responsiveness of species to attractants used (Semter and Hair 1975, Kinzer et al. 1990, Holscher et al. 1980, Ginsberg and Ewing 1989, Falco and Fish 1992, Solberg et al. 1992, Schulze et al. 1997, Petry et al. 2010). Challenges for use of different sampling methods include the variation and complexity of the habitat sampled (such as open grassy meadows, low and sparse understory vegetation, high dense brambles) (Ginsberg and Ewing 1989) and differences in behavior between tick species (‘hunter’ versus ‘ambush’ strategists) (Waladde and Rice 1982, Petry et al. 2010). Biotic and abiotic parameters described by Barnard (1991) impact tick-host contact and can be exploited to increase collection rates of host-seeking ticks. Additional factors affecting the success of a surveillance method include efficacy of the method for contacting the questing tick, response of the tick to the attractant provided, and the responsiveness of particular stages, sexes, and species to attractants used. An effective surveillance plan will consider a wide range of variables optimized for the species and stage of interest.
Optimizing Quadrat Length for Estimating the Abundance of Dermacentor andersoni (Kateryn Rochon)
Disease vector population monitoring generates the data needed to conduct risk analysis and establish disease prevention strategies. Therefore, careful attention to sampling effort is necessary to ensure valid population-level inferences. Larger sample sizes yield more reliable estimates; however, optimal sampling yields precise and repeatable estimates, with reduced time and resource costs. Data collected across the landscape at different locations can also be compared when using a well designed sampling program.
Investigators can determine the minimum sample size required to obtain an estimate with a given level of precision (Karandinos 1976). The general formula requires three parameters: the mean, the variance, and a coefficient of variability. The coefficient of variability represents the ratio of error-to-mean the investigator is willing to accept in the estimate. This method was applied in the development of a sampling plan to estimate the abundance of the Rocky Mountain wood tick (Dermacentor andersoni Stiles) in southern Alberta. Preliminary sampling provided an estimate of the mean tick abundance and the variance was estimated by applying Taylor’s power law (equation 2 in Taylor 1961, Taylor et al. 1978), which describes the relationship between the mean and the variance for a given organism and sampling method. Log-transformation of Taylor’s power law generates a linear relationship, allowing for parameters a and b to be estimated by simple linear regression, where a is the intercept and b is the slope.
This step gives the investigator an estimate of the variance, given a mean, that can be used to calculate the optimum sample size needed to obtain an estimate with a given degree of reliability (Taylor 1984, Lysyk and Axtell 1986). The greater the variability of the estimate around the true mean, the higher the coefficient of variability, and the lower the precision. The acceptable ratio of error-to-mean depends on the purpose of sampling, but 0.25 is acceptable for management purposes in populations exhibiting high variability (Southwood and Henderson 2000).
Although increasing the precision level is intuitively desirable, investigators must evaluate if the added effort (more samples, more traps, increased hours, greater surveillance area, larger data set, etc.) is necessary to adequately address the original purpose of the sampling plan. In the previous example of tick sampling in southern Alberta, each sample (n) is a 10-m2 quadrat. In this example, increasing the precision level by 10% (i.e., changing the coefficient of variability from 0.25 to 0.15) when tick density (the given mean) is one tick per 100 m2 will require almost three times as many samples, leading to an increase in sampling area from 2.2 km2 to 6.2 km2 for each sampling event. This demonstrates the importance of developing a proper sampling scheme: insufficient sampling will yield unreliable data and too much sampling will consume time and resources without providing added value.
Trap Technology
To gather the most accurate population estimates, target insect-capture efficiency must be maximized. The most efficient trap type will use technologies designed to take advantage of the organism’s natural behavioral responses to draw the organism to the trap. Visual, olfactory, tactile, and auditory attractants will change depending on the age (immature stages or adult) and life history stage (feeding, mating, or ovipositing) of the target arthropod. Targeting specific attractants to the physiology and behavior of the organism will maximize trapping rates. Adding new technologies to existing traps, such as light emitting diodes (LEDs) or synthetic olfactory lures, can further increase trapping efficiency. Advancements in trapping technology are discussed for the red flour beetle [Tribolium castaneum (Herbst)], small hive beetle (Aethina tumida Murray), and bed bug (Cimex lectularius L.). The limitations of artificial lures, which can be biased for specific species, are discussed in relation to animal baited traps for Culicoides and mosquitoes. Regardless of the trap composition, competition from surrounding abiotic and biotic factors in addition to human disturbances will influence capture rates and must be considered when analyzing the data.
Visual Attraction of Arthropods (Lee Cohnstaedt)
Arthropod attraction to colors is well studied and has been used to produce attractive visual traps (reviewed in Allan et al. 1987 and Briscoe and Chittka 2001). Sudia and Chamberlain (1962) designed the first Centers for Disease Control (CDC) light trap by using a low-watt incandescent bulb to produce a full spectrum of visible colors or white light. Although white light is attractive to arthropods, the broad spectrum can be decomposed into narrow bands of light by using newer technologies such as fluorescent bulbs and light emitting diodes (Burkett et al. 1998, Burkett and Butler 2005, Cohnstaedt et al. 2008). The wavelengths of light that may cause the greatest photo-attraction are likely in multiple locations within the target arthropod’s visual spectrum. The first location is at the target arthropod’s visual maxima for each receptor. This is the color or wavelength (nm) of light for which a photo receptor is most easily detected (maximally sensitive), therefore, the arthropod perceives this wave length as having the greatest intensity. The second location is where two or more receptors overlap, allowing more than one receptor to respond to the color or wavelength. The overlapping coverage provides the greatest contrast between wavelengths because two or more color receptors will be detecting the light at different intensities. Regardless of the basis for positive photo-attraction, narrow wavelengths of light increase genera and species-specific collection rates and can decrease nontarget species attraction (Wilton and Fay 1972, Bishop et al. 2004). Where light is abundant, diurnal visual cues consist of details distinguishable to insects with high visual acuity. In contrast, nocturnal insects are more sensitive to light and therefore light intensity is perceived better than specific details based on eye construction (reviewed by Land 1997). Light traps placed in urban areas must compete with light pollution from street lamps and houses. Similarly, visual traps in natural settings must be more attractive than the native plants they are mimicking; therefore, in theory multiple LEDs may be necessary to increase trap capture by more accurately projecting the visual cues that enhance the trap’s contrast with its surroundings.
Using New Technology and Insect Behavior in Novel Terrestrial and Flying Insect Traps (Adrian Duehl)
In theory, a trap that perfectly mimics an attractive object (food, mate, shelter, etc.) will be most effective at luring that arthropod to the trap. For example, if an insect uses visual cues to locate shelter, then an attractive trap will be more effective if it includes elements associated with shelter, such as shape or color.
