Abstract
As our patients become more physically active at all ages, the incidence of injuries to articular cartilage is increasing and is causing patients significant pain and disability at a younger age. The intrinsic healing response of articular cartilage is poor, because of its limited vascular supply and capacity for chondrocyte division. Nonsurgical management for the focal cartilage lesion is successful in the majority of patients. Those patients that fail conservative management may be candidates for a cartilage reparative or reconstructive procedure. The type of treatment available depends on a multitude of lesion-specific and patient-specific variables. First-line therapies for isolated cartilage lesions have demonstrated good clinical results in the correct patient but typically repair cartilage with fibrocartilage, which has inferior stiffness, inferior resilience, and poorer wear characteristics. Advances in cell-based cartilage restoration have provided the surgeon a means to address focal cartilage lesions utilizing mesenchymal stem cells, chondrocytes, and biomimetic scaffolds to restore hyaline cartilage.
Keywords: Hyaline, Cartilage, Chondrocyte, Matrix, Scaffold
Introduction
Due to the increase in physical activity among patients of all ages, injury to articular cartilage is increasing causing significant pain and disability. One study of 31,516 knee arthroscopies found that 63 % of patients had chondral injury [1]. Another review of 993 knee arthroscopies of patients with a mean age of 35 years old, found an 11 % incidence of full thickness cartilage lesions that could have benefited from surgical treatment [2]. Even though we do not know what factors lead asymptomatic cartilage lesions to eventually become symptomatic, we do know that chondral lesions further degenerate within the knee over time [3, 4].
The intrinsic healing response of articular cartilage is poor, because of its limited vascular supply and capacity for chondrocyte division and migration [5]. Superficial damage will injure chondrocytes, limit their metabolic capacity for repair, and lead to decreased proteoglycan concentration, increased hydration, and altered fibrillar organization of collagen [6, 7]. This leads to increased force transmission to the damaged cartilage causing damage to the neighboring healthy cartilage. This vicious cycle is thought to contribute to the progression of partial-thickness articular cartilage injuries to full-thickness injuries and eventually diffuse osteoarthritis [8, 9].
A defect that penetrates the subchondral plate has a higher capacity to heal with a normal healing response beginning with hematoma formation, stem cell migration, and synthesis of type 1 cartilage [10]. Once the subchondral plate has been violated, an influx of marrow contents including inflammatory cells, undifferentiated mesenchymal cells, cytokines, and growth factors bathe the injured area and stimulate cartilage formation [11]. However, the resultant repair typically resembles fibrocartilage instead of hyaline cartilage, which has inferior stiffness, inferior resilience, and poorer wear characteristics [12].
Nonsurgical management of articular cartilage injury consisting of rest, analgesics to control pain, and anti-inflammatory medications has remained largely the same over many decades. There is little to no evidence in the literature supporting corticosteroid or viscosupplementation injections in the setting of focal cartilage lesions. Failure of a trial of nonoperative treatment for 4–6 months is an indication for surgery to address the focal cartilage defect. However, surgical treatment of chondral injuries continues to evolve, and there are many techniques currently at the surgeon’s disposal. Autologous chondrocyte implantation is one of these techniques that are currently being refined to restore the more durable hyaline cartilage.
Evaluation of chondral injuries
Most patients will present with pain in the affected joint and not always recall a specific injury. A patient who recalls a single injury or series of injuries is likely to have incurred a focal chondral or osteochondral lesion, whereas an atraumatic injury is likely a degenerative chondral lesion or diffuse osteoarthritis. A thorough exam should be performed assessing gait, limb alignment, range of motion, presence of an effusion, and ligamentous stability at the time of initial presentation.
Initial evaluation should include radiographs. Radiographic examination not only may show the specific osteochondral defect, it may also show associated pathology such as osteophytes, joint space narrowing, fractures, or signs of ligamentous injury which may affect treatment options. MRI can assist with preoperative planning to determine the size and location of the lesion and can better access the other compartments and soft tissue components of the knee.
