Abstract
Purpose
The purpose of this study was to evaluate the usefulness of contrast-enhanced ultrasonography (CEUS) in the bioptic sampling of soft tissue tumors (STT) compared with unenhanced ultrasonography alone.
Methods
This is a prospective longitudinal study of 40 patients subjected to ultrasonography (US)-guided core needle biopsy (CNB) to characterize a suspected STT. Three series of bioptic samplings were carried out on each patient, respectively using unenhanced US alone and CEUS in both the areas of the tumor enhanced or not by the contrast medium. All bioptic samples underwent a histological evaluation and the results were analyzed by comparing the histology of the biopsy with the definitive diagnosis in 15 surgically excised samples.
Results
27 (67.5 %) of the 40 patients completed the entire study procedure; in 19 cases (70.3 %) the three bioptic samplings gave unanimous results, also when compared to the surgical specimen; in seven cases (25.9 %) use of CEUS allowed to obtain additional or more accurate information about the mass in question, compared to simple US guidance without contrast; in one patient (3.7 %) sampling obtained using unenhanced ultrasonography guidance and in the areas enhanced by the contrast agent had precisely the same results of the surgical specimen.
Conclusions
CEUS, due to its ability to evaluate microvascular areas, has proven to be a promising method in guiding bioptic sampling of soft tissue tumor, directing the needle to the most significant areas of the tumor. Given the small number of patients evaluated in our study, to achieve statistically significant results, it would be appropriate to obtain a larger sample size, since the very first results seem to be encouraging and to justify the increase of the population.
Keywords: Contrast-enhanced ultrasonography, Soft tissue tumors, Core needle biopsy
Riassunto
Scopo
Lo scopo di questo studio è stato quello di valutare l’utilità dell’ecografia conmdc (CEUS) rispetto alla ecografia tradizionale, nel prelievo bioptico dei tumori dei tessuti molli (STT).
Metodi
si tratta di uno studio longitudinale prospettico di 40 pazienti sottoposti ad ago-biopsia ecoguidata (US-CNB) per la caratterizzazione di un STT sospetto. Sono stati prelevati 3 campioni bioptici su ogni paziente, utilizzando ecografia b-mode e CEUS sia nelle aree del tumore non evidenziate dal mezzo di contrasto che in quelle dotate di contrast-enhancement. Tutti i campioni bioptici sono stati sottoposti a valutazione istologica e sono stati analizzati i risultati confrontando l’istologico della biopsia con i 15 campioni asportati chirurgicamente, con diagnosi definitiva.
Risultati
27 (67,5%) dei 40 pazienti hanno completato l’intera procedura di studio; in 19 casi (70,3%) i tre campioni bioptici hanno dato risultati unanimi, anche rispetto al modello chirurgico; in 7 casi (25,9%) l’uso della CEUS ha permesso di ottenere informazioni supplementari, o più precise, circa la massa in questione, rispetto al semplice esame ecografico senza mezzo di contrasto; in 1 paziente (3,7%) i campioniottenuti usando sia l’ecografia tradizionale che quella con contrasto sono risultati esattamente corrispondenti ai campioni chirurgici.
Conclusioni
la CEUS, grazie alla sua capacità di valutare la microvascolarizzazione, ha dimostrato di essere un metodo promettente nel guidare il prelievo bioptico negli STT, dirigendo l’ago nelle zone più caratteristiche del tumore. Secondo i risultati di questo studio pilota, sarebbe pertanto opportuno esaminare un maggior numero di pazienti per confermare le potenzialità della metodica.
