Abstract
Osteochondritis dissecans (OCD) of the knee is a disease of the subchondral bone with secondary injury to the overlying articular cartilage. OCD lesions are generally categorized as juvenile—growth plates open—or adult—growth plates closed. This maturity-based classification scheme has a prognostic value in that many juvenile OCD lesions will heal with conservative care while most symptomatic adult OCD lesions need surgical intervention. OCD can result in pain, knee joint effusions, loose body formation, and arthritis. Short-term treatment goals include pain and symptom resolution while the long-term goal is to minimize arthritis. Surgical options include debridement, drilling, microfracture, reduction and fixation, autograft osteochondral transplantation, autologous chondrocyte implantation, and allograft osteochondreal transplantation.
Keywords: Osteochondritis dissecans, Cartilage, Knee, Drilling, Microfracture, Juvenile, Chondrocyte
Introduction
Osteochondritis dissecans of the knee is an idiopathic disorder primarily affecting subchondral bone with secondary effects on the overlying articular cartilage [1]. The most common locations of osteochondritis dissecans (OCD) of the knee are the medial femoral condyle (70–80 %), lateral femoral condyle (15–20 %), and patella (5–10 %) [2, 3]. The etiology of OCD is unknown. OCD is postulated to primarily stem from repetitive microtrauma; however, ischemia, genetic influences, and growth disturbances may play a role [1]. The two main categories of OCD are juvenile (open physes) and adult (closed physes). The maturity distinction has clinical significance. Most cases of juvenile OCD will heal without surgery, while adult OCD often requires surgical intervention [2, 4].
Patients with an OCD of the knee will typically present with aching or activity-related knee pain [5]. In patients with advanced disease or unstable lesions, the patient may complain of locking, swelling, and catching. In 1967, Wilson described a clinical sign that he thought was diagnostic of medial femoral OCD [6]. He suggested that impingement of the tibial eminence on the OCD lesion caused pain and a resulting external rotation gait. “Wilson’s sign” has been described in which the examiner extends the knee from 90° to 30° against resistance while internally rotating the tibia. The test is considered positive when symptoms are reproduced by internal rotation and relieved by external rotation of the tibia. Wilson correlated healing of the lesion with conversion of the sign from positive to negative. Conrad and Stanitski demonstrated that Wilson’s sign lacked diagnostic specificity but was useful as a clinical monitoring test during treatment [7].
Patients with a suspected OCD of the knee should be evaluated with radiographs including: AP, lateral, sunrise/merchant, and tunnel views (Fig. 1a, b). Although intuitively obvious, the utilization of radiographs in the work up of a patient with possible OCD was given a “weak” recommendation by the 2011 American Academy of Orthopaedic Surgeons (AAOS) Clinical Practice Guidelines (CPG), due to only one level of evidence II study supporting this recommendation [8]. Bilateral OCD of the knee has been reported in 12–30 % of cases [9, 10]. A recent study of only juvenile OCD patients noted the prevalence of bilateral disease at 29 % [10]. These authors recommended contralateral radiographs in all juvenile OCD patients. However, the AAOS 2011 CPG was unable to recommend for or against radiographs on the contralateral asymptomatic knee because they were unable to find any high-quality evidence on this theme [8].
Fig. 1.
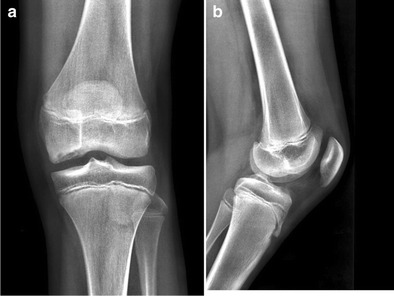
a AP radiographic view of the right knee in a 13-year-old travel soccer athlete with OCD medial femoral condyle. b Lateral radiographic view of the right knee in a 13-year-old travel soccer athlete with OCD medial femoral condyle
After a patient has been diagnosed with an OCD of the knee, a magnetic resonance imaging (MRI) is most commonly used to further evaluate the lesion. MRI allows characterization of the lesion as stable or unstable [11]. MRI indications of OCD instability include: a high-signal-intensity line beneath the lesion, a cystic area beneath the lesion, a high-signal-intensity line through the articular cartilage, and a focal articular defect (Fig. 2). MRI has sensitivity for determining OCD instability of 97–100 % in both juveniles and adults [11–13]; however, specificity is only 11–55 % in juvenile compared with 100 % in adults [12–14]. The AAOS 2011 CPG graded the recommendation to obtain an MRI to characterize the OCD lesion as “weak” due to only two levels of evidence II studies supporting this recommendation [8]. We utilize MRI routinely prior to surgery.
Fig. 2.