Arthroscopy is the most accurate way to access the location, size, depth, shape, and stability of articular cartilage. Currently, the most used classification system for describing chondral injuries was initially described by Dr. Outerbridge [13], which was designed to describe chondromalacia of the patella. This system classifies chondral injury in four grades: grade 1, softening and swelling of the cartilage; grade 2, partial thickness fragmentation and fissuring of the cartilage in an area less than 1.5 cm in diameter; grade 3, full thickness fracturing and fissuring involving greater than 1.5 cm in diameter of cartilage; and grade 4, erosion of cartilage down to bone.
Recently, the International Cartilage Repair Society (ICRS) introduced a universal grading system that offers a more precise description of the damaged cartilage [14, 15]. In this system, grade 1 is normal, grade 1a has some mild softening or fibrillations, and grade 2 has more involvement but still less than 1/2 the cartilage depth. Grade 3 lesions involve more than 1/2 the cartilage depth and include subgroups a, b, c, and d with increasing severity of damage (3a, 50 % cartilage thickness damage; 3b, damage to calcified cartilage; 3c, exposed subchondral bone; and 3d, full-thickness delamination). A grade 4 lesion is an osteochondral lesion violating the subchondral plate (4a, superficial, and 4b, deep bony involvement).
Chondral injuries cannot be treated in a vacuum. Concomitant ligamentous insufficiency, mechanical malalignment, patellar maltracking, and dysfunctional menisci must be addressed to maximize patient outcomes and provide a suitable environment for the cartilage restorative procedure [5, 16]. Failure to do so may overload the restored cartilage, as the concomitant pathology did to the native cartilage, leading to surgical failure even when the biology may have otherwise been successful. Even a partial meniscectomy leads to increased tibiofemoral contact pressures [17], and concomitant meniscal repair or in more severe cases allograft transplantation should be considered, as opposed to meniscectomy, as these patients have shown equivalent or superior results when compared to patients undergoing cartilage restoration procedures alone [18, 19].
Cultured autologous chondrocyte implantation
Autologous chondrocyte implantation (ACI) is a technique, developed by Dr. Lars Peterson and coworkers in Sweden during the 1980s, that attempts to repair the damaged chondral tissue by replacing it with viable autogenous chondrocytes [20]. As originally described, this is done in two separate procedures. The first stage involves an arthroscopic evaluation of the focal chondral lesion to assess containment, depth, and potential bone loss. During the first stage, biopsy of normal hyaline cartilage is performed from a nonweight-bearing region of the knee. The typical harvest sites include the lateral trochlea near the sulcus terminalis, the intercondylar notch, and the medial trochlea [21, 22]. The total volume of the biopsy should be approximately 200 to 300 mg, preferably in three “Tic-Tac-sized” fragments [1A]. This tissue is then sent to a lab to be enzymatically treated to release the chondrocytes, which are subsequently expanded in culture for a period of 2–4 weeks to create more than 10 million cells from only the few hundred thousand cells originally in the biopsy.
The second stage of the procedure is cell implantation, which typically takes place between 6 weeks and 18 months after the biopsy, and is done through an arthrotomy. The surgical exposure depends on defect location. Patellofemoral lesions are approached through a midline incision, allowing a simultaneously performed tibial tubercle osteotomy, and femoral condyle lesions are addressed through limited parapatellar arthrotomies. Circular- or oval-shaped prepared defects are biomechanically more stable [23] (Fig. 1b). The diseased cartilage is debrided down to the subchondral layer, leaving healthy surrounding hyaline cartilage to form stable vertical walls shouldering the lesion. Care must be taken not to penetrate the subchondral bone, and the site should be packed with thrombin-soaked gauze to prevent bleeding into the area of repair, which could impede chondrocyte growth. Additionally, preoperative imaging and initial arthroscopy should provide the surgeon with evidence of subchondral endplate disruption, and if the depth of the lesion is >8 mm, then it is recommended to perform bone grafting of the lesion at the time of ACI or in a staged fashion. According to the original description, the cultured cells are then implanted under an autologous periosteal patch taken from the proximal medial tibial cortex using a separate incision. This periosteal patch is then secured with a 6–0 vicryl suture on a cutting needle, and a watertight seal is created with fibrin glue. Prior to sealing the top of the patch and injecting the chondrocytes, a water–seal test should be first performed by completely drying the surgical site and injecting a small volume of sterile saline. There should be a complete containment of the saline without leaking. In cases where the cartilage lesion is located near the edge of the articular surface, the periosteal patch repair may be augmented with a small suture anchor or bone tunnels in order to contain the injected chondrocytes. This original description of ACI is now known as first-generation technique or periosteum-based autologous chondrocyte implementation (P-ACI).