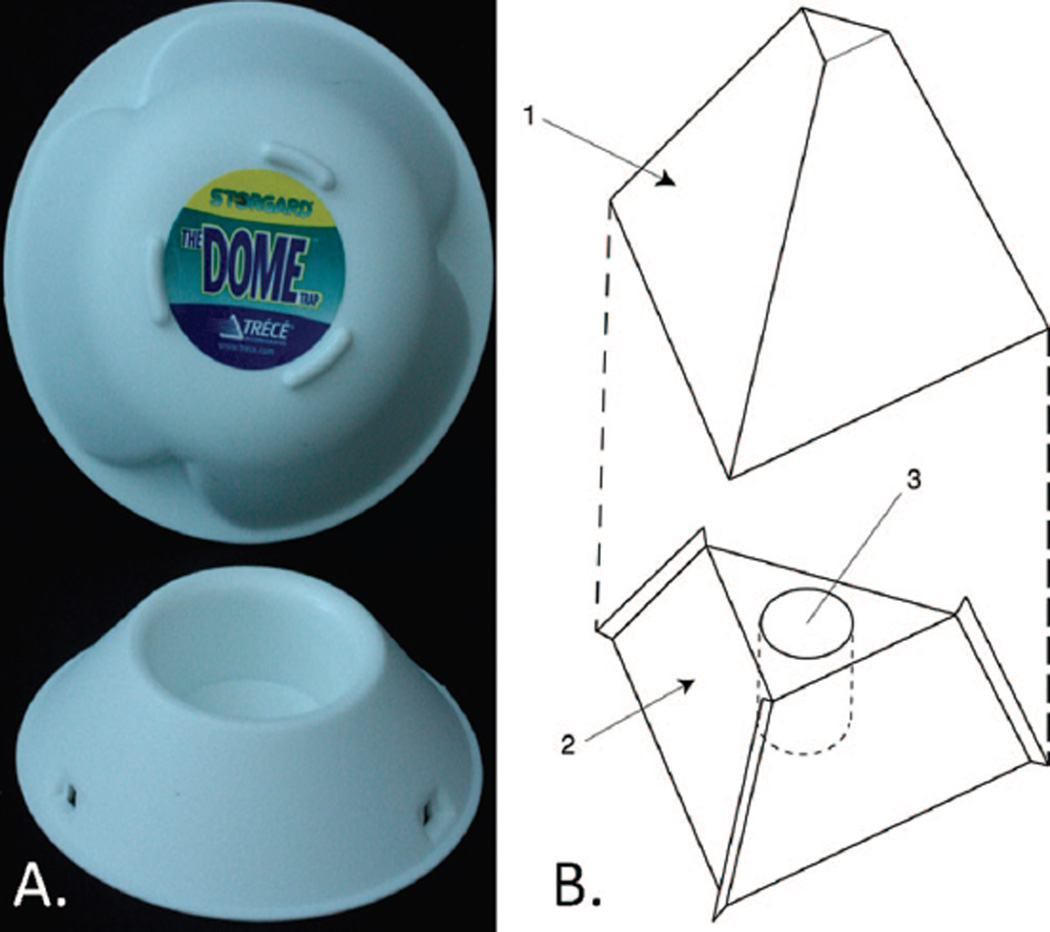
Bioassays evaluating the red flour beetles visual preferences showed the greatest response was to wavelengths of 390 nm (Duehl et al. 2011), in the near UV and one of their spectral perception peaks (Jackowska et al. 2007). Light emitting diodes (LEDs) transmitting light at this dominant wavelength were incorporated into a commonly used stored product pest trap, the Dome trap (Trècè, Adair, OK). When tested competitively against an unlit trap in a darkened shed, the added light component increased trap efficiency twenty fold (Duehl et al. 2011). Further observations of the beetle’s preference for edges and corners inspired a new trap design that incorporated the pitfall and cover elements of the Dome trap, but added features to take advantage of the beetle’s thig-motaxis (Fig. 1). The triangular shape and fins increase edges and effectively guide the beetles to the center pitfall area. This trap’s visual attractiveness was further improved by its dark color and the addition of LEDs, which increased contrast with the surroundings (Semeao et al. 2011) in both light and dark situations and maximized its appeal to beetles that prefer refuges in dark cracks.
Fig. 1.
The two traps used for stored product insects, A) the commonly used Dome trap (Trècè) and B) the newly developed pyramid trap. The pyramid trap is made up of two elements: a cover (1) andabase (2) with a center pitfall (3). (Online figure in color.)
The visual preferences of various developmental stages of a second insect, the small hive beetle and a pest of honey bee colonies, were also examined to improve trap design. Adult beetles locate and enter hives, and their offspring eat bee larvae and stored pollen. The evaluation of photospectal attraction revealed both wandering larvae searching for a pupation location outside the hive and host seeking adults were most attracted to the same 390-nm wavelength as the red flour beetle (Duehl et al. 2011). The addition of LEDs to an open pitfall trap for the larvae and to a hanging trap for the adults increased trap capture at least tenfold when tested in a dark shed (A. J. Duehl, unpublished data). When tested in outdoor bee yard settings, LED-augmented adult traps did not improve the capture of the target insect over control traps, but the same adult traps placed in a honey house did increase trap capture (A. J. Duehl, unpublished data). This suggests that light is attractive to adult small hive beetles when trying to move out of enclosed spaces, but not important when in naturally illuminated open areas. Larval traps were effective in the honey house, although optimal trap placement needs further investigation. These examples demonstrate the importance of insect developmental stage and progress through that stage in behavioral response. For trap development, early field testing is recommended to determine if the attractive cue evaluated in the lab can be effectively mimicked to improve capture in a more heterogeneous environment.
Bed Bug (Cimex lectularius) Sampling Techniques (John F. Anderson)
Semiochemicals may be used to attract bed bugs into traps as they leave their refuges to seek bloodmeals or after they completed blood-feeding and are seeking harborages away from the host (Weeks et al. 2010). Host-seeking bed bugs use a combination of heat and kairomones to locate hosts, and bed bugs aggregate within refuges during the day. Aggregation is likely mediated by pheromones and maintained by thigmotaxis.
The role of pheromones in mating behavior is unclear, but alarm and aggregation pheromones have been identified (Levinson and Bar Ilan 1971, Siljander et al. 2008a). Alarm pheromones are released when disturbed bed bugs discharge contents from their dorsal scent glands. This pheromone alerts others and results in dispersal from the site of discharge, but it has not been used to help detect bed bugs, although a laboratory study has demonstrated its possible usefulness in scattering bed bugs and enhancing effectiveness of insecticidal dusts (Benoit et al. 2009). The components of the aggregation pheromone have been identified, and a patent application has been filed that covers using a trap baited with the synthetic pheromone and infrared radiation, but data have not been published on effectiveness (Siljander et al. 2008b). Feldlaufer et al. (2010) reported two different 4-oxy-aldehydes that are present in the dorsal abdominal glands and suggested that the role of these compounds in the behavior of bed bugs needs to be determined.
Carbon dioxide and heat are attractants for host-seeking bed bugs (Anderson et al. 2009, Wang et al. 2009). A trap combining heat, CO2, proprionic acid, butyric acid, valeric acid, octenol, and L-lactic acid was reported to be attractive in both laboratory and field studies. A trap using these baits, the NightWatch Bed Bug Monitor (BioSensory Inc., Putnam, CT) is available commercially. The CLIMBUP Insect Interceptor (Susan McKnight, Inc., Memphis, TN) uses no attractant, but has been reported to be effective in detecting bed bugs in apartments and hallways (Wang et al. 2010). Additional traps, with and without heat or CO2 currently being advertised include 1) BB Alert active bed bug monitor (Midmos Solutions, Brierly Hills, West Midlands, United Kingdom); 2) BB Alert passive bed bug monitor (Midmos Solutions, Brierly Hills, West Midlands, United Kingdom); 3) Bedbug Beacon CO2 active monitor (Nuvenco LLC, Fort Collins, CO); 4) Silvatronic Bug Dome bed bug heat monitor (Nuvenco LLC, Fort Collins, CO); and 5) Eco-Keeper bed bug monitor and glue trap (Anteater Pest Control, Inc., Duluth, GA). The BB Alert passive bed bug monitor is designed as a harborage to be used by bed bugs. Live bed bugs, fecal stains, or cast skins are indications of an active infestation.
In the future, monitors baited with semiochemicals are likely to be used in combination with current detection methods, including nonbaited monitors, visual inspections (Pinto et al. 2007), and trained bed bug sniffing dogs (Pfiester et al. 2008).