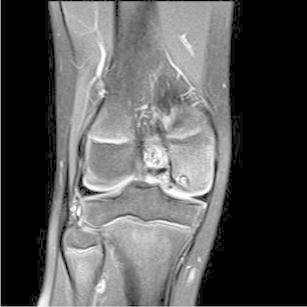
Coronal T2-weighted MRI showing a cystic area beneath the lesion
Non-operative treatment of OCD of the knee is commonly utilized for juvenile with stable lesions. Non-operative treatment includes: activity modification, weight-bearing limitations, anti-inflammatory medication, casting, bracing, splinting, unloader bracing, electrical or ultrasound, or bone stimulation. Outcomes of non-operative treatment in juvenile OCD without a loose body have generally been good [2, 5, 15–17]. However, the AAOS 2011 CPG was unable to recommend for or against non-surgical treatment for symptomatic or asymptomatic juvenile OCD because they were unable to find any quality evidence to support non-surgical treatment [8]. In general, we recommend surgery for symptomatic juvenile lesions that have failed initial non-operative treatment and non-surgical management for asymptomatic lesions.
Indications/contraindications
The stable juvenile OCD should be initially treated non-operatively. The treatment of unstable and displaced OCD does not depend on the presence of an open growth plate. Surgery is recommended. General indications for surgical treatment include: failure of non-operative treatment greater than 6 months for juvenile cases or 3–6 months for adults cases, symptomatic loose bodies, or an unstable fragment.
Techniques
Surgical techniques for OCD of the knee can be classified as palliative, reparative, and restorative/reconstructive [18]. Palliative techniques including loose body removal or debridement of the defect aim to relieve mechanical symptoms without restoring the articular surface. Reparative techniques aim to either stabilize the osteochondral lesion or generate fibrocartilage. These include transarticular and retroarticular drilling, open reduction and internal fixation, arthroscopic-assisted fixation, and microfracture. Restorative techniques include autologous chondrocyte implantation and auto- or allograft transplantation into the osteochondral lesion to fill in the bone and articular cartilage defect.
The AAOS 2011 CPG guidelines gave a consensus recommendation that both skeletally immature and mature patient’s salvageable unstable or displaced OCD lesions be given the option of surgery [8]. A consensus recommendation was indicated because of the absence of reliable quality evidence on this theme. The AAOS 2011 CPG guidelines were also unable to recommend for or against any specific cartilage repair technique in patients with an unsalvageable OCD lesion [8]. The guidelines suggest that in the absence of reliable evidence supporting surgical treatment of OCD, the choice to proceed with surgery is part of a shared-decision making process between the patient, family, and physician [8].
Palliative
Loose body removal/chondroplasty
Osteochondral defects that are loose may cause pain, locking, and swelling of the knee. Arthroscopic removal of the loose body will provide short- term relief of symptoms [19, 20]. However, degenerative changes have been noted later on in life in patients with adult OCD [21, 22]. For example, Shelbourne and associates [23] reported that a role still exists for arthroscopic loose body removal and chondroplasty. The study involved 33 patients with a mean lesion size of 2.7 cm [2] with a mean follow-up time of 7.7 years and found that 24 % of patients had joint space narrowing on radiographs. As an alternative, additional reparative, restorative/reconstructive techniques can be combined with the loose body removal or chondroplasty to promote healing of the remaining full-thickness cartilage defect.
Reparative
Transarticular or retroarticular drilling
Drilling with a smooth K-wire is commonly performed for stable juvenile OCD lesions that are less than 2.5 cm [2]. The goal of drilling is to create vascular channels from the underlying marrow to promote a healing response of the subchondral bone. Transarticular or retroarticular drilling are equally efficacious [24]. Transarticular drilling involves perforation of the articular cartilage under arthroscopic visualization of the OCD lesion (Fig. 3). Retroarticular drilling occurs through the affected condyle sparing the articular cartilage and requires intraoperative fluoroscopy to direct the K-wire to the back of the OCD lesion. Both techniques involve a diagnostic arthroscopy of the knee to address any concurrent pathology, followed by evaluation of the lesion size and grade.
Fig. 3.
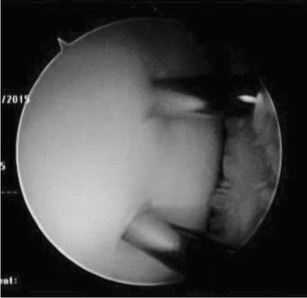
Arthroscopic image of transarticular drilling with two 0.062 K-wires through the articular surface across the OCD lesion and into the cancellous bone
Retroarticular drilling can be performed with an ACL reconstruction transtibial guide and intraoperative use of fluoroscopy to ensure proper placement of a K-wire. Pennock [24] suggests aiming the first K-wire at the center of the OCD lesion and using this as a guide for ten drillings utilizing a parallel wire guide. The benefits of the procedure include theoretically not violating the articular cartilage. The retroarticular technique is technically more challenging than the transarticular technique and requires more radiation to the patient and surgeon. Excellent results have been documented by multiple authors with this technique [25–28]. For example, Edmonds et al. [25] reported on 59 juvenile OCD patients treated with retroarticular drilling. The average time to return to sports was 3 months. Seventy-five percent of lesions completely healed radiographically at 12 months and 98 % at 36 months [25].
