Abstract
Acute limb ischemia (ALI) is a limb-threatening and life-threatening disease process. Mural aortic thrombosis (MAT) is a rare cause of ALI. While there is limited evidence on the use of bedside ultrasound for the detection of ALI or MAT, duplex ultrasound remains the standard in the diagnosis and ultimate medical decision-making in patients with acute and chronic limb ischemia. Point-of-care ultrasound may be used in the evaluation of patients with signs and symptoms of this disease entity. This is a case of a 79-year-old female with a complicated medical history, who presented with a pulseless right leg and abdominal tenderness. The patient quickly decompensated requiring intubation for airway protection. A post-intubation arterial blood gas (ABG) was unsuccessfully attempted in the right femoral artery, prompting an ultrasound-guided ABG. On B-mode ultrasound evaluation, echogenic material was visualized in the right common femoral artery without evidence of Doppler flow signal. Additionally, a partially obstructing echogenic material was also noted at the femoro-saphenous vein junction with only partial compressibility by compression sonography. A computed tomography angiography of the aorta was performed indicating extensive infrarenal aortic thrombosis. The patient expired despite the relatively prompt diagnosis, highlighting the importance of early identification of acute arterial occlusion.
Keywords: Mural aortic thrombus, Acute limb ischemia, Point-of-care ultrasound, Bedside ultrasound, Arterial thrombus, Deep vein thrombosis
Riassunto
L’ischemia acuta degli arti inferiori (ALI) è una malattia che mette in pericolo gli arti e la vita stessa del paziente. La trombosi parietale dell’aorta (MAT) è una causa rara di ALI. Mentre vi è una limitata evidenza sull’uso dell’ecografia al letto del paziente per la rilevazione di ALI o MAT, il Doppler rimane lo standard nella diagnosi e nel processo decisionale medico finale, in pazienti con ischemia acuta e cronica degli arti. L’ecografia può essere utilizzata nella valutazione dei pazienti con segni e sintomi di questa malattia. Presentiamo il caso di una donna di 79 anni, con una complicata storia clinica che si è presentata senza polso nella gamba destra e tensione addominale. La paziente si scompensava rapidamente, richiedendo l’intubazione per la protezione delle vie respiratorie. Dopo l’intubazione è stato tentato un arterial blood gas test (ABG) nell’arteria femorale destra, senza successo spingendo verso un ABG con guida ecografica. La valutazione ecografica in B-mode ha evidenziato materiale ecogeno nell’arteria femorale comune destra senza evidenza di segnali Doppler. Inoltre materiale ecogeno ostruente è stato osservato a livello della giunzione femoro-safena con solo parziale compressibilità con la compressione ecografica. E’ stata effettuata un’angio tomografia computerizzata dell’aorta che ha evidenziato un’ampia trombosi dell’aorta sotto-renale. Il paziente moriva, nonostante la diagnosi in tempi relativamente brevi, mettendo in evidenza l’importanza di individuare tempestivamente le occlusioni arteriose acute.
Introduction
A mural aortic thrombus (MAT) is an extremely rare condition, with less than 250 cases reported in the literature [1, 2] and remains an infrequent cause of distal arterial thromboembolism. As a MAT is usually seen in the setting of an aneurysm or dissection, distal extremity embolus originating from a thrombus found in a morphologically normal aorta, as described in our case below, represents a rare event. [3–6] While one study found an incidence of only 0.45 % in MAT without aneurysm, the overall true incidence of MAT is unknown [3]. More recent evidence seems to indicate that underlying occult endothelial vessel disease, however, may more likely be the major contributing factor for a MAT and may be related to a constellation of atherosclerotic risk factors including nicotine use, diabetes, hypertension and hypercholesterolemia. [3, 4, 7].
Most patients later found to have a MAT initially present with clinical findings consistent with acute limb ischemia (ALI) of the lower extremity including the “six Ps” of ischemia—pain, pallor, paresthesia, paralysis, poikilothermia and pulselessness. [2, 8, 9–11] As ALI remains a time-sensitive condition, rapid diagnosis and management must be attained, as further management is based upon the clinical degree of ischemic insult and may range from simple anticoagulation to surgical amputation. Primary amputation of an ischemic limb ranges from 10 to 40 %. Mortality rates in this group are around 20 % at 1 year and range from 40 to 70 % in 5 years [12].
Lower extremity point-of-care ultrasound (POCUS) has regularly been used to identify the femoral vein for purposes of central catheter placement or compression sonography in the diagnosis of deep vein thrombosis (DVT). While the aorto-ilio-femoral arteries are easily distinguishable on ultrasound, POCUS is rarely used to localize peripheral arterial thrombosis.