Animal Baited Trapping (Alec Gerry)
Animal-baited trapping systems may be used to examine host associations of animal-biting insects and to determine the seasonal activity or geographic distribution of these insect species. For vector species, animal-baited trapping also is useful to measure parameters of pathogen transmission, including host feeding preference, pathogen infection prevalence, and host biting rate (bites/ host/ time). The biting rate is a product of vector abundance and host-seeking activity related to available breeding sites as well as to past and current environmental conditions specific to each sampling site. An increase in the biting rate would be expected to result in increased pathogen transmission to susceptible hosts, all other conditions being equal. Although studies demonstrating a direct association between biting rate and disease incidence are few, Gerry et al. (2001) showed that an increase in bluetongue virus infection in cattle was associated with an increase in the biting rate of the primary midge vector Culicoides sonorensis Wirth and Jones. Similarly, an increase in the human biting rate of Anopheles mosquitoes has been associated with an increase in the incidence of malaria (e.g., Rosenberg et al. 1990, Moreno et al. 2007). Monitoring changes in biting rate therefore should provide “early warning” of increased pathogen transmission or disease outbreaks.
Given the association of biting rate and pathogen transmission, any effort to model pathogen transmission risk or gauge effectiveness of control measures should include a measurement of vector biting rate. But, accurate measurement of biting rate can be difficult for hematophagous insects. Traps exposing and then enclosing host animals for defined periods may be used (e.g., Jones 1961, Muller and Murray 1977, Schmidtmann et al. 1980, Mullens and Gerry 1998) (Fig. 2), but they may not strictly discriminate between biting insects and those simply attracted to the vicinity of the host but that will not feed. An assessment of the engorgement rate of species captured in this way can provide additional information on host feeding. Using an enclosure trap placed over dairy calves in southern California, Mullens and Gerry (1998) showed that both C. sonorensis and Cx. quinquefasciatus were attracted to cattle, but only C. sonorensis fed on the restrained animals. The lack of feeding by Cx. quinquefasciatus indicated that cattle were not a suitable host for the mosquito population sampled. Biting rates also can be estimated through direct removal of biting insects from the animal using mechanical aspiration (Jones et al. 1977, Campbell and Kettle 1979, Gerry et al. 2008) (Fig. 3). Although this requires the presence and possible bias of a human collector in close proximity to the target host, engorged insects aspirated from the animal are very likely to have fed on that host (Muller and Murray 1977) and collections from different body locations can provide useful information on biting site selection.
Fig. 2.
Enclosure trap to capture host-seeking insects. The trap is open for an exposure period and then closed to allow feeding insects time to complete engorgement, after which insects are collected from the interior wall of the enclosure netting. (Online figure in color.)
Fig. 3.
Capture of blood-feeding insects by direct removal from the host using a mechanical aspirator. (Online figure in color.)
Use of bait animals for collecting biting insects is not without difficulties. Use of bait animals is labor intensive and there is risk of injury to the handlers as well as to the animal. Animals may need to be transported if suitable bait animals are not present at the time and place where sampling is desired, and there may be costs associated with procuring or caring for these animals. Finally, the need for an animal use protocol approved by an institutional oversight may be enough to keep even dedicated researchers from pursuing studies using bait animals.
For all of the reasons above, the relative activity of biting insects typically is measured using a trap that is baited artificially with host semiochemicals or with light of an appropriate wavelength. For pest species where host-seeking is limited to females, light traps also may be used to capture males; a particularly useful feature to identify members of a species complex where females are difficult to separate by morphology.
However, the relationship of insect capture by these traps to the true animal biting rate is usually purely speculative. An animal is likely to be far more attractive to host-seeking insects than a trap baited with light or with a limited number of host semiochemicals. Trap designs also may reduce capture of insects attracted to the vicinity by the artificial bait. For example, C. sonorensis were captured from cattle in numbers that were 3.7 × greater than their capture from suction traps baited with the bait-animal equivalent amount of C02 (Mullens and Gerry 1998), and Carpenter et al. (2008) showed that Culicoides chiopterus (Meigen 1830) was abundant on sheep in southern England, although relatively uncommon in nearby light traps. Furthermore, attraction to or capture with semiochemical or light baited traps may vary substantially between biting insect species, making comparisons of activity between biting insect species problematic (Gerry et al. 2009) (Table 1).
Table 1.
Total midges collected over eight trapping days by collection method
| Species captured | Sheepa | CO2 trapa | UV trap |
|---|---|---|---|
| Culicoides obsoletus | 313a | 2b | 16♀b |
| Culicoides scoticus | 4ab | 0b | 7♀a |
| Culicoides parroti | 387a | 38b | 10♂54♀b |
| Culicoides imicola | lb | 0b | 2♂8♀a |
| Culicoides circumscripta | 0b | lb | 91 ♂ 589♀a |
| Culicoides punctatusb | 4 | 0 | 3♀ |
| Culicoides flavipulicarisb | 0 | 0 | 2♂1♀ |
Only female midges were captured. Female midge captures within arow followed by the same letter are not significantly different by Kruskal-Wallis Nonparametric ANOVA Test with medians separated by Dunn’s Multiple Comparison Test.
Too few midges were collected for analysis. Data from Gerry et al. 2009
Semiochemical or light baited traps are further limited in that some biting insects may not readily be collected in them. Mullens and Dada (1992a,b) noted that bird-feeding, mostly cactiphilic Culicoides in a southern California desert mountain habitat were rare in CO2-baited (dry ice) traps, but were abundant at light or could be collected using birds as bait. Dry ice-baited suction traps release amounts of CO2 (500–1,500 ml/min) far in excess of that produced by birds. Perhaps the large amounts of released CO2 repel such species, or their trap approach behavior may prevent their capture. However, even some midge species known to feed on large mammals, such as those in the C. obsoletus complex, can be rare in CO2-baited suction traps, although being very common on a nearby mammalian host (Mullens et al. 2005, Carpenter et al. 2008, Gerry et al. 2009). Again, trap approach behavior may have prevented their capture in the suction traps, or it may be that supplemental cues such as other semiochemicals, body shape, or heat are important for host-seeking bythese midges.
The use of trap baited with semiochemicals or light may provide a poor estimate of biting rate, and reliance solely on these trap collections may lead to a poor understanding of pathogen transmission risk by vector species (see Gerry et al. 2009) that may even result in control efforts aimed at the wrong biting insect species. Thus, it is vital that some animal-based collecting also be done to interpret the epidemiological significance of the artificially-baited trap collections.
Although labor and cost considerations may necessitate the use of artificial lures over the use of animal hosts for research or surveillance studies, it is important to recognize the limitations associated with artificial lures. Some of these limitations may be mitigated through direct comparison of insect capture by using an animal host paired against the artificial lure; a significant correlation between the counts would allow for estimation of one from the other.
Animal-baited traps provide many important advantages over the use of artificial lures. Although artificial lures are appropriate for routine surveillance of biting insect activity, for most other purposes it would be wise to consider the use of bait animals to provide more meaningful data.
Analysis Techniques
After collecting the target arthropods, proper analysis techniques are necessary to extrapolate the “real” abundance and distribution of the target population from the trap capture data. Accurately interpreting the data are important if it is to be used to better understand the larger environmental processes. In this way, trap-capture data can be used to understand abiotic and biotic processes, to make predictive models, and to spatially target control measures. In a temporal sense, if action thresholds in population size are triggered, population control measures may be preemptive. Spatial, temporal analysis, or both of subterranean termite, stored-product pests, and mosquito population dynamics are discussed.
Estimating the Abundance and Boundary of Closed Populations From Mark-Recapture Data (Nan-Yao Su)
Reliable information on the abundance of arthropods, for both pest and beneficial species, is vital to their management. Literature regarding the use of mark-recapture methods to estimate population density is extensive, and all models essentially are based on the simplest Lincoln index initially advocated by Petersen (1896), i.e., N = M/P, where N: population size, M: number of marked and released animals, and P: capture probability. Because of the heterogeneity of the capture probability of marked individuals (P = m/n, where n is the total number of recaptured individuals, and m is the number of marked individuals in n), generally the Lincoln index does not provide accurate estimates of the size of target populations. Heterogeneity in capture probability remains an unsolved problem (Huggins and Yip 2001), and the common solution is to assign a conditional probability (Pij) for capturing an i-th individual at j-th sample (Seber 1992) so as to use one of many models to account for the source of heterogeneity. Choice of proper model, however, may be challenging because the precise source of heterogeneity is often unknown. Despite the shortcomings, the mark-recapture method remains the only practical option for estimating the abundance of many animal species, especially cryptic species such as subterranean termites.