Fig. 1.
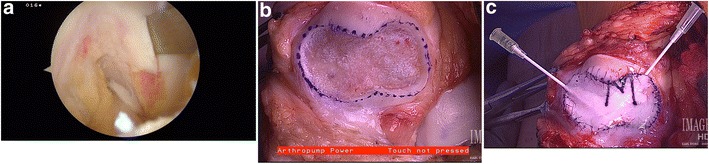
a Arthroscopic photograph of a chondral harvest of a nonweight-bearing portion of the femoral condyle. b Prepared lesion of the patella articular surface. c Patellar lesion that has been prepared with autologous chondrocyte implantation using collagen membrane (C-ACI) technique and ready for chondrocyte implantation
Limitations of the periosteal patch (such as periosteal hypertrophy, periosteum suturing, calcification, delamination, intra-articular adhesions, another surgery to harvest the periosteum, and patch integrity in older patients) have led to the development of synthetic substitutes [24•]. Second-generation ACI substitutes the periosteum with a membrane containing type I/III collagen [25] (Fig. 1c). The advantages of second-generation ACI are the availability of the biopatch, usability, decreased risk of patch hypertrophy, and decreased surgical time. Third-generation ACI uses biomimetic membranes or scaffolds that are seeded with the harvested chondrocytes prior to implantation [26]. The scaffolds have a nanofiber architecture that maximizes the potential for chondrocyte ingrowth and maintenance of the extracellular matrix. These surgical techniques are also called autologous chondrocyte implantation using collagen membrane (C-ACI) and membrane-associated autologous chondrocyte implantation (M-ACI), respectively.
Results of autologous chondrocyte implantation
Cultured autologous chondrocyte implantation has also shown to improve pain and function in patients with second-look biopsies showing hyaline-like cartilage [27–29, 30•]. Good results have also been reported at 85 % on patellar lesions as long as an anteromedialization tibial tubercle osteotomy was done concurrently [31]. While there has been evidence that autologous cultured chondrocyte implantation has a higher failure rate and worse clinical outcome following a failed microfracture procedure [32, 33], other investigators have shown clinical improvement following marrow-stimulating procedures [34]. Ideally, cultured chondrocyte implementation is used on focal unipolar defect measuring 2–10 cm2 with minimal subchondral bone loss.
Dr. Peterson et al. [27] reported 10–20-year follow-up (mean 12.8 years) on 224 patients who underwent ACI for cartilage lesions measuring about 5.3 cm2. Seventy-four percent of these patients reported their status as better or the same, and 92 % of patients were satisfied and would have ACI again. They also noted an increase in all functional knee scores (Lysholm, Tegner–Wallgren, Brittberg–Peterson, modified Cincinnati (Noyes), and Knee Injury and Osteoarthritis Outcome Score (KOOS)) compared to their preoperative values. In the USA, Dr. Minas et al. [30•] reported a 10-year outcome data (mean follow-up of 12 years) on 210 patients who had received ACI for lesions with a mean surface area of 8.4 cm2. He reported a 25 % (53 patients) failure rate of the graft at 10 years with 19 of these patients going on to arthroplasty, 27 getting a revision ACI procedure, and 7 declining further treatment. Seventy-five percent of his patients had an increase of function, and he reported increases in all of the function knee scores compared to their preoperative values. He also reported that a previous marrow-stimulating procedure and lesions larger than 15 cm in patients were predictors for failure in his cohort.
