Abstract
Revision total knee arthroplasty (TKA) is the treatment of choice in patients with periprosthetic joint infection. It may be performed in either a single stage or two stages. In the latter option, between stages, an antibiotic-loaded spacer may be used to maintain a certain amount of joint stability and mobility after the infected implant is removed, adding an intra-articular concentration of antibiotics. There are two types of antibiotic-loaded cement spacers: static and dynamic. Static spacers basically create a temporary arthrodesis with antibiotic-loaded cement and usually are handmade within the surgical field. Dynamic spacers can be created intraoperatively by using different tools or may be prepackaged by the manufacturer; they allow range of motion between stages. In this article, the authors review the indications, surgical techniques, and results for static and dynamic spacers in two-stage revision TKA.
Keywords: Revision total knee arthroplasty, Infection, Spacer, Cement, Treatment
Introduction
Periprosthetic joint infection (PJI), a complication much feared by the orthopedic surgeon, is now one of the most common indications for revision in total knee arthroplasty (TKA) [1, 2].
The latest and best accepted definition of PJI is based on two major criteria: two positive periprosthetic cultures with phenotypically identical organisms and the presence of a sinus tract communicating with the joint. Furthermore, PJI is diagnosed if at least three of six minor criteria are present: elevated serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), elevated synovial fluid white blood cell count (WBC), elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%), positive histologic analysis of periprosthetic tissue, or a single positive culture [3•, 4, 5].
Revision surgery in infected TKA may be very challenging for the orthopedic surgeon and result in inferior clinical outcomes compared with primary TKA [6]. Furthermore, compared with revision TKA from other causes, surgery performed for septic loosening results in higher costs [7]. Revision TKA for PJI may be performed as either a one- or two-stage procedure. One-stage revision TKA exposes the patient to only one operation consisting of removal of the old prosthesis, aggressive and complete tissue debridement, and immediate positioning of the new implant [8]. Several authors have reported decreased morbidity, reduced health care costs, and improved functional results as major advantages of one-stage revision TKA [9, 10].
Two-stage revision TKA, on the other hand, begins with surgery to expose the knee articulation, remove the infected components, completely debride the infected tissue, and implant an antibiotic-loaded cement spacer. Patients receive specific oral or intravenous antibiotic therapy, or both, targeted toward bacteria isolated during the first-stage surgery [11]. Although the ideal duration for antibiotic therapy is not defined, most of the recent literature recommends a 6- to 12-week course [12–15]. Once the infection is eradicated, and CRP and ESR levels return to normal, the second surgery is performed to remove the spacer and implant the revision prosthesis. The latter approach may be performed in most cases of PJI [16, 17] and has a success rate ranging from 37.1 to 100 % [18–20].
In this article, we focus on antibiotic-loaded cement spacers and review the literature regarding indications and results.
Antibiotic-loaded cement spacers
Antibiotic-loaded cement spacers are used routinely in two-stage revision TKA. The goal of two-stage revision TKA is to radically eliminate the infection and to create the healthiest tissue possible for the new implant [21]. Between the two stages, the surgeon’s main goal is to prevent soft tissue contraction, which may be difficult to treat during the second-stage implantation [22]. Therefore, it is crucial for the surgeon to maintain a sufficient grade of joint stability while adding an intra-articular concentration of antibiotics and keeping the patient’s knee joint free of any foreign infected prosthetic material [23, 24].
Antibiotic-loaded cement spacers may be classified in two types:
Static spacers, which do not allow motion through the knee joint
Articulating or dynamic spacers, which allow range of motion (ROM) of the knee
Static cement spacers
Definition
Static spacers keep the knee joint in full extension or minimal flexion. Although they prevent movement of the knee, they preserve the joint space and deliver local antibiotic. The static spacer may be considered a temporary antibiotic-loaded knee arthrodesis.
Indications and contraindications
Some authors state that static spacers provide greater relief to infected and congested soft tissues, allowing for better eradication of the infection [25]. Others report that these devices cost significantly less than articulating spacers [26]. However, several disadvantages have been described that affect both the patient postoperatively and the surgeon intraoperatively during the second stage of the revision.
The main drawback of static spacers is joint stiffness with poor ROM after the second stage of the revision; however, instability and wound healing problems also have been associated with static spacers [27], although less frequently than with use of dynamic spacers. Technical concerns also exist, such as accurate insertion of the rods into the intramedullary canals, the failure of which is associated with exposure difficulty during revision surgery [28]. Furthermore, some authors assert that static spacers usually do not restore the normal anatomic joint contours, particularly in heavier patients, leading to significant bone loss with a higher risk for spacer displacement [29].
Considering all the advantages and disadvantages of static spacers, these devices may be indicated in patients with ligamentous instability, insufficient extensor mechanism, and massive bone loss after infected prosthesis removal, or in cases of a compromised overlying soft tissue envelope [30–32].
Surgical technique
The surgical approach to an infected knee is similar to most revision procedures and is the same for static and dynamic spacers [33]. After the patient is given anesthesia and intravenous prophylactic antibiotics, the knee is prepared for surgery. A longitudinal incision is made along the previous scar, and a medial parapatellar arthrotomy is performed. Tissue and fluid samples should be obtained from representative areas; tissue cultures are preferable because of their increased sensitivity (93 vs. 70 %) and specificity (98 vs. 89 %) compared with swab cultures [34]. Although there is no consensus regarding the number of samples to be taken, sensitivity and specificity are greater with three to six periprosthetic swabs, preferably obtained from the bone interface [35]. Culture specificity should not be reduced by taking more than five samples, especially in cases of less virulent organisms or in patients with recent antibiotic treatment, in whom up to 10 samples may be collected [36]. After samples are obtained, wide surgical debridement and complete synovectomy are performed and all previous components and any remaining cement and necrotic bone are removed [28, 37].