One source of heterogeneity is the nonequilibrium state in the spatial distribution of marked animals after their release. Depending on the recapture timing and population size, capture probability is time- (t) and spatial- (r, θ) dependent, and can be described as the function of time and space, or P(r,θ,t); where r and θ are distance and angle from release point, respectively. The capture probabilities at distance r in all directions can be averaged (or “directional averaging”), to eliminate the angle variable, θ. This was first reported by Turchin and Odendaal (1996), who used the directional averaging technique to estimate the “effective sampling area” of pheromone traps for southern pine beetles. The rearranging process averaged out capture probabilities for all directions at distance r from release point, and transformed a heterogeneously-distributed capture probability into a symmetrical distribution, thus one cross section of the rearranged capture probability represents the probability density function. Because marked individuals continue to move into the population, given sufficient time, the rearranged capture probability may reach an equilibrium Pe, where Pe is a constant across the distance, and thus satisfy the equal mixing assumption of the mark-recapture protocol. The equilibrium capture probability (Pe) can be then applied in the Lincoln index to estimate the size of closed populations (Be-gonl979).
Another application of this equilibrium model is to estimate the boundary of closed populations. When the heterogeneously-distributed capture probability was transformed to a symmetrical distribution, the total volume of either distribution remains the same (i.e., the total capture probability of one, or 100% if the probability is expressed as percentage). Hence, the area beneath the probability density function, which is one cross section of the rearranged capture probability, is a constant (K), or.
where c is the x-intercept of the probability density function P(x). Before the capture probability reaches the equilibrium (Pe), P(x) has to intersect with the x-axis to define K. When the capture probability reaches Pe, however, P(x) no longer intersects the x-axis. Instead, the area beneath the function P(x) is defined by the distance between the release point and the population boundary (l). Thus,
Because K can be calculated using the pre-equilibrium data, and Pe is known, l can be calculated as l = K/Pe.
The equilibrium model was tested by using a 50-m extended laboratory foraging arena that was designed to simulate the distance factor of subterranean termite colonies (Su 2005). Over the 42-d test period, marked termites continued to redistribute in the arenas, resulting in four phases of probability density functions: exponential decline phase, linear decline phase, equilibrium phase, and postequilibrium phase. The equilibrium capture probability Pe, derived as the intercept of the linear regression during the equilibrium phase, correctly projected N estimates that were not significantly different from the known number of workers in the arena (Su and Lee 2008). In most cases, the model also correctly predicted the population boundary distance of 25 m, which is the distance between the release point and the end of the extended arena (Su and Lee 2008).
Advances in the Implementation and Interpretation of Stored-product Insect Monitoring Programs in Food Facilities (James Campbell)
Implementing and interpreting monitoring programs for stored-product insects in food facilities (e.g., mills, processing plants, warehouses, retail stores) have inherent problems similar to other indoor surveillance programs such as 1) The structural and spatial complexity of buildings creates hidden and difficult to monitor resource patches for pest populations; 2) pest populations are inherently spatially patchy and temporally dynamic; 3) pest populations which can be distributed over a spatial area larger than a given facility. In addition, food facilities present some unique sampling challenges such as 4) the unknown relationship between trap captures and economic injury, 5) low tolerance for insect activity, and 6) production schedules that limit flexibility in terms of scheduling major pest control interventions. Therefore surveillance plans must use highly attractive traps and carefully analyze the data to compensate for the trapping environment and limited control options.
Pheromone and kairomone baited traps are widely used to monitor pest populations because they provide quantitative information on pest trends and spatial distribution, however, they primarily capture individuals moving between resource patches rather than within a patch. Rapid trap capture increases may indicate increased risk of population spread, reduced treatment effectiveness (Campbell et al. 2010a), the need for alternative management options, or a combination of the three. However, high trap captures lead to increased management intensity and subsequent target insect mortality, thus making it difficult to determine how unmanaged population increases would relate to trap capture. One way to compensate for uncertainty in population abundance is the use of action thresholds and EILs based on the increase in mean trap captures from one monitoring period to the next. This approach is based on three simple assumptions: 1) the number of insects increases the risk of negative consequences and the cost of suppression tactics, 2) the rate of insect population increase is positively correlated with risk, and 3) increases in insect trap captures will reflect the overall population growth rate and possible expansion.
Long-term red flour beetle monitoring datasets generated from two flour mills were used to evaluate how different management tactics impacted the pattern of beetle capture in traps (Campbell et al. 2010a). In this analysis, the percent reduction in beetle captures after fumigation treatments was evaluated and a threshold value of 2.5 beetles per trap per 2-wk monitoring period was developed. This threshold is based on the median capture level when fumigations were performed, and is used to measure population rebound after these fumigation treatments. Comparing the average change in captures above and below this threshold revealed that the average change was not significantly different, however, for intervals with increases above this threshold, the magnitude of increase was significantly greater (Campbell et al. 2010b). An expanded monitoring dataset that included other wheat and rice mills (J. F. Campbell, unpublished data) has revealed that below this threshold the average change from one monitoring period to the next was −0.4 ± 0.1 beetles, essentially no change, whereas above this threshold the average change was 1.3 ± 0.7 beetles, a significant difference by using a Mann-Whitney Rank Sum Test. Furthermore, above the management threshold, significantly more beetles were recovered in product samples from a flour mill (J. F. Campbell, unpublished data).
Management thresholds enable food facility personnel and pest control professionals to develop management targets that can be used to evaluate program success. This approach is feasible with any combination of pest control tactics and provides feedback on the impact of each element of the program. This expanded analysis of flour mills is still in the early stages, and additional analyses using more facilities and expansion to include other pest species are needed. At this stage the results support this strategy as a useful management tool for risk adverse food facilities.
Modeling Seasonal Activity of Biting Flies (Tim J. Lysyk)
Data collected by routine monitoring programs often consists of serial estimates of abundance, denoted as yi, collected at various times i. Such time series may be of varying lengths depending on how long populations are active throughout the year and the frequency of abundance estimates. These series often are matched with meteorological and other environmental data to develop models with purposes ranging from providing simple descriptions of the timing of population events, identifying factors correlated with population growth, and forecasting future numbers, outbreaks, or pest damage. Many of these purposes can be accomplished simultaneously; however, it is important for investigators to clearly define the objectives of the analysis, identify appropriate transformations to the response variable of insect abundance, and select appropriate, biologically meaningful explanatory variables.
The response variables usually are a measure of insect numbers captured or seen during a time interval. Visual estimates, such as numbers landing or biting, provide useful measures of attack but are usually measured over relatively short time periods (minutes or multiples of) and are subject to environmental variation at the time of capture. Estimates based on traps left in the field for intervals of a day or longer may be more appropriate for population analyses as traps collected over longer periods of time may be less subject to short-term environmental fluctuations (Lysyk and Moon 1994). Abundance measured using traps usually is expressed as a measure of catch per unit length of the sample interval, such as numbers per trap night or numbers per trap week. However, although samples may be collected at relatively uniform intervals of calendar time, temperatures between intervals may vary over a season and a compelling argument can be made to standardize numbers in terms of capture per unit of physiological time such as degree-days lapsed during the interval (Beresford and Sutcliffe 2009). Before analyses, response variables can be further transformed using log(yi) to meet assumptions of least-squares analyses, or can even be reduced to a series of binary values indicating whether or not an abundance threshold was exceeded.