Few randomized controlled trials comparing the various cartilage restoration procedures exist; however, there are a few comparing ACI to microfracture, each reporting differing results. Knutsen et al. [35] compared microfracture to ACI with histology ranging from fibrous to hyaline-like in both groups with no correlation with clinical outcome at 5 years. On the other hand, Saris et al. [36] reported superior histology of biopsies of a chondrocyte-cell-based technology compared to microfracture at 5 years, with those patients implanted with the chondrocyte-cell-based technology procedure demonstrating superior outcomes. Criticisms of the Knutsen series included inadequate numbers for statistical power and lack of subset analysis of smaller versus larger lesions.
Second- and third-generation ACIs have also shown promising restoration of hyaline-like cartilage and similar results while mitigating the limitations of P-ACI [37–39] (Table 1). First, Gomoll et al. [19] reported the results of 300 consecutive patients who underwent P-ACI to the results of the next 100 consecutive patients who underwent C-ACI for failure rates and reoperation rates due to graft hypertrophy. They saw a decrease from 23 to 5 % in reoperation rates due to graft hypertrophy after switching to C-ACI with no significant difference in failure rates. Gooding et al. [40] reported the 2-year results of P-ACI (33 patients) compared with those of C-ACI (35 patients) in a randomized controlled study. The mean age of the patients was 30.5 years, and the mean lesion size was 4.54 cm2. They did not show any statistical difference in results between the two groups; however, a significant number of patients required a subsequent arthroscopy and periosteal shaving in the P-ACI group.
Table 1.
Table summarizing the current available literature for autologous chondrocyte implantation technique (ACI)
| Author, year | Type of study | Technique evaluated | Follow-up | Subjective results | Objective results |
|---|---|---|---|---|---|
| Peterson et al. 2010 [27] | Case series | ACI (224 patients) | 12.8 years | 74 % reported same or better than before surgery | Significant increase in Lysholm, Tegner, Brittberg–Peterson, and KOOS scores |
| 92 % were satisfied and would do again | |||||
| Minas et al. 2014 [30•] | Prospective cohort | ACI (210 patients) | 12 years | 75 % reported improved function | Improved modified Cincinnati, WOMAC, KSS, SF-36 scores |
| 71 % did not need another surgery | |||||
| Knutsen et al. 2007 [35] | Randomized controlled trial | ACI (40 patients) versus Microfracture (40 patients) | 5 years | Both techniques had a success rate of 77 % | No difference in histological quality on second look biopsies |
| 33 % of patients in both groups had radiographic findings of OA | |||||
| Saris et al. 2011 [36] | Randomized controlled trial | ACI (51 patients) versus Microfracture (61 patients) | 5 years | 7 failures in ACI and 10 failures in MF | Better increases in KOOS scores are noted in the ACI group, especially if onset of the symptoms was less than 3 years |
| Gomoll et al. 2009 [19] | Retrospective cohort | P-ACI (300 patients) versus C-ACI (100 patients) | 1 year | P-ACI had 2.3 % failure rate and 25.7 % reoperation rate due to graft hypertrophy | |
| C-ACI had 4 % failure rate and 5 % reoperation rate due to graft hypertrophy | |||||
| Gooding et al. 2006 [40] | Randomized controlled trial | P-ACI (33 patients) versus C-ACI (35 patients) | 2 years | P-ACI had 67 % good to excellent results C-ACI had 74 % good to excellent results | Second look arthroscopies showed similar results |
| 36.4 % in P-ACI group required shaving for graft hypertrophy | |||||
| Bartlett et al. 2005 [39] | Randomized controlled trial | C-ACI (44 patients) versus M-ACI (47 patients) | 1 year | Comparable increases in modified Cincinnati and CRS scores. Comparable graft hypertrophy and reoperation rates. Both techniques had hyaline-like cartilage on second-look biopsies | |
| Zeifang et al. 2010 [41] | Randomized controlled trial | P-ACI (10 patients) versus M-ACI (11 patients) | 2 years | No differences in IKDC, SF-36, and Tegner scores between groups. Lysholm score were significantly better in the P-ACI group. MOCART scores on postoperative MRI were significantly better in P-ACI group | |