Introduction
Soft tissue tumors (STT) comprise a large heterogeneous group of mesenchymal neoplasms that are classified according to their normal tissue counterpart [1]; most of them are benign, but they represent the 15 % of the malign tumors of the pediatric age and little less than 1 % of the adulthood [2]. The annual incidence of benign STT has been estimated to be about 3,000/million population, whereas that of malign ones is about 30/million [1]. The most affected anatomical sites, in order of frequency, are the extremities (59 %, with prevailing interest of the lower limbs, especially the thighs), the trunk (19 %), the retroperitoneum (15 %), the visceral district and the head and neck region (9 %) [3]. Approximately one-third of STT has superficial location, with an average diameter of about 5 cm, while the others are deeper and larger (average 9 cm) [4]. The structure of the lesions can be extremely variable, and may be solid, liquid, calcified or hemorrhagic, but more frequently all these aspects are variously represented in the same mass. Although these lesions are divided into four categories [benign, intermediate (locally aggressive), intermediate (rarely metastasising) and malignant] [5], many of them have an intermediate nature, displaying a locally aggressive behavior with a low-to-moderate tendency to metastasize [6]. Imaging studies that are used to evaluate STT include ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI). Of these modalities, ultrasonography is the easiest to perform and the least invasive; it is important in the initial study of a mass, providing information on the shape, size, margins, and eco-structure (solid, liquid, mixed, fibrous) differentiating between tumor lesions and tumor-like lesions presenting a specific benign cystic pattern, and allows to express a judgment of first suspicion of malignancy or benignity of the lesion. Furthermore US allows a proper evaluation of the tumor vasculature, both from macroscopic and microscopic point of view; in particular, in some type of tumors, contrast-enhanced ultrasonography (CEUS) is useful in the identification of the tumor vascularisation and neo-angiogenesis [7–9] (Fig. 1). The gold standard for the diagnosis of a STT, however, remains histology that makes use of bioptic samples; the importance and accuracy of percutaneous needle biopsy in the workup of a soft tissue tumor have been described previously in the medical literature [10]. Among the different bioptic techniques, core needle biopsy (CNB) takes advantage to provide adequate tissue for histopathologic analysis [11] and most pathologists are in agreement, dealing with STT, to prefer it rather than samples taken from cytology [12]. Sonographically guided CNB is an accurate and safe means to obtain tissue samples for the histopathologic diagnosis of soft tissue masses [13]. Ultrasonography and in particular CEUS have provided very promising results from this point of view and it has been demonstrated that CEUS-guided CNB has 97.1 % sensitivity and 92.5 % specificity in relation to the adequacy and accuracy of diagnosis [14]. CEUS, allowing the visualization of micro-vasculature and areas of angiogenesis, may allow to perform the bioptic sampling at the most significant areas, to obtain a correct histological diagnosis of the tumor, in particular when the lesion has huge dimensions and shows heterogenous areas at imaging, making difficult to identify the most representative areas for sampling (Fig. 2). Although potentially a very useful imaging tool [15], CEUS is still not widely used in clinical practice, and few reports have discussed its use in the evaluation of STT. The purposes of this pilot prospective longitudinal study were: evaluating the correlation between the results of histological examinations obtained by the simple unenhanced ultrasonography guidance and those obtained by CEUS guidance; comparing these results with the reference sample given from histopathological examination of the resected surgical mass. All this is done to justify the use of CEUS as a method of choice in the course of ultrasound-guided biopsy of STT.
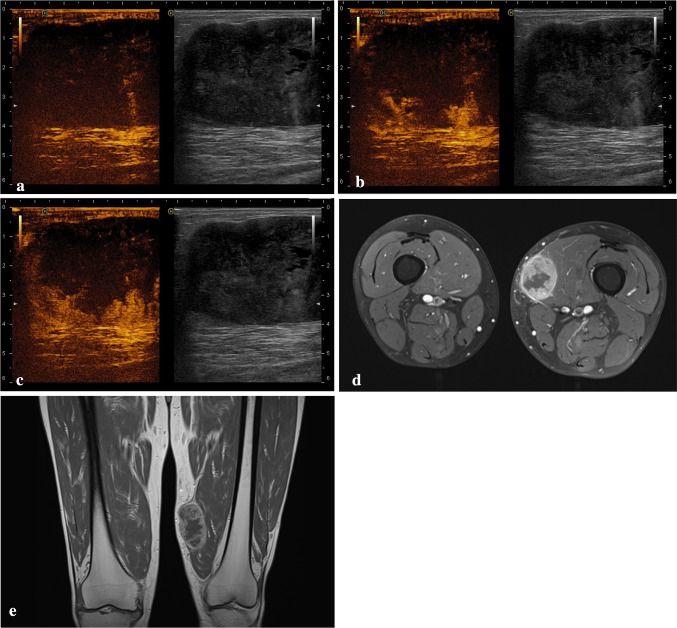
Fig. 1.
