Abstract
Receptor activator of nuclear factor-κB ligand (RANKL) is a tumor necrosis factor (TNF) family member, which signals through the osteoclast surface RANK. As such, RANKL is required for osteoclast differentiation and function, namely bone resorption. There is now growing evidence that RANKL is a therapeutic target for musculoskeletal neoplasms, namely giant cell tumor of bone (GCTB) and osteosarcoma.
Keywords: RANKL, RANK, Giant cell tumor of bone, Osteosarcoma
Introduction
Musculoskeletal neoplasms are a heterogeneous group of benign and malignant tumors of mesenchymal origin [1]. Treatment for benign neoplasms relies principally on surgery whereas treatment of malignant musculoskeletal neoplasms (sarcomas) involves a multidisciplinary approach involving surgery, chemotherapy, and radiation, depending on the subtype, grade, location, and staging. The principle oncologic problem with benign musculoskeletal neoplasms is local recurrence, whereas in the sarcomas, developing metastatic disease is the most common cause of death. Moreover, response rates to conventional chemotherapy in the metastatic setting remain poor. Given these outcomes, there is much interest in identifying new treatment targets for these patients. Despite the successes of targeted small molecule inhibitors in the last decade (for example, imatinib/cKIT for gastrointestinal stromal tumor, erlotinib/EGFR in non-small cell lung cancer or vemurafenib/Braf in melnaoma), most sarcomas typically lack these classic kinase mutations characteristic of epithelial and neural crest-derived tumors. Thus, sarcoma drug development now often focuses on novel anti-tumor strategies or exploring alternate applications for existing compounds. The fascinating story of the RANKL inhibitor denosumab (Amgen, Inc.), a drug developed for osteoporosis and carcinomas with bone metastases, and giant cell tumor of bone (GCTB) is an example of this strategy.
Bone homeostasis and RANK ligand inhibitors
In the human skeleton, the bone is continuously remodeled throughout life. This involves highly regulated homeostasis between osteoblasts, a mesenchymal cell which forms bone, and osteoclasts, which are multinucleated giant cells of monocyte/macrophage origin, that function to resorb bone [2]. In simplest terms, osteoblasts synthesize a precursor matrix called osteoid which is later mineralized with hydroxyapatite to form mature bone while osteoclasts function to resorb bone in the remodeling process. Interestingly, it has been elucidated that osteoblasts are able to regulate osteoclast differentiation, migration, and activity, at least in part, through expression of RANKL signaling through osteoclast surface RANK. Thus, RANKL is one of the signaling molecules required for osteoclast bone resorptive function; alternative pathways of osteoclast activation exist, however (e.g., lysyl oxidase in breast cancer [3]).
Denosumab is a humanized monoclonal IgG2 antibody that targets RANKL with high affinity and inhibits its signaling through RANK. It was hypothesized that RANKL inhibition would function to block osteoclast activity through the above model. In early phase studies, denosumab clearly reduced bone resorption [4], and this later led to subsequent clinical trials showing improvement in osteoporosis [5] and reducing skeletal events in multiple non-sarcoma cancers [6–9].
Giant cell tumor of bone and RANKL inhibition as a therapeutic strategy
Giant cell tumor of bone (GCTB) is an uncommon benign, albeit locally aggressive, skeletal neoplasm (for review see WHO Classification of Tumors and Soft Tissue and Bone [1] and Raskin et al. 2013 [10]). GCTB predominantly arises in the long bones of the mature skeleton where it represents less than 5 % of bone tumors [11]. GCTB most commonly occurs in the 3rd to 5th decade of life, though pediatric and geriatric cases have been described [12]. These tumors clinically present with lytic lesions on imaging and symptoms including pain, fracture, mass, limited limb function, or nerve injury. The most common locations are epiphyseal, including the femur and tibia. Other sites including the axial skeleton/sacrum, hands, feet, and jaw are seen as well. GCTB is unusual in that it is a benign tumor yet it does rarely metastasize to the lungs (<7 % of cases), even in the absence of malignant transformation [13]. Malignant sarcoma transformation is seen, typically in 1 % or less of tumors, and this has been described both with and without radiation therapy [14–17].