| Niethammer et al. 2015 [42•] | Retrospective cohort | M-ACI (143 patients) | 2 years | Revision rate was 23.4 % for symptomatic bone marrow edema (8.3 %, n = 3), arthrofibrosis (22.2 %, n = 8), and partial graft cartilage deficiency (47.2 %, n = 17) |
ACI autologous chondrocyte implantation, KOOS Knee Injury and Osteoarthritis Outcome Score, P-ACI periosteum-based autologous chondrocyte implementation, C-ACI collagen membrane autologous chondrocyte implantation, M-ACI membrane-associated autologous chondrocyte implantation, MOCART magnetic resonance observation of cartilage repair tissue, CRS cincinnati rating system, IKDC international knee documentation committee, KSS knee society score, OA osteoarthritis, SF-36 short form 36, MF Microfracture, WOMAC Western Ontario and McMaster Universities Arthritis Index
M-ACI was developed to mitigate some of the disadvantages of P-ACI and C-ACI, namely, unequal distribution of cells in the chamber, delamination, and suturing of the membrane to surrounding cartilage. A collagen I/III matrix is infused with the harvested chondrocytes and implanted as a unit, with early reported results comparable to previous generations of ACI, although inconclusive. Bartlett et al. [39] preformed a randomized, prospective trial comparing C-ACI (44 patients; mean age, 33.7 years) to M-ACI (47 patients; mean age, 33.4 years) with a mean lesion size of 6 cm2 and a mean follow-up period of 1 year. He reported improvement in all clinical scores with both the techniques, and histologic assessments showed no significant difference between the groups. Zeifang et al. [41] compared full-thickness cartilage lesions treated with either P-ACI or M-ACI techniques in 21 patients with a mean defect size of 4.1 cm2 on the femoral condyle. At 2 years, they found similar IKDC, Tegner, and SF-36 scores; however, the Lysholm and Gillquist scores were significantly lower in the M-ACI group. On MRI examination, the M-ACI group had significantly lower magnetic resonance observation of cartilage repair tissue (MOCART) scores. Recently, a revision rate of 23 % has been reported in M-ACI within the first 2 years for arthrofibrosis, symptomatic bone marrow edema, and partial graft cartilage deficiency [42•]. Also, many of these patients have graft hypertrophy within the first 2 years with an unknown clinical significance [43•].
Because these techniques are relatively new, there are only a few short-term follow-up studies with small patient cohorts, and predominance of young patients with medium-sized defects. More studies are needed with larger sample sizes and longer follow-up going forward with these newer techniques to demonstrate improved outcomes compared to first-generation ACI. In our practice, we perform C-ACI using a noncrossed-linked type I/III collagen porcine collagen patch (Bio-Gide® Geistlich). This patch is commonly used in oral-maxillary facial procedures and has a natural bilayer with a smooth layer that is placed facing the joint and a dense porous layer that acts as a guide for chondrocyte attachment. The patch can be easily handled by the surgeon and has resiliency ideal for suture placement. There are several commercially available collagen membrane patches, some of which utilize bovine collagen. The ideal patch should have acceptable biodegradation time (Bio-Gide 4–6 weeks), high vascularization and ingrowth, and limited potential for foreign body reaction. Patch overgrowth has not been an issue utilizing this commercially available collagen patch.
In our active duty military patient population, it is critical to select the appropriate patient for success of the procedure and return to full duty. In addition to the aforementioned indications and contraindications, one of the most important factors leading to success is selecting a patient who can be cooperative with weight-bearing restrictions and for a long rehabilitation protocol (12–18 months). Surgeons should be aware that all generations of ACI are considered experimental in the patellofemoral joint and on the talus. C-ACI and M-ACI are also considered experimental due to the lack of conclusive evidence of improved outcomes over first-generation ACI.