Transarticular drilling is performed arthroscopically under direct visualization with the use of standard portals. Increased knee flexion may be utilized to obtain access to posterior lesions; 0.45 or 0.62 mm K-wires are used to drill multiple points in the lesion. Adequate depth is confirmed when blood and fat are visualized through the drill hole. The theoretical disadvantage of transarticular drilling is the necessary violation of the articular surface. Results of drilling are dependent on the status of the physis with far better results in patients with open physes [29–31]. Louisia et al. [29] reported on 24 patients and found 82 % good or excellent results in juvenile OCD compared with 50 % good results in adult OCD. A recent systematic review reported that transarticular drilling is the most common surgical technique for treating stable OCD lesions [32•].
The AAOS 2011 CPG was unable to recommend for or against arthroscopic drilling for symptomatic or asymptomatic juvenile OCD due to the lack of quality evidence [8].
Open reduction internal fixation and arthroscopic-assisted internal fixation
Stable OCD lesions with intact cartilage after failed non-operative management or unstable OCD lesions with adequate bone are candidates for internal fixation. The goals of internal fixation for an OCD lesion are to re-approximate the articular cartilage surfaces, stimulate vascular perfusion to the defect, and provide compression to the fragment to maximize healing. Fixation may be done via an open or arthroscopic approach (Fig. 4a, b). Arthroscopic techniques theoretically decrease operative morbidity and lead to a quicker recovery. In either case, a diagnostic arthroscopy may be performed to obtain further information about the size and character of the lesion (Fig. 5).
Fig. 4.
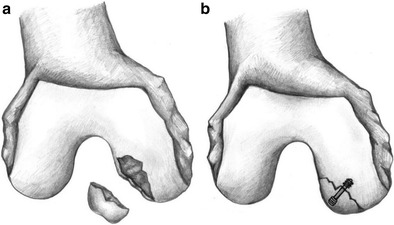
a An unstable OCD lesion. b An open reduction and fixation of the OCD lesion using a screw with head buried under the articular surface
Fig. 5.
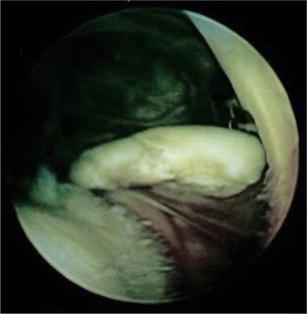
Arthroscopic image of large osteochondral loose body in a high school basketball athlete
Once the lesion is determined to be amenable to fixation, the base is exposed and prepared with debridement with a curette or motorized shaver to remove any fibrous tissue. Microfracture of the base is then performed to obtain a bleeding base (Fig. 6). The fragment is then reduced to an anatomic position and provisionally fixed with a K-wire. Final fixation may be performed with bioabsorbable or non-absorbable pins, nails, or screws (Fig. 7). The fixation device must be buried below the articular surface to prevent cartilage damage. Metallic or non-absorbable devices are removed after 8 to 12 weeks. A recent systematic review noted that bioabsorbable pin fixation is the most common surgical technique for treating unstable OCD lesions [32•]. Adachi et al. [33•] used bioabsorbable pins for fixation of unstable OCD lesions and noted 97 % healing with good to excellent clinical outcomes confirmed by radiographs and MRI. Camp et al. [34] recently published a technique using biocompression screws (Arthrex, Naples, FL) countersunk 2 to 3 mm deep to the articular cartilage surface.
Fig. 6.
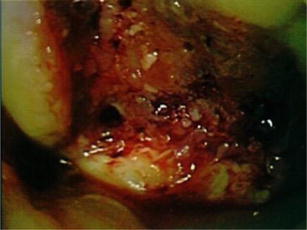
Arthroscopic image of the bed of the ODC lesion after debridement with a motorized shaver and curette and prepared with microfracture
Fig. 7.
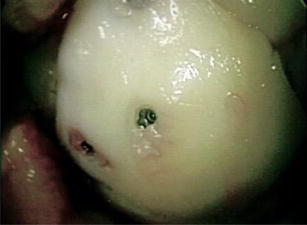
Arthroscopic image of the loose body repaired to the OCD bed using metallic screws with the head of the screws buried 2 mm under the surface of the articular cartilage
The perceived benefit of bioabsorbable implants is not requiring an additional surgery to remove the hardware. However, complications with bioabsorbable implants have been reported, including non-union [35], osteolysis, screw breakage [36], and synovitis [37]. Screw breakage was found in 23 % of bioabsorbable screws used for fixation of OCD lesions in skeletally immature patients. The authors noted some of these failures were based on MRI alone and had no clinical symptoms [36].