The first step in implanting a static antibiotic-loaded cement spacer is to construct it, a procedure that may be done directly in the surgical field. The typical static spacer consists of two rods fitting in both the femoral and tibial canals and overlapping across the joint space, as well as a parallelepiped cement block to fill the joint space left empty after the implant is removed. For the intramedullary rods, two large Steinman 3-mm pins are completely covered by cement; then, the correct-sized static element is assembled with a cement spacer block and positioned between the femoral and tibial surfaces. More antibiotic-loaded cement is applied to the femoral surface of the suprapatellar pouch to minimize the quadriceps tendon from scarring down to the distal femur. To obtain the volume of cement needed, two to three 40-g bags of cement typically are required (a total of 80 to 120 g); however, there is no consensus regarding the best method of preparing high-dose antibiotic cement spacers. The cement spacer should have enough antibiotic agent to deliver relatively high doses to local tissues. At the same time, the antibiotic dose should be low enough to prevent weakening of the cement’s mechanical properties [38]. Most infections can be treated with a spacer containing vancomycin (1 to 4 g per 40-g package of cement) and gentamicin or tobramycin (2.4 to 4.8 g per 40-g package of cement) [23]. It is crucial to achieve the right tension on the soft tissues, ensuring the implant is neither too loose nor too tight, thus avoiding the increased risk of bone loss during the second step (Fig. 1). A drain is positioned intra-articularly and left in situ for 24 h after surgery.
Fig. 1.
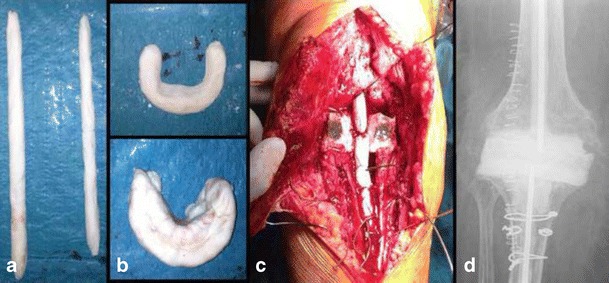
Antibiotic-loaded cemented static spacer. a Handmade intramedullary rods. b Handmade spacers for maintaining joint tension. c Definitive static spacers. d Postoperative radiograph, anteroposterior view
Articulating cement spacers
Definition
The main characteristic of dynamic antibiotic-loaded cement spacers is that they allow flexion and extension of the knee between the two surgical stages. As with static spacers, maintenance of the joint space and local delivery of antibiotic are the main functions.
Indications and contraindications
The main advantage of an articulating spacer is that it efficiently eradicates infection while allowing range of knee motion during the interval between surgical stages [39].
Revision surgery in patients with an articulating spacer may be easier than in those with a static one [39, 40]. Allowing motion during the surgical stages is useful in maintaining adequate length and elasticity of the extensor mechanism, preventing scar tissue formation around the knee joint, quadriceps shortening, and capsular thickening and contracture [41], all of which may explain the easier reimplantation during revision surgery [42]. Moreover, the patient’s ability to bend the knee increases his or her quality of life between stages, especially if a long period of antibiotic therapy is necessary to eradicate the infection.
No clear contraindications exist regarding the use of an articulating spacer, except in cases in which there is concern over wound healing. Disadvantages of commercially available off-the-shelf dynamic spacers include a limited choice of implant sizes and antibiotic dosages. Handmade mobile spacers also have drawbacks, such as difficulty in maintaining stability and a well-shaped and congruent articular surface [37].
Surgical techniques
Several techniques have been described for creating a dynamic spacer using different types of interfaces, including cement-on-cement, prosthesis-on-polyethylene, and metal-on-polyethylene constructs [23]. The first steps are the same as those described for static spacer implantation: the surgical approach, extended debridement, synovectomy, and removal of components, with particular care taken to preserve as much healthy bone as possible.
Cement-on-cement interface: molded or preformed spacers
Cement-on-cement spacers fall into two major categories: molded and preformed [43].
Different molds are available commercially in various sizes and dimensions. Custom-made molds can be assembled with standard posterior stabilized TKA provisional components (trials) the same size as the original prosthesis [44]. The cement is loaded with gentamicin (0.5 g per 40-g package) or vancomycin antibiotic powder (3 g per 40-g package); usually, two to three cement packages are enough to complete the spacer, depending on the requested size. After the correct mold is chosen, the antibiotic-loaded cement is poured into the mold in the late doughy phase until polymerization is achieved. Finally, the mold is removed carefully and the spacers are ready to be implanted [29].
Preformed gentamicin- and/or vancomycin-loaded cement knee spacers (Spacer-K® or Vancogenx-Space Knee®, Tecres, Sommacampagna, Italy) are another valid option. These spacers are available in four different femoral and tibial sizes (small, medium, large, and extra large, respectively, of 60-, 70-, 80-, and 90-mm tibial plateau dimensions) that may be selected intraoperatively. The cement is preloaded with antibiotics by the manufacturer [45].
Regardless of whether the molded or the preformed construct is used, the tibial component is inserted first and cemented to the proximal tibia with additional antibiotic-loaded cement in efforts to preserve the joint line. The femoral unit then is positioned and cemented to the distal femur with more antibiotic-loaded cement. The spacer must adhere strongly to the bone surface while the cement is still in a doughy state, remaining in position until the cement is completely polymerized [46]. At the same time, excessive penetration of the cement into the surrounding bone must be avoided, because it may further damage residual bone stock when the spacer is removed during the second stage.
ROM, patellar tracking, and knee stability are checked carefully [47]. Following the same technique described for static spacers, a drain is inserted in articulation to reduce postoperative hematoma formation.
A slight variation in this technique consists of screwing two 3-mm K-wires in the middle of both femoral and tibial components and covering them with antibiotic-loaded cement. The cement-loaded K-wires act as prosthetic stems and are inserted into the femoral and tibial intramedullary canals. Only the prosthetic components and the proximal part of the stems are then cemented to the articular surfaces, whereas the rest of the stems are left uncemented (Fig. 2). There are two rationales for this slightly different technique. First, it has been demonstrated that up to about one third of infectious processes of the knee are expanded along the intramedullary femoral and tibial canals; therefore, placing a cemented intramedullary stem may help control the infection at this level [48•]. Second, in some cases, the lack of a stem may lead to dislocation of the components, eventually leading to wound and vascular problems [49].