Pleomorphic rhabdomyosarcoma of the lower limbs: contrast-enhanced sonograms a before injection of SonoVue and b twenty and c forty seconds after bolus injection of SonoVue, show an intense enhancement of the inferior and lateral portions of the mass, whereas the rest of the mass shows no enhancement. d–e Axial T2-weighted and coronal T1-weighted images of the same mass
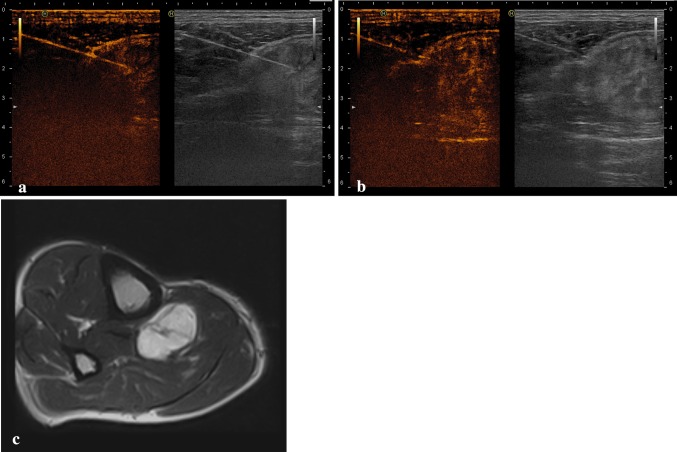
Fig. 2.
a, b Contrast-enhanced ultrasonographically guided biopsy of a myxoid mesenchymal tumor: the needle tip has correctly been inserted into the enhanced area of the mass. c Axial T1-weighted image of the same mass
Materials and methods
The subjects were 40 patients with a suspected STT. Patients were recruited from the outpatient services related to Melanoma and Sarcoma Oncology Unit of a regional referral centre for diagnosis and treatment of bone and soft tissue tumors. Everyone was examined with US, CT and MRI to establish if they presented indication to perform the bioptic sampling using US-guided CNB, in accordance with the guidelines on STT established by the local Regional Health Service. Informed consent was obtained from all patients. In the period between November 2012 and July 2014 were evaluated 40 patients, including 22 males and 18 females: all underwent evaluation with US- and CEUS-guided CNB, and histological findings were compared with each other and, in case of surgical resection, with the final pathologic diagnosis, obtained from the resected masses. The patients enrolled had a range age 25–88 years, average age 57.8 years (women range age 32–80, mean age 56.3 years; men range age 25–88 years, average age 59). Exclusion criteria were not belonging to the STT group and the absence of the histological diagnosis on surgically excised mass. The median tumor size calculated on the surgically excised masses was 9.2 cm (range, 4.2–13.6 cm). US and CEUS were performed by the same radiologist using the same ultrasound (Hi Vision Ascendus, Hitachi® Medical Corporation, Inc, Tokyo, Japan); CEUS was performed evaluating the contrast enhancement through contrast tuned-imaging (Hi Vision, Hitachi® Medical Corporation, Inc, Tokyo, Japan) and using an 8-μL/mL solution of sulfur hexafluoride microbubbles stabilized by a phospholipid shell (SonoVue, Bracco® SpA, Milan, Italy) as contrast medium. Scans were performed along the whole extension of the mass, according to the longitudinal and transversal axes, with linear and/or convex probes, using frequency transmission of 3.5 or 7.5 MHz, according to the size and depth of the lesions, trying to identify the most significant points to perform the biopsy, avoiding areas of necrosis or fibrosis, which could alter histological examination and identification of the tumor. A single use soft polyurethane cover with gel was used for sterile technique. Completed a preliminary study of the mass, the surgeon, who then would have performed the biopsy, administered a local anesthesia by subcutaneous injection of 10 ml solution of 1 % lidocaine; then he created a gap skin to insert a 14-gauge Tru-cut biopsy needle with an automated biopsy system (Covidien®). No patient experienced any side effects, nor complained of discomfort associated to the administration of the contrast agent or to CNB. Exploiting the only US guidance (without contrast) were collected 2–3 samples, immediately placed in a solution of 10 % formalin; then, after injecting 5 ml of ultrasound contrast agent intravenously, followed by an injection of 20 ml of saline solution, two other series of bioptic samples were done: the former collected in the points of the mass enhanced by the contrast agent (probably those with a greater neo-angiogenesis), the other samples collected in the points were not enhanced by the contrast agent. All three series of samplings were put in three different test tubes and properly labeled, respectively with the following tags: pre-contrast agent, contrast agent +, contrast agent −. The samples were submitted for histopathological examination and evaluated by the same pathologist. The results obtained from the evaluation of pathologic biopsy were then verified by comparing them with the pathologic evaluation of the mass removed, in case of indication for resection. Every case has been discussed at the weekly Interdisciplinary Sarcoma Group meeting and clinical strategy took place by dedicated radiologists, surgeons, pathologists and medical oncologists; all specialists worked in this study were expert in sarcoma as an active part of the interdisciplinary group. This research was performed following the principles of the Declaration of Helsinki.