When technically feasible, GCTB is managed surgically with en block excision or, perhaps now more commonly, curettage with or without local adjuvant therapies (e.g., liquid nitrogen, phenol, ethanol, hydrogen peroxide, cement, etc.) [10]. Local control rates are excellent with this strategy with recurrence typically less than 20 %, depending on the anatomical features of the tumor [10, 18]. However, despite these outcomes, there are cases with recurrent disease, highly morbid procedures, or patients who develop lung metastases, as described above. Until recently, treatment options for these patients were quite limited. For the unresectable or recurrent tumor, radiation therapy is active and a reasonable option for palliation. Radiation therapy has very good rates of local control in up to 75 to 90 % of cases. The typical dosing is 35 Gy or higher [16, 17, 19–22]. However, in this relatively young population, caution should be taken with radiation therapy given the risks of secondary malignancies or theoretical transformation of the GCTB [16, 17]. Although clinical trials are lacking, additionally strategies include embolization, cryotherapy, interferon, and bisphosphonates.
Histologically, GCTB is characterized by large multinucleated osteoclasts dispersed in a background of ovoid to spindle-shaped tumor stromal cells [1, 10]. Interestingly, it has now been shown that the GCTB stromal cells express high levels of RANKL, among other cytokines, which are thought then to directly stimulate the recruitment of the benign, non-tumor monocytes. These monocytes then differentiate into multinucleated osteoclasts where they secrete factors to destroy bone [23–29]. Though clearly present and integral to the pathobiology of GCTB, the mechanism of RANKL overexpression, however, remains unknown.
As above, RANKL is required for osteoclast differentiation, function, and survival, and denosumab is a high affinity antibody, which inhibits RANKL binding to RANK. Thus, given the proposed model of stromal RANKL-expressing cells recruiting RANK-expressing monocyte/osteoclast cells, it was hypothesized that denosumab could interrupt this paracrine process which drives tumor growth and bone destruction.
In an initial phase II study, Thomas et al. treated 37 patients with denosumab for recurrent or unresectable GCTB [30]. Dosing was aggressive with injections in 3 of 4 weeks in the first month followed by monthly treatments thereafter. Thirty of 35 (86 %) patients derived clinical benefit as assessed by either histological response or no tumor progression. Interestingly, for those patients with histology available for review, all had a reduction in RANK-expressing giant cells and in RANKL-expressing stromal cells; there was evidence of new bone formation in some as well [31].
In a follow-up study, denosumab was evaluated in over 280 patients with arms for unresectable GCTB (cohort 1), resectable GCTB (cohort 2), and patients continuing from the above pilot study (cohort 3) [32]. The interim analysis was published in 2013, and the results are striking. By investigator analysis, in cohort 1, unresectable GCTB, 96 % of patients did not progress during the study, and there were 8 patients (5 %) with a complete response and 57 (36 %) with partial response. In cohort 2, the resectable GCTB, the data is somewhat more difficult to analyze as at the time of reporting only 26 of 100 patients had planned surgeries. Though subjective, per the investigators, more than half did undergo a less morbid surgery. Of these 100 patients in cohort 2, again by investigator assessment, 17 had a complete response and 37 a partial response with only one patient progressing. Median time to response was 95 weeks in the unresectable cohort and 28 weeks in the resectable cohort. Clinical benefit ranged 40–60 % between cohorts. Using standard clinical trial objective response criteria, the response rates were 25 % (modified RECIST criteria), 96 % EORTC criteria, and 76 % by Choi criteria. Median time to response was 3.1 months by these objective criteria. Importantly, these responses were durable with 94 % of patients remaining progression free on study.
The safety profile for denosumab in GCTB was very good in this study with constitutional symptoms and minor electrolyte abnormalities being relatively common (e.g., arthralgias, headache, fatigue, hypocalcemia). Rare but serious events such as osteonecrosis of the jaw, grade 3 hypocalcemia, and grade 3 hypophosphatemia occurred in 3 % or less of patients [32].
Based on these findings, denosumab was approved by the FDA in 2013 for the GCTB which is unresectable or associated with a highly morbid procedure/outcome.
In our group, the practice has been to offer denosumab in the following clinical situations. In patients with initial or recurrent GCTB where the surgical morbidity would be potentially unacceptably high, we will lead in with neo-adjuvant denosumab to maximal clinical response. Patients should have imaging of the primary site, a chest x-ray, or CT (risk is highest in recurrent GCTB) and possibly a bone scan depending on the clinical scenario. Before and on therapy, they must have adequate vitamin D, calcium, and phosphorous levels as well as routine dental maintenance. As described in the studies above, the median time to objective response was 3 months; thus, the patient should be followed clinically and with sequential imaging. We typically discontinue treatment prior to surgery, and no adjuvant denosumab is given. A representative patient is shown in Fig. 1.