Fig. 2.
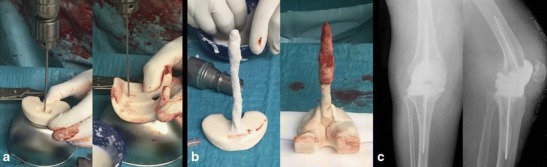
Antibiotic-loaded dynamic cement spacer. a Preparing the intramedullary rods using a K-wire. b The intramedullary handmade rod is covered by antibiotic-loaded cement. c Postoperative anteroposterior and lateral view radiographs
In some cases, the premolded spacers may be adapted. Figure 3 shows a premolded articulating spacer used in a case of uni-compartimental knee arthroplasty septic loosening.
Fig. 3.
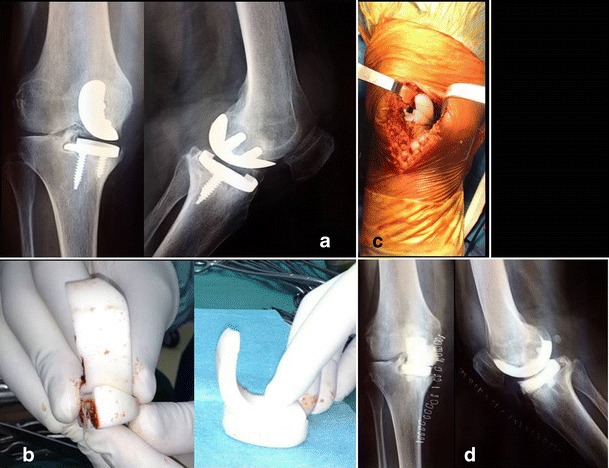
Antibiotic-loaded cemented dynamic spacer used as a uni-compartimental spacer. a Preoperative X-rays (antero-posterior and lateral view). b Intraoperative preparation of the uni-compartimental spacer dividing the premolded one in two parts. c Intraoperative picture showing the mono-compartimental spacer in situ. d Postoperative X-rays of the dynamic mono-compartimental spacer (anterior and lateral views)
Prosthesis-on-polyethylene interface: the Hofmann technique
The history of articulating cement spacers of the knee began in 1995 with Hofmann and colleagues, who described this self-named technique [50].
After standard exposure, irrigation, debridement of all dead tissue, synovectomy, and removal of all components and cement, the infected femoral prosthesis is cleaned and sent for autoclaving [27]. A new tibial all-polyethylene insert is placed on the tibial plateau and in same surgical time; when the femoral component returns from sterilization, it is reimplanted and articulated with the polyethylene insert. Both components then are cemented in place loosely to avoid rigid fixation [50].
Metal-on-polyethylene interface: PROSTALAC®
The metal-on-polyethylene technique consists of a special dynamic knee spacer called PROSTALAC® (acronym for prosthesis with antibiotic-loaded acrylic cement; DePuy Synthes, Warsaw, IN) made up of two parts: a femoral and a tibial component. Each component contains antibiotic-loaded cement, associated with a bicondylar metal shell on the femoral component and a complementary polyethylene section on the tibial one. These spacers are available in different sizes and thicknesses and result in low-friction articulation of metal on polyethylene [51]. This system, developed in 1987, is derived from a posterior-stabilized design that currently is in use [52].
Results of static versus articulating spacers
The literature contains many studies comparing the results of static versus dynamic spacers in terms of ROM, clinical function scores, infection eradication rate, amount of bone loss, surgical ease, and complications.
In the past decades, two-stage knee revision surgeries in patients with PJI were performed using static spacers and a less than satisfactory knee ROM was observed [53]. In 2013, Voleti et al. [30] presented a systematic review comparing outcomes from the use of static and articulating spacers. They evaluated reinfection rates, ROM after second-stage surgery, functional scores, and wound-related and spacer-related problems. The authors analyzed combined level III and level IV comparative studies including a total of 1526 patients, 654 of whom were treated with static spacers and 872 of whom were treated with articulating ones. None of the studies demonstrated a statistically significant difference in terms of reinfection rates between the two treatment groups (mean of 12 % for static and 8 % for articulating spacers, with P = 0.1 and confidence intervals set to 95 %). However, the authors noted a statistically significant variation in knee ROM after second-stage surgery, with better ROM in the articulating spacer group (91° vs. 101°). Despite this difference in terms of motion, though, no significant differences were detected in functional outcomes assessment (Hospital for Special Surgery Score [HSS] and Knee Society Scoring [KSS]) or wound-related complications (8 % in static spacers and 2 % in dynamic ones) among the different spacers. However, because of the low rate of complications in the analyzed studies, the review was underpowered for evaluating a significant difference in complication rates after static or mobile spacer implantation.
Pivec et al. [54] studied a population of 707 patients with static spacers and 962 with articulating spacers and detected a statistically significant difference in the average ROM finally achieved by the patients. However, they found no statistically significant differences in KSS between the groups. Regarding infection eradication rate and mean percentage of reinfection (PRI) between the two groups, the authors noted no statistically significant differences (mean PRI, 9.7 % for static and 7.9 % for articulating spacers; P = 0.35). In this casuistry, the most relevant complications resulted in delayed wound healing, aseptic loosening, deep venous thrombosis, and patellar injuries, with no statistically significant differences between static and articulated spacers (10.7 % for the statics, 6.9 % for the complex articulating spacers, and 5.8 % for the articulating spacers). Furthermore, no differences in terms of additional complications were detected between the groups (2 vs. 3 % reoperation rate).