Results
In the period between November 2012 and July 2014 were enrolled in the study 40 patients, a significant number if compared to the rarity of this disease. Results are tabulated in Tables 1 and 2. 27 of 40 patients underwent surgical resection of the mass and completed the entire protocol of the study, allowing us the comparison between the biopsy and the surgical specimen. Five cases were excluded because the biopsy showed they did not belong to the group of STT (three cases of localization of melanoma and two of non-Hodgkin lymphoma). In six patients it was found a benign tumor of the STT group that did not have indication for surgery (desmoid fibromatosis, two cases of elastofibroma dorsi, granular cell tumor, decubital fasciitis, lipoma). In the other two cases not surgically treated, the reason was the need to make further investigation, performing additional diagnostic tests, still under development (chondroma, myxoid mesenchymal tumor). The masses surgically removed were located in the upper limbs in ten cases, in the lower limbs in 15 cases, one at the level of the chest wall and one on the buttock. The whole procedure established by the protocol of the study was completed in 27 patients (67.5 % of total recruited): in 19 cases (70.4 %) of these the histological result of the bioptic sampling gave concordant results for all three methods and with the pathologic analysis of the surgical specimen. In one patient (3.7 %), in which the analysis of the surgical specimen revealed the presence of an angioma, histological evaluation of the sample taken at level of the non-contrast medium had highlighted the presence of a vascular proliferation without obvious characters of malignancy, concordant with the final diagnosis; the evaluation of samples obtained with ultrasound guidance alone and by sampling in the points enhanced by the contrast medium, respectively noted blood material and material-fibrin blood. In one case (3.7 %) there was discordance between the three methods: a desmoid fibromatosis (determined by the evaluation of the surgical specimen), detected by the study of biopsies by ultrasound without contrast agent and samplings at the level of the area absorbing the contrast agent, but not by histological examination of the portion of the mass not enhanced by the contrast medium. In six cases (22.2 %) histological studies of samples taken using CEUS guidance, while agreeing about histotype with both the biopsy performed by ultrasound guidance alone and by the surgical specimen, has allowed the addition of useful information for the evaluation of grading of the tumor (three cases of neurofibroma, myxoid liposarcoma, angioma, desmoid fibromatosis). Such information, although less significant than that inherent histotype, is however important to evaluate subsequent stages of treatment. The sample size of patients who followed the entire protocol of the study, even high if given the rarity of the disease, not allows to give statistical significance to the results obtained by this pilot study. The preliminary assessment of the results of the bioptic samples is however possible and show the usefulness of the CEUS-guided biopsy. CEUS permitted to evaluate the most appropriate place in which sampling is performed in 25.9 % of cases (7 of 27 patients), allowing to obtain additional or more accurate information about the mass in question, compared to simple US guidance without contrast.