Conclusion
Significant chondral injury is a major clinical challenge to bring relief and increased function to patients. There are many surgical options available to treat this difficult condition, and many of these techniques are continuing to be developed and refined. It is imperative for a provider treating these conditions to keep up on current literature. Cell-based therapies, including ACI, offer promising and attractive treatment options for patients with the isolated cartilage lesion or as salvage procedures for other failed treatments. Further randomized controlled studies to investigate the long-term outcomes of these procedures are needed to help guide treatment in this continually evolving field of cartilage reconstruction.
Acknowledgments
The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US Government.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Dr. Giuliani and Dr. Pickett have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cartilage Repair Techniques in the Knee
Contributor Information
Jeffrey R. Giuliani, Phone: 301-512-5551, Email: Jeffrey.R.Giuliani.mil@mail.mil
Adam Pickett, Phone: 801-310-3057, Email: Adam.M.Pickett@gmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–60. doi: 10.1016/S0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 2.Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–5. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 3.Linden B. Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am. 1977;59(6):769–76. [PubMed] [Google Scholar]
- 4.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996;67(2):165–8. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 5.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 6.Lohmander LS, Dahlberg L, Ryd L, Heinegård D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989;32(11):1434–42. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- 7.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–6. [PubMed] [Google Scholar]
- 8.Mow VC, Ratcliffe A, Rosenwasser MP, Buckwalter JA. Experimental studies on repair of large osteochondral defects at a high weight bearing area of the knee joint: a tissue engineering study. J Biomech Eng. 1991;113(2):198–207. doi: 10.1115/1.2891235. [DOI] [PubMed] [Google Scholar]
- 9.Hunziker EB, Quinn TM. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am. 2003;85-A(Suppl 2):85–92. doi: 10.2106/00004623-200300002-00011. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg VM, Caplan AI. Biologic restoration of articular surfaces. Instr Course Lect. 1999;48:623–7. [PubMed] [Google Scholar]
- 11.Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62(1):79–89. [PubMed] [Google Scholar]
- 12.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–62. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Outerbridge RE. The Etiology of Chondromalacia Patellae. 1961;2001:5–8. doi: 10.1097/00003086-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 15.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85(A Suppl 2):45–57. [PubMed] [Google Scholar]
- 16.Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696–705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SJ, Aadalen KJ, Malaviya P, Lorenz EP, Hayden JK, Farr J, et al. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. 2006;34(8):1334–44. doi: 10.1177/0363546506286786. [DOI] [PubMed] [Google Scholar]
- 18.Rue J-PH, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770–8. doi: 10.1177/0363546508317122. [DOI] [PubMed] [Google Scholar]
- 19.Gomoll AH, Kang RW, Chen AL, Cole BJ. Triad of cartilage restoration for unicompartmental arthritis treatment in young patients: meniscus allograft transplantation, cartilage repair and osteotomy. J Knee Surg. 2009;22(2):137–41. doi: 10.1055/s-0030-1247738. [DOI] [PubMed] [Google Scholar]
- 20.Peterson L. Articular cartilage injuries treated with autologous chondrocyte transplantation in the human knee. Acta Orthop Belg. 1996;62(Suppl 1):196–200. [PubMed] [Google Scholar]
- 21.Ahmad CS, Cohen ZA, Levine WN, Ateshian GA, Mow VC. Biomechanical and topographic considerations for autologous osteochondral grafting in the knee. Am J Sports Med. 2001;29(2):201–6. doi: 10.1177/03635465010290021401. [DOI] [PubMed] [Google Scholar]
- 22.Garretson RB, Katolik LI, Verma N, Beck PR, Bach BR, Cole BJ. Contact pressure at osteochondral donor sites in the patellofemoral joint. Am J Sports Med. 2004;32(4):967–74. doi: 10.1177/0363546503261706. [DOI] [PubMed] [Google Scholar]
- 23.Cole BJ, D’Amato M. Autologous chondrocyte implantation. Operative techniques in orthopaedics 2001;11(2):115–31.