Postoperative rehabilitation for open or arthroscopic internal fixation for OCD lesions include protected weight bearing for the first 6 to 8 weeks. Physical therapy may include an emphasis on quadriceps strengthening. Range of motion can begin immediately. After 6 weeks, weight bearing is increased until as tolerated without crutches at 12 weeks. The AAOS 2011 CPG guidelines made a consensus recommendation that those patients who have received surgical treatment for OCD be offered the option of postoperative physical therapy. The AAOS 2011 CPG workgroup noted the paucity of evidence on the effectiveness of physical therapy; however, the AAOS CPG recommendation was consistent with current practice [8].
If the patient is indicated for a second surgery for removal of hardware, the patient may limit their activities for a few weeks following the second procedure to allow adequate time for healing. Overall, either open or arthroscopic reduction and internal fixation for unstable or displaced osteochondral lesions has demonstrated good outcomes [18].
Restorative/reconstructive
Microfracture
The goal of microfracture is to stimulate fibrocartilage to fill a chondral defect. Microfracture was originally designed for posttraumatic full thickness cartilage lesions and has also been applied to patients with osteochondritis dissecans. Small (<2.5 cm2) unstable lesions in low-demand patients are often indicated for microfracture. The lesion is debrided to a stable rim to enable a well-shouldered/contained lesion, followed by removal of the calcified cartilage layer with a curette [38] or motorized shaver. Care must be taken to maintain the integrity of the subchondral plate. Microfracture awls are then used to penetrate the subchondral bone with minimal force. Three to four perforations per square centimeter are recommended. The awl should penetrate to a far-enough depth to allow fat and blood droplets to extrude through. These droplets contain the stem cells and growth factors from the bone marrow [38].
Postoperatively, patients are partial weight bearing for 6 to 8 weeks. They may also utilize continuous passive motion (CPM) machines and begin physical therapy to work on quadricep-strengthening exercises. Weight bearing is increased after 8 weeks with gradual weaning of crutches. Low-impact exercises are started between 8 and 12 weeks postoperatively and sport-specific exercise may begin at 3 to 4 months. Depending on the size and location of the lesion, as well as the level of activity, return to sport may be anywhere from 4 to 9 months.
The outcomes of this technique for traumatic chondral defects have generally been good [39, 40]. However, long-term outcomes in patients treated with microfracture for OCD lesions specifically has not been as promising. For example, in a study by Gudas [41], patients with juvenile OCD were randomized to microfracture or osteochondral autograft transplant systems (OATS). Both groups did well at 1 year postoperative but failure rate increased to 41 % in the microfracture group compared with 0 % in the OATS group when the patients were followed to 4 years postoperative. Fourteen percent of patients in the microfracture group returned to preinjury activities compared with 81 % in the OATS group. The authors concluded that articular cartilage is better able to withstand shear stress than fibrocartilage [41]. The AAOS 2011 CPG guidelines were unable to recommend for or against a specific repair technique including microfracture, autologous chondrocyte transplantation, and osetochondral transplantation, in symptomatic unsalvageable adult OCD lesions. Four level IV studies addressed this theme, and the work group judged the evidence inconclusive [8].
Autologous chondrocyte implantation
Autologous chondrocyte implantation (ACI) involves a diagnostic arthroscopy and harvesting a small amount of healthy cartilage from the non-weight bearing area of the knee for cell culture. The cells are then grown in vitro for about 6 weeks. A second-stage procedure is then performed in which the cells are re-implanted into the defect (Fig. 8). ACI is most effective in OCD lesions larger than 2.5 cm2 in low-demand patients. At time of re-implantation, the calcified cartilage layer is debrided and the defect is covered with a synthetic collagen membrane or periosteal flap that is sutured in place. Fibrin glue is used to seal the edges, and the cells are injected beneath the patch. If the depth of the lesion is greater than 8–10 mm, autogenous bone graft may be placed in the bony defect [42], covered with an additional patch prior to the placement of the cultured cells. This is known as the sandwich technique. Postoperatively, the patients undergo a similar program as for microfracture with limited weight bearing initially, early range of motion, possible CPM, and quad-strengthening exercises.
Fig. 8.
The steps involved autologous chondrocyte implantation (ACI), including diagnostic arthroscopy, harvesting a small amount of cartilage for cell culture, and later at a second stage re-implant, the cells into the defect and sealing the defect with a periosteal patch
Peterson et al. [43] reported on 58 patients who underwent ACI for an OCD lesion and found 91 % good to excellent results at 2–10 years. Other authors have found similar results [35, 42–45]. The AAOS 2011 CPG guidelines were unable to recommend for or against a specific repair technique, including microfracture, autologous chondrocyte transplantation, and osteochondral transplantation, in symptomatic unsalvageable adult OCD lesions. Four level IV studies addressed this theme, and the work group judged the evidence inconclusive [8].
Other biologic techniques
Biologic biphasic scaffolds have been developed and may obviate potential risks and complications associated with current chondral restorative techniques.