In their systematic review, Guild et al. [55] found comparable results regarding ROM but slightly different outcomes in reinfection rates. The authors analyzed 47 studies, including 2011 two-stage revision TKAs, 924 using static and 1087 using dynamic spacers. A subanalysis was added to the study that separated complex cases (positive culture for a virulent organism such as methicillin-resistant Staphylococcus aureus, Gram negative or polymicrobial; Anderson Orthopaedic Research Institution [AORI] grade II or III bone loss; or the presence of a draining sinus tract) from the simple ones. The results showed a statistically significant difference in suitability favoring static spacers for use in complex cases. With regard to HSS and KSS function scores, however, there was no significant difference between the static and mobile spacer groups, either preoperatively or postoperatively. Similar to the previously mentioned review, a statistically significant difference in ROM was reported in favor of dynamic spacers (100.1° ± 1.6° vs. 89.7° ± 2°). The Guild group also evaluated infection eradication rates and reported a statistically significant difference in favor of articulating spacers. Furthermore, the authors noted a minor adoption of associated technique, such as quadriceps snips or tibial tubercle osteotomies, during the second-stage surgery after use of an articulated spacer. Regarding further bone loss during the period of cement spacer use, the authors noted a highly remarkable lack of extra bone loss with dynamic spacer use (3.3 % of additional bone deficit detected vs. 47.4 %).
Romanò et al. [18] in 2012 presented a review that showed a better infection eradication rate for articulating spacers compared with static ones (91.2 vs. 87 % at 43.5 ± 20.1 months of follow-up).
Table 1 summarizes the most recent and relevant literature on static and dynamic spacers.
Table 1.
Summary of the most recent and relevant literature on outcomes of antibiotic-loaded cement spacers
| Author | Year | Knees | Spacers (A or S) | Average ROM at follow-up | Infection eradication rate (%) | Complications |
|---|---|---|---|---|---|---|
| Anderson et al. [57] | 2009 | 25 | A | 115° | 96 | No mention |
| Cai et al. [42] | 2012 | 23 | A | 100° | 91,3 | No complications |
| Chiang et al. [58] | 2011 | 43 | 22 A 21 S |
113° in A 85° in S |
95 in A 90 in S |
Lower incidence and easier re-implant in A |
| Choi et al. [40] | 2012 | 47 | 14 A 33 S |
Improved | 71 in A 67 in S |
Less frequent extensile surgical approaches in A |
| Cuckler et al. [59] | 2005 | 44 | A | 110° | 97 | No mention |
| Durbhakula et al. [46] | 2004 | 24 | A | 104° | 92 | No mention |
| Emerson et al. [60] | 2002 | 48 | 22 A 26 S |
107,8° in A 93,7° in S |
91 in A 92,4 in S |
Late reinfection rate of 23% in S |
| Evans et al. [61] | 2004 | 31 | A | Improved | 93,5 | No mention |
| Fehring et al. [62] | 2000 | 55 | 30 A 25 S |
105° in A 98° in S |
93 in A 88 in S |
Important bone losses in S and easier re-implant in A |
| Freeman et al. [63] | 2007 | 76 | 48 A 28 S |
108° in A 98° in S |
94,7 in A 92,1 in S |
Less frequent extensile surgical approaches in A |
| Gacon et al. [26] | 1997 | 29 | S | 95° | 82 | No mention |
| Garg et al. [43] | 2011 | 36 | A | Improved | 100 | Two fracture of the spacers |
| Gooding et al. [51] | 2011 | 115 | A | 93,2° | 98 | Surgical complications in 17% of cases |
| Haddad et al. [52] | 2000 | 45 | A | 94,5° | 91 | Related to the extensor mechanism and stability of the knee between stages |
| Haleem et al. [64] | 2004 | 96 | S | No mention | 90 at 5 years 77,3 at 10 years |
Modest rate of late recurrent infection or mechanical implant failure |
| Hart et al. [65] | 2006 | 48 | A | 92° | 88 | No mention |
| Hoad-Reddick et al. [66] | 2005 | 59 | S | No mention | 89 | No mention |
| Hofmann et al.[27] | 2005 | 50 | A | 104° | 90 | One patient had a fusion |
| Hofmann et al. [50] | 1995 | 26 | A | 106° | 100 | No mention |
| Hsu et al. [67] | 2008 | 32 | S | 88° | 86 | 37.5% of VY quadricepsplasty, 15.6% of quadriceps snip, 10% of patella baja, 10% of extension lag |
| Hsu et al. [39] | 2007 | 28 | 21 A 7 S |
95° in A 78° in S |
91 in A 86 in S |
28% of S vs 5% of A required a more extensile approach. 100% bone loss in S 14% of S vs 1% of A of common peroneal nerve palsy |
| Huang et al. [68] | 2006 | 21 | A | 97,6° | 95 | Sic cases of VY quadricepsplasty |
| Hwang et al. [69] | 2012 | 30 | A | No mention | 93 | One arthrodesis |
| Jaekel et al. [70] | 2012 | 36 | 22 A 14 S | No mention | No mention | No mention |
| Jämsen et al. [71] | 2006 | 34 | 24 A 10 S | 103,7° in A 92° in S |
91 in A 75 in S |
One amputation in S |
| Jia et al. [72] | 2012 | 21 | A | 94,3° | 100 | No complications |
| Johnson et al. [49] | 2012 | 115 | 81 A 34 S | 99° in A 95° in S |
83 in A 83 in S |
More complications in A (subluxation, mechanical failure, fracturated component) |
| Kalore et al. [73] | 2012 | 53 | A | 96,3° | 88,6 | No mention |