Fig. 1.
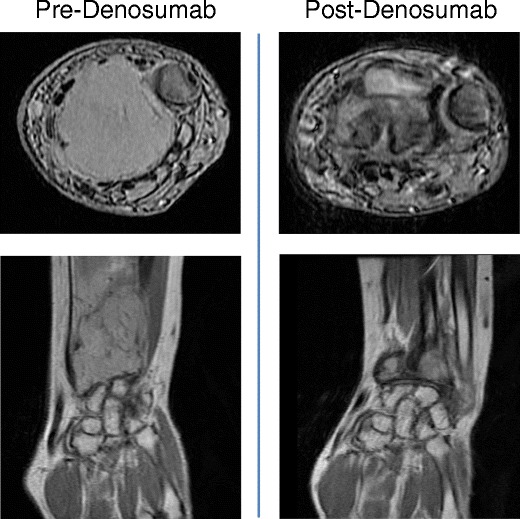
Shown are axial and coronal MRI images of a GCTB of the wrist in a patient treated with denosumab for 3 months
As above, less than 7 % of GCTB patients will develop lung metastases. In these patients, options include surgery, ablation (if feasible), or systemic treatment with denosumab. We will follow patients with serial chest imaging (e.g., every 3–6 months) and treat up to 3 years, depending on the clinical scenario. Again, levels of calcium, vitamin D, phosphorous and adequate dental hygiene needs to be followed.
It is unknown what length of time of denosumab is safe for GCTB patients. This is of particular concern for young patients where the risk of osteonecrosis of the jaw or unusual bone fractures could occur.
RANKL inhibitors in other sarcomas
The exciting data above raises the question as to whether the RANK/RANKL signaling pathway is important in other sarcomas, particularly those of bone. Much of the work is preclinical, but early evidence suggests that the RANK/RANKL is at least present and may have prognostic or therapeutic value.
Osteosarcoma is a highly aggressive malignancy of bone. Modern chemotherapy has dramatically improved survival; however, up to 30 % of patients will have metastatic disease at diagnosis [33]. Osteosarcoma does form lytic bone lesions, and this process may be important for tumor growth, invasion, and ultimately metastasis. Additionally, osteosarcoma can histologically show osteoclastic-type giant cells perhaps linking the RANK/RANKL to tumor physiology [1, 34].
Evidence now confirms that RANK and RANKL can be expressed in animal and human cell lines as well as human osteosarcoma tissue. There is some discrepancy as to which is present on the tumor cells, the ligand or receptor, perhaps suggesting that either or both can be expressed to function as either a paracrine or autocrine signaling pathway. Early cell line work detected RANK is present in the POS-1 [35], the Saos-2 [36], and MOTO [37] osteosarcoma lines and that RANKL can alter downstream kinase signaling and gene expression (implying it may be functional). In contrast, other cell lines (e.g., K7M3) and in human tumor samples suggest that RANKL rather is expressed by the tumor cells themselves and that these may contribute to osteolysis and aggressive behavior of the tumor [34, 38, 39].
In a small case-control study, Avnet et al. show that 5 of 10 patients with osteoclasts in their tumor samples had metastases at diagnosis while 6 of 6 without osteoclasts did not have metastases at diagnosis [34]. In these samples, they detect osteoclast-differentiating factors (RANKL, PTHrP, M-CSF, IL6 as well as the metalloproteinase MMP-9). The numbers are small yet certainly intriguing and mechanistically plausible as a contributor to invasion. In a subsequent study from Korea of 40 patient samples, RANKL expression in tumors inversely correlated with survival and chemotherapy response [39]. A third study examined RANK and RANKL expression 91 human osteosarcoma samples, and nearly 70 % expressed RANK while only 9 % RANKL. In their cohort, again, RANK was associated with shorter disease-free survival but without correlation of chemotherapy sensitivity or overall survival [40].
Can targeting RANKL or osteoclasts be therapeutic in osteosarcoma models? Assays exploring small interfering RNA inhibition of RANKL in mouse models showed possible slowing of tumor progression when combined with chemotherapy [41]. Similarly, in a mouse osteosarcoma model, a RANK-Fc antagonist of RANKL reduced lung metastases [42]. Additionally, bisophosphonates, which inhibit osteoclast function, was shown to inhibit tumor growth in cell lines and murine models [38, 43, 44].