Explanatory variables usually consist of meteorological data collected on-site in the case of planned studies, or obtained from nearby weather stations. Raw variables often include daily observations of maximum and minimum temperature, precipitation, humidity, and so on. Trap captures from previous sampling occasions also can be incorporated. The variables can be summarized across the same time interval as the response variable is measured, and the two correlated. Meteorological conditions during the same period i, may be interpreted as having an effect on trap capture or insect activity during interval i (Lysyk 1993), whereas lagged variables may be interpreted as influencing longer-term population growth. Goulson et al. (2005) matched weekly fly counts for three species with meteorological variables lagged up to 4 wk to account for the duration of the insect’s life cycle. Taylor et al. (2007) and Lysyk (2007) used a similar approach for stable flies and Culicoides sonorensis. Although this approach has merit in identifying environmental correlates with population growth and can be used for forecasting, more refined procedures can be used to examine the influence these variables may have on population dynamics. Curriero et al. (2005) suggested that using the same interval length for both the response and lagged environmental variables might not be appropriate because the duration of exposure to an environmental variable may be important. These workers introduced the use of cross-correlation maps that can help identify both the beginning of the lag period, and the duration of the interval, and used these to describe the effects of weather on mosquito populations during the season (Shone et al. 2006) and before the season (Walsh et al. 2008). Because trap captures usually only measure the adult stage, consideration also should be given to the fact that adults at one time period are a function of the numbers present during the previous generation, not sampling interval, and that lagging weather variables by a fixed time period also ignores variation in developmental time caused by changing environmental conditions. Castro et al. (2008) indicated this in their analysis of horn fly populations, and demonstrated an elegant method for developing more biologically meaningful models.
Multiple regression, Poisson regression, and logistic regression have been used to analyze trap data. Multiple regression is the most common method. Both it and Poisson regression (used for count data) make quantitative predictions of numbers that will generally predict annual trends but may not capture the magnitude of population peaks (Ailes 1998). Predictions made using multiple regression may be imprecise (often because of collinearity) and unsuitable for management decisions (Lysyk 1990). Parameter sets also may be site, year specific, or both (Shaman et al. 2002). Logistic regression is a useful alternative, as rather than focusing on predicting the magnitude of the population, logistic models predict the probability of the population exceeding a threshold (Shaman et al. 2002) or reaching outbreak levels (Kokkinn et al. 2009, Williams et al. 2009). The choice of threshold is arbitrary and should reflect the aims of the study. Lysyk (2010) used a threshold of one to define the beginning and end of seasonal activity for five species of mosquito in southern Alberta. A similar approach can be used to define meteorological conditions necessary to declare vector-free periods when these are defined by levels of insect abundance such as for Culicoides in Europe (Carpenter et al. 2009).
There are some mathematical and statistical considerations when fitting models, including the questions of autocorrelation and collinearity. These can be dealt with using appropriate model-fitting techniques. Careful consideration should be given to the method used for model-building and selection. In many cases, some type of stepwise selection routine is employed so that large numbers of variables can be screened rapidly. Although this is advantageous, more recent approaches to model evaluation should be considered. Anderson et al. (2000) and Burnham and Anderson (2002) advocate the use of an information-theoretic approach that ranks sets of well-conceived, a priori models rather than relying on models developed by “data-dredging”. A concise outline of this approach is given in Anderson (2008) and could be adopted in future modeling endeavors.
Conclusions
Entomological sampling gathers actionable information for management planning and intervention strategies. Without understanding arthropod distributions, administrative actions at best waste time and resources and at worst harm individuals and the environment. Therefore, regardless of the surveillance purpose, a good plan will consider the method, trap technology, and analysis technique to ensure collected arthropods accurately represent the natural or perhaps wild population. The purpose for the monitoring program will determine the surveillance method and dictate the temporal frequency and spatial distribution of sampling. Sampling methods may require multiple types of traps that have different capture efficiencies for the various life history stages and species of interest. Detecting small populations or rare individuals will require traps that incorporate additional sensory cues associated with positive taxis to efficiently attract individuals based on their natural behavior and physiological needs. Ultimately, the plan’s analysis techniques must extrapolate the sampled area (a room, building, habitat, or landscape) to the larger environment to accurately inform and guide future actions of the surveillance managers based on population fluctuations or distribution changes. If one of the three components fails or is inefficient, managers will be unable to respond in an effective, targeted, and timely manner. Careful planning based on research and attention to the target organism’s biology will improve the sampling efficiency and sensitivity of entomological surveillance for arthropods of urban, medical, and veterinary importance.
References Cited
- Ailes MC. Failure to predict abundance of saltmarsh mosquitoes Aedes sollicitans and A. taeniorhynchus (Dip-tera: Culicidae) by using variables of tide and weather. J. Med. Entomol. 1998;35:200–204. doi: 10.1093/jmedent/35.3.200. [DOI] [PubMed] [Google Scholar]
- Allan SA. Chemical ecology of tick-vector interactions. In: Takken W, Knols GJ, editors. Olfaction of vector-host interactions. Wageningen, Netherlands: Wageningen Academic; 2010. pp. 327–348. [Google Scholar]
- Allan SA, Day JF, Edman JD. Visual ecology of biting flies. Annu. Rev. Entomol. 1987;32:297–316. doi: 10.1146/annurev.en.32.010187.001501. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Model based inference in the life sciences: a primer on evidence. New York: Springer; 2008. [Google Scholar]
- Anderson DR, Burnham KP, Thompsom WL. Null hypothesis testing: problems, prevalence, and alternative. J. Wildlife Manage. 2000;64:912–923. [Google Scholar]
- Anderson JF, Ferrandino FJ, McKnight S, Nolen J, Miller J. A carbon dioxide, heat and chemical lure trap for the bedbug, Cimex lectularius . Med. Vet. Entomol. 2009;23:99–105. doi: 10.1111/j.1365-2915.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- Barnard DR. Mechanisms of host-tick contact with special reference to Amhlyomma americanum (Acari: Ix-odidae) in beef cattle forage areas. J. Med. Entomol. 1991;28:557–564. doi: 10.1093/jmedent/28.5.557. [DOI] [PubMed] [Google Scholar]