Delcogliano et al. reported implanting a type 1 collagen-hydroxyapatite nanostructural biomimetic osteochondral scaffold in 23 patients with symptomatic knee OCD. The average lesion size was 3.5 cm2. The results were encouraging. The authors found statistically significant improvements in subjective and objective measures at 12 and 24 months postoperative [46]. Additional authors have also reported that biological scaffold implantation is a viable option for treating symptomatic OCD [47, 48].
Osteochondral autograft transplant
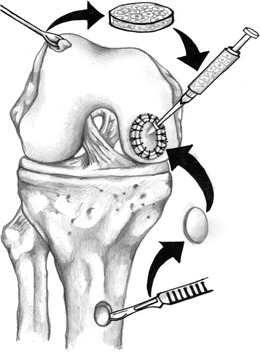
OATS are indicated for high-demand patients with small (<2.5 cm2) lesions. This technique may be performed arthroscopically or via an open approach if needed. A diagnostic arthroscopy is performed first. Next, the OCD lesion is prepared by removing loose debris and preparing the edges with curette or knife. Any fibrous tissue or cartilage is removed from the subchondral bone. The size and depth of the lesion is then measured, and the need for additional bone plugs or bone grafting is determined. A guide is used to remove the recipient bone plug, careful to not damage the surrounding healthy cartilage. An appropriately matched donor plug from the medial or lateral trochlea is harvested and then press fit into the recipient site (Fig. 9). Chondrocyte damage is minimized with an increased number of impacts but with less force [49]. The grafts should not be left proud, as this can lead to increased contact pressures and potential graft demise [50].
Fig. 9.
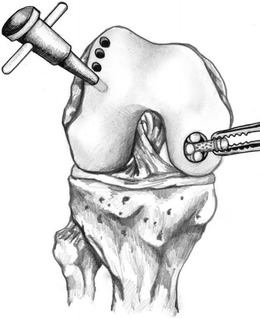
The steps involved in osteochondral autograft transplant, including cell harvest, cell growth in vitro, injection of the cells into the OCD defect, and OCD coverage. (Illustrations drawn by Zachary Winthrop BS)
Postoperatively, patients are kept non-weight bearing for 3 weeks, followed by progressive weight bearing at weeks 3–6, and then full weight bearing at 6 weeks. Range of motion exercises and CPM may begin in the immediate postoperative period. Return to sports may be expected at 4–6 months postoperatively.
Limitations of the OATS technique include donor site morbidity, lack of donor site cartilage, and inability to restore the normal contour of the condyle. Outcomes for OATS procedures in patients with OCD of the knee are better in patients with smaller lesions and patients with lesions in the medial femoral condyle [50]. One study of 142 OATS procedures, 61 for OCD lesions reported 72.5 % good or excellent results at 8-year follow-up [50]. Gudas et al. [51] published data on 10-year follow-up of patients treated with OATS versus microfracture. Twenty-six of the 60 patients included in the study had OCD lesions with a mean lesion size of 2.7 cm2. Patients in both groups had significant improvement in clinical outcomes at year follow-up, but OATS patients had better ICRS scores and higher athletic activity levels at 10 years compared with the microfracture group.
Osteochondral allografting
Typically, osteochondral allografts are indicated for high-demand patients with large lesions (>2.5 cm2) or patients that have failed other surgical treatments. The basic fundamentals of fresh osteochondral allograft transplantation involve the transplantation of mature hyaline cartilage containing viable chondrocytes along with a variable amount of supporting subchondral bone to replace a chondral or osteochondral defect [52]. An open approach is performed via a medial or lateral parapatellar arthrotomy determined by where the location of the lesion. Lesion is debrided and a cylindrical hole is created with healthy cartilage and bone surrounding the hole. A fresh (14–21 days) allograft is used to obtain the donor cylindrical plug to match the recipient. The graft is press fit into the hole and may be supplemented with metal or bioabsorbable screws if necessary. This technique has been successful in several studies [20, 53, 54]. A recent study examined the use of osteochondral allografts in 11 juveniles with OCD of the knee and found 100 % of patients were able to return to sports with improved functional scores at an average of 24 months postsurgery [55]. This study suggests osteochondral allografts are an option for patients with juvenile OCD. Similarly, Murphy et al. [56] reported on 26 patients with juvenile OCD lesions (average age, 16.4 years) treated with osteochondral allografts and found an 85 % success rate at an average of 8.9 years of follow-up.
Patellofemoral management
There is a paucity of literature regarding management of OCD lesions of the patella and trochlea. A recent retrospective review of a series of 26 patients treated with surgical management was published [57••]. The treatment included transarticular drilling, reduction and fixation, microfracture, and lesion excision with marrow stimulation. Eighty-five percent of patients were able to return to play at an average of 5 months after surgery. All but one patient had improvement in the postoperative pain scores. This study emphasizes that although patellofemoral OCD lesions are rare, surgical treatment resulted in a high level of satisfaction and return to sports.