At the moment, there is virtually no human published experience of denosumab in osteosarcoma patients. One case report describes a patient who received denosumab with a multikinase inhibitor, sorafenib, where there was clinical benefit [45]. However, this should be interpreted with caution as it was a single case, and sorafenib could be the active treatment. Controlled clinical studies are currently planned through the Children’s Oncology Group (NCT02470091) with anticipated opening in 2015–2016.
Conclusions
The RANK/RANKL signaling system is a key regulator of osteoclast function. There is evidence that it is integral to the pathogenesis of GCTB and perhaps important for bone invasion and microenvironment in other sarcomas. Phase II clinical studies show a remarkable disease control rate for patients with advanced GCTB, and it is now an FDA-approved therapy for those with metastatic or difficult to resect tumors. There is also growing evidence that the RANK/RANKL pathway may be important in other connective tissue malignancies, including osteosarcoma. Clinical trials are currently planned to determine the safety and anti-cancer activity in osteosarcoma patients.
Compliance with Ethical Standards
Conflict of Interest
Dr. Cote is an investigator on the denosumab/giant cell tumor of bone registration phase II study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Orthopedic Oncology: New Concepts and Techniques
References
- 1.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology; 46:95–104. [DOI] [PubMed]
- 2.Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256:449–55. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]
- 3.Cox TR, Rumney RM, Schoof EM, Perryman L, Hoye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature; 522:106–10. [DOI] [PMC free article] [PubMed] [Retracted]
- 4.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–66. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 5.Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab; 98:4483–92. [DOI] [PMC free article] [PubMed]
- 6.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 7.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol; 29:1125–32. [DOI] [PubMed]
- 8.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol; 28:5132–9. [DOI] [PubMed]
- 9.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res; 25:440–6. [DOI] [PubMed]
- 10.Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ. Giant cell tumor of bone. J Am Acad Orthop Surg; 21:118–26. [DOI] [PubMed]
- 11.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–14. [PubMed] [Google Scholar]
- 12.Federman N, Brien EW, Narasimhan V, Dry SM, Sodhi M, Chawla SP. Giant cell tumor of bone in childhood: clinical aspects and novel therapeutic targets. Paediatr Drugs; 16:21–8. [DOI] [PubMed]
- 13.Chan CM, Adler Z, Reith JD, Gibbs CP, Jr. Risk factors for pulmonary metastases from giant cell tumor of bone. J Bone Joint Surg Am; 97:420–8. [DOI] [PubMed]
- 14.Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22:19–26. doi: 10.1007/s002640050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grote HJ, Braun M, Kalinski T, Pomjanski N, Back W, Bleyl U, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skelet Radiol. 2004;33:169–75. doi: 10.1007/s00256-003-0682-5. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarti A, Spiro IJ, Hug EB, Mankin HJ, Efird JT, Suit HD. Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone. J Bone Joint Surg Am. 1999;81:1566–73. doi: 10.2106/00004623-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Caudell JJ, Ballo MT, Zagars GK, Lewis VO, Weber KL, Lin PP, et al. Radiotherapy in the management of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 2003;57:158–65. doi: 10.1016/S0360-3016(03)00416-4. [DOI] [PubMed] [Google Scholar]
- 18.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res; 469:591–9. [DOI] [PMC free article] [PubMed]
- 19.Chen ZX, Gu DZ, Yu ZH, Qian TN, Huang YR, Hu YH, et al. Radiation therapy of giant cell tumor of bone: analysis of 35 patients. Int J Radiat Oncol Biol Phys. 1986;12:329–34. doi: 10.1016/0360-3016(86)90346-9. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CJ, Jr, Marcus RB, Jr, Million RR, Enneking WF. Radiation therapy for giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 1993;26:299–304. doi: 10.1016/0360-3016(93)90210-M. [DOI] [PubMed] [Google Scholar]