- 24.•.Goyal D, Goyal A, Keyhani S, Lee EH, Hui JHP. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy 2013;29(11):1872–1878. doi:10.1016/j.arthro.2013.07.271. A systemic review of only level I and level II studies comparing first generation ACI to newer generation ACI techniques. This study highlights the weak evidence supporting C-ACI (collagen based) over P-ACI (periosteum based) and no evidence to support arthroscopic or scaffoldbased ACI. This study points out the paucity of studies with large patient population and long term follow-up. [DOI] [PubMed]
- 25.Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11(1):51–5. doi: 10.1016/S0968-0160(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 26.Steinwachs M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009;25(2):208–11. doi: 10.1016/j.arthro.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–24. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 28.Cole BJ, DeBerardino T, Brewster R, Farr J, Levine DW, Nissen C, et al. Outcomes of autologous chondrocyte implantation in study of the treatment of articular repair (STAR) patients with osteochondritis dissecans. Am J Sports Med. 2012;40(9):2015–22. doi: 10.1177/0363546512453292. [DOI] [PubMed] [Google Scholar]
- 29.Micheli LJ, Browne JE, Erggelet C, Fu F, Mandelbaum B, Moseley JB, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11(4):223–8. doi: 10.1097/00042752-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 30.•.Minas T, Keudell Von A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472(1):41–51. doi:10.1007/s11999-013-3146-9. This is a Level IV evidence study that is the first to look at the long-term survivorship of ACI grafts as well as functional outcomes using validated scoring tools after ACI. Most importantly, it highlights the most common cause of failure of ACI to be previous microfracture procedure and larger lesions. [DOI] [PMC free article] [PubMed]
- 31.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–34. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325–31. doi: 10.1177/0363546511425651. [DOI] [PubMed] [Google Scholar]
- 33.Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–8. doi: 10.1177/0363546508330137. [DOI] [PubMed] [Google Scholar]
- 34.Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. STAR study principal investigators. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the study of the treatment of articular repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55. doi: 10.1177/0363546508322897. [DOI] [PubMed] [Google Scholar]
- 35.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–12. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 36.Vanlauwe J, Saris DBF, Victor J, Almqvist KF, Bellemans J, Luyten FP. TIG/ACT/01/2000&EXT Study Group. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39(12):2566–74. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 37.Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37 Suppl 1(1_suppl):20S–23S. doi: 10.1177/0363546509348477. [DOI] [PubMed] [Google Scholar]
- 38.Samuelson EM, Brown DE. Cost-effectiveness analysis of autologous chondrocyte implantation: a comparison of periosteal patch versus type I/III collagen membrane. Am J Sports Med. 2012;40(6):1252–8. doi: 10.1177/0363546512441586. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 40.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13(3):203–10. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38(5):924–33. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 42.•.Niethammer T, Valentin S, Ficklscherer A, Gülecyüz M, Pietschmann M, Müller P. Revision surgery after third generation autologous chondrocyte implantation in the knee. International Orthopaedics (SICOT) 2015;39(8):1615–1622. doi:10.1007/s00264-015-2792-9. Study looking at the revision rates and their risk factors with third generation Collage I/III scaffold ACI. This study is unique in that it is one of the first to demonstrate a high revision rate (23.4%) with third generation technique and it points out the common causes for revision surgery and the improved clinical outcomes from those surgeries. [DOI] [PubMed]
- 43.•.Ebert JR, Smith A, Fallon M, Butler R, Nairn R, Breidahl W, Wood DJ. Incidence, degree, and development of graft hypertrophy 24 months after matrix-induced autologous chondrocyte implantation: association with clinical outcomes. Am J Sports Med July 2015:0363546515591257. doi:10.1177/0363546515591257. Level IV study looking at the incidence of graft hypertrophy with M-ACI (Matrix–Induced) adcorrelation with clinical outcomes. Despite a high rate of graft hypertrophy, this study demonstrated no difference in clinical outcome scores and/or correlation of severity of graft hypertrophy with outcomes. [DOI] [PubMed]