Here, we present a case of an unstable patient who was found to have a common femoral artery occlusion via prompt use of point-of-care ultrasound, which led to the expedited discovery of a mural aortic thrombus and the incidental discovery of a common femoral vein DVT. To the authors’ best knowledge, there have been no previous reports of the concurrent discovery of arterial and venous thrombosis in the same lower extremity by bedside sonography.
Case report
A 79-year-old female, never smoker, with a past medical history of morbid obesity, diabetes mellitus type 2, hypertension, atrial fibrillation on Coumadin, and past surgical history of left femoral-popliteal bypass graft and below-the-knee amputation presented to the emergency department (ED) with a poor appetite and pain in the right leg for 3 days. There were no associated fevers, chills, cough, nausea, vomiting, diarrhea, abdominal pain, chest pain, trauma, numbness or tingling. The patient reported chronic shortness of breath, but it was noted to be worse that day.
On physical examination, the patient’s vital signs were: temperature 36.3 °C (97.5 °F) orally, pulse 108 beats/min, blood pressure 142/99 mm Hg, respirations 18 breaths/min, and room air pulse oxygenation 92 %. The patient was noted to be in mild–moderate respiratory distress, but speaking in full sentences. The lungs demonstrated wheezing bilaterally. The abdomen was mildly tender diffusely, without rebound or guarding. The left below-the-knee amputation was noted to have a well-healed surgical wound. The patient’s right lower extremity was noted to be pale appearing and cold to touch. The right dorsalis pedis, posterior tibial and popliteal pulses were nonpalpable. Sensation was normal to light touch.
The patient was placed on a nasal cannula and an emergent surgical consult was obtained for concern for ischemic limb and a confirmation of the absence of flow to the right dorsalis pedis, posterior tibial and popliteal arteries was made by handheld Doppler. At approximately 30 min after initial presentation, the patient became acutely more tachypneic with a respiratory rate noted at 33 breaths/min. Vital signs on reassessment were: pulse 118 beats/min, blood pressure 137/68 mmHg, respirations 40 breaths/min, and a pulse oxygenation was unattainable on a nonrebreather mask. A repeat abdominal examination was significant for increased tenderness diffusely. An initial arterial blood gas (ABG) showed a pH of 7.39, PaCo2 of 20.3 mmHg, PaO2 of 84.4 mmHg, HCO3 of 12.1 mmol/L, SaO2 of 97 %, and base excess of −10.7 mmol/L. A mental status examination revealed increased lethargy and the patient was intubated for increased work of breathing and airway protection. A 7.5 mm endotracheal tube was placed by rapid sequence intubation using 10 mg of IV vecuronium and 20 mg of IV etomidate. A chest X-ray confirmed tube placement, as well as a right pulmonary infiltrate consistent with pneumonia. Significant laboratory results included lactic acid 8.5 mmol/L, creatine kinase 21, 969 IU/L, INR 1.6, blood urea nitrogen 24 mg/dL, creatinine 2.45 mg/dL, potassium 6.3 mmol/L, glucose 392 mg/dL, troponin 3.04 ng/mL, creatine kinase/myoglobin fraction 0.8 %, white blood cell count 10,200 K/uL and hemoglobin 13.2 g/dL. An electrocardiogram revealed normal sinus rhythm without ischemic changes, evidence of peaked T waves or interval changes.
The patient was begun on broad-spectrum antibiotic coverage for sepsis and pneumonia and treatment for hyperkalemia was begun. An emergent ultrasound-guided right internal jugular central line was placed as the patient was noted to have poor peripheral intravenous access.