- 21.Nair MK, Jyothirmayi R. Radiation therapy in the treatment of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 1999;43:1065–9. doi: 10.1016/S0360-3016(98)00526-4. [DOI] [PubMed] [Google Scholar]
- 22.Shi W, Indelicato DJ, Reith J, Smith KB, Morris CG, Scarborough MT, et al. Radiotherapy in the management of giant cell tumor of bone. Am J Clin Oncol; 36:505–8. [DOI] [PubMed]
- 23.Atkins GJ, Haynes DR, Graves SE, Evdokiou A, Hay S, Bouralexis S, et al. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res. 2000;15:640–9. doi: 10.1359/jbmr.2000.15.4.640. [DOI] [PubMed] [Google Scholar]
- 24.Atkins GJ, Bouralexis S, Haynes DR, Graves SE, Geary SM, Evdokiou A, et al. Osteoprotegerin inhibits osteoclast formation and bone resorbing activity in giant cell tumors of bone. Bone. 2001;28:370–7. doi: 10.1016/S8756-3282(01)00404-5. [DOI] [PubMed] [Google Scholar]
- 25.Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol. 2002;117:210–6. doi: 10.1309/BPET-F2PE-P2BD-J3P3. [DOI] [PubMed] [Google Scholar]
- 26.Morgan T, Atkins GJ, Trivett MK, Johnson SA, Kansara M, Schlicht SL, et al. Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB. Am J Pathol. 2005;167:117–28. doi: 10.1016/S0002-9440(10)62959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156:761–7. doi: 10.1016/S0002-9440(10)64942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skubitz KM, Cheng EY, Clohisy DR, Thompson RC, Skubitz AP. Gene expression in giant-cell tumors. J Lab Clin Med. 2004;144:193–200. doi: 10.1016/j.lab.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, et al. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res. 2005;23:203–9. doi: 10.1016/j.orthres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol; 11:275–80. [DOI] [PubMed]
- 31.Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res; 18:4415–24. [DOI] [PubMed]
- 32.Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol; 14:901–8. [DOI] [PubMed]
- 33.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 34.Avnet S, Longhi A, Salerno M, Halleen JM, Perut F, Granchi D, et al. Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int J Oncol. 2008;33:1231–8. [PubMed] [Google Scholar]
- 35.Wittrant Y, Lamoureux F, Mori K, Riet A, Kamijo A, Heymann D, et al. RANKL directly induces bone morphogenetic protein-2 expression in RANK-expressing POS-1 osteosarcoma cells. Int J Oncol. 2006;28:261–9. [PubMed] [Google Scholar]
- 36.Mori K, Berreur M, Blanchard F, Chevalier C, Guisle-Marsollier I, Masson M, et al. Receptor activator of nuclear factor-kappaB ligand (RANKL) directly modulates the gene expression profile of RANK-positive Saos-2 human osteosarcoma cells. Oncol Rep. 2007;18:1365–71. [PubMed] [Google Scholar]
- 37.Beristain AG, Narala SR, Di Grappa MA, Khokha R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci; 125:943–55. [DOI] [PubMed]
- 38.Ohba T, Cole HA, Cates JM, Slosky DA, Haro H, Ando T, et al. Bisphosphonates inhibit osteosarcoma-mediated osteolysis via attenuation of tumor expression of MCP-1 and RANKL. J Bone Miner Res; 29:1431–45. [DOI] [PubMed]
- 39.Lee JA, Jung JS, Kim DH, Lim JS, Kim MS, Kong CB, et al. RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatr Blood Cancer; 56:738–43. [DOI] [PubMed]
- 40.Bago-Horvath Z, Schmid K, Rossler F, Nagy-Bojarszky K, Funovics P, Sulzbacher I. Impact of RANK signalling on survival and chemotherapy response in osteosarcoma. Pathology; 46:411–5. [DOI] [PubMed]
- 41.Rousseau J, Escriou V, Lamoureux F, Brion R, Chesneau J, Battaglia S, et al. Formulated siRNAs targeting Rankl prevent osteolysis and enhance chemotherapeutic response in osteosarcoma models. J Bone Miner Res; 26:2452–62. [DOI] [PubMed]
- 42.Akiyama T, Dass CR, Shinoda Y, Kawano H, Tanaka S, Choong PF. Systemic RANK-Fc protein therapy is efficacious against primary osteosarcoma growth in a murine model via activity against osteoclasts. J Pharm Pharmacol; 62:470–6. [DOI] [PubMed]
- 43.Kubista B, Trieb K, Sevelda F, Toma C, Arrich F, Heffeter P, et al. Anticancer effects of zoledronic acid against human osteosarcoma cells. J Orthop Res. 2006;24:1145–52. doi: 10.1002/jor.20129. [DOI] [PubMed] [Google Scholar]
- 44.Dass CR, Choong PF. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol Cancer Ther. 2007;6:3263–70. doi: 10.1158/1535-7163.MCT-07-0546. [DOI] [PubMed] [Google Scholar]
- 45.Cathomas R, Rothermundt C, Bode B, Fuchs B, von Moos R, Schwitter M. RANK ligand blockade with denosumab in combination with sorafenib in chemorefractory osteosarcoma: a possible step forward? Oncology; 88:257–60. [DOI] [PubMed]