Conclusions
Osteochondritis remains a poorly understood condition. The AAOS 2011 CPG guidelines have clearly demonstrated that high-level evidence does not exist regarding the diagnosis and treatment of OCD. Although thousands of studies were reviewed, only 16 studies were rated of sufficient quality to be included in the AAOS 2011 CPG for OCD [8]. Therefore, well-designed studies about the natural history of OCD of the knee and appropriate treatment are clearly needed. Recently, a multicenter orthopaedic study group, Research in Osteochondritis of the Knee (ROCK), was created to study questions regarding OCD. These studies, as well as others, will hopefully improve our understanding and treatment of OCD in the future.
Acknowledgments
Compliance with Ethics Guidelines
ᅟ
Conflict of interest
The authors have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cartilage Repair Techniques in the Knee
Contributor Information
Zachary Winthrop, Email: Zwinthrop@hmc.psu.edu.
Gregory Pinkowsky, Email: Gpinkowsky@gmail.com.
William Hennrikus, Phone: 717.531-7006, Email: Whennrikus@hmc.psu.edu, Email: Whennnrikus@hmc.psu.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. J Am Acad Orthop Surg. 2006;14(2):90–100. doi: 10.5435/00124635-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cahill BR, Phillips MR, Navarro R. The results of conservative osteochondritis dissecans using joint scintigraphy. A prospective study. Am J Sports Med. 1989;17(5):601–605. doi: 10.1177/036354658901700502. [DOI] [PubMed] [Google Scholar]
- 3.Linden B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664–667. doi: 10.3109/17453677608988756. [DOI] [PubMed] [Google Scholar]
- 4.Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102–108. [PubMed] [Google Scholar]
- 5.de Gauzy Sales J, Mansat C, Darodes PH, Cahuzac JP. Natural course of osteochondritis dissecans in children. J Pediatr Orthop B. 1999;8(1):26–28. [PubMed] [Google Scholar]
- 6.Wilson JN. A diagnostic sign in osteochondritis dissecans of the knee. J Bone Joint Surg (Am Vol) 1967;49(3):477–480. [PubMed] [Google Scholar]
- 7.Conrad JM, Stanitski CL. Osteochondritis dissecans: Wilson’s sign revisited. Am J Sports Med. 2003;31(5):777–778. doi: 10.1177/03635465030310052301. [DOI] [PubMed] [Google Scholar]
- 8.Chambers HG, Shea KG, Carey JL. AAOS Clinical Practice Guideline: diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19(5):307–309. doi: 10.5435/00124635-201105000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hefti F, Beguiristain J, Krauspe R, Moller-Madsen B, Riccio V, Tschauner C, et al. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop B. 1999;8(4):231–245. [PubMed] [Google Scholar]
- 10.Cooper T, Boyles A, Samora WP, Klingele KE. Prevalence of bilateral JOCD of the knee and associated risk factors. J Pediatr Orthop. 2014. [DOI] [PubMed]
- 11.De Smet AA, Ilahi OA, Graf BK. Reassessment of the MR criteria for stability of osteochondritis dissecans in the knee and ankle. Skelet Radiol. 1996;25(2):159–163. doi: 10.1007/s002560050054. [DOI] [PubMed] [Google Scholar]
- 12.Heywood CS, Benke MT, Brindle K, Fine KM. Correlation of magnetic resonance imaging to arthroscopic findings of stability in juvenile osteochondritis dissecans. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthosc Assoc. 2011;27(2):194–199. doi: 10.1016/j.arthro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Kijowski R, Blankenbaker DG, Shinki J, Fine JP, Graf B, De Smet AA. Juvenile versus adult osteochondritis dissecans of the knee: appropriate MR imaging criteria for instability. Radiology. 2008;248(2):571–578. doi: 10.1148/radiol.2482071234. [DOI] [PubMed] [Google Scholar]
- 14.Samora WP, Chevillet J, Adler B, Young GS, Klingele KE. Juvenile osteochondritis dissecans of the knee: predictors of lesion stability. J Pediatr Orthop. 2012;32(1):1–4. doi: 10.1097/BPO.0b013e31823d8312. [DOI] [PubMed] [Google Scholar]
- 15.Schenck RC, Jr, Goodnigh JM. Osteochondritis dissecans. J Bone Joint Surg (Am Vol) 1996;78(3):439–456. [PubMed] [Google Scholar]
- 16.Williams JS, Jr, Bush-Joseph CA, Bach BR., Jr Osteochondritis dissecans of the knee. Am J Knee Surg. 1998;11(4):221–232. [PubMed] [Google Scholar]
- 17.Detterline AJ, Goldstein JL, Rue JP, Bach BR., Jr Evaluation and treatment of osteochondritis dissecans lesions of the knee. J Knee Surg. 2008;21(2):106–115. doi: 10.1055/s-0030-1247804. [DOI] [PubMed] [Google Scholar]
- 18.Erickson BJ, Chalmers PN, Yanke AB, Cole BJ. Surgical management of osteochondritis dissecans of the knee. Curr Rev Musculoskelet Med. 2013;6:102–114. doi: 10.1007/s12178-013-9156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim HC, Bae JH, Park YE, Park YH, Park JH, Park JW, et al. Long-term results of arthroscopic excision of unstable osteochondral lesions of the lateral femoral condyle. J Bone Joint Surg Br Vol. 2012;94(2):185–189. doi: 10.1302/0301-620X.94B2.27212. [DOI] [PubMed] [Google Scholar]
- 20.Pascual-Garrido C, Friel NA, Kirk SS, McNickle AG, Bach BR, Jr, Bush-Jospeh CA, et al. Midterm results of surgical treatment for osteochondritis dissecans of the knee. Am J Sports Med. 2009;37(Suppl 1):125S–130S. doi: 10.1177/0363546509350833. [DOI] [PubMed] [Google Scholar]
- 21.Wright RW, McLean M, Matava MJ, Shivelt RA. Osteochondritis dissecans of the knee: long-term results of excision of the fragment. Clin Orthop Relat Res. 2004;424:239–243. doi: 10.1097/01.blo.0000128216.10732.d8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AF, Pagnani MJ. Osteochondritis dissecans of the femoral condyles. Long-term results of excision of the fragment. Am J Sports Med. 1997;25(6):830–834. doi: 10.1177/036354659702500617. [DOI] [PubMed] [Google Scholar]
- 23.Shelbourne KD. Presented abstract at Arthroscopy Association of North America annual meeting, February 7, 2013; Sun Valley, Idaho.
- 24.Pennock AT, Bomar JD, Chambers HG. Extra-articular, intraepiphyseal drilling for osteochondritis dissecans of the knee. Arthrosc Technol. 2013;2(3):e231–e235. doi: 10.1016/j.eats.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edmonds EW, Albright J, Bastrom T, Chambers HG. Outcomes of extra-articular, intra-epiphyseal drilling for osteochondritis. J Pediatr Orthop. 2010;20:870–878. doi: 10.1097/BPO.0b013e3181f5a216. [DOI] [PubMed] [Google Scholar]
- 26.Boughanem J, Riaz R, Patel RM, Sarwark JF. Functional and radiographic outcomes of juvenile osteochondritis dissecans of the knee. Am J Sports Med. 2011;39:2212–2217. doi: 10.1177/0363546511416594. [DOI] [PubMed] [Google Scholar]
- 27.Adachi N, Deie M, Nakamae A, Ishikawa M, Motoyama M, Ochi M. Functional and radiographic outcome of stable juvenile osteochondritis dissecans. Arthroscopy. 2009;25:145–152. doi: 10.1016/j.arthro.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Adachi N, Ochi M, Deie M, Nakamae A. Paper 65; functional and radiographic outcome of juvenile osteochondritis dissecans of the knee treated with retroarticular drilling. Arthroscopy. 2012;28:e372. doi: 10.1016/j.arthro.2012.05.548. [DOI] [PubMed] [Google Scholar]
- 29.Louisia S, Beaufils P, Katabi M, Robert H. Transchondral drilling for osteochondritis dissecans of the medial condyle of the knee. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2003;11(1):33–39. doi: 10.1007/s00167-002-0320-0. [DOI] [PubMed] [Google Scholar]
- 30.Yonetani Y, Tanaka Y, Shiozaki Y, Kanamoto T, Kusano M, Tsuji A, et al. Transarticular drilling for stable juvenile osteochondritis dissecans of the medial femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2011. [DOI] [PubMed]
- 31.Kocher MS, Micheli LJ, Yaniv M, Zurakowski D, Ames A, Adrignolo AA. Functional and radiographic outcome of juvenile osteochondritis dissecans of the knee treated with transarticular arthroscopic drilling. Am J Sports Med. 2001;29(5):562–566. doi: 10.1177/03635465010290050701. [DOI] [PubMed] [Google Scholar]
- 32.•.Abouassaly M, Peterson D, Salci L, Farrokhyar F, D’Souza J, Bhandari M, et al. Surgical management of osteochondritis dissecans of the knee in the pediatric population: a systematic review addressing surgical techniques. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1216–1224. doi: 10.1007/s00167-013-2531-y. [DOI] [PubMed] [Google Scholar]
- 33.•.Adachi C, Deie M, Nakamae A, Okuhara A, Kamei G, Ochi M. Functional and radiographic outcomes of unstable juvenile osteochondritis dissecans lesion fixation using bioabsorbable pins. J Pediatr Orthop. 2015;35(1):82–88. doi: 10.1097/BPO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 34.Camp CL, Krych AJ, Stuart MJ. Arthroscopic preparation and internal fixation of an unstable osteochondritis dissecans lesion of the knee. Arthrosc Technol. 2013;2(4):e461–e465. doi: 10.1016/j.eats.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochs BG, Muller-Horvat C, Albrecht D, Schewe B, Weise K, Aicher WK, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. Am J Sports Med. 2011;39(4):764–773. doi: 10.1177/0363546510388896. [DOI] [PubMed] [Google Scholar]
- 36.Camathias C, Gogus U, Hirschmann MT, Rutz E, Brunner R, Haeni D, et al. Implant failure after biodegradable screw fixation in osteochondritis dissecans of the knee in skeletally immature patients. Arthroscopy. 2014;31(3):410–415. doi: 10.1016/j.arthro.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 37.Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443–460. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 38.Frisbie DD, Trotter GW, Powers BE, Rodkey WG, Steadman JR, Howard RD, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242–255. doi: 10.1053/jvet.1999.0242. [DOI] [PubMed] [Google Scholar]
- 39.Steadman JR, Briggs KK, Rodrigo JJ, Gill TJ, et al. Outcomes of patients treated arthroscopically by microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 40.Steadman JR, Karas SG, Miller BS, Schlegel TM, Briggs KK, Hawkins RJ. The Microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. Am J Knee Surg. 2003;16(2):83086. [PubMed] [Google Scholar]
- 41.Gudas R, Simonaityte R, Cekanauskas E, Tamosiunas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29(7):741–748. doi: 10.1097/BPO.0b013e3181b8f6c7. [DOI] [PubMed] [Google Scholar]
- 42.Vijayan S, Bartlett W, Bentley G, Carrington RW, Skinner JA, Pollock RC, et al. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: a two- to eight-year follow up study. J Bone Joint Surg Br Col. 2012;94(4):488–492. doi: 10.1302/0301-620X.94B4.27117. [DOI] [PubMed] [Google Scholar]
- 43.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A(Suppl 2):17–24. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 44.Cole BJ, Deberardino T, Brewster R, Farr J, Levine DW, Nissen C, et al. Outcomes of autologous chondrocyte implantation in Study of the Treatment of Articular Repair (STAR) patients with osteochondritis dissecans. Am J Sports Med. 2012. [DOI] [PubMed]
- 45.Steinhagen J, Bruns J, Deuretzbacher G, Ruether W, Fuerst M, Niggemeyer O. Treatment of osteochondritis dissecans of the femoral condyle with autologous bone grafts and matrix supported autologous chondrocytes. Int Orthop. 2010;34(6):819–825. doi: 10.1007/s00264-009-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delcogliano M, Menghi A, Lacella G, Speziali A, Cerulli G, et al. Treatment of osteochondritis dissecans of the knee with a biomimetic scaffold. A prospective multicenter study. Joints. 2014;2(3):102–108. doi: 10.11138/jts/2014.2.3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filardo G, Kon E, Di Martino A, et al. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med. 2013;41:1786–1793. doi: 10.1177/0363546513490658. [DOI] [PubMed] [Google Scholar]
- 48.Berruto M, Delcogliano M, de Caro F, et al. Treatment of large knee osteochondral lesions with a biomimetic scaffold: results of a multicenter study of 49 patients at 2-year follow-up. Am J Sports Med. 2014;42:1607–1617. doi: 10.1177/0363546514530292. [DOI] [PubMed] [Google Scholar]
- 49.Kang RW, Friel NA, Williams JM, Cole BJ, Wimmer MA. Effect of impaction sequence on osteochondral graft damage: the role of repeated and varying loads. Am J Sports Med. 2010;38(1):105–113. doi: 10.1177/0363546509349038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ollat D, Lebel B, Thaunat M, Jones D, Mainard L, Dubrana F, et al. Mosaic osteochondral transplantations in the knee joint, mid-term results of the SFA multicenter study. Orthop Traumatol Surg Res OTSR. 2011;97(8 Suppl):S160–S166. doi: 10.1016/j.otsr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40:2499–2508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 52.Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 53.McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411–420. doi: 10.1177/0363546506295178. [DOI] [PubMed] [Google Scholar]
- 54.Emmerson BC, Gortz S, Jamali AA, Chung C, CAmiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 55.Lyon R, Nissen C, Liu XC, Curtin B. Can fresh osteochondral allografts restore function in juveniles with osteochondritis dissecans of the knee. Clin Orthop Relat Res. 2013;471:1166–1173. doi: 10.1007/s11999-012-2523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635–640. doi: 10.1177/0363546513516747. [DOI] [PubMed] [Google Scholar]
- 57.••.Kramer DE, Yen YM, Simoni MK, Miller PE, Micheli LJ, Kocher MS, et al. Surgical management of osteochondritis dissecans lesions of the patella and trochlea in the pediatric and adolescent population. Am J Sports Med. 2015;43:654–662. doi: 10.1177/0363546514562174. [DOI] [PubMed] [Google Scholar]