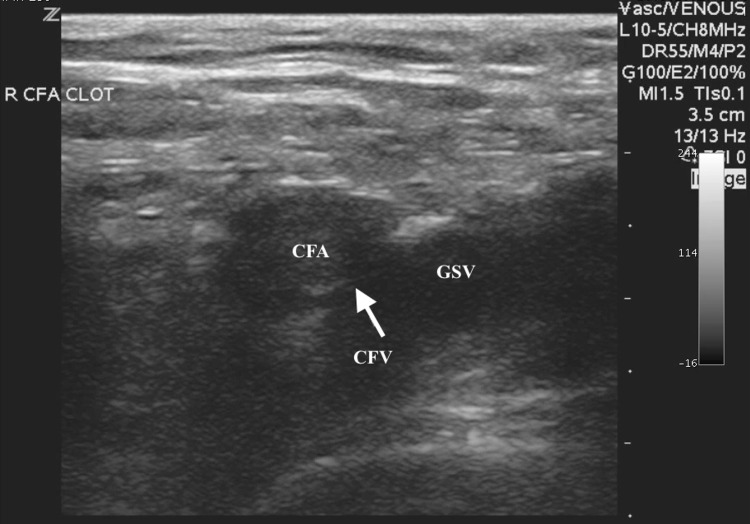
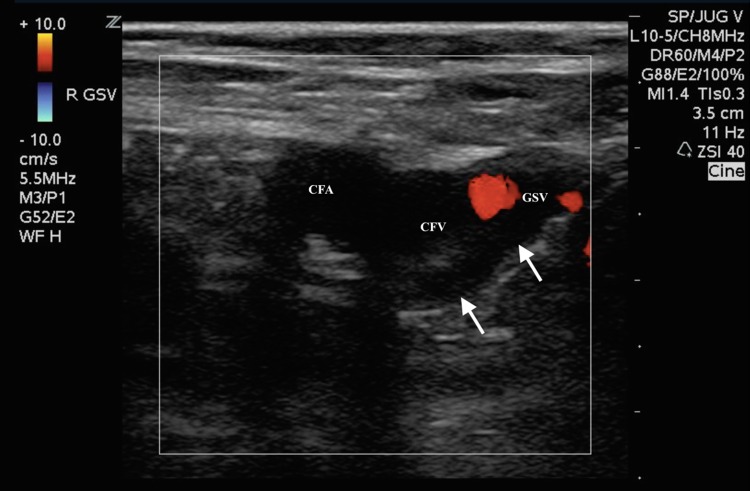
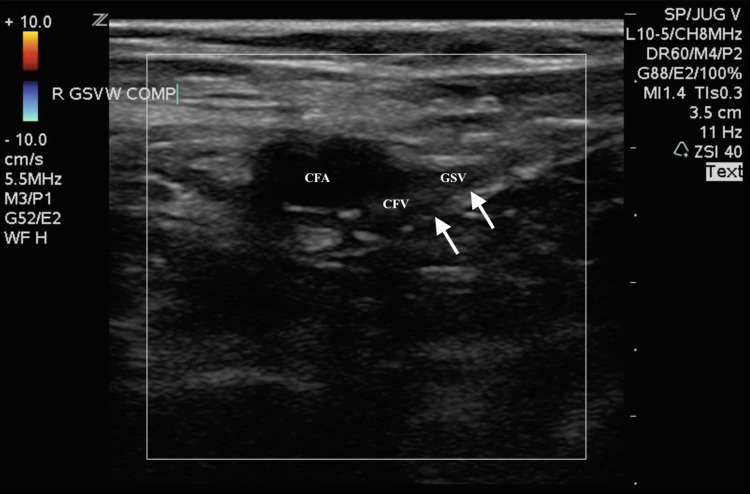
An unsuccessful repeat ABG was attempted in the right femoral artery by the emergency medicine senior resident yielding no blood aspirate, and no palpable pulses were noted despite adequate mean arterial blood pressure. The supervising emergency medicine attending then performed an ultrasound-guided right femoral arterial blood gas, as prior attempts using the landmark method were unsuccessful. The examining emergency physician placed a high-frequency linear transducer in the area of the right inguinal ligament to visualize the common femoral vessels (Fig. 1). On B-mode, hyperechoic material in the right common femoral artery most consistent with a complete obstructing arterial thrombus was noted, which likely extended to the deep and superficial femoral arteries. This finding was further confirmed by color Doppler when no flow was visualized in the common femoral artery (Fig. 2). Additionally, a small, partially obstructing hyperlucent material adhering to the posterior aspect of the common femoral and great saphenous vein was also noted, most consistent with a deep venous thrombus, at the point of entry of the great saphenous vein (Fig. 3). This finding was then confirmed by color Doppler and compression sonography, indicating a partially compressible right common femoral and great saphenous vein (Fig. 4). As there were concerns of a more proximal arterial thrombus, which was likely extending distally, attempts were made to visualize more proximally at the level of the iliacs and distal aorta, but were unsuccessful due to bowel gas and large body habitus.
Fig. 1.
Transverse B-mode ultrasound image of the right common femoral artery (CFA) and vein (CFV) at the level of the great saphenous vein (GSV). Arrow pointing to echogenic material that can be seen in the right common femoral artery (CFA)
Fig. 2.