| Kohl et al. [74] | 2011 | 16 | A | 102° | 100 | No complications |
| Kotwal et al. [31] | 2012 | 58 | S | No mention | 83,8 | 5,4% of quadriceps snips |
| Lee et al. [75] | 2012 | 20 | A | 107,6° | 95 | No complications |
| MacAvoy et al. [76] | 2005 | 13 | A | 98° | 69 | No mention |
| Macheras et al. [77] | 2011 | 34 | A | No mention | 91,1 | One aseptic loosening |
| Macmull et al. [32] | 2010 | 19 | A | No mention | 63 | Two amputations |
| Meek et al. [78] | 2004 | 54 | A | 87,1° | 96 | One cement spacer broke |
| Ocguder et al. [79] | 2010 | 17 | A | 95° | 94,1 | Six femoral components broke |
| Park et al. [28] | 2010 | 36 | 16 A 20 S |
108° in A 92° in S | 93,7 in A 85 in S |
75% of bone losses in S vs 0% in A |
| Pascale et al. [80] | 2007 | 14 | A | 120° | 100 | No mention |
| Pietsch et al. [81] | 2006 | 33 | A | No mention | 91 | One dislocation of the spacer, one tibia fracture |
| Pitto et al. [44] | 2005 | 19 | A | 94° | 100 | No complications |
| Qiu et al. [82] | 2010 | 10 | A | 110° | 90 | One arthrodesis |
| Shaikh et al. [83] | 2014 | 15 | A | 115° | 100 | One secundary debridement |
| Shen et al. [29] | 2010 | 17 | A | 96,7° | 94,1 | One arthrodesis and one amputation |
| Siebel et al. [84] | 2002 | 10 | A | No mention | 100 | No mention |
| Souillac et al. [85] | 2006 | 18 | A | Improved | 85,7 | One dislocation of the spacer |
| Su et al. [86] | 2009 | 15 | A | 110° | 93,3 | No complications |
| Thabe et al. [41] | 2007 | 20 | A | 107° | 100 | No mention |
| Tigani et al. [19] | 2013 | 38 | A | 101° | 76,4 | No mention |
| Trezies et al. [87] | 2006 | 11 | A | No mention | 90,9 | No mention |
| Van Thiel et al. [88] | 2011 | 60 | A | 101,3° | 88 | One femoral component broke |
| Villanueva-Martinez et al. [89] | 2008 | 30 | A | 80° | 100 | No mention |
| Wan et al. [45] | 2012 | 33 | A | No mention | 90,9 | No signs of breakage or loosening |
A articulated spacers, S static spacers, ROM range of motion
At the end of July 2013 in Philadelphia, Dr. Javad Parvizi of the Rothman Institute in Philadelphia and Dr. Thorsten Gehrke of the ENDO Clinic in Germany led a special conference on PJI. After deep analysis and evaluation of all the available literature on the topic, the participants drafted and finalized a consensus document. Among the various topics covered, the one concerning antibiotic-loaded cement spacers showed some interesting results [56•]. Strong consensus emerged regarding the existence of a functional difference in the use of nonarticulating versus articulating spacers in two-stage exchange arthroplasty, with a superior outcome observed in patients receiving articulating spacers. Functional differences also were evaluated 2 years after reimplantation, resulting in a nonsignificant tendency toward an ROM increase after use of articulating spacers compared with static ones. The technical ease of reimplantation surgery also was debated, and despite the lack of studies making direct comparisons, based on anecdotal reports, it appears that use of a dynamic spacer might facilitate second-stage surgery. With the agreement of 89 % of the participants (strong consensus), it appears the type of spacer implanted does not influence the infection eradication rate in TKA revision for infection. Also, among the various types of mobile spacers in use (PROSTALAC, Hofmann technique, cemented molds, and Spacer-K), control of infection does not appear to differ significantly, even between manufactured spacers and surgeon-made dynamic spacers.
Conclusions
Antibiotic-loaded cement spacers are fundamental in two-stage revision TKA. They help maintain a grade of joint stability, add an intra-articular concentration of antibiotics, and keep the patient’s knee joint free of any foreign infected prosthetic material during infection eradication. Both static and dynamic spacers are effective in eradicating the infection.
According to the literature, no clear differences exist between the two types of spacers; however, articulating spacers appear to have superior functional outcomes and result in easier reimplantation, allowing a degree of ROM between the stages. There is some agreement in the literature regarding the conclusion that the type of spacer implanted does not influence the infection eradication rate in TKA revision for infection.
Compliance with Ethics Guidelines
Conflict of Interest
The authors have no conflict of interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Revision Knee Arthroplasty
Investigation was performed at the Hospital Mauriziano “Umberto I”, Torino, Italy.
Contributor Information
Luca Mazzucchelli, Phone: 0115085011, Email: lucamazzucchelli@hotmail.it.
Federica Rosso, Phone: 0115085011, Email: federica.rosso@yahoo.it.
Antongiulio Marmotti, Phone: 0115085011, Email: antonio.marmotti@inwind.it.
Davide Edoardo Bonasia, Phone: 0115085011, Email: davidebonasia@virgilio.it.
Matteo Bruzzone, Phone: 0115085011, Email: matteo.bruzzone@fastwebnet.it.
Roberto Rossi, Phone: 0115085011, Email: rossir@fastwebnet.it.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg (Am Vol) 2012;94:e104. doi: 10.2106/JBJS.K.01417. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.•.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–4. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workgroup Convened by the Musculoskeletal Infection S. New definition for periprosthetic joint infection. The Journal of arthroplasty. 2011;26:1136-8 [DOI] [PubMed]
- 5.Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg (Am Vol) 2011;93:1355–7. doi: 10.2106/JBJS.9314ebo. [DOI] [PubMed] [Google Scholar]
- 6.Lombardi AV, Jr, Berend KR, Adams JB. Why knee replacements fail in 2013: patient, surgeon, or implant? J Bone Joint. 2014;96-B:101–4. doi: 10.1302/0301-620X.96B11.34350. [DOI] [PubMed] [Google Scholar]
- 7.Oduwole KO, Molony DC, Walls RJ, Bashir SP, Mulhall KJ. Increasing financial burden of revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2010;18:945–8. doi: 10.1007/s00167-010-1074-8. [DOI] [PubMed] [Google Scholar]
- 8.Jamsen E, Stogiannidis I, Malmivaara A, Pajamaki J, Puolakka T, Konttinen YT. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oussedik SI, Dodd MB, Haddad FS. Outcomes of revision total hip replacement for infection after grading according to a standard protocol. J Bone Joint Surg Br Vol. 2010;92:1222–6. doi: 10.1302/0301-620X.92B9.23663. [DOI] [PubMed] [Google Scholar]
- 10.Singer J, Merz A, Frommelt L, Fink B. High rate of infection control with one-stage revision of septic knee prostheses excluding MRSA and MRSE. Clin Orthop Relat Res. 2012;470:1461–71. doi: 10.1007/s11999-011-2174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 12.Dubee V, Zeller V, Lhotellier L, Kitzis MD, Ziza JM, Mamoudy P, et al. Continuous high-dose vancomycin combination therapy for methicillin-resistant staphylococcal prosthetic hip infection: a prospective cohort study. Clin Microbiol Infect. 2013;19:E98–105. doi: 10.1111/1469-0691.12071. [DOI] [PubMed] [Google Scholar]
- 13.Esposito S, Esposito I, Leone S. Considerations of antibiotic therapy duration in community- and hospital-acquired bacterial infections. J Antimicrob Chemother. 2012;67:2570–5. doi: 10.1093/jac/dks277. [DOI] [PubMed] [Google Scholar]
- 14.Bernard L, Legout L, Zurcher-Pfund L, Stern R, Rohner P, Peter R, et al. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J Infect. 2010;61:125–32. doi: 10.1016/j.jinf.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker JP, Warren RE, Jones RS, Gregson PA. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br Vol. 2009;91:44–51. doi: 10.1302/0301-620X.91B1.20930. [DOI] [PubMed] [Google Scholar]
- 16.Cooper HJ, Della Valle CJ. The two-stage standard in revision total hip replacement. J Bone Joint. 2013;95-B:84–7. doi: 10.1302/0301-620X.95B11.32906. [DOI] [PubMed] [Google Scholar]
- 17.Masters JP, Smith NA, Foguet P, Reed M, Parsons H, Sprowson AP. A systematic review of the evidence for single stage and two stage revision of infected knee replacement. BMC Musculoskelet Disord. 2013;14:222. doi: 10.1186/1471-2474-14-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romanò CL, Gala L, Logoluso N, Romanò D, Drago L. Two-stage revision of septic knee prosthesis with articulating knee spacers yields better infection eradication rate than one-stage or two-stage revision with static spacers. Knee Surg Sports Traumatol Arthrosc. 2012;20:2445–53. doi: 10.1007/s00167-012-1885-x. [DOI] [PubMed] [Google Scholar]
- 19.Tigani D, Trisolino G, Fosco M, Ben Ayad R, Costigliola P. Two-stage reimplantation for periprosthetic knee infection: Influence of host health status and infecting microorganism. Knee. 2013;20:9–18. doi: 10.1016/j.knee.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Stammers J, Kahane S, Ranawat V, Miles J, Pollock R, Carrington RW, et al. Outcomes of infected revision knee arthroplasty managed by two-stage revision in a tertiary referral centre. Knee. 2015;22:56–62. doi: 10.1016/j.knee.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Iarikov D, Demian H, Rubin D, Alexander J, Nambiar S. Choice and doses of antibacterial agents for cement spacers in treatment of prosthetic joint infections: review of published studies. Clin Infect Dis. 2012;55:1474–80. doi: 10.1093/cid/cis735. [DOI] [PubMed] [Google Scholar]
- 22.Antoci V, Phillips MJ, Antoci V, Jr, Krackow KA. The treatment of recurrent chronic infected knee arthroplasty with a 2-stage procedure. J Arthroplast. 2009;24(159):e13–7. doi: 10.1016/j.arth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356–68. doi: 10.5435/00124635-200906000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Incavo SJ, Russell RD, Mathis KB, Adams H. Initial results of managing severe bone loss in infected total joint arthroplasty using customized articulating spacers. J Arthroplast. 2009;24:607–13. doi: 10.1016/j.arth.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Faschingbauer M, Bieger R, Reichel H, Weiner C, Kappe T. Complications associated with 133 static, antibiotic-laden spacers after TKA. Knee Surg, Sports Traumatol Arthrosc 2015. [DOI] [PubMed]
- 26.Antoci V, Phillips MJ, Antoci V, Jr, Krackow KA. Using an antibiotic-impregnated cement rod-spacer in the treatment of infected total knee arthroplasty. Am J Orthop. 2009;38:31–3. [PubMed] [Google Scholar]
- 27.Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–31. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 28.Park SJ, Song EK, Seon JK, Yoon TR, Park GH. Comparison of static and mobile antibiotic-impregnated cement spacers for the treatment of infected total knee arthroplasty. Int Orthop. 2010;34:1181–6. doi: 10.1007/s00264-009-0907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen H, Zhang X, Jiang Y, Wang Q, Chen Y, Wang Q, et al. Intraoperatively-made cement-on-cement antibiotic-loaded articulating spacer for infected total knee arthroplasty. Knee. 2010;17:407–11. doi: 10.1016/j.knee.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Voleti PB, Baldwin KD, Lee GC. Use of static or articulating spacers for infection following total knee arthroplasty: a systematic literature review. J Bone Joint Surg Am Vol. 2013;95:1594–9. doi: 10.2106/JBJS.L.01461. [DOI] [PubMed] [Google Scholar]
- 31.Kotwal SY, Farid YR, Patil SS, Alden KJ, Finn HA. Intramedullary rod and cement static spacer construct in chronically infected total knee arthroplasty. J Arthroplast. 2012;27:253–9. doi: 10.1016/j.arth.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Macmull S, Bartlett W, Miles J, Blunn GW, Pollock RC, Carrington RW, et al. Custom-made hinged spacers in revision knee surgery for patients with infection, bone loss and instability. Knee. 2010;17:403–6. doi: 10.1016/j.knee.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Silvestre A, Almeida F, Renovell P, Morante E, Lopez R. Revision of infected total knee arthroplasty: two-stage reimplantation using an antibiotic-impregnated static spacer. Clin Orthop Surg. 2013;5:180–7. doi: 10.4055/cios.2013.5.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal VK, Higuera C, Deirmengian G, Parvizi J, Austin MS. Swab cultures are not as effective as tissue cultures for diagnosis of periprosthetic joint infection. Clin Orthop Relat Res. 2013;471:3196–203. doi: 10.1007/s11999-013-2974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–9. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128:1039–46. doi: 10.1007/s00402-007-0454-0. [DOI] [PubMed] [Google Scholar]
- 37.Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res. 2007;464:164–78. doi: 10.1097/BLO.0b013e318157eb1e. [DOI] [PubMed] [Google Scholar]
- 38.Paz E, Sanz-Ruiz P, Abenojar J, Vaquero-Martin J, Forriol F, Del Real JC. Evaluation of elution and mechanical properties of high-dose antibiotic-loaded bone cement: comparative “in vitro” study of the influence of vancomycin and cefazolin. J Arthroplast. 2015;30:1423–9. doi: 10.1016/j.arth.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 39.Hsu YC, Cheng HC, Ng TP, Chiu KY. Antibiotic-loaded cement articulating spacer for 2-stage reimplantation in infected total knee arthroplasty: a simple and economic method. J Arthroplast. 2007;22:1060–6. doi: 10.1016/j.arth.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 40.Choi HR, Malchau H, Bedair H. Are prosthetic spacers safe to use in 2-stage treatment for infected total knee arthroplasty? J Arthroplast. 2012;27:1474–9. doi: 10.1016/j.arth.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Thabe H, Schill S. Two-stage reimplantation with an application spacer and combined with delivery of antibiotics in the management of prosthetic joint infection. Oper Orthop Traumatol. 2007;19:78–100. doi: 10.1007/s00064-007-1196-4. [DOI] [PubMed] [Google Scholar]
- 42.Cai P, Hu Y, Xie L, Wang L. Two-stage revision of infected total knee arthroplasty using antibiotic-impregnated articulating cement spacer. zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26:1169–73. [PubMed] [Google Scholar]
- 43.Garg P, Ranjan R, Bandyopadhyay U, Chouksey S, Mitra S, Gupta SK. Antibiotic-impregnated articulating cement spacer for infected total knee arthroplasty. Indian J Orthop. 2011;45:535–40. doi: 10.4103/0019-5413.87126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–8. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan Z, Karim A, Momaya A, Incavo SJ, Mathis KB. Preformed articulating knee spacers in 2-stage total knee revision arthroplasty: minimum 2-year follow-up. J Arthroplast. 2012;27:1469–73. doi: 10.1016/j.arth.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 46.Durbhakula SM, Czajka J, Fuchs MD, Uhl RL. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplast. 2004;19:768–74. doi: 10.1016/j.arth.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 47.Villanueva M, Rios A, Pereiro J, Chana F, Fahandez-Saddi H. Hand-made articulating spacers for infected total knee arthroplasty: a technical note. Acta Orthop. 2006;77:329–32. doi: 10.1080/17453670610046190. [DOI] [PubMed] [Google Scholar]
- 48.•.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 49.Johnson AJ, Sayeed SA, Naziri Q, Khanuja HS, Mont MA. Minimizing dynamic knee spacer complications in infected revision arthroplasty. Clin Orthop Relat Res. 2012;470:220–7. doi: 10.1007/s11999-011-2095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 51.Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:985–93. doi: 10.1007/s11999-010-1579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br Vol. 2000;82:807–12. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 53.Wang CJ, Hsieh MC, Huang TW, Wang JW, Chen HS, Liu CY. Clinical outcome and patient satisfaction in aseptic and septic revision total knee arthroplasty. Knee. 2004;11:45–9. doi: 10.1016/S0968-0160(02)00094-7. [DOI] [PubMed] [Google Scholar]
- 54.Pivec R, Naziri Q, Issa K, Banerjee S, Mont MA. Systematic review comparing static and articulating spacers used for revision of infected total knee arthroplasty. J Arthroplast. 2014;29:553–7. doi: 10.1016/j.arth.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 55.Guild GN, 3rd, Wu B, Scuderi GR. Articulating vs. static antibiotic impregnated spacers in revision total knee arthroplasty for sepsis. A systematic review. J Arthroplast. 2014;29:558–63. doi: 10.1016/j.arth.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 56.•.Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. J Bone Joint. 2013;95-B:1450–2. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 57.Anderson JA, Sculco PK, Heitkemper S, Mayman DJ, Bostrom MP, Sculco TP. An articulating spacer to treat and mobilize patients with infected total knee arthroplasty. J Arthroplasty. 2009;24:631–5. doi: 10.1016/j.arth.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Chiang ER, Su YP, Chen TH, Chiu FY, Chen WM. Comparison of articulating and static spacers regarding infection with resistant organisms in total knee arthroplasty. Acta Orthop. 2011;82:460–4. doi: 10.3109/17453674.2011.581266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuckler JM. The infected total knee: management options. J Arthroplasty. 2005;20:33–6. doi: 10.1016/j.arth.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Emerson RH, Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–8. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 61.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 62.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Freeman MG, Fehring TK, Odum SM, Fehring K, Griffin WL, Mason JB. Functional advantage of articulating versus static spacers in 2-stage revision for total knee arthroplasty infection. J Arthroplasty. 2007;22:1116–21. doi: 10.1016/j.arth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–9. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 65.Hart WJ, Jones RS. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J Bone Joint Surg (Br) 2006;88:1011–5. doi: 10.1302/0301-620X.88B8.17445. [DOI] [PubMed] [Google Scholar]
- 66.Hoad-Reddick DA, Evans CR, Norman P, Stockley I. Is there a role for extended antibiotic therapy in a two-stage revision of the infected knee arthroplasty? J Bone Joint Surg (Br) 2005;87:171–4. doi: 10.1302/0301-620X.87B2.15640. [DOI] [PubMed] [Google Scholar]
- 67.Hsu CS, Hsu CC, Wang JW, Lin PC. Two-stage revision of infected total knee arthroplasty using an antibiotic-impregnated static cement-spacer. Chang Gung Med J. 2008;31:583–91. [PubMed] [Google Scholar]
- 68.Huang HT, Su JY, Chen SK. The results of articulating spacer technique for infected total knee arthroplasty. J Arthroplasty. 2006;21:1163–8. doi: 10.1016/j.arth.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 69.Hwang BH, Yoon JY, Nam CH, Jung KA, Lee SC, Han CD, et al. Fungal peri-prosthetic joint infection after primary total knee replacement. J Bone Joint Surg (Br) 2012;94:656–9. doi: 10.1302/0301-620X.94B5.28125. [DOI] [PubMed] [Google Scholar]
- 70.Jaekel DJ, Day JS, Klein GR, Levine H, Parvizi J, Kurtz SM. Do dynamic cement-on-cement knee spacers provide better function and activity during two-stage exchange? Clin Orthop Relat Res. 2012;470:2599–604. doi: 10.1007/s11999-012-2332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jämsen E, Sheng P, Halonen P, Lehto MU, Moilanen T, Pajamäki J, et al. Spacer prostheses in two-stage revision of infected knee arthroplasty. Int Orthop. 2006;30:257–61. doi: 10.1007/s00264-006-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia YT, Zhang Y, Ding C, Zhang N, Zhang DL, Sun ZH, et al. Antibiotic-loaded articulating cement spacers in two-stage revision for infected total knee arthroplasty: individual antibiotic treatment and early results of 21 cases. Chin J Traumatol. 2012;15:212–21. [PubMed] [Google Scholar]
- 73.Kalore NV, Maheshwari A, Sharma A, Cheng E, Gioe TJ. Is there a preferred articulating spacer technique for infected knee arthroplasty? A preliminary study. Clin Orthop Relat Res. 2012;470:228–35. doi: 10.1007/s11999-011-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kohl S, Evangelopoulos DS, Kohlhof H, Krueger A, Hartel M, Roeder C, et al. An intraoperatively moulded PMMA prostheses like spacer for two-stage revision of infected total knee arthroplasty. Knee. 2011;18:464–9. doi: 10.1016/j.knee.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 75.Lee JK, Choi CH. Two-stage reimplantation in infected total knee arthroplasty using a re-sterilized tibial polyethylene insert and femoral component. J Arthroplasty. 2012;27:1701–6. doi: 10.1016/j.arth.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 76.MacAvoy MC, Ries MD. The ball and socket articulating spacer for infected total knee arthroplasty. J Arthroplasty. 2005;20:757–62. doi: 10.1016/j.arth.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Macheras GA, Kateros K, Galanakos SP, Koutsostathis SD, Kontou E, Papadakis SA. The long-term results of a two-stage protocol for revision of an infected total knee replacement. J Bone Joint Surg (Br) 2011;93:1487–92. doi: 10.1302/0301-620X.93B11.27319. [DOI] [PubMed] [Google Scholar]
- 78.Meek RM, Dunlop D, Garbuz DS, McGraw R, Greidanus NV, Masri BA. Patient satisfaction and functional status after aseptic versus septic revision total knee arthroplasty using the PROSTALAC articulating spacer. J Arthroplasty. 2004;19:874–9. doi: 10.1016/j.arth.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 79.Ocguder A, Firat A, Tecimel O, Solak S, Bozkurt M. Two-stage total infected knee arthroplasty treatment with articulating cement spacer. Arch Orthop Trauma Surg. 2010;130:719–25. doi: 10.1007/s00402-010-1054-y. [DOI] [PubMed] [Google Scholar]
- 80.Pascale V, Pascale W. Custom-made articulating spacer in two-stage revision total knee arthroplasty. An early follow-up of 14 cases of at least 1 year after surgery. HSS J. 2007;3:159–63. doi: 10.1007/s11420-007-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pietsch M, Hofmann S, Wenisch C. Treatment of deep infection of total knee arthroplasty using a two-stage procedure. Oper Orthop Traumatol. 2006;18:66–87. doi: 10.1007/s00064-006-1163-5. [DOI] [PubMed] [Google Scholar]
- 82.Qiu XS, Sun X, Chen DY, Xu ZH, Jiang Q. Application of an articulating spacer in two-stage revision for severe infection after total knee arthroplasty. Orthop Surg. 2010;2:299–304. doi: 10.1111/j.1757-7861.2010.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shaikh AA, Ha CW, Park YG, Park YB. Two-stage approach to primary TKA in infected arthritic knees using intraoperatively molded articulating cement spacers. Clin Orthop Relat Res. 2014;472:2201–7. doi: 10.1007/s11999-014-3545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siebel T, Kelm J, Porsch M, Regitz T, Neumann WH. Two-stage exchange of infected knee arthroplasty with an prosthesis-like interim cement spacer. Acta Orthop Belg. 2002;68:150–6. [PubMed] [Google Scholar]
- 85.Souillac V, Costes S, Aunoble S, Langlois V, Dutronc H, Chauveaux D. Evaluation of an articulated spacer for two-stage reimplantation for infected total knee arthroplasty: 28 cases. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:485–9. doi: 10.1016/S0035-1040(06)75835-4. [DOI] [PubMed] [Google Scholar]
- 86.Su YP, Lee OK, Chen WM, Chen TH. A facile technique to make articulating spacers for infected total knee arthroplasty. J Chin Med Assoc. 2009;72:138–45. doi: 10.1016/S1726-4901(09)70039-5. [DOI] [PubMed] [Google Scholar]
- 87.Van Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:994–1001. doi: 10.1007/s11999-010-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trezies A, Parish E, Dixon P, Cross M. The use of an articulating spacer in the management of infected total knee arthroplasties. J Arthroplasty. 2006;21:702–4. doi: 10.1016/j.arth.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 89.Villanueva-Martínez M, Ríos-Luna A, Pereiro J, Fahandez-Saddi H, Villamor A. Hand-made articulating spacers in two-stage revision for infected total knee arthroplasty: good outcome in 30 patients. Acta Orthop. 2008;79:674–82. doi: 10.1080/17453670810016704. [DOI] [PubMed] [Google Scholar]