Table 1.
Characteristics of the study population and results of histological examinations of the samples obtained by unenhanced ultrasonography guidance (pre-contrast agent), and those obtained by CEUS guidance collected in the points of the mass enhanced by the contrast agent (contrast agent +) and in the points not enhanced by the contrast agent (contrast agent −)
| Patient ID | Age | Sex | Pre-contrast agent | Contrast agent + | Contrast agent − |
|---|---|---|---|---|---|
| 1 | 52 | M | Pleomorphic rhabdomyosarcoma | Pleomorphic rhabdomyosarcoma | Pleomorphic rhabdomyosarcoma |
| 2 | 52 | M | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) |
| 3 | 72 | M | Skeletal muscle tissue | Desmoid fibromatosis | Skeletal muscle tissue |
| 4 | 56 | M | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma |
| 5 | 32 | M | Neurofibroma | Neurofibroma with focal atypia and no mitotic activity | Neurofibroma with focal atypia and no mitotic activity |
| 6 | 35 | F | Desmoid fibromatosis | Desmoid fibromatosis | Desmoid fibromatosis |
| 7 | 56 | F | Nodular fascitis | Nodular fascitis | Nodular fascitis |
| 8 | 36 | M | Nodular fascitis | Nodular fascitis | Nodular fascitis |
| 9 | 35 | M | Neurofibroma | Neurofibroma with focal atypia and no mitotic activity | Neurofibroma |
| 10 | 32 | F | Localization of melanoma | Localization of melanoma | Localization of melanoma |
| 11 | 72 | M | Localization of melanoma | Localization of melanoma | Localization of melanoma |
| 12 | 80 | F | Adipose tissue without atypia | Adipose tissue without atypia | Adipose tissue without atypia |
| 13 | 76 | M | Blood material | Fibrin-blood material | Non-malignant vascular proliferation |
| 14 | 25 | M | Cartilaginous neoplasm without atypia | Cartilaginous neoplasm without atypia | Cartilaginous neoplasm without atypia |
| 15 | 75 | F | Atypical lipomatous tumor | Atypical lipomatous tumor | Atypical lipomatous tumor |
| 16 | 77 | M | Desmoid fibromatosis | Desmoid fibromatosis | Skeletal muscle tissue |
| 17 | 54 | M | Decubital fascitis | Decubital fascitis | Decubital fascitis |
| 18 | 72 | F | Granular cell tumor | Granular cell tumor | Granular cell tumor |
| 19 | 58 | F | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma |
| 20 | 62 | F | Elastofibroma dorsi | Elastofibroma dorsi | Elastofibroma dorsi |
| 21 | 66 | M | Schwannoma | Schwannoma | Schwannoma |
| 22 | 47 | F | Chondroma | Chondroma | Chondroma |
| 23 | 59 | M | Skeletal muscle and adipose tissue | Adipose tissue and fibrin leukocyte material | Adipose tissue and fibrin leukocyte material |
| 24 | 77 | F | Solitary fibrous tumor | Solitary fibrous tumor | Solitary fibrous tumor |
| 25 | 61 | F | Myxoid mesenchymal tumor | Myxoid mesenchymal tumor | Myxoid mesenchymal tumor |
| 26 | 71 | M | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma |
| 27 | 48 | M | Neurofibroma | Neurofibroma with focal atypia and non-mytotic activity | Neurofibroma |
| 28 | 48 | F | Nodular fascitis | Nodular fascitis | Nodular fascitis |
| 29 | 57 | M | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma |
| 30 | 58 | F | Localization of melanoma | Localization of melanoma | Localization of melanoma |
| 31 | 55 | F | Elastofibroma dorsi | Elastofibroma dorsi | Elastofibroma dorsi |
| 32 | 68 | M | Atypical lipomatous tumor | Myxoid liposarcoma | Atypical lipomatous tumor |
| 33 | 58 | M | Blood material | Non-malignant vascular proliferation | Fibrin-blood material |
| 34 | 60 | F | Schwannoma | Schwannoma | Schwannoma |
| 35 | 88 | M | Undifferentiated pleomorphic sarcoma | Undifferentiated pleomorphic sarcoma | Undifferentiated pleomorphic sarcoma |
| 36 | 43 | F | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma | Non-Hodgkin lymphoma |
| 37 | 68 | M | Solitary fibrous tumor | Solitary fibrous tumor | Solitary fibrous tumor |
| 38 | 76 | M | Mesenchymal malign neoplasm | Mesenchymal malign neoplasm | Mesenchymal malign neoplasm |
| 39 | 41 | F | Chondroma | Chondroma | Chondroma |
| 40 | 54 | F | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) |
Table 2.