- Barrera R. Simplified pupal surveys of Aedes aegypti (L.) for entomologic surveillance and dengue control. Am. J. Trop. Med. Hyg. 2009;81:100–107. [PubMed] [Google Scholar]
- Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implication for dengue control. Med. Vet. Entomol. 2008;22:62–69. doi: 10.1111/j.1365-2915.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Begon M. Investigating animal abundance: capture-recapture for biologists. Baltimore, MD: University Park Press; 1979. [Google Scholar]
- Benoit JB, Phillips SA, Croxall TJ, Christensen BS, Yoder JA, Denlinger DL. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius . J. Med. Entomol. 2009;46:572–579. doi: 10.1603/033.046.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford DV, Sutcliffe JF. Sampling designs of insect time series data: are they all irregularly spaced? Oikos. 2009;118:115–121. [Google Scholar]
- Bishop AL, Worrall R, Spohr LJ, McKenzie HJ, Barchia IM. Response of Culicoides spp. (Diptera: Ceratopogonidae) to light-emitting diodes. Aust. J. Entomol. 2004;43:184–188. [Google Scholar]
- Breteau H. La fièvre jaune en Afrique occidentale française: un aspect de la médicine préventive massive. B. World Health Org. 1954;11:453–481. [PMC free article] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Burkett DA, F.Butler J. Laboratory evaluation of colored light as an attractant for female Aedes aegypti, Aedes alhopictus, Anopheles quadrimaculatus, and Culex nigripalpus . Fla. Entomol. 2005;88:383–389. [Google Scholar]
- Burkett DA, Butler JF, Kline DL. Field evaluation of colored light-emitting diodes as attractants for woodland mosquitoes and other dipteral in north central Florida. J. Am. Mosq. Control Assoc. 1998;14:186–195. [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer; 2002. [Google Scholar]
- Campbell JF, Toews MD, Arthur FH, Arbogast RT. Long term monitoring of Tribolium castaneum populations in two flour mills: seasonal patterns and impact of fumigation. J. Econ. Entomol. 2010a;103:991–1001. doi: 10.1603/ec09347. [DOI] [PubMed] [Google Scholar]
- Campbell JF, Toews MD, Arthur FH, Arbo-gast RT. Long term monitoring of Tribolium castaneum populations in two flour mills: rebound after fumigation. J. Econ. Entomol. 2010b;103:1002–1011. doi: 10.1603/ec09348. [DOI] [PubMed] [Google Scholar]
- Campbell MM, Kettle DS. Abundance and temporal and spatial distribution of Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) on cattle in southeast Queensland. Aust. J. Zool. 1979;27:251–260. [Google Scholar]
- Carpenter S, Szmaragd C, Barber J, Labuschagne K, Gubbins S, Mellor P. An assessment of Culicoides surveillance techniques in northern Europe: have we underestimated a potential bluetongue vector? J. Appl. Ecol. 2008;45:1237–1245. [Google Scholar]
- Carpenter S, Wilson A, Mellor PS. Culicoides and the emergence of bluetongue virus in northern Europe. Trends Microbiol. 2009;17:172–178. doi: 10.1016/j.tim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Castro E, Gil A, Piaggio J, Chifflet L, Farias NA, Solari MA, Moon RD. Population dynamics of horn fly, Haematobia irritans irritans (L.) (Diptera: Mus-cidae), on Hereford cattle in Uruguay. Vet. Parasitol. 2008;151:286–299. doi: 10.1016/j.vetpar.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Clark GG, Seda H, Gubler DJ. Use of the “CDC backpack aspirator” for surveillance of Aedes ae-gypti in San Juan, Puerto Rico. J. Am. Mosq. Control Assoc. 1994;10:119–124. [PubMed] [Google Scholar]
- Cohnstaedt L, Gillen JI, Munstermann LE. Light-emitting diode technology improves insect trapping. J. Am. Mosq. Control Assoc. 2008;24:331–334. doi: 10.2987/5619.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner ME, Monroe WM. Stegomyia indices and their value in yellow fever control. Am. J. Trop. Med. 1923;4:9–19. [Google Scholar]
- Curriero FC, Shone SM, Glass GE. Cross correlation maps: a tool for visualizing and modeling time lagged associations. Vector Borne Zoonotic Dis. 2005;5:267–275. doi: 10.1089/vbz.2005.5.267. [DOI] [PubMed] [Google Scholar]
- Duehl AJ, Cohnstaedt LW, Arbogast RT, Teal PEA. Evaluating light attraction to increase trap efficiency for Tribolium castaneum (Coleoptera: Tenebri-onidae) J. Econ. Entomol. 2011;104:1430–1435. doi: 10.1603/ec10458. [DOI] [PubMed] [Google Scholar]
- Facchinelli L, Valerio L, Pombi M, Reiter P, Con-stantini A. Development of a novel sticky trap for container-breeding mosquitoes and evaluation of its sampling properties to monitor urban populations of Aedes albopictus . Med. Vet. Entomol. 2007;21:183–195. doi: 10.1111/j.1365-2915.2007.00680.x. [DOI] [PubMed] [Google Scholar]
- Falco RC, Fish D. A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp. Appl. Acarol. 1992;14:165–173. doi: 10.1007/BF01219108. [DOI] [PubMed] [Google Scholar]
- Fay RW, Eliason DA. A preferred oviposition site as a surveillance method for Aedes aegypti . Mosq. News. 1966;26:531–535. [Google Scholar]
- Fay RW, Prince WH. A modified visual trap for Aedes aegypti . Mosq. News. 1970;30:20–23. [Google Scholar]
- Feldlaufer MF, Domingue MJ, Chauhan KR, Aldrich JR. 4-oxo-aldehydes from the dorsal abdominal glands of the bed bug (Hemiptera: Cimicidae) J. Med. Entomol. 2010;47:140–143. doi: 10.1603/me09210. [DOI] [PubMed] [Google Scholar]
- Focks DA. A review of entomological sampling methods and indicators for dengue vectors. Special Program for Research and Training in Tropical Diseases (TDR), UNICEF, UNDP, World Bank, World Health Organization. 2003 http://www.who.int/tdr/publications/publications/pdf/dengue_vectors.pdf.
- Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am. J. Trop. Med. Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 2000;62:11–18. [PubMed] [Google Scholar]
- Geier M, Rose A, Grunewald J, Jones O. New mosquito traps improve the monitoring of disease vectors. Int. Pest Control. 2006;48:124–126. [Google Scholar]
- Gerry AC, Mullens BA, Maclachlan NJ, Mecham JO. Seasonal transmission of bluetongue virus by Culicoides sonorensis (Diptera: Ceratopogonidae) at a Southern California dairy and evaluation of vectorial capacity as a predictor of bluetongue virus transmission. J. Med. Entomol. 2001;38:197–209. doi: 10.1603/0022-2585-38.2.197. [DOI] [PubMed] [Google Scholar]
- Gerry AC, Nawaey TM, Sanghrajka PB, Wisniewska J, Hullinger P. Hematophagous Diptera collected from a horse and paired carbon dioxide-baited suction trap in southern California: relevance to West Nile virus epizootiology. J. Med. Entomol. 2008;45:115–124. doi: 10.1603/0022-2585(2008)45[115:hdcfah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gerry AC, Sarto I, Monteys V, Moreno Vidal J-O, Francino O, Mullens BA. Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in northeastern Spain in relation to midge capture using UV light and carbon dioxide baited traps. J. Med. Entomol. 2009;46:615–624. doi: 10.1603/033.046.0329. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Ewing CP. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini. and lone-star ticks, Amblyomma americanum (Acari: Ix-odidae) Exp. Appl. Acarol. 1989;7:313–322. doi: 10.1007/BF01197925. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Suarez MF. Sewers: the principal Aedes aegypti breeding sites in Cali, Colombia. Am. J. Trop. Med. Hyg. 1995;53:160. [Google Scholar]
- Goulson D, Derwent LC, Hanley ME, Dunn DW, Abolins SR. Predicting calyptrate fly populations from the weather, and probable consequences of climate change. J. Appl. Ecol. 2005;42:795–804. [Google Scholar]
- Gray JS. A carbon dioxide trap for prolonged sampling of Ixodes ricinus L. populations. Exp. Appl. Acarol. 1985;1:35–44. doi: 10.1007/BF01262198. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus . J. Am. Mosq. Control Assoc. 1988;4:1–40. [PubMed] [Google Scholar]
