Abstract
Importance
Hemispheric specialization of the human brain is a marker of successful neurodevelopment. Altered brain asymmetry that has been repeatedly reported in schizophrenia may represent consequences of disrupted neurodevelopment in the disorder. However, a complete picture of functional specialization in the schizophrenic brain and its connectional substrates are yet to be unveiled.
Objective
We aimed to quantify intrinsic hemispheric specialization at a cortical and subcortical level and to reveal potential disease effects in schizophrenia.
Design/Participants
Resting-state functional connectivity MRI has been previously used to quantitatively measure hemispheric specialization in healthy subjects, in a reliable manner. Here we quantified the intrinsic hemispheric specialization at the whole brain level in 31 patients with schizophrenia and 37 demographically matched healthy control subjects using resting-state functional connectivity MRI.
Results
The caudate nucleus, and cortical regions with connections to the caudate nucleus, showed markedly abnormal hemispheric specialization in schizophrenia. Compared to healthy controls, patients exhibited weaker specialization in the left, but the opposite pattern in the right, caudate nucleus. Schizophrenia patients also displayed a disruption of the inter-hemispheric coordination among the cortical regions with connections to the caudate nucleus. A linear classifier based on the specialization of the caudate nucleus distinguished patients from controls with a classification accuracy of 74%.
Conclusions and Relevance
These data suggested that hemispheric specialization could serve as a potential imaging biomarker of schizophrenia that, compared to task-based fMRI measures, is less prone to the confounding effects of variation in task compliance, cognitive ability, and command of language.
Introduction
Hemispheric specialization is a prominent feature of the human brain and appears to be a marker of successful neurodevelopment 1–3. It has been shown that higher order cognitive functions, such as memory and language, are lateralized 3 (i.e., subserved by one hemisphere more than the other) early on in neurodevelopment and that there are associated differences between the two hemispheres in anatomy, activity and connections 2, 4. Because hemispheric asymmetry may occur as early as in the second trimester of pregnancy 5, 6, abnormalities in hemispheric specialization may provide crucial evidence for neurodevelopmental mechanisms of disease and clues about their timing. Schizophrenia is suggested to be a disorder of neurodevelopment 7–9 and abnormal development of hemispheric specialization could play an important role in the pathophysiology of the illness 10–12. Over the past several decades, hemispheric specialization in schizophrenia has been investigated with increasingly powerful neuroanatomical, neurobehavioral and neurophysiological measures 13, 14.
Emerging from this work, convergent evidence indicates that cerebral asymmetry is altered in schizophrenia. The prevalence of non-right-handedness has been reported to be significantly higher in patients with schizophrenia than in healthy subjects 15, 16, which may indicate a failure to establish cerebral asymmetry (but also see 17). Diminished laterality in gray matter volume of the planum temporale has been reported in schizophrenia 18–23; however, negative findings (i.e., normal planum temporale laterality in schizophrenia) have also been reported 17, 20, 24, 25. Task-dependent functional magnetic resonance imaging (fMRI) studies have demonstrated, more consistently than structural studies, reduced left lateralization in schizophrenia 21, 26–28, as well as impairment of functions that are known to depend on right hemispheric specialization such as attention modulation 29. Moreover, reduced functional laterality in schizophrenia has been repeatedly shown to correlate with symptom severity 15, 21, 30. Importantly, impaired functional laterality has been found in schizophrenia patients experiencing their first episode of illness 28, 31 and in first-degree relatives of people with schizophrenia 32, suggesting that abnormal hemispheric specialization occurs early on (most likely during neural development) in people who go on to develop (or are at risk for) schizophrenia. Thus, a reliable and quantitative measure of intrinsic hemispheric specialization could represent a highly useful marker of neurodevelopmental injury in people at risk for, or affected by, the illness.
Here we apply a novel approach for the exploration of whole brain cerebral specialization based on resting-state functional connectivity MRI (rs-fcMRI) 33. The degree of specialization is quantified using the autonomy index (AI), defined as the difference between intra- and inter-hemispheric connectivity. Regions with preferential intra-hemispheric connectivity rather than inter-hemispheric connectivity are considered more specialized. This approach does not rely on comparing homologous regions of interest (ROIs) in two hemispheres thereby avoiding the potential confound of anatomical asymmetry. AI was recently evaluated in a large cohort of 1000 healthy subjects and was able to predict asymmetric brain activation during language processing 33. With this novel approach, we explored the connectional underpinnings of hemispheric specialization in schizophrenia.
Materials and Methods
Participants
31 patients with DSM-IV diagnosed schizophrenia and 37 control subjects completed the study. All patients were outpatients at the time of the scan. 23 patients were being treated with an anti-psychotic medication, but had been off medication for at least four weeks prior to the scan. All subjects were right-handed 34 and were native speakers of English. The healthy control subjects were screened using the Structured Clinical Interview for DSM-IV Axis I Disorders 35. Written informed consent was obtained from all subjects in accordance with the guidelines of the Partners Healthcare Institutional Review Board. The two groups were matched with respect to age, gender, pre-morbid verbal IQ, and parental socioeconomic status (see Table S1). Severity of schizophrenia symptoms was assessed using the Positive and Negative Syndrome Scale 36. Chlorpromazine equivalents were calculated based on Woods and colleagues 37.
MRI Data Acquisition and Preprocessing
Resting-state fMRI data were collected on a 3T Tim Trio scanner (Siemens Healthcare, Erlangen, Germany) using a 12-channel head coil. Functional images were acquired using an echo planar imaging (EPI) pulse sequence (TR = 5000 ms, TE = 30 ms, flip angle = 90°, 2 × 2 × 2 mm3 voxels, 76 time points per run). 13 healthy controls and 15 patients performed two resting state scans, all other subjects performed one resting state scan. Only resting state scans with an average slice SNR above 100 were included in the analyses. All but one scan (of a schizophrenia patient) passed this criterion. Structural MRI scans were acquired using a sagittal 3D MPRAGE sequence. Resting-state and structural fMRI data were processed according to procedures previously described 33, 38 (also see supplemental information).
Hemispheric Autonomy Index
We uniformly sub-sampled the cerebrum in the FreeSurfer nonlinear volumetric space (voxel size 8 × 8 × 8mm3). For each seed voxel, the degree of within- and cross-hemisphere connectivity were computed by summing up the number of voxels strongly correlated (r > 0.25) to the seed in the ipsi-lateral hemisphere, and in the contra-lateral hemisphere, respectively. We have tested different selections of correlation threshold and found the distribution of AI could be robustly estimated 33.
AI was calculated as the difference between the normalized within- and cross-hemisphere connectivity according to the following equation:
where Ni and Nc are the numbers of voxels significantly correlated to the seed in the ipsi- and contra-lateral hemisphere, respectively. Hi and Hc are the total number of voxels in the ipsi- and contra-lateral hemisphere, respectively. As the connectivity degree is normalized by the total number of vertices in each hemisphere AI is denoted as a percentage. For further details see supplemental information.
For each subject, AI values were averaged within important subcortical structures, including the caudate nucleus, the putamen, the thalamus, the amygdala, and the hippocampus. The anatomical masks for these subcortical structures were generated using FreeSurfer segmentations 39. AI values were also averaged within a cortical mask, which was generated by identifying the cerebral voxels strongly correlated (r > 0.25) to the caudate nucleus based on 1000 healthy subjects.
Group Effects
For any between-group comparisons reported in this paper, the overall threshold for significance is p< 0.05. In Figure 1, the AI maps of both the patient group and control group only show voxels with p<0.05 (F-test, FDR corrected). The uncorrected mean difference between these two groups is shown in Figure 1C. The group comparison based on the AI values averaged within each of the five subcortical ROIs (caudate nucleus, putamen, thalamus, amygdala, hippocampus) was performed using a nonparametric Wilcoxon rank sum test at p<0.05. To test whether hemispheric specialization is a sensitive marker that differentiates patients and controls, we trained a support vector machine (SVM) classifier based on the subcortical AI values (for a detailed explanation please refer to the supplemental information). As a control analysis, the same strategy was applied using traditional anatomical markers (volumes of subcortical structures), including hippocampal volume.
Figure 1. Hemispheric specialization in specific cortical regions is altered in schizophrenia.
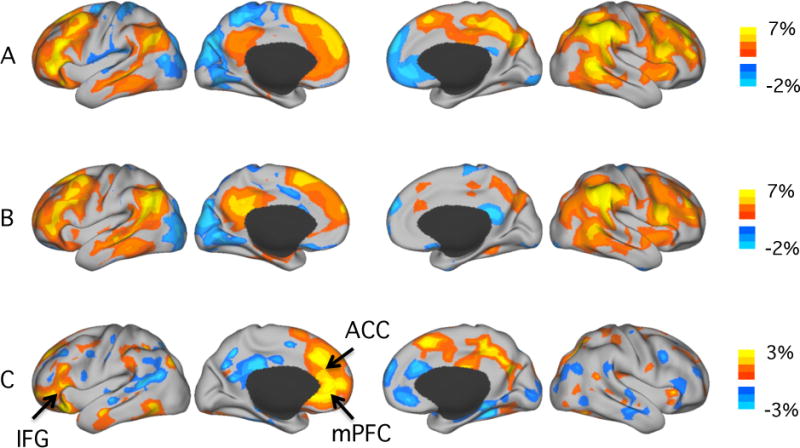
A. Hemispheric specialization in the healthy control group (n=37). Strong hemispheric specialization was observed in the association cortices including the lateral prefrontal, inferior parietal and temporal regions. Visual, somatosensory and motor cortices exhibited minimal autonomy. In the left hemisphere, strong specialization was observed in inferior prefrontal and temporal regions overlapping the default network and areas involved in language processing. In the right hemisphere, strong specialization was observed in the insula, angular gyrus and supramarginal gyrus that are engaged in attention control.
B. Hemispheric specialization in the patient group (n=31). The patterns of hemispheric specialization largely replicate between the patient and the control sample. However, subtle differences seem to emerge in the frontal and the midline regions.
C. Cortical regions showing patient/control difference in specialization. For each voxel, the mean AI value in the patient group was subtracted from the mean AI value in the control group. The maps show uncorrected mean difference between the two groups. Schizophrenia patients showed decreased AI values in the left anterior cingulated cortex (ACC), left medial prefrontal cortex (mPFC), left inferior frontal gyrus (IFG) and in right frontal and parietal midline regions. Increased AI values in schizophrenia patients could be detected in the right ACC and mPFC.
Relation to Developmental Cortical Expansion
A map of regional developmental cortical expansion between term born infants and young adults was published previously 40, 41 and made publicly available. The map of right hemispheric developmental cortical expansion and the map of hemispheric specialization in 1000 healthy subjects 33 were projected to the Conte69 164k_fs_LR mesh 42 (http://sumsdb.wustl.edu/sums/directory.do?id=8291494&dir_name=CONTE69). The data were extracted using the Caret Surface Statistics Toolbox 43 for correlation analysis.
Results
1. Hemispheric specialization of the cerebral cortex in schizophrenia
Hemispheric specialization of the cerebral cortex showed a characteristic spatial distribution in both the schizophrenic patients and healthy controls (Figure 1A & B). Strong hemispheric specialization was observed in the association cortices, including the lateral prefrontal, inferior parietal and temporal regions, whereas visual, somatosensory and motor cortices exhibited minimal specialization. Additionally, hemispheric specialization in the two hemispheres demonstrated different patterns, replicating previous findings based on 1000 healthy subjects 33. In the left hemisphere, strong specialization was observed in regions overlapping the default network and involved in language processing. In the right hemisphere, strong specialization was observed in regions engaged in attention control. This characteristic distribution was grossly preserved in the patient group (Figure 1B). However, when contrasting the patient group with the control group, several brain regions showed a moderate difference. Compared to schizophrenia patients, healthy controls showed stronger specialization in the left anterior cingulate cortex (ACC) and the left medial prefrontal cortex (mPFC). Additionally, the inferior frontal gyrus (likely Broca’s area) showed reduced specialization in schizophrenia patients compared to healthy controls, possibly related to prior evidence of altered language specialization in this illness 44–46. In contrast, schizophrenia patients showed stronger specialization in the right ACC and the right mPFC (Figure 1C).
2. Decreased left-hemispheric and increased right-hemispheric specialization of the caudate nucleus in schizophrenia
We then investigated the hemispheric specialization of subcortical structures. AI was quantified within the caudate nucleus, putamen, amygdala, hippocampus and thalamus. Hemispheric specialization of the right and left caudate nucleus and right thalamus was significantly altered in patients (Figure 2). While the patients showed significantly weaker specialization in the left caudate nucleus (p<0.005), specialization of the right caudate nucleus and right thalamus was significantly stronger (p<0.005) in the patient than in the control group. Accordingly, a significant group by hemisphere interaction of AI in the caudate nucleus (p<0.0001, univariate ANOVA in SPSS) and in the thalamus (p<0.02) was found.
Figure 2. Schizophrenia patients show decreased left-hemispheric and increased right-hemispheric specialization of the caudate nucleus.
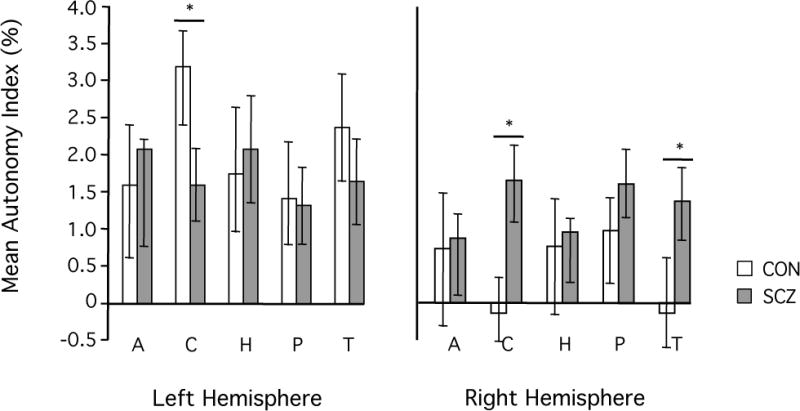
AI values were averaged within five subcortical regions, including two striatal structures (caudate nucleus and putamen), two limbic structures (hippocampus and amygdala), and the thalamus. Bars represent the mean AI value across subjects with the respective subcortical structure. Error bars indicate two standard errors. In the healthy control group (white bars) AI values were generally higher in the left than in the right hemisphere. In the patient group (gray bars) this overall pattern was generally less pronounced. Specifically, schizophrenia patients exhibited significantly decreased left- but increased right- hemispheric specialization of the caudate nucleus as compared to the healthy controls (p<0.005, respectively).
For any functional network with a strong inter-hemispheric interaction, a strong negative correlation between the AI values in the left and right hemisphere portion of the network would be expected (for a detailed explanation please refer to the supplemental information). However, if the sub-regions in two hemispheres become disconnected, the negative correlation will diminish. In the healthy control group, all five subcortical structures showed strong negative correlations between left- and right-hemisphere AI values (Figure 3, solid lines), suggesting that the functions of the subcortical structures in the two hemispheres are tightly integrated. Remarkably, schizophrenia patients failed to show this characteristic anti-correlation in the caudate nucleus and the putamen (p<0.05, respectively, whereat the significance of the difference between the anti-correlations of the two groups was established by interactive analysis of covariance using MATLAB (http://www.mathworks.com/help/stats/aoctool.html). However, the patients did show the normal pattern of anti-correlated AI values in the thalamus, amygdala and hippocampus (Figure 3, dotted lines). These results suggest that in schizophrenia, the network associated with the left striatum may be segregated from the network associated with the right striatum.
Figure 3. Schizophrenia patients show segregated left-hemispheric and right-hemispheric networks connected to the caudate nucleus and the putamen.
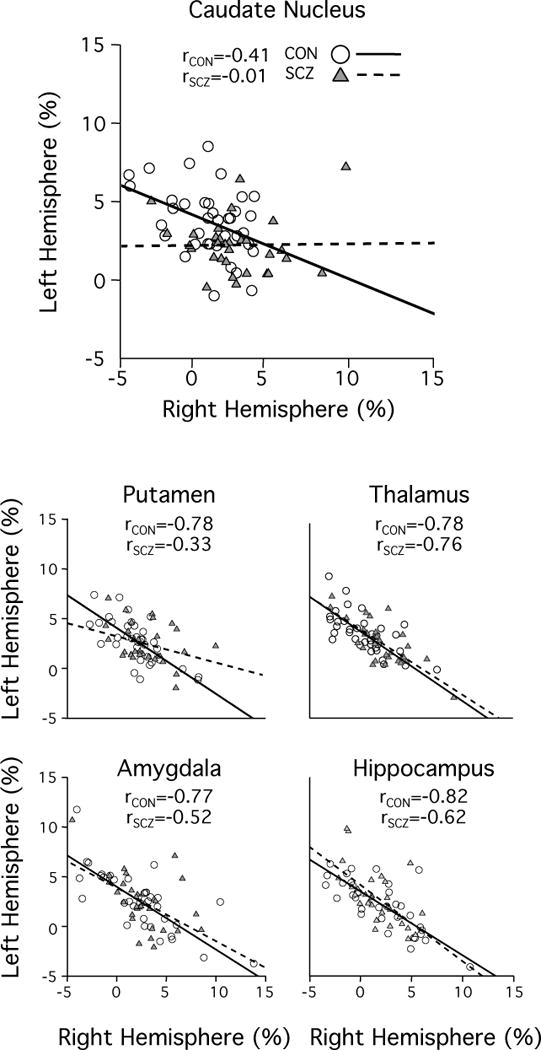
According to the mathematical definition of the autonomy index, for a specific network spanning across two hemispheres, a negative correlation between left and right hemispheric AI values should be expected. All subcortical structures of interest showed this strong negative correlation in the healthy control group (solid line, white circles). In schizophrenia patients, this characteristic anti-correlation was reduced in the caudate nucleus and the putamen as compared to their healthy controls (dotted line, gray triangles), indicating a disruption of interconnection and coordination between the two hemispheres.
We then investigated whether this loss of AI anti-correlation in the caudate nucleus is driven by specific sub-division(s) of the caudate nucleus. Choi and colleagues segmented the human caudate nucleus 47 according to its connection strength to different cortical networks 38 and found that ventromedial parts of the caudate nucleus are strongly connected to limbic cortical regions, while dorsal portions of the caudate nucleus are connected to association networks, specifically to the default network (DN) and to the frontoparietal network (FPN), as seen in the monkey 48. We quantified the specialization in these sub-divisions of the caudate nucleus. The absence of AI anti-correlation in schizophrenia patients was more prominent in the association relative to the limbic, portion of the caudate nucleus (Figure S1). The strongest group effect within the association portion of the caudate nucleus was in the segment showing connectivity to the DN, where schizophrenia patients showed a complete absence of AI anti-correlation, in comparison to the healthy controls (p<0.01). Group comparisons of the FPN- and limbic- connected segments of the caudate nucleus revealed no significant differences (p=0.20 and p=0.56, respectively). To control for potential outliers driving this differential AI anti-correlation of the caudate nucleus or subportions of the caudate nucleus we have performed a standardized analysis of outliers (for detailed methods and results see supplemental information).
3. Hemispheric specialization is altered in schizophrenia in the large-scale functional network connected to the caudate nucleus
Hemispheric specialization of the caudate nucleus demonstrated significant changes in schizophrenia, with patients showing decreased left- hemispheric but increased right- hemispheric specialization. Consequently, the difference between left-and-right caudate nucleus specialization was significantly decreased in patients as compared to the healthy controls (Figure 4A; p<0.0005).
Figure 4. Hemispheric specialization is altered in subcortical and cortical networks associated with the caudate nucleus.
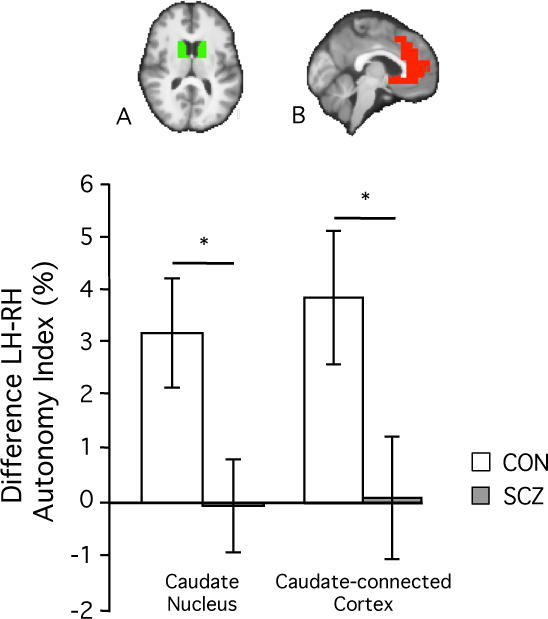
A. Due to the decreased left- and increased right-hemispheric caudate specialization, the difference between left and right AI values was significantly diminished in schizophrenia patients (gray bar) as compared to healthy controls (white bar) (p<0.0005).
B. This diminished difference between left- and right- hemispheric AI values could be replicated in caudate-connected cortical regions (p<0.01). The cortical mask was derived from a correlation analysis in 1000 healthy subjects, in which the caudate nucleus served as the seed region. Cortical AI values were averaged within the boundaries of this mask for each individual subject, and the individual mean values were averaged within each of the two groups.
Previous studies have indicated that the medial prefrontal regions that showed reduced AI values in schizophrenia (Figure 1C) are tightly connected to the caudate nucleus 47, 49. As a post-hoc analysis here we investigated if hemispheric specialization was similarly altered in the cortical regions connected to the caudate nucleus. The cortical regions functionally associated with the caudate nucleus included the ACC and mPFC (Figure 4B, top row), and therefore mainly fell within the default network (see Figure S2 for the cortical regions connected to the caudate nucleus and the boundaries of the 7-network cortical parcellation described in 38). Compared to healthy controls, patients showed significantly weaker left-right differences in specialization in these cortical regions (Figure 4B, bottom row, p<0.01), a pattern similar to that seen in the caudate nucleus (Figure 4A). Additionally, similar to the caudate nucleus findings, this difference in left-right cortical specialization was driven by both a decrease in left-sided specialization (p<0.01) and an increase in right-sided specialization (p<0.05) in the patient group (Figure S3). For a summary of group differences of hemispheric specialization of the caudate nucleus see Table S2.
4. Abnormal hemispheric specialization of the caudate nucleus may represent a specific marker of schizophrenia
Next we determined whether the specialization difference between the left and right caudate nucleus could accurately categorize the subjects into the patient and control groups. We trained a SVM classifier based on the AI values in the caudate nucleus and tested the classification accuracy. Using the difference between left and right hemispheric caudate nucleus AI values as the input, the classifier yielded an accuracy rate of 74% (with a sensitivity of 68% and a specificity of 78%). In contrast, classification accuracy based on volume estimates of several subcortical structures derived from Freesurfer parcellation 50 ranged from 43% to 63% (Table 1).
Table 1.
Classification accuracy of functional autonomy and anatomy imaging markers
| Imaging marker | Classification accuracy |
|---|---|
| Caudate Autonomy difference (LH-RH) | 74 % |
| LH Caudate volume | 57 % |
| RH Caudate volume | 52 % |
| Difference (LH-RH) Caudate volume | 43 % |
| LH Putamen volume | 57 % |
| RH Putamen volume | 57 % |
| Difference (LH-RH) Putamen volume | 48 % |
| LH Thalamus volume | 61 % |
| RH Thalamus volume | 59 % |
| Difference (LH-RH) Thalamus volume | 50 % |
| LH Amygdala volume | 61 % |
| RH Amygdala volume | 63 % |
| Difference (LH-RH) Amygdala volume | 52 % |
| LH Hippocampus volume | 63 % |
| RH Hippocampus volume | 61 % |
| Difference (LH-RH) Hippocampus volume | 51 % |
5. Association of hemispheric specialization and cortical expansion during neurodevelopment
To test if hemispheric specialization is associated with cortical expansion during neurodevelopment we compared the hemispheric specialization map derived from 1000 healthy subjects 33 to a map of regional developmental cortical expansion between term born infants and healthy young adults (Figure S4) provided by David van Essen and colleagues 40, 41 (http://sumsdb.wustl.edu/sums/directory.do?id=7601585). On a whole surface level developmental expansion and hemispheric specialization showed a moderate, yet significant correlation (Spearman rank correlation r=0.39, p<0.0001), indicating that the extent of hemispheric specialization is related to the developmental cortical expansion.
6. Examination of potential confounds
To investigate a potential anatomical confound, we compared intra-cranial volume (ICV) corrected caudate nucleus volumes of all subjects. No significant patient/control differences were found (p > 0.3 for the left and right caudate nucleus, respectively). We further explored whether duration of illness or antipsychotic medication dose confounded our findings. The AI values in the left/right caudate nucleus and their difference showed no correlation with the duration of illness or medication dose (p > 0.05 for each of these 6 correlations). Furthermore, we tested whether there were any differences in our outcomes between the unmedicated (n=8) and medicated (n=23) schizophrenia patients of our sample. The absence of anti-correlation between left and right caudate nucleus AI, and the weakened difference between them, was found in both the unmedicated and medicated patients (see Table S3). To ensure that our conclusions were not affected by variable data acquisition length across the subjects, we replicated the main results in 26 patients and 24 healthy controls who had a single resting state run (see Table S4). Lastly, we performed an additional correlation analysis that showed that there was no significant correlation between head motion (mean relative displacement in millimeters 51) and AI of the caudate nucleus (left caudate nucleus: r=-0.16, p=0.2, right caudate nucleus: r=0.17, p=0.15; but see recent discussions on the potential biological basis of head motion52). To provide a rough estimate of test-retest reliability of cortical and subcortical AI estimates we performed additional analyses (see Figure S5 and supplemental information), which resulted in a mean reliability of cortical AI values of around 0.87 and a mean reliability of subcortical AI values of around 0.75.
7. Correlation with clinical measures
Symptom severity of the patients was evaluated using clinical measures including PANSS total, PANSS negative symptoms, PANSS positive symptoms and trait anxiety. However, their associations with cortical and subcortical AI measures failed to reach statistical significance. One possible reason is that the patients involved in the present study were stable and compliant outpatients with little variability in symptom severity. Most schizophrenia patients had a PANSS total of around 50, which resembles a rather mild symptom severity 53 (mean=50.0, std=13.6).
Discussion
This study investigated hemispheric specialization in schizophrenia. First, we found that the physiologic left-over-right asymmetry of hemispheric specialization in the caudate nucleus and in caudate-connected prefrontal regions was reduced in schizophrenia patients. Second, schizophrenia patients also displayed disrupted coordination between the two hemispheres, as manifested by the weakened inverse correlation between left and right hemispheric specialization in the caudate nucleus. Finally, within the caudate nucleus, the subdivision with connections to association cortex showed the strongest differences between the two groups, while subdivisions connected to limbic regions did not show significant group effects.
Caudate nucleus and prefrontal function in schizophrenia
In the present study the strongest alterations in hemispheric specialization in schizophrenia were found in the caudate nucleus, as well as in the medial prefrontal regions connected to the caudate nucleus. These findings are generally consistent with prior findings of structural 20, 54, functional 55, 56 and post-mortem 57–59 abnormalities of the striatum in schizophrenia. It has been long noted that the striatum and the associated cortical circuits play a key role in many of the cognitive and affective processes that are disrupted in schizophrenia 59,60,61. Deficits in these functional domains have been linked to the classic symptoms of schizophrenia: cognitive impairment, negative symptoms and positive symptoms. Thus, altered function of fronto-striatal circuitry may represent a fundamental pathophysiological feature of schizophrenia. While previous studies have reported both decreased 62 and increased 63 fronto-striatal connectivity in schizophrenia, our results suggest that the changes in caudate nucleus and medial prefrontal connectivity may correspond to the alteration in hemispheric specialization in schizophrenia. This very specific alteration is the result of both increased cross-hemispheric connectivity and reduced within-hemispheric connectivity, rather than an absolute change in magnitude. One structural correlate for the reduced left-sided within-hemispheric connectivity found in this study might be the decreased integrity of the frontal and temporal white matter, affecting important intra-hemispheric connections like the cingulate bundle and the inferior longitudinal fasciculus 64. Although our findings in the caudate nucleus depend on the precision of anatomical parcellation, a prior study on the reliability of subcortical segmentations has found caudate nucleus results to be most reliable, followed by the hippocampus, the putamen, the thalamus, and the amygdala 65.
Relating alterations in hemispheric specialization to dopamine asymmetry in schizophrenia
Our study revealed that the left caudate nucleus is more specialized than the right caudate nucleus in healthy controls, but this asymmetry was significantly diminished in patients. In healthy individuals presynaptic dopamine levels, dopamine transporters, and D2 receptors density show a right over left lateralization 66. Previous studies have indicated a loss of this physiologic asymmetry of presynaptic striatal dopamine 67, striatal dopamine transporters 68, 69 and striatal D2 receptor density 70–72 in schizophrenia. Our results suggest that the right-over-left asymmetry of striatal dopamine metabolism is accompanied by a left-over-right asymmetry of striatal hemispheric specialization in healthy individuals, and these asymmetries are significantly diminished in patients. The fact that dopaminergic drugs model fronto-striatal connectivity 73 further supports the assumption that altered specialization of the caudate nucleus and connected frontal regions might relate to changes in dopamine signaling.
Abnormalities of the associative striatum in schizophrenia
We found that changes in hemispheric specialization of the caudate nucleus in schizophrenia are mainly limited to alterations in the associative subdivision of the caudate nucleus, whereas the limbic subdivision does not show a significant effect. This finding is in line with the results of several previous molecular imaging studies. Woodward and colleagues found that a correlation between schizotypal traits in healthy individuals and striatal dopamine release was particularly strong in the associative subdivision of the striatum 74. Similarly, among individuals at ultra-high risk for psychosis, those who transitioned to psychosis later on had a significantly higher level of striatal dopamine synthesis than those who did not, again with the greatest effect size seen in the associative striatum 75. Furthermore, another study found that compared to healthy controls, schizophrenia patients showed greater D2 receptor availability increase in the associative striatum following dopamine depletion, whereas there were no between-group differences observed in the limbic and sensory-motor striatum 76. These findings of selective abnormalities of the associative striatum in schizophrenia converge with evidence (including the current data) for abnormalities in the cortical regions projecting to this segment of the striatum, such as the ACC 77, 78. Thus, a dorsomedial fronto-caudate associative network may be particularly affected in schizophrenia, accounting for the abnormalities seen in higher order cognitive and affective functions subserved by this network.
Neurodevelopment of hemispheric specialization and its relation to schizophrenia as a developmental disorder
Multiple lines of evidence suggest that schizophrenia is a neurodevelopmental disorder 7, 8, that might be characterized by impaired establishment of cerebral asymmetry 11. Here we investigated abnormalities in hemispheric specialization that may provide crucial evidence for a neurodevelopmental mechanism of the illness and clues about its timing. Asymmetry in brain structures become recognizable by 31 weeks of gestation 5. Differential gene expression levels between the left and right hemisphere can even be detected as early as at 12 weeks of gestation 6, suggesting that transcriptional asymmetry might set the stage for hemispheric specialization at very early stages of brain development. Successful development of hemispheric specialization requires asymmetric pruning of dispensable connections and maturation of functionally specialized connections. Thus, failure to establish hemispheric specialization may occur very early in development but only become clinically apparent much later, when highly specialized connectional hubs such as the prefrontal cortex would normally have reached their full functionality.
Alterations in hemispheric specialization as an imaging biomarker for the exploration of schizophrenia risk genes
Neuroimaging measures are increasingly used as “intermediate phenotypes” to investigate the impact of genetic variants on brain structure and function 79. As hemispheric specialization per se is a heritable trait 1, 2, 6, 80 that has been related to symptom severity in schizophrenia 27, 30, 46, hemispheric specialization of the caudate nucleus might serve as an imaging endophenotype for schizophrenia risk variants. The classification accuracy and specificity indicate that caudate AI alone is already a strong discriminative feature that might be a valuable contributor to a multivariate classifier or prediction model in the future. Hemispheric specialization of medial prefrontal regions connected to the associative striatum might also be a meaningful endophenotype, especially as functional network efficiency of the medial frontal cortex is known to be highly heritable 81, 82. As this measurement does not depend on the performance of a cognitive task, it is less prone, compared to task-based fMRI measures, to the confounding effects of variation in task compliance, cognitive ability, and command of language. Thus, if abnormal caudate nucleus specialization is present prior to the onset of the illness and linked to genetic variants associated with schizophrenia risk, this phenotype may prove useful for detecting the illness early and understanding how risk genes contribute to neurodevelopment changes that lead to the emergence of symptoms. Although the AI is a meaningful measure of hemispheric specialization that captures biologically plausible associations with task-based language lateralization 33, evolution 33, and neurodevelopment, its association with symptom severity in schizophrenia remains to be investigated.
Supplementary Material
Acknowledgments
This work was supported by NIH grant 1K25NS069805 (H.L.), NARSAD Young Investigator Grant (H.L.), German Research Foundation grant MU 3222/2-1(S.M.), NIH grant K23MH076054 (D.H.), and NIH grant R01MH095904 (D.H.).
The authors thank Randy Buckner, PhD (Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, MGH, Charlestown, MA and Department of Psychology and Center for Brain Science, Harvard University, Cambridge, Massachusetts) for discussions and comments on the manuscript.
Hesheng Liu and Sophia Mueller had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Sophia Mueller and Hesheng Liu (Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, MGH, Charlestown, MA) conducted the analyses.
Footnotes
The Authors declare no conflict of interests.
References
- 1.Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003 Jan;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 3.Gazzaniga MS. Cerebral specialization and interhemispheric communication. Brain. 2000 Jul 1;123(7):1293–1326. doi: 10.1093/brain/123.7.1293. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977 Jun;34(6):346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- 6.Sun T, Patoine C, Abu-Khalil A, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005 Jun 17;308(5729):1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987 Jul;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 8.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000 Summer;12(3):501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 9.Ruby E, Polito S, McMahon K, Gorovitz M, Corcoran C, Malaspina D. Pathways Associating Childhood Trauma to the Neurobiology of Schizophrenia. Frontiers in Psychological and Behavioral Science. 2014;3(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- 10.Gruzelier J, Venables P. Bimodality and lateral asymmetry of skin conductance orienting activity in schizophrenics: replication and evidence of lateral asymmetry in patients with depression and disorders of personality. Biol Psychiatry. 1974 Feb;8(1):55–73. [PubMed] [Google Scholar]
- 11.Crow TJ, Ball J, Bloom SR, et al. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989 Dec;46(12):1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- 12.Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol. 1978 Apr;87(2):226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- 13.Gur RE. Is schizophrenia a lateralized brain disorder? Editor’s introduction. Schizophrenia Bulletin. 1999;25(1):7–9. doi: 10.1093/oxfordjournals.schbul.a033368. [DOI] [PubMed] [Google Scholar]
- 14.Oertel-Knochel V, Linden DE. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011 Oct;17(5):456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- 15.Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001 Apr;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 16.Satz P, Green MF. Atypical handedness in schizophrenia: some methodological and theoretical issues. Schizophr Bull. 1999;25(1):63–78. doi: 10.1093/oxfordjournals.schbul.a033367. [DOI] [PubMed] [Google Scholar]
- 17.Deep-Soboslay A, Hyde TM, Callicott JP, et al. Handedness, heritability, neurocognition and brain asymmetry in schizophrenia. Brain. 2010 Oct;133(10):3113–3122. doi: 10.1093/brain/awq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki Y, Suzuki M, Takahashi T, et al. Anomalous cerebral asymmetry in patients with schizophrenia demonstrated by voxel-based morphometry. Biol Psychiatry. 2008 Apr 15;63(8):793–800. doi: 10.1016/j.biopsych.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Kwon JS, McCarley RW, Hirayasu Y, et al. Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry. 1999 Feb;56(2):142–148. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- 20.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001 Apr 15;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oertel V, Knochel C, Rotarska-Jagiela A, et al. Reduced Laterality as a Trait Marker of Schizophrenia-Evidence from Structural and Functional Neuroimaging. Journal of Neuroscience. 2010 Feb 10;30(6):2289–2299. doi: 10.1523/JNEUROSCI.4575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003 Jan;160(1):156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petty RG, Barta PE, Pearlson GD, et al. Reversal of asymmetry of the planum temporale in schizophrenia. Am J Psychiatry. 1995 May;152(5):715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- 24.Kulynych JJ, Vladar K, Fantie BD, Jones DW, Weinberger DR. Normal asymmetry of the planum temporale in patients with schizophrenia. Three-dimensional cortical morphometry with MRI. Br J Psychiatry. 1995 Jun;166(6):742–749. doi: 10.1192/bjp.166.6.742. [DOI] [PubMed] [Google Scholar]
- 25.Barta PE, Pearlson GD, Brill LB, 2nd, et al. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry. 1997 May;154(5):661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- 26.Razafimandimby A, Maiza O, Herve PY, et al. Stability of functional language lateralization over time in schizophrenia patients. Schizophrenia Research. 2007 Aug;94(1–3):197–206. doi: 10.1016/j.schres.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Sommer IE, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001 Oct 1;52(1–2):57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 28.Bleich-Cohen M, Hendler T, Kotler M, Strous RD. Reduced language lateralization in first-episode schizophrenia: An fMRI index of functional asymmetry. Psychiatry Research-Neuroimaging. 2009 Feb 28;171(2):82–93. doi: 10.1016/j.pscychresns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Honey GD, Pomarol-Clotet E, Corlett PR, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005 Nov;128(Pt 11):2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artiges E, Martinot JL, Verdys M, et al. Altered hemispheric functional dominance during word generation in negative schizophrenia. Schizophr Bull. 2000;26(3):709–721. doi: 10.1093/oxfordjournals.schbul.a033488. [DOI] [PubMed] [Google Scholar]
- 31.van Veelen NM, Vink M, Ramsey NF, et al. Reduced language lateralization in first-episode medication-naive schizophrenia. Schizophr Res. 2011 Apr;127(1–3):195–201. doi: 10.1016/j.schres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Oertel-Knochel V, Knochel C, Matura S, Prvulovic D, Linden DE, van de Ven V. Reduced functional connectivity and asymmetry of the planum temporale in patients with schizophrenia and first-degree relatives. Schizophr Res. 2013 Jul;147(2–3):331–338. doi: 10.1016/j.schres.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Buckner RL, Liu H. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J Neurosci. 2014 Sep 10;34(37):12341–12352. doi: 10.1523/JNEUROSCI.0787-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: 1995. [Google Scholar]
- 36.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 37.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003 Jun;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 38.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011 Sep;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan 31;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 40.Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007 Oct 25;56(2):209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Van Essen DC, Glasser MF, Dierker DL, Harwell J. Cortical Parcellations of the Macaque Monkey Analyzed on Surface-Based Atlases. Cereb Cortex. 2011 Nov 2; doi: 10.1093/cercor/bhr290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diedrichsen J. Surface statistics using Caret. 2005;2005 [Google Scholar]
- 44.Bleich-Cohen M, Sharon H, Weizman R, Poyurovsky M, Faragian S, Hendler T. Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr Res. 2012 Feb;134(2–3):131–136. doi: 10.1016/j.schres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997 Aug;20(8):339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 46.Oertel V, Knochel C, Rotarska-Jagiela A, et al. Reduced laterality as a trait marker of schizophrenia–evidence from structural and functional neuroimaging. J Neurosci. 2010 Feb 10;30(6):2289–2299. doi: 10.1523/JNEUROSCI.4575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi EY, Yeo BT, Buckner RL. The Organization of the Human Striatum Estimated By Intrinsic Functional Connectivity. J Neurophysiol. 2012 Jul 25; doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000 Mar 15;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008 Dec;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 50.Buchsbaum MS, Friedman J, Buchsbaum BR, et al. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2006 Dec 1;60(11):1181–1187. doi: 10.1016/j.biopsych.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012 Jan 2;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng LL, Wang D, Fox MD, et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):6058–6062. doi: 10.1073/pnas.1317424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research. 2005;79(2):231–238. doi: 10.1016/j.schres.2005.04.008. 2014/07/23. [DOI] [PubMed] [Google Scholar]
- 54.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008 Aug;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010 Mar 11;65(5):585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehrlich S, Yendiki A, Greve DN, et al. Striatal function in relation to negative symptoms in schizophrenia. Psychol Med. 2011 Jul 7;:1–16. doi: 10.1017/S003329171100119X. [DOI] [PubMed] [Google Scholar]
- 57.Roberts RC, Conley R, Kung L, Peretti FJ, Chute DJ. Reduced striatal spine size in schizophrenia: a postmortem ultrastructural study. Neuroreport. 1996 Apr 26;7(6):1214–1218. doi: 10.1097/00001756-199604260-00024. [DOI] [PubMed] [Google Scholar]
- 58.Holt DJ, Hersh LB, Saper CB. Cholinergic innervation in the human striatum: a three-compartment model. Neuroscience. 1996 Sep;74(1):67–87. doi: 10.1016/0306-4522(96)00094-2. [DOI] [PubMed] [Google Scholar]
- 59.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010 Jan;35(1):258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006 Jan 15;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 61.Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005 May;30(5):984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- 62.Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010 Jul;36(4):713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salvador R, Sarro S, Gomar JJ, et al. Overall Brain Connectivity Maps Show Cortico-Subcortical Abnormalities in Schizophrenia. Human Brain Mapping. 2010 Dec;31(12):2003–2014. doi: 10.1002/hbm.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 65.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Sullivan EV. Combining atlas-based parcellation of regional brain data acquired across scanners at 1.5 T and 3.0 T field strengths. NeuroImage. 2012 Apr 2;60(2):940–951. doi: 10.1016/j.neuroimage.2012.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun. 1998 Aug;19(8):781–787. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995 Oct 28;346(8983):1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 68.Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophr Res. 2003 Dec 1;65(1):39–46. doi: 10.1016/s0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 69.Laakso A, Vilkman H, Alakare B, et al. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000 Feb;157(2):269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 70.Acton PD, Pilowsky LS, Costa DC, Ell PJ. Multivariate cluster analysis of dynamic iodine-123 iodobenzamide SPET dopamine D2 receptor images in schizophrenia. Eur J Nucl Med. 1997 Feb;24(2):111–118. doi: 10.1007/BF02439541. [DOI] [PubMed] [Google Scholar]
- 71.Farde L, Wiesel FA, Stone-Elander S, et al. D2 dopamine receptors in neuroleptic-naive schizophrenic patients. A positron emission tomography study with [11C]raclopride. Arch Gen Psychiatry. 1990 Mar;47(3):213–219. doi: 10.1001/archpsyc.1990.01810150013003. [DOI] [PubMed] [Google Scholar]
- 72.Pilowsky LS, Costa DC, Ell PJ, Verhoeff NP, Murray RM, Kerwin RW. D2 dopamine receptor binding in the basal ganglia of antipsychotic-free schizophrenic patients. An 123I-IBZM single photon emission computerised tomography study. Br J Psychiatry. 1994 Jan;164(1):16–26. doi: 10.1192/bjp.164.1.16. [DOI] [PubMed] [Google Scholar]
- 73.Honey GD, Suckling J, Zelaya F, et al. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003 Aug;126(Pt 8):1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodward ND, Cowan RL, Park S, et al. Correlation of Individual Differences in Schizotypal Personality Traits With Amphetamine-Induced Dopamine Release in Striatal and Extrastriatal Brain Regions. American Journal of Psychiatry. 2011 Apr;168(4):418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howes OD, Bose SK, Turkheimer F, et al. Dopamine Synthesis Capacity Before Onset of Psychosis: A Prospective [(18)F]-DOPA PET Imaging Study. American Journal of Psychiatry. 2011 Dec;168(12):1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010 Mar;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 77.Holt DJ, Cassidy BS, Andrews-Hanna JR, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011 Mar 1;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008 Apr;131(Pt 4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 79.Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Curr Opin Genet Dev. 2011 Jun;21(3):340–348. doi: 10.1016/j.gde.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006 Aug;7(8):655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 81.Fornito A, Bullmore ET. Connectomic intermediate phenotypes for psychiatric disorders. Front Psychiatry. 2012;3:32. doi: 10.3389/fpsyt.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fornito A, Zalesky A, Bassett DS, et al. Genetic influences on cost-efficient organization of human cortical functional networks. J Neurosci. 2011 Mar 2;31(9):3261–3270. doi: 10.1523/JNEUROSCI.4858-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