Results of histological examinations of the samples obtained by unenhanced ultrasonography guidance (pre-contrast agent), and those obtained by CEUS guidance collected in the points of the mass enhanced by the contrast agent (contrast agent +) and in the points not enhanced by the contrast agent (contrast agent −), compared with the histopathological evaluation of the mass removed (surgical specimen), in the 15 cases surgical resected and their locations
| Patient ID | Pre-contrast | Contrast medium + | Contrast medium − | Surgical specimen | Location |
|---|---|---|---|---|---|
| 1 | Pleomorphic rhabdomyosarcoma | Pleomorphic rhabdomyosarcoma | Pleomorphic rhabdomyosarcoma | Pleomorphic rhabdomyosarcoma | Lower limbs |
| 2 | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) | Lipoma | Upper limbs |
| 3 | Skeletal muscle tissue | Desmoid fibromatosis | Skeletal muscle tissue | Desmoid fibromatosis | Upper limbs |
| 4 | Myxoid liposarcoma; | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma | Buttock |
| 5 | Neurofibroma | Neurofibroma with focal atypia and no mitotic activity | Neurofibroma with focal atypia and no mitotic activity | Neurofibroma | Lower limbs |
| 7 | Nodular fascitis | Nodular fascitis | Nodular fascitis | Nodular fascitis | Upper limbs |
| 8 | Nodular fascitis | Nodular fascitis | Nodular fascitis | Nodular fascitis | Upper limbs |
| 9 | Neurofibroma | Neurofibroma with focal atypia and no mitotic activity | Neurofibroma | Neurofibroma | Upper limbs |
| 13 | Blood material | Fibrin-blood material | Non-malignant vascular proliferation | Angioma | Upper limbs |
| 14 | Cartilaginous neoplasm without atypia | Cartilaginous neoplasm without atypia | Cartilaginous neoplasm without atypia | Chondroma | Chest wall |
| 15 | Atypical lipomatous tumor | Atypical lipomatous tumor | Atypical lipomatous tumor | Atypical liposarcoma | Lower limbs |
| 16 | Desmoid fibromatosis | Desmoid fibromatosis | Skeletal muscle tissue | Desmoid fibromatosis | Lower limbs |
| 21 | Schwannoma | Schwannoma | Schwannoma | Schwannoma | Upper limbs |
| 23 | Skeletal muscle and adipose tissue | Adipose tissue and fibrin leukocyte material | Adipose tissue and fibrin leukocyte material | Cavernous hemangioma | Lower limbs |
| 24 | Solitary fibrous tumor | Solitary fibrous tumor | Solitary fibrous tumor | Solitary fibrous tumor | Lower limbs |
| 26 | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma | Lower limbs |
| 27 | Neurofibroma | Neurofibroma with focal atypia and non-mytotic activity | Neurofibroma | Neurofibroma | Lower limbs |
| 28 | Nodular fascitis | Nodular fascitis | Nodular fascitis | Nodular fascitis | Upper limbs |
| 29 | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma | Myxoid liposarcoma | Lower limbs |
| 32 | Atypical lipomatous tumor | Myxoid liposarcoma | Atypical lipomatous tumor | Myxoid liposarcoma | Lower limbs |
| 33 | Blood material | Non-malignant vascular proliferation | Fibrin-blood material | Angioma | Upper limbs |
| 34 | Schwannoma | Schwannoma | Schwannoma | Schwannoma | Lower limbs |
| 35 | Undifferentiated pleomorphic sarcoma |
Undifferentiated pleomorphic sarcoma | Undifferentiated pleomorphic sarcoma | Undifferentiated pleomorphic sarcoma | Lower limbs |
| 37 | Solitary fibrous tumor | Solitary fibrous tumor | Solitary fibrous tumor | Solitary fibrous tumor | Lower limbs |
| 38 | Mesenchymal malign neoplasm | Mesenchymal malign neoplasm | Mesenchymal malign neoplasm | Chondrosarcoma | Lower limbs |
| 39 | Chondroma | Chondroma | Chondroma | Chondroma | Lower limbs |
| 40 | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) | Fibroadipose tissue (lipoma) | Lipoma | Upper limbs |
Discussion
The function of diagnostic imaging, in detecting STT, is to provide useful information for the diagnosis and the staging of the neoplasia, to guide bioptic samples and to evaluate the response to therapy. STT can have high variability and many different morphological characteristics; this means that different imaging methods play a role in their study and among these US offers significant advantages such as low cost, ease of execution, repeatability and adequate morphological resolution. CEUS, through the administration of a contrast medium with an exclusively blood pool, is used to display the areas of micro-vascularization and neoangiogenesis in the lesion [7–9]. US can also be used to provide a guide for the execution of a biopsy, giving the material for the histological study. CEUS has shown promising results in providing a guide for biopsy. CNB combines the advantages of minimally invasive, like FNAB, to the possibility of obtaining a tissue sample that provides histological information. It is important to take the vital part, and not the necrotic one, of the tumor and for this reason both US guidance and CEUS guidance can help [16]. With the increase of pathological knowledge of these diseases, the orientation is to identify as much as possible the correct histology, just to be able to act in the best manner, identifying the proper diagnostic process, whether it be medical or surgical. It is mandatory to obtain a correct presurgical staging, identifying the vital component of the mass, where the bioptic sampling is performed, to provide the pathologist as much material as possible to reach a correct diagnosis. It should be considered, in fact, that STT (especially malignant) are almost never uniform formations, but inhomogeneous masses with large necrotic and/or fibrous components, which, if taken as a single sample, preventing the pathologist’s diagnosis. This problem leads to a delay in diagnosis, which will result in a worse outcome of the patient, or even worse, to incorrect diagnosis, on the basis of which may be formulated inappropriate therapeutic indications, with the consequences [17]. The purpose of this pilot prospective longitudinal study is to provide preliminary data regarding the useful of CEUS, compared with unenhanced US, as imaging modality to guide the CNB in patients with suspected STT. CEUS could improve the precision of sampling due to its ability to identify the micro-vasculature and neoangiogenesis area, allowing a greater number of correct histological diagnoses. Our study, even if with a low population, having a prospective nature, could provide additional information in the evaluation of the effectiveness of CEUS guidance. Overall, in 25.9 % of cases the use of CEUS has been useful in determining the most appropriate area in which to perform the CNB, to obtain more accurate histological results respect to the simple US guidance. CEUS-guided CNB has provided promising preliminary results regarding its effectiveness in providing guidance for sampling biopsy of STT. The results obtained in our study seem to confirm the importance of getting bioptic samplings from the portion of the mass provided of a greater micro-vasculature and neo-angiogenetic processes. This area, whose visualization is made possible thanks to the use of the method CEUS, should be the most vital part of the tumor and therefore the most important component to achieve the correct histologically diagnosis. To date, the paucity of data in the literature and the limited diffusion of this method do not allow to define its use as an absolute indication. Given the small number of patients evaluated in our study, to achieve statistically significant results, it would be appropriate to obtain a larger sample size, since the very first results seem to be encouraging and to justify the increase of the population.
Conflict of interest
The authors have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). All patients provided written informed consent to enrolment in the study and to the inclusion in this article of information that could potentially lead to their identification.
Human and animal studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
Abbreviations
- CEUS
Contrast-enhanced ultrasonography
- STT
Soft tissue tumors
- CNB
Core-needle biopsy
References
- 1.Doyle LA, Nascimento AF (2011) Soft tissue tumors. Essentials of anatomic pathology, 3rd edn, pp 995–1045
- 2.Fletcher CD, Krishnan K, Mertens F (2002) Pathology and Genetics of tumours of soft tissue and bone
- 3.De Vita VT, Jr, Hellman S, Rosenberg SA. Cancer: Principles and practice of oncology. 6. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1841–1891. [Google Scholar]
- 4.Grimer RJ. Size matters for sarcomas. Ann R Coll Surg Engl. 2006;88:519–524. doi: 10.1308/003588406X130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer VK. Cytology of soft tissue tumors: benign soft tissue tumors including reactive, non-neoplastic lesions. J Cytol. 2008;25:81–86. doi: 10.4103/0970-9371.44034. [DOI] [Google Scholar]
- 6.Shidham VB, Acker SM, Hackbarth DA, et al. (2012) Benign and malignant soft tissue tumors. Emedicine
- 7.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49:113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 8.Lassau N, Lamuraglia M, Leclere J, et al. Functional and early evaluation of treatment in oncology: interest of ultrasonographic contrast agents. J Radiol. 2004;85:704–712. doi: 10.1016/S0221-0363(04)97651-2. [DOI] [PubMed] [Google Scholar]
- 9.Lassau N, Koscielny S, Opolon P, et al. Evaluation of contrast enhanced color Doppler ultrasound for the quantification of angiogenesis in vivo. Invest Radiol. 2001;36:50–55. doi: 10.1097/00004424-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kissin MW, Fisher C, Carter RL, et al. Value of Tru-cut biopsy in the diagnosis of soft tissue tumours. Br J Surg. 1986;73:742–744. doi: 10.1002/bjs.1800730921. [DOI] [PubMed] [Google Scholar]
- 11.Torriani M, Etchebehere M, Amstalden E. Sonographically guided core needle biopsy of bone and soft tissue tumors. J Ultrasound Med. 2002;21(3):275–281. doi: 10.7863/jum.2002.21.3.275. [DOI] [PubMed] [Google Scholar]
- 12.Simon MA. Biopsy of musculoskeletal tumors. J Bone Jt Surg Am. 1982;64:1253–1257. [PubMed] [Google Scholar]
- 13.Soudack M, Nachtigal A, Vladovski E, et al. Sonographically guided percutaneous needle biopsy of soft tissue masses with histopathologic correlation. J Ultrasound Med. 2006;25(10):1271–1277. doi: 10.7863/jum.2006.25.10.1271. [DOI] [PubMed] [Google Scholar]
- 14.De Marchi A, Branch del Prever EM, et al. Accuracy of core needle biopsy after contrast-enhanced ultrasound in soft-tissue tumours. Eur Radiol. 2010;20(11):2740–2748. doi: 10.1007/s00330-010-1847-y. [DOI] [PubMed] [Google Scholar]
- 15.Stramare R, Gazzola M, Coran A, et al. Contrast-enhanced ultrasound findings in soft-tissue lesions: preliminary results. J Ultrasound. 2013;16(1):21–27. doi: 10.1007/s40477-013-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mocellin S, Rossi CR, Brandes A, et al. Adult soft tissue sarcomas: conventional therapies and molecularly targeted approaches. Cancer Treat Rev. 2006;32(1):9–27. doi: 10.1016/j.ctrv.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]