- Holscher KH, Gearhart HL, Barker RW. Electrophysiological responses of three tick species to carbon dioxide in the laboratory and field. Ann. Entomol. Soc. Am. 1980;73:288–292. [Google Scholar]
- Huffaker CB, Back RC. A study of methods of sampling mosquito populations. J. Econ. Entomol. 1943;36:561–569. [Google Scholar]
- Huggins RM, Yip PSF. A note on nonpara-metric inference for capture-recapture experiments with heterogeneous capture probabilities. Stat. Sin. 2001;11:843–853. [Google Scholar]
- Jackowska M, Bao R, Liu Z, McDonald EC, Cook TA, Friedrich M. Genomic and gene regulatory signatures of cryptozoic adaptation: loss of blue sensitive photoreceptors through expansion of long wavelength-opsin expression in the red flour beetle Tribolium castaneum . Front. Zool. 2007;4:24. doi: 10.1186/1742-9994-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RH. Some observations on biting flies attacking sheep. Mosq. News. 1961;21:113–115. [Google Scholar]
- Jones RH, Hayes RO, Potter HW, Jr, Francy DB. A survey of biting flies attacking equines in three states of the southwestern United States, 1972. J. Med. Entomol. 1977;14:441–447. doi: 10.1093/jmedent/14.4.441. [DOI] [PubMed] [Google Scholar]
- Karandinos MG. Optimum sample size and comments on some published formulae. Bull. Entomol. Soc. Am. 1976;22:417–421. [Google Scholar]
- Kay BH, Ryan PA, Russell BM, Holt JS, Lyons SA, Foley PN. The importance of subterranean mosquito habitat to arbovirus vector control strategies in North Queensland, Australia. J. Med. Entomol. 2000;37:846–853. doi: 10.1603/0022-2585-37.6.846. [DOI] [PubMed] [Google Scholar]
- Kinzer DR, Presley SM, Hair JA. Comparative efficacy of flagging and carbon dioxide-baited sticky traps for collecting the lone star ticks, Amblyomma americanum (Acarina:Ixodidae) J. Med. Entomol. 1990;27:750–755. doi: 10.1093/jmedent/27.5.750. [DOI] [PubMed] [Google Scholar]
- Kokkinn MJ, Duval DJ, R Williams C. Modelling the ecology of the coastal mosquitoes Aedes vigilax and Aedes camptorhynchus at Port Pirie, South Australia. Med. Vet. Entomol. 2009;23:85–91. doi: 10.1111/j.1365-2915.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Land MF. Visual acuity in insects. Annu. Rev. Entomol. 1997;42:147–177. doi: 10.1146/annurev.ento.42.1.147. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. The biology of blood sucking insects. 2nd ed. Cambridge, United Kingdom: Cambridge University Press; 2005. [Google Scholar]
- Levinson HZ, Bar Ran AR. Assembling and alerting scents produced by the bedbug Cimex lectularius L. Experientia. 1971;27:102–103. doi: 10.1007/BF02137766. [DOI] [PubMed] [Google Scholar]
- Lysyk TJ. Relationships between spruce budworm (Lepidoptera: Tortricidae) egg mass density and resultant defoliation of balsam fir and white spruce. Can. Entomol. 1990;122:253–262. [Google Scholar]
- Lysyk TJ. Seasonal abundance of stable flies and house flies (Diptera: Muscidae) in dairies in Alberta, Canada. J. Med. Entomol. 1993;30:888–895. doi: 10.1093/jmedent/30.5.888. [DOI] [PubMed] [Google Scholar]
- Lysyk TJ. Seasonal abundance, parity, and survival of adult Culicoides sonorensis (Diptera: Ceratopogonidae) in southern Alberta, Canada. J. Med. Entomol. 2007;44:959–969. doi: 10.1603/0022-2585(2007)44[959:sapaso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lysyk TJ. Species abundance and seasonal activity of mosquitoes at cattle facilities in southern Alberta, Canada. J. Med. Entomol. 2010;47:32–42. doi: 10.1603/033.047.0105. [DOI] [PubMed] [Google Scholar]
- Lysyk TJ, Axtell RC. Field evaluation of three methods for monitoring populations of house flies (Musca domestica) (Diptera: Muscidae) and other filth flies in three types of poultry housing systems. J. Econ. Entomol. 1986;79:144–151. doi: 10.1093/jee/79.1.144. [DOI] [PubMed] [Google Scholar]
- Lysyk TJ, Moon RD. Sampling arthropods in livestock management systems. In: Pedigo L, Buntin D, editors. Handbook of sampling methods for arthropod pests in agriculture. Boca Raton, FL: CRC; 1994. pp. 515–538. [Google Scholar]
- Maranga RO, Hassanali A, Kaaya GP, Mueke JM. Attraction of Amblyomma variegatum (ticks) to the attraction-aggregation-attachment-pheromone with or without carbon dioxide. Exp. Appl. Acarol. 2003;29:121–130. doi: 10.1023/a:1024265529030. [DOI] [PubMed] [Google Scholar]
- Mogi M, Choochote W, Khamboonruang C, Suwan-panit P. Applicability of presence - absence and sequential sampling for ovitrap surveillance of Aedes (Diptera: Culicidae) in Chiang Mai, northern Thailand. J. Med. Entomol. 1990;27:509–514. doi: 10.1093/jmedent/27.4.509. [DOI] [PubMed] [Google Scholar]
- Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behavior and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med. Vet. Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Mullens BA, Dada CE. Spatial and seasonal distribution of potential vectors of hemorrhagic disease viruses to peninsular bighorn sheep in the Santa Rosa mountains of southern California. J. Wildlife Dis. 1992a;28:192–205. doi: 10.7589/0090-3558-28.2.192. [DOI] [PubMed] [Google Scholar]
- Mullens BA, Dada CE. Insects feeding on desert bighorn sheep, domestic rabbits, and Japanese quail in the Santa Rosa mountains of southern California. J. Wildlife Dis. 1992b;28:476–480. doi: 10.7589/0090-3558-28.3.476. [DOI] [PubMed] [Google Scholar]
- Mullens BA, Gerry AC. Comparison of bait cattle and carbon dioxide baited suction traps for collecting Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and Culex quinquefasciatus (Diptera: Culicidae) J. Med. Entomol. 1998;35:245–250. doi: 10.1093/jmedent/35.3.245. [DOI] [PubMed] [Google Scholar]
- Mullens BA, Owen JP, Heft DE, Sobeck RV. Culicoides and other biting flies on the Palos Verdes Peninsula of southern California, and their possible relationship to equine dermatitis. J. Am. Mosq. Control Assoc. 2005;21:90–95. doi: 10.2987/8756-971X(2005)21[90:CAOBFO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Murray MD. Blood-sucking flies feeding on sheep in eastern Australia. Aust. J. Zool. 1977;25:75–85. [Google Scholar]
- Muturi EJ, Mwangangi J, Shililu J, Muriu S, Jacob B, Mbogo CM, John G, Novak R. Evaluation of four sampling techniques for surveillance of Culex quinquefasciatus (Diptera: Culicidae) and other mosquitoes in African rice agroecosystems. J. Med. Entomol. 2007;44:503–508. doi: 10.1603/0022-2585(2007)44[503:eofstf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Obenauer PJ, Kaufman PE, Kline DL, Allan SA. Detection of and monitoring for Aedes albopictus (Diptera: Culicidae) in suburban and sylvatic habitats in north central Florida using four sampling techniques. Environ. Entomol. 2010;39:1608–1616. doi: 10.1603/EN09322. [DOI] [PubMed] [Google Scholar]
- Petersen CGJ. The yearly immigration of young plaice into Limfjord from the German Sea. Report Danish Biol. Stn. 1896;6:1–48. [Google Scholar]
- Petry WK, Fore SA, Fielden LJ, Kim J. A quantitative comparison of two sample methods for collecting Amblyomma americanum and Dermacentor variabilis (Acari: Ixodidae) in Missouri. Exp. Appl. Acarol. 2010;52:427–438. doi: 10.1007/s10493-010-9373-9. [DOI] [PubMed] [Google Scholar]
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J. Econ. Entomol. 2008;101:1389–1396. doi: 10.1603/0022-0493(2008)101[1389:aobbct]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pinto LJ, Cooper RK, Kraft S. Bed bug handbook: the complete guide to bed bugs and their control. Mechanicsville, MD: Pinto & Associates, Inc; 2007. [Google Scholar]
- Reiter P, Gubler DJ. Surveillance and control of urban dengue vectors. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. New York: CAB International; 1997. pp. 425–462. [Google Scholar]
- Reiter P, Amador M, Colon N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J. Am. Mosq. Control Assoc. 1991;7:52–55. [PubMed] [Google Scholar]
- Ritchie SA, Long S, Hart A, Webb CE, Russell RC. An adulticidal sticky ovitrap for sampling container breeding mosquitoes J. Am. Mosq. Control Assoc. 2003;19:235–242. [PubMed] [Google Scholar]
- Rosenberg R, GAndre R, Somchit L. Highly efficient dry season transmission of malaria in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1990;84:22–28. doi: 10.1016/0035-9203(90)90367-n. [DOI] [PubMed] [Google Scholar]
- Schmidtmann ET, Jones JFCJ, Gollands B. Comparative host-seeking activity of Culicoides (Diptera: Ceratopogonidae) attracted to pastured livestock in central New York State, USA. J. Med. Entomol. 1980;17:221–231. [Google Scholar]
- Schulze TL, Jordan RA, Hung RW. Biases associated with several sampling methods used to estimate abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) J. Med. Entomol. 1997;34:615–623. doi: 10.1093/jmedent/34.6.615. [DOI] [PubMed] [Google Scholar]
- Seber GAF. A review of estimating animal abundance II. Int. Stat. Rev. 1992;60:129–166. [Google Scholar]
- Semeao AA, Campbell JF, Whitworth RJ, Sloderbeck PE. Response of Tribolium castaneum and Tribolium confusum adults to vertical black shapes and its potential to improve trap capture. J. Stored Prod. Res. 2011;47:88–94. [Google Scholar]
- Semter PJ, Hair JA. Evaluation of CO2-baited traps for survey of Amblyomma maculatum Koch and Dermacentor variabilis Say (Acarina: Ixodidae) J. Med. Entomol. 1975;12:1137–138. doi: 10.1093/jmedent/12.1.137. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquitoes (Culicidae) In: Lane RP, Crosskey RW, editors. Medical Insects and Arachnids. London, United Kingdom: Chapman & Hall; 1993. [Google Scholar]
- Shaman J, Stieglitz M, Stark C, Blancq SL, Cane M. Using a dynamic hydrology model to predict mosquito abundances in flood and swamp water. Emerg. Infect. Dis. 2002;8:6–13. doi: 10.3201/eid0801.010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard PM, Macdonald WW, Tonn RJ, Grab B. The dynamics of an adult population of Aedes ae-gypti in relation to dengue haemorrhagic fever in Bangkok. J. Anim. Ecol. 1969;38:661–702. [Google Scholar]
- Shone SM, Curriero FC, Lesser CR, Glass GE. Characterizing population dynamics of Aedes sol-licitans (Diptera: Culicidae) using meteorological data. J. Med. Entomol. 2006;43:393–402. doi: 10.1603/0022-2585(2006)043[0393:cpdoas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Siljander E, Gries R, Khaskin G, Gries G. Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius . J. Chem. Ecol. 2008a;34:708–718. doi: 10.1007/s10886-008-9446-y. [DOI] [PubMed] [Google Scholar]
- Siljander ED, Takacs S, Gries R, Gries G. US Patent: 7. Controlling bedbugs with synthetic pheromones and/or infrared radiation. 2008b;892:528. 2011.
- Solberg VG, Neidhart K, Sardelis MR, Hildebrandt C, Hoffmann FJ, R Boobar L. Quantitative evaluation of sampling methods for Ixodes dammini and Amhlyomma americanum (Acari: Ixodidae) J. Med. Entomol. 1992;29:451–456. doi: 10.1093/jmedent/29.3.451. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of ticks. Vol. 2. New York: Oxford University Press; 1993. [Google Scholar]
- Soper FL. The prospects for Aedes aegypti eradication in Asia in the light of its eradication in Brazil. B. World Health Org. 1967;36:645–647. [PMC free article] [PubMed] [Google Scholar]
- Southwood R, Henderson PA. Ecological methods. 3rd ed. Maiden, MA: Wiley-Blackwell; 2000. [Google Scholar]
- Su N-Y. Response of the Formosan subterranean termites (Isoptera: Rhinotermitidae) to baits or nonrepel-lent termiticides in extended foraging arenas. J. Econ. Entomol. 2005;98:2143–2152. doi: 10.1603/0022-0493-98.6.2143. [DOI] [PubMed] [Google Scholar]
- Su N-Y, Lee S-H. Estimating the population size and colony boundary of subterranean termites by using the density functions of directionally-averaged capture probability. J. Econ. Entomol. 2008;101:592–604. doi: 10.1603/0022-0493(2008)101[592:etpsac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sudia WD, Chamberlain RW. Battery-operated light trap, an improved model. Mosq. News. 1962;22:126–29. [Google Scholar]
- Taylor LR. Aggregation, variance and the mean. Nature. 1961;189:732–735. [Google Scholar]
- Taylor LR. Assessing and interpreting the spatial distributions of insect populations. Annu. Rev. Entomol. 1984;29:321–357. [Google Scholar]
- Taylor DB, Berkebile DR, Scholl PJ. Stable fly population dynamics in eastern Nebraska in relation to climatic variables. J. Med. Entomol. 2007;44:765–771. [PubMed] [Google Scholar]
- Taylor LR, Woiwod IP, Perry JN. The density-dependence of spatial behaviour and the rarity of randomness. J. Anim. Ecol. 1978;47:383–406. [Google Scholar]
- Turchin P, Odendaal FJ. Measuring the effective sampling area of a pheromone trap for monitoring population density of southern pine beetle (Coleoptera: Scolytidae) Environ. Entomol. 1996;25:582–588. [Google Scholar]
- Waladde SM, Rice MJ. The sensory basis of tick feeding behaviour. In: Obenchain FD, Galun R, editors. Physiology of ticks. New York: Pergamon; 1982. pp. 71–118. [Google Scholar]
- Walsh A, Glass G, Lesser C, Curriero F. Predicting seasonal abundance of mosquitoes based on offseason meteorological conditions. Environ. Ecol. Stat. 2008;15:279–291. [Google Scholar]
- Wang C, Gibb T, Bennett GW, McKnight S. Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J. Econ. Entomol. 2009;102:1580–1585. doi: 10.1603/029.102.0423. [DOI] [PubMed] [Google Scholar]
- Wang C, Saltzmann K, Chin E, Bennett GW, Gibb T. Characteristics of Cimex lectularius (Hemiptera: Cimicidae), infestation and dispersal in a high-rise apartment building. J. Econ. Entomol. 2010;103:172–177. doi: 10.1603/ec09230. [DOI] [PubMed] [Google Scholar]
- Weeks EN, Birkett MA, Cameron JA, Pickett MM, Logan JG. Semiochemicals of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae), and their potential for use in monitoring and control. Pest Manag. Sci. 2010;67:10–20. doi: 10.1002/ps.2024. [DOI] [PubMed] [Google Scholar]
- Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-sentinel compared with CDC backpack aspirators and CO2-baited EVS traps for collections of adult Aedes aegypti in Cairns, Queensland, Australia. J. Am. Mosq. Control Assoc. 2006;22:296–300. doi: 10.2987/8756-971X(2006)22[296:FEOTBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Williams CR, R Williams S, Nicholson J, Little SM, Riordan J, Fricker SR, Kokkinn MJ. Diversity and seasonal succession of coastal mosquitoes (Diptera: Culicidae) in the northern Adelaide region of South Australia. Aust. J. Entomol. 2009;48:107–112. [Google Scholar]
- Wilson JG, Kinzer DR, Sauer JH, Hair JA. Chemo-attraction in the lone star tick (Acarina: Ixodidae): 1. Response of different developmental stages to carbon dioxide administered via traps. J. Med. Entomol. 1972;9:245–252. doi: 10.1093/jmedent/9.3.245. [DOI] [PubMed] [Google Scholar]
- Wilson ML. Population ecology of tick vectors: interaction, measurement and analysis. In: Sonenshine DE, Mather TN, editors. Ecological dynamics of tick-borne zoonoses. New York: Oxford University Press; 1994. [Google Scholar]
- Wilton DP, Fay RW. Responses of adult Anopheles stephensi to light of various wavelengths. J. Med. Entomol. 1972;9:301–304. doi: 10.1093/jmedent/9.4.301. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. Multi country study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. Geneva, Switzerland: WHO; 2006. [Google Scholar]