Transverse color flow Doppler ultrasound image of the right common femoral artery (CFA) and great saphenous (GSV) and common femoral vein (CFV) junction. Partial flow can be seen in the common femoral (CFV) and great saphenous veins (GSV), but common femoral arterial (CFA) flow is absent
Fig. 3.
Transverse color flow Doppler ultrasound image of the right common femoral artery (CFA) and great saphenous (GSV) and common femoral vein (CFV) junction. Arrow pointing to partially obstructing echogenic material that can be seen in both the right common femoral artery and on the posterior aspect of the common femoral and great saphenous vein. No flow is demonstrated in the common femoral artery and only partial color Doppler flow is noted in the common femoral vein, at the level of the junction with the great saphenous vein
Fig. 4.
Transverse color flow Doppler ultrasound image of the right common femoral artery (CFA) and great saphenous (GSV) and common femoral vein (CFV) junction with compression sonography. Arrow pointing to echogenic material that is noted with incomplete compression of the common femoral vein (CFV), at the level of the junction with the great saphenous vein (GSV)
Emergent computed tomography angiography (CTA) scan with intravenous contrast of the aorta was then performed which revealed an occlusion in the infrarenal aorta extending to the right renal artery with notably dilated loops of bowel. An emergent consultation with the attending vascular surgeon was had at that time and indicated that, given the length of time of symptoms, likely infarction of significant portions of corresponding bowel and kidney and necrosis of the entire right lower extremity, the prognosis was extremely grave and the patient was a poor surgical candidate.
A final radiology attending read of the CTA demonstrated a complete thrombotic occlusion of the infrarenal abdominal aorta extending into the right renal artery, distal to the area of inferior mesenteric artery and a complete thrombotic occlusion to the distal portion of the superior mesenteric artery; areas of right renal, splenic and hepatic infarcts; distension of the small and large loops of bowel, likely ischemia/infarction in the setting of vascular disease; right lower lobe pulmonary artery emboli with several small emboli noted in segmental and subsegmental branches; as well as moderate right-sided and small left-sided pleural effusions and bilateral pulmonary consolidations.
The patient was placed on supportive care, begun on a heparin drip and eventually required pressors for bouts of hypotension. Repeat laboratory values indicated worsening renal failure, rhabdomyolysis and lactic acidosis, despite aggressive fluid resuscitation and treatment of hyperkalemia. The patient was admitted to the medical intensive care unit and eventually expired due to severe hyperkalemia and metabolic acidosis, 2 days after initial presentation to the emergency department.
Discussion
Acute limb ischemia (ALI) typically presents with one of six classic findings titled the “six Ps”—pain, pallor, paresthesia, paralysis, poikilothermia and pulselessness. Clinicians should keep a very high index of suspicion for ALI in any patient who presents with any of the above symptoms, a history of cardioembolic disease and vascular claudication and should be wary of the timing of onset, as limb salvage has traditionally been associated with rapid intervention within 4–6 h. This time period, however, has recently been called into question, as hypoperfusion, resulting in muscle damage, may occur in a shorter period and, conversely, many patients may present with a history of chronic limb ischemia, so that timing of exact onset may be difficult to pinpoint. Nonetheless, rapid diagnosis remains crucial as biotoxins accumulate within the tissue bed, distal to occlusion, and compartment syndrome or reperfusion syndrome may occur and is associated with profound and life-threatening acidosis, hyperkalemia, myoglobinuria, renal failure and death. Therefore, often, primary amputation may be life saving [8, 13].
Arterial and venous thromboembolism in a patient with no known history of atherosclerosis is incredibly rare. The above patient was found to have a strong history of peripheral vascular disease, a femoral-popliteal bypass graft and below-the-knee amputation. She acutely decompensated during her short stay in the emergency department, likely secondary to a combination of acute reperfusion injury and multi-organ ischemia and failure. Interestingly, the patient had already been on Coumadin therapy to treat her atrial fibrillation, but was noted to have a subtherapeutic INR, likely also contributing to worsening thrombosis.
Commonly, magnetic resonance angiography (MRA) or CTA has been employed to evaluate acute limb ischemia. While one systemic review and another meta-analysis indicate a lower sensitivity for detecting lower limb arterial thrombosis of >50 % by ultrasound (median sensitivity 90 %, specificity 99 %), duplex sonography was found to have a similarly high specificity as CTA (median sensitivity 97 %, specificity 99.6 %) and MRA (median sensitivity 94 %, specificity 99.2 %) [14, 15].
During resuscitative efforts for this patient, point-of-care ultrasound was used to quickly identify an arterial occlusion, when the landmark method for ABG was found on repeat attempts to be unsuccