Abstract
Purpose
Recent evidence suggests the urothelium functions as a sensory transducer of chemical, mechanical or thermal stimuli that signals to nerve terminals and other cells in the bladder wall. The cellular and molecular basis of neuro-urothelial communication is not easily studied in the intact bladder, which led us to establish a method of co-culturing dorsal root ganglion (DRG) sensory neurons and bladder urothelial cells.
Materials and Methods
Sensory neurons and urothelial cells obtained from DRG and bladders dissected from adult female Sprague-Dawley rats were isolated by enzyme treatment and mechanical dissociation. These were plated together, or separately, on collagen-coated substrate and cultured in keratinocyte medium for 48–72 h. Retrograde tracer labeling was performed to identify bladder afferents used for functional testing.
Results
Neurite growth and complexity in neurons co-cultured with urothelial cells was increased relative to neuronal monocultures. The growth-promoting effect of urothelial cells was reduced by the tyrosine kinase inhibitor, K252a, but upstream inhibition of NGF signaling with TrkA-Fc had no effect. Fura-2 calcium imaging of urothelial cells showed responses to ATP (100μM) and activation of TRPV4 (4alpha-PDD, 10μM), but not TRPV1 (capsaicin, 1μM), TRPV3 (farnesyl pyrophosphate, 1μM), or TRPA1 (mustard oil, 100μM). In contrast, co-cultured neurons were activated by all agonists except farnesyl pyrophosphate.
Conclusions
Co-culturing provides a new methodology for investigating neuro-urothelial interactions in animal models of urological conditions. Our results suggest that neuronal properties are not only maintained in the presence of urothelium but that neurite growth is potentiated by an NGF-independent mechanism.
Introduction
The specialized epithelial cells in the urothelium lining the lower urinary tract respond rapidly to environmental changes by altering their structure and chemistry, and releasing signaling molecules to influence nearby cells1,2. Neuroactive substances (ATP, nitric oxide) released by urothelial cells in response to stretch and chemical stimulation are suggested to communicate with sensory nerve terminals in normal bladder functions (e.g. activation of afferents during bladder filling) and pathological states (e.g. bladder hyperexcitability and pain)1–4.
Further progress in understanding the molecular basis of functional interactions of the urothelium and bladder innervation has been hindered by the complexity of both tissue types and the technical difficulty of directly measuring interactions in vivo. Many populations of sensory nerves innervate the bladder—expressing different transmitters, neuropeptides, and other markers—and urothelial cells are also heterogeneous2,3. Functional studies have shown that numerous channels and receptors are expressed by both neurons and urothelial cells, limiting the interpretation of experiments using conventional pharmacological or global gene deletion approaches.
Cultures containing two distinct cell types (“co-cultures”) provide a powerful way to examine the mechanisms of intercellular communication. This approach has been adopted for a number of neuronal targets, such as skin, tracheal epithelium, cardiac muscle and vascular smooth muscle5–8. To our knowledge, this method has not yet been applied to studies of the urothelium and its sensory nerve supply. We have previously refined a culture method to study the physiological, pharmacological and growth properties of isolated adult rat sensory neurons9,10. Methods for culturing isolated urothelial cells from adult rodents are also well established11. We sought to establish co-cultures of bladder sensory neurons and urothelial cells, isolating each cell type from adult rats, using two types of output measures, analyses of neuronal growth and live cell imaging to determine functional expression of key receptors and channels. The outcome of our study was establishment of a viable co-culture system and identification of a robust growth-promoting effect of urothelial cells on sensory neurons.
Materials and Methods
Animals
Female Sprague-Dawley rats (8–12wks; Animal Resources Centre, Perth, Australia) were used according to procedures approved by the institutional ethics committee and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (NHMRC, Australia). Prior to tissue removal, rats were anesthetised (sodium pentobarbitone, 80 mg/kg, i.p.) and decapitated. Estrus cycle was monitored but not controlled for as no effect was detected.
DRG cell culture
DRGs containing bladder-projecting sensory neurons12,13 were removed from spinal levels L6/S1, and neurons were isolated and cultured10. Briefly, ganglia collected in Tyrode’s solution (mmol/l: NaCl 130, NaHCO3, 20, KCl 3, CaCl2 4, MgCl2 1, HEPES 10, glucose 1 with antibiotic-antimycotic), were dissociated (collagenase Type I; Worthington, West Chester, PA / 0.25% trypsin, Invitrogen, Mulgrave, Australia, at 37°C for 1h), washed, and triturated prior to centrifugation (900 rpm, 10 min) in 15% bovine serum albumin (Sigma-Aldrich, Sydney, Australia). The pellet was resuspended in Neurobasal-A containing B27 supplement, glutamine and antibiotic-antimycotic (Invitrogen), and plated onto polyornithine (500 μg/ml; Sigma-Aldrich) /laminin (5 μg/ml; Invitrogen) coated glass coverslips. All cultures were incubated in 5% CO2 and used 48–72h after isolation.
Urothelial cell culture
Urothelial cell isolation and culture was based on a published protocol14. Dissected bladders with urothelium uppermost were pinned flat to a Sylgard® plate in Krebs solution (mmol/l: NaCl 6.90, KCl 0.37, MgSO4 0.27, NaH2PO4 0.17, NaHCO3 2.10, glucose 0.99, CaCl2 0.18, 5% CO2 in O2). After washing with minimum essential media (MEM) and incubating at 37°C for 3–4 h in MEM/dispase (0.25 mg/ml; Sigma-Aldrich), the urothelium was removed by gentle scraping with a scalpel blade, resuspended in 0.25% trypsin/EDTA solution (Invitrogen) and incubated at 37°C for 30 min, with gentle trituration every 10min. The cell suspension was then centrifuged (1500rpm, 15min) in a tube containing MEM/10% fetal bovine serum (Invitrogen). The pellet was then resuspended in keratinocyte medium (KSFM; Invitrogen), centrifuged, resuspended in KSFM and plated onto collagen-coated (250 μg collagen/ml 0.02N acetic acid; Sigma-Aldrich) glass coverslips.
Urothelial-neuronal co-cultures
Pilot studies (see Results) determined KSFM media was most suitable for co-culture experiments, most of which used the following protocol. Urothelial and DRG cells from the same animal were isolated using the above protocols for monocultures, but substituting KSFM for final resuspension. Urothelial cells were plated 45min after neurons on collagen-coated coverslips, and maintained for 16h (37°C) before washing in KSFM and incubated until use. Some cultures were treated with nerve growth factor (NGF; 10 ng/ml, Sigma), K252a (100nM, Biomol Research Laboratories, Plymouth Meeting, PA) or recombinant rat TrkA/Fc chimera (4 ng/ml, R&D Systems, Minneapolis, MN); these concentrations are effective in cultures of adult rodent neurons15. For some qualitative immunocytochemical studies, co-cultures were maintained for 72h (n=2), used neurons and urothelial cells from different rats (n=3), or grew urothelial cells 24h prior to adding neurons from a second rat (n=2).
Retrograde labelling of bladder afferent neurons
Bladder-projecting DRG neurons were labelled by injecting fluorescent tracer into the bladder 1–2 weeks prior to tissue removal16. DiI (2 mg/ml in DMSO) was chosen because the excitation/emission wavelengths (549/565 nm) are compatible with Fura-2 live cell calcium imaging. Under anesthesia (60mg/kg ketamine, 10mg/kg xylazine, i.p.), the bladder was exposed via an abdominal incision and DiI microinjected into four sites (total 10–20μl). The musculature and skin were sutured separately with silk and tissues removed for culture 1–2 weeks later.
Immunocytochemistry
Cultures were fixed (4% paraformaldehyde in 0.1M phosphate buffer) and processed for immunocytochemistry (Table 1) as described previously9. Digital analysis was performed on 8-bit monochrome images captured using an Olympus BX-51 fluorescence microscope. Neurite initiation was defined as neurons with ≥ 1 Tuj1+ neurites longer than the soma diameter. Total neurite length and neurite branch points were quantified using HCA-Vision software (CSIRO, North Ryde, Australia, http://www.hca-vision.com). Parameters measured in ≥30 neurons per culture, and cultures from ≥3 animals were used for statistical analysis.
Table 1.
Antibodies and labels used for visualization of cultured cells.
| Antibody or label | Purpose | Working dilution | Host species | Source |
|---|---|---|---|---|
| Anti-β-tubulin isotype III (Tuj1) | General neuronal marker | 1:200 | Mouse | Sigma-Aldrich, Sydney, Australia |
| Anti-calcitonin gene-related peptide (CGRP) | Marker of peptidergic nociceptor sensory neurons | 1:2000 | Goat | Biogenesis, Poole, UK |
| Cytokeratin 17 (CK17) | Marker of basal urothelial cells | 1:1000 | Mouse | DakoCytomation, Campbellfield, Australia |
| Cytokeratin 20 (CK20) | Marker of umbrella cells in urothelium | 1:100 | Mouse | DakoCytomation |
| Cy2-conjugated anti-mouse IgG | Visualisation of cells binding anti-Tuj1 and -CK antibodies | 1:400 | Donkey | Jackson Immunoresearch Laboratories, West Grove, PA |
| Cy3-conjugated anti-goat IgG | Visualisation of cells binding anti-CGRP antibody | 1:2000 | Donkey | Jackson Immunoresearch Laboratories |
| Alexa488-phalloidin | Labels filamentous actin, showing all cell types | 1:40 | N/A | Invitrogen, Mulgrave, Australia |
| 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) | Labels nuclei of all cell types | 1 μg/ml | N/A | Sigma-Aldrich |
Calcium imaging of cultured cells
Cells were loaded with 5μM Fura-2 (fura-2-acetoxymethyl ester; Anaspec, Fremont, CA) in 0.02% pluronic (Invitrogen) at RT for 45 min in culture medium17. Intracellular calcium concentration ([Ca2+]in) was measured as the ratio of fluorescence emitted by alternating 340/380nm excitation. Isolated neurons and groups of 5–10 urothelial cells were imaged on coverslips in a superfusion chamber using an Olympus IX-71 microscope, TILL Polychrome V monochromator (Rochester, NY) and an Andor iXonEM+ camera (Belfast, Ireland). Cells were constantly superfused at RT with (mmol/l): NaCl 140, KCl 4, HEPES 10, MgCl2 2, CaCl2 2, glucose 10, pH 7.4, and pharmacological agents applied with a Perfusion Fast Step SF77B system (Warner, Hamden, CT). Fura-2 ratios were calculated using Andor IQ 1.10.2 software. Farnesyl pyrophosphate, phorbol 12,13-didecanoate and icilin were purchased from Enzo (Plymouth Meeting, PA) and all other agents from Sigma-Aldrich. Pharmacological agents were tested on neurons in which depolarisation by high K+ (30mM) increased [Ca2+]in by greater than 20% over baseline17.
Statistical analysis and figure production
SPSS v20 or GraphPad Prism 6.0b were used for statistical analysis. Planned Holm-Sidak multiple comparisons were used for neurite initiation data. Two-way mixed-model ANOVA was used for separate comparisons of total neurite length and neurite branch points — factors being culture type (two levels: neuronal culture, neuron/urothelial co-culture) and neuron class (two levels: CGRP-positive and -negative). All factorial combinations were randomly assigned to one or more culture plates obtained from one rat, which were analysed as within-subject or matched data using repeated-measurements ANOVA.
Results
1. Structural features of DRG and urothelium monocultures
The growth pattern of L6-S1 DRG neurons cultured on laminin-polyornithine was similar to previous studies of adult rat DRG cultures9. Neurons grown without neurotrophic factor showed diverse morphologies after 48h — most having grown one or more neurites, with morphologies ranging from highly complex branched neurites to a simple structure with few branches (Fig. 1a). Glial cells were found closely associated with the soma and primary neurites of most neurons, or in small clumps distant from neurons (Fig. 1b). NGF (10 ng/ml, 48h) stimulated exuberant outgrowth of neurites from many neurons (Fig. 1c,d).
Figure 1.
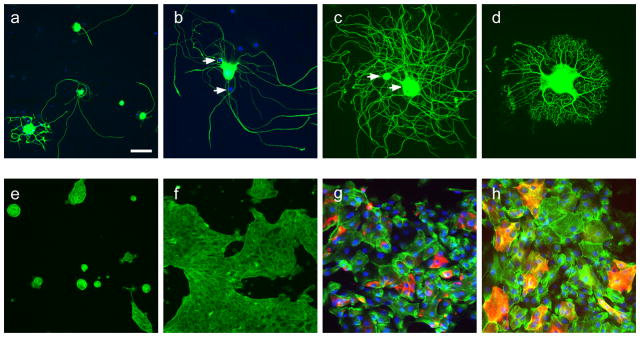
Structural and immunohistochemical properties of neuronal and urothelial monocultures
a. Dorsal root ganglion (DRG) monoculture, grown on polyornithine-laminin substrate for 48h. Five neurons are shown, immunostained for β-tubulin (green) and contrast slightly enhanced to enable better visualization of neurites. The nuclear stain, DAPI (blue), shows the distribution of glial cells but is not readily visible in neurons due to the intensity of immunolabelling. b. DRG neuron grown and labeled as for a, but at higher magnification to show the typical glial cell distribution (examples shown by arrows). c, d. DRG neurons grown and labeled as for a, but with nerve growth factor (NGF, 10ng/ml) present in the culture. In c, two neuronal somata are indicated (arrows). e. Urothelium monocultures grown for 48h on collagen, and stained with phalloidin-FITC to demonstrate filamentous actin. f. Urothelium monocultures grown on collagen for 72h. g, h. Urothelium monoculture grown for 72 h, immunolabelled for (g) cytokeratin 17 (red) or cytokeratin 20 (red) and co-stained for phalloidin-FITC (green). Calibration bar in panel a represents 60μm (a), 30μm (b–f), or 20μm (g,h).
Urothelial cell monocultures resembled those in previous studies11,14. Single or small islands of cells (<10 cells per island) predominated after 24h in culture, but by 72h, larger areas of closely associated urothelial cells had formed (Fig. 1e,f), including cells that were cytokeratin 17+ (marker of basal cells) or cytokeratin 20+ (marker of umbrella cells)2 (Fig. 1g,h).
2. Structural features of co-cultures
In pilot studies, urothelial cells adhered poorly to neuronal substrate (laminin-polyornithine) and did not grow in neuronal culture medium (Neurobasal A), with few cells surviving after 24h. However, neurons did adhere to the collagen substrate used for urothelial cell cultures and grew in the urothelium culture media, KSFM. As neurons and urothelial cells isolated from the same or different animals grew equally well (see below), subsequent experiments used the former approach to minimize use of animals.
There was a great diversity in the types of neuronal growth and in the spatial relationships between neurons and urothelial cells (Fig. 2). Most neurons grew long, branched neurites (Fig. 2a) but the exuberance of neurite growth and proximity to urothelial cells did not appear correlated, which suggested the trophic effect of urothelium could be due to a factor(s) secreted into the medium. Many neurons located close to islands of urothelial cells extended neurites to envelope them (Figs. 2b–e). Varicose neurites were often closely opposed to urothelial cells (Figs. 2b,c,e). Some appeared to form terminations (Fig. 2f,g) but others traversed islands of urothelial cells without terminating (Fig. 2h). These features were comparable in co-cultures where urothelial cells and neurons were from different rats, and were independent of the timing of neurons added to the culture. Neurons and urothelial cells containing retrograde tracer dye had similar structural features to unlabeled cells (Figs. 2i–k).
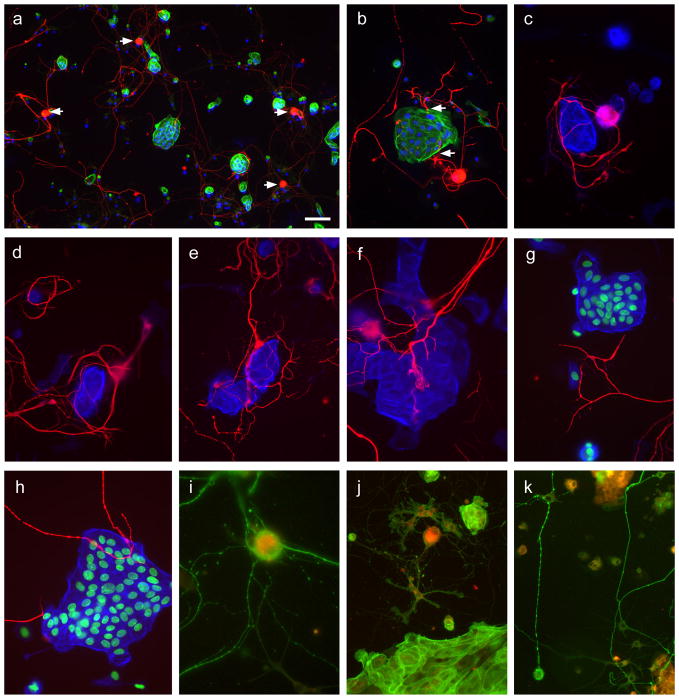
Figure 2.
Structural and immunohistochemical properties of neuronal and urothelial co-cultures
Panels a–h show co-cultures (48–72h) of cells isolated from control animals. In panels a and b, neurons were immunolabelled for β-tubulin (red), urothelial cells identified with phalloidin-FITC (green) and nuclei labelled with DAPI (blue). In c–h, urothelial cells have been digitally recolorised blue and DAPI green to facilitate visualisation of the closely associated nerve fibers (red) and, in some cases nuclear staining omitted for simplicity. a. Low magnification view of a typical co-culture (arrows show examples of neuronal somata). Glial cell nuclei (cytoplasm unstained) are also shown. b. Neurites enveloping an island of urothelial cells. c. Neurites enveloping a small cluster of urothelial cells but not approaching other single urothelial cells that are growing nearby. d. Neurites enveloping both clusters and single urothelial cells. e, f. Dense networks of neurites associated with urothelial cells, showing potential sites of termination. g, h. Neurites travelling near urothelial cells but forming few or no sites of close association with urothelial cells. Panels i–k show co-cultures from animals in which the urinary bladder had previously been microinjected in vivo with DiI (red) that is apparent in many of the neurons (i, j) and urothelial cells (j,k). Neurons are labelled with β-tubulin (green) in panels i and k; urothelial cells are labelled with phalloidin-FITC (j). Calibration bar in panel a represents 50μm (a), 30μm (b, j, k), or 20μm (c–i).
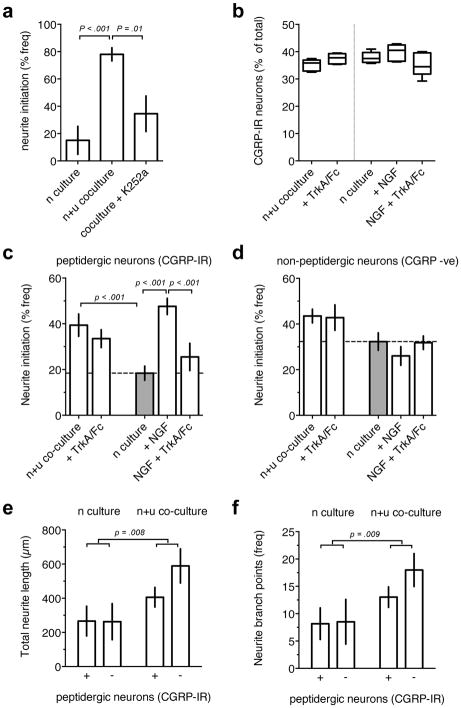
Neurite initiation was low (15±10%) in DRG monocultures grown for 48h under urothelial culture conditions or in the absence of exogenous NGF, but increased to 78±4.8% when neurons were cultured with urothelial cells under identical conditions (Fig. 3a). The structure of peptidergic unmyelinated bladder afferents is regulated by NGF derived from the urothelium and other tissues in normal and diseased bladder18. To determine if the neurotrophic effect of urothelial cells was dependent on tyrosine kinase signaling, which is required for downstream signaling by the NGF receptor TrkA19, we tested the broad spectrum inhibitor, K252a (100nM). This significantly reduced neurite initiation to 34±13% in co-cultures (Fig. 3a). We next used a recombinant TrkA-Fc chimera (4 ng/ml)15 as an NGF decoy to determine if upstream neuronal NGF/TrkA receptors were activated in co-cultures. In this experiment, identification of neuronal phenotype revealed no significant effect of TrkA/Fc treatment on neurite initiation in co-cultured peptidergic (p = .546, 4 df) or non-peptidergic neurons (p = .171, 4 df). However, in a positive control, treatment of monocultures with TrkA/Fc attenuated neurite initiation in peptidergic neurons treated with NGF (Fig. 3c). Neither TrkA/Fc nor NGF had a significant effect on the proportion of CGRP+ neurons in co-cultures (Fig. 3b) and there was no effect on neurite initiation in non-peptidergic neurons in monoculture (Fig. 3d). Analysis of untreated control co-cultures showed the neurotrophic effect of urothelial cells was caused by increased neurite initiation in CGRP+ neurons (Fig. 3c) but not CGRP− non-peptidergic neurons (Fig. 3d). In summary, co-culturing with urothelial cells preferentially stimulated neurite initiation in peptidergic sensory neurons. This was dependent on tyrosine kinase signaling but not NGF/TrkA.
Figure 3. Enhanced neurite initiation and growth in sensory neurons co-cultured with urothelial cells.
Data in a–c analysed by Holm’s-Sidak multiple comparisons test. Group data, a, showed co-culturing significantly increased mean neurite initiation (as percent frequency of neurons with neurites; df =10). Neurite initiation was significantly decreased in co-cultures after 24h treatment with K252a (100nM, df = 10). Boxplots, b, showed the percent frequency of CGRP-immunoreactive neurons was not significantly affected by co-culturing, and 24h treatments with NGF (10ng/ml) or NGF plus TrkA/Fc (4 ng/ml) (df =4). Group data, c, showed co-culturing significantly increased mean neurite initiation in peptidergic (CGRP-immunoreactive) sensory neurons but not in non-peptidergic neurons (df =16). Neurite initiation in co-cultured sensory neurons was not significantly affected by 24h treatment with TrkA/Fc (4 ng/ml). In neuronal monocultures, NGF (10 ng/ml, 24h) significantly increased neurite initiation in peptidergic neurons but not non-peptidergic neurons, and this effect of NGF was reduced by TrkA/Fc. Image analysis of neurite growth, d, showed that co-culturing significantly increased the mean total neurite length (F(1,6) = 15.67) and number of branch points (F(1,6) = 14.62; Two-way RM ANOVA, Main Effect of type of culture). Peptidergic and non-peptidergic neurons showed no significant difference in neurite length (F(1,6) = 2.844, p = .143) or branch points (F(1,6) = 2.513, p = 0.164, Main Effect of neuron class). Summary data are the mean, and standard error of the mean.
Further analysis of neurite outgrowth did not detect any significant differences in the effect of co-culturing on peptidergic and non-peptidergic sensory neurons. There was a significant main effect of culture type (neuronal culture, or neuronal/urothelial co-culture) on total neurite length and number of branch points (Fig. 3e,f), however neither a main effect of neuronal class nor a significant interaction was detected.
3. Functional characterisation of neurons and urothelial cells in co-cultures
Sensory neurons and urothelial cells in co-cultures were also assessed using ratiometric Fura-2 imaging to detect agonist induced changes in intracellular calcium ([Ca2+]in).Neuronal recordings were restricted to neurons with a soma diameter < 30μm. Rapid local superfusion with high K+ (30mM) transiently increased [Ca2+]in in 53 DRG neurons in cocultures (ΔF: 0.94±0.05 a.u.). These Ca2+ transients typically peaked during the 60s period of high K+ superfusion, and returned to baseline within 2–3 mins of washout. Typical recordings are shown in Fig. 4 and group data summarised in Fig. 5. Ca2+ transients were induced by selective agonists of TRPV120 (1μM capsaicin), TRPV421,22 (10μM 4α-PDD) and TRPA120 (100μM mustard oil). Ca2+ transients were also induced by a cannabinoid that is a non-selective TRPA1 agonist (30μM Δ9–THC). Agonists of TRPV323 (1μM farnesyl pyrophosphate; 0 of 3) and TRPM822 (1μM icilin, 0 of 6) did not induce Ca2+ transients in these recordings. The purinoceptor agonist ATP (100μM) induced small Ca2+ transients and only 2 of 6 neurons showed Ca2+ transients in response to the selective P2X1/3 agonist α,β,MeATP (ΔF 0.20 and 0.32).
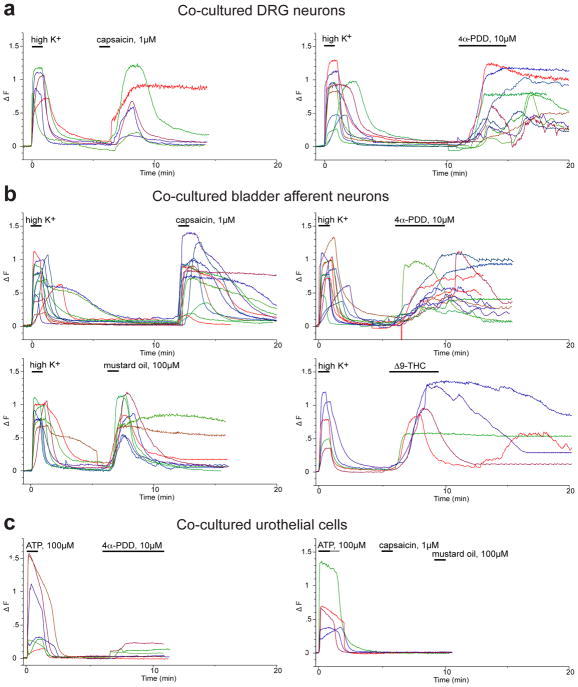
Figure 4.
Fura-2 ratiometric imaging of [Ca2+]i in co-cultured sensory neurons and urothelial cells. Example plots of ΔF (change in peak 340nm/380nm fluorescence emission ratio of Fura-2 from baseline) over time (min), which illustrate the effect of agonists on, a, unlabelled DRG neurons, b, bladder afferent neurons labelled with retrograde tracer dye (DiI), and, c, urothelial cells. All agonists were locally applied by rapid superfusion. High K+ was applied at 30 mM concentration in an isosmotic solution and all other agonists were applied at the concentrations shown. Data are from single neurons and from >5 contiguous urothelial cells within larger islands of cells.
Figure 5. Calcium transients activated by high potassium and agonists in co-cultured sensory neurons and urothelial cells.
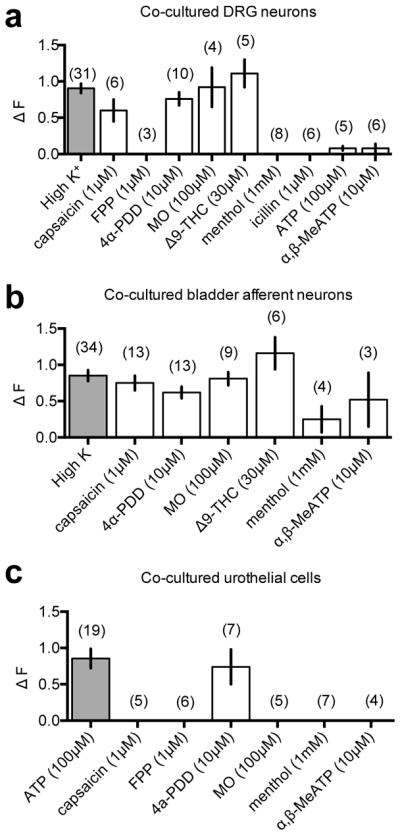
Plots of group data (a–c) summarise the effect of agonists on ΔF (change in peak 340nm/380nm fluorescence emission ratio of Fura-2 from baseline) in (a) unidentified DRG neurons, (b) retrogradely labelled bladder neurons, and (c) urothelial cells. Shown are the mean, and standard error of the mean with the number of replicates indicated in brackets.
Recordings were also made from sacral bladder afferents co-cultured with urothelial cells (Figs. 4, 5), in which high K+ induced Ca2+ transients (0.84 ± 0.05 a.u.) in 48 DiI-labelled neurons. Ca2+ transients were induced in co-cultured bladder afferents by capsaicin (1μM), 4α-PDD (10μM), and mustard oil (100μM). The non-selective TRPA1 agonist Δ9–THC (30μM) also induced Ca2+ transients and the TRPM8 agonist menthol (1mM) induced Ca2+ transients in 2 of 4 neurons (ΔF: 0.77 and 0.13 a.u.).
ATP (100μM) reliably induced Ca2+ transients in urothelial cells in co-cultures (Figs. 4 and 5). These responses were measured in islands of 5 or more urothelial cells. The time-to-peak and the amplitude of Ca2+ transients were variable. In some recordings, the responses peaked early and decayed during agonist application, whereas others plateaued or were still rising at the end of the agonist application. The P2X2/3 selective agonist agonist α,β,MeATP had no effect on the [Ca2+]in in 4 recordings from co-cultured urothelial cells. Only urothelial cells that responded to ATP were used to evaluate TRP channel expression. The TRPV4 agonist 4α-PDD (10μM) induced amplitude Ca2+ transients (1.8±0.65 a.u.) after a delay in 4 of 8 recordings from co-cultured urothelial cells. Ca2+ transients were not induced in recordings from co-cultured urothelial cells by any of the other TRP agonists tested.
Discussion
The urothelium is suggested to interact with the bladder sensory innervation and function in sensory transduction2. The molecular mechanism of this interaction has been difficult to define. Here we report a co-culture method whereby neurons and urothelial cells grown together retain structural, immunohistochemical and functional properties previously identified in acutely dissociated preparations. This offers a new approach for understanding neuro-urothelial communication in the healthy bladder and animal models of clinical conditions (e.g. cystitis, overactive bladder, spinal cord injury). While this opens up many opportunities, it will also be important to assess whether urothelial cells and/or neurons in these co-cultures undergo plastic changes during the culture period.
The strong neurotrophic effect of urothelial cells was similar to the trophic effects of keratinocytes on sensory neurons8,24. Our preliminary attempts to identify the factor responsible showed that tyrosine kinase but not TrkA signalling was involved. A study on neonatal sensory neurons also identified a K252a-sensitive neurotrophic effect of keratinocytes that was reduced by steroidogenesis inhibitors24; a possible role for urothelium-derived steroids in our adult neuron cultures would be of great interest in the context of bladder modulation.
Functional responses to agonists were detected in small diameter, presumed unmyelinated neurons25 and urothelial cells in co-culture by measuring changes in [Ca2+]in with Fura-2 ratiometric imaging. The robust Ca2+ transients induced by high K+ in most neurons suggested that a hyperpolarised resting membrane potential and the excitability of the neurons was maintained in co-culture. This is consistent with evidence that Ca2+ transients activated by high K+ in rat lumbar DRG sensory neurons are caused by membrane depolarisation and the subsequent influx of extracellular Ca2+ through voltage-activated calcium channels (VACCs)17. Agonists of TRPV1, TRPA1 and TRPV4 also induced Ca2+ transients in sacral DRG neurons and bladder afferents in co-culture, but these channels are permeable to calcium and a broad range of monovalent cations. Our experiments using the TRPV1 agonist, capsaicin, were in agreement with electrophysiological data that suggests 70% of rat bladder afferents are capsaicin sensitive12. Functional expression of TRPA1 in bladder afferents has not been previously demonstrated in primary cultures, but is inferred from immunohistochemistry, in situ hybridisation, and physiological studiesreviewed by 26. The TRPA1 agonist used in our experiments, mustard oil, can activate TRPV1 at millimolar concentrations but is selective for TRPA1 at the micromolar concentration used here20. We are unaware of prior evidence of TRPV4 expression in bladder afferents, but strong evidence suggests TRPV4 expressed by urothelial cells regulates urinary tract function26. TRPV4 is a “thermo-TRP” but the functioning of TRPV4 in mechanosensation may have greater functional relevance in bladder afferents27.
Neuronal Ca2+ transients activated by Δ9–THC, which elevates [Ca2+]in in DRG sensory neurons by activating TRPV1 and TRPA1 may be clinically relevant, as desensitisation of these TRPs by cannabinoids induces peripheral antinociception and antihyperalgesia28. Only two bladder afferents (of 10 recordings) responded to the TRPM8 agonist, menthol, but this channel is mostly expressed in the Aδ class of DRG neurons26, which have a soma diameter greater than 30μm and were excluded from our recordings. TRPV3 has not been reported in bladder afferents, and farnesyl pyrophosphate (FPP), a selective agonist of TRPV323 was without effect.
Ca2+ transients in co-cultured urothelial cells were consistently activated by ATP—previously used as a positive control in calcium imaging studies of mouse urothelial cells22— but not by α,β,MeATP, which selectively activates P2X receptor homomers or P2X1 or P2X2 heteromers29,30. Consistent with a recent functional characterisation of TRP channels in mouse urothelial cells22, we detected TRPV4 channels in rat urothelial cells maintained in co-culture but did not detect TRPV1, TRPA1 or TRPM8. FPP did not detect TRPV3, which is detected in mouse urothelium22. Further experimentation will be required to determine the functional expression of TRPV3 in rat urothelium.
Conclusions
We have refined and characterised a method for co-culturing lumbosacral sensory neurons and bladder urothelium. The presence of urothelial cells promoted the growth of neurons. This new approach will be valuable for probing neuro-urothelial communication in health and disease.
Acknowledgments
This study was supported by Award Number R01DK069351 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. It was also supported by Project Grant 1003512 and Senior Research Fellowship 632903 from the National Health and Medical Research Council of Australia. We thank Dr Lori Birder for her early input to development of the co-culture concept and Dr Mitchell Quinlivan for his participation in the neural growth experiments.
Abbreviations
- CGRP
calcitonin gene-related peptide
- CK
cytokeratin
- DRG
dorsal root ganglion/ganglia
- KSFM
keratinocyte serum free medium
- NGF
nerve growth factor
- PBS
phosphate buffered saline
References
- 1.Birder LA. Urothelial signaling. Handb Exp Pharmacol. 2011:207–231. doi: 10.1007/978-3-642-16499-6_10. [DOI] [PubMed] [Google Scholar]
- 2.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297:F1477–501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011:395–423. doi: 10.1007/978-3-642-16499-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damon DH. NGF-independent survival of postganglionic sympathetic neurons in neuronal-vascular smooth muscle cocultures. Am J Physiol Heart Circ Physiol. 2001;280:H1722–8. doi: 10.1152/ajpheart.2001.280.4.H1722. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi S, Fujishita K, Inoue K, et al. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Liu H, Li Z. Formation of neuromuscular junctions and synthesis of sensory neuropeptides in the co-cultures of dorsal root ganglion and cardiac myocytes. Cell Mol Neurobiol. 2008;28:939–947. doi: 10.1007/s10571-008-9268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulmann L, Rodeau J-L, Danoux L, et al. Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur J Neurosci. 2007;26:113–125. doi: 10.1111/j.1460-9568.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- 9.Wanigasekara Y, Keast JR. Nerve growth factor, glial cell line-derived neurotrophic factor and neurturin prevent semaphorin 3A-mediated growth cone collapse in adult sensory neurons. Neuroscience. 2006;142:369–379. doi: 10.1016/j.neuroscience.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Xu S, Cheng Y, Keast JR, et al. 17beta-estradiol activates estrogen receptor beta-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149:5540–5548. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truschel ST, Ruiz WG, Shulman T, et al. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura N, Seki S, Erickson KA, et al. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullmann FA, Artim D, Beckel J, et al. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol. 2008;294:F971–81. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalous A, Nangle MR, Anastasia A, et al. Neurotrophic actions initiated by proNGF in adult sensory neurons may require peri-somatic glia to drive local cleavage to NGF. J Neurochem. 2012;122:523–536. doi: 10.1111/j.1471-4159.2012.07799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest SL, Forrest SL, Keast JR, et al. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu S, Gold MS. Inflammation-induced increase in evoked calcium transients in subpopulations of rat dorsal root ganglion neurons. Neuroscience. 2008;153:279–288. doi: 10.1016/j.neuroscience.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steers WD, Tuttle JB. Mechanisms of Disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3:101–110. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- 19.Ernsberger U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res. 2009;336:349–384. doi: 10.1007/s00441-009-0784-z. [DOI] [PubMed] [Google Scholar]
- 20.Everaerts W, Gees M, Alpizar YA, et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuki T, Sokabe T, Araki I, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everaerts W, Vriens J, Owsianik G, et al. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298:F692–701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang S, Yoo S, Yang T-J, et al. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem. 2010 doi: 10.1074/jbc.M109.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulmann L, Rodeau J-L, Danoux L, et al. Dehydroepiandrosterone and neurotrophins favor axonal growth in a sensory neuron-keratinocyte coculture model. Neuroscience. 2009;159:514–525. doi: 10.1016/j.neuroscience.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Gabella G. Structure of the intramural nerves of the rat bladder. J Neurocytol. 1999;28:615–637. doi: 10.1023/a:1007084130642. [DOI] [PubMed] [Google Scholar]
- 26.Skryma R, Prevarskaya N, Gkika D, et al. From urgency to frequency: facts and controversies of TRPs in the lower urinary tract. Nat Rev Urol. 2011;8:617–630. doi: 10.1038/nrurol.2011.142. [DOI] [PubMed] [Google Scholar]
- 27.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Akopian AN, Ruparel NB, Jeske NA, et al. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2009;30:79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford APDW, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol. 2011:485–526. doi: 10.1007/978-3-642-16499-6_22. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Hill WG, Apodaca G, et al. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal Physiol. 2011;300:F49–59. doi: 10.1152/ajprenal.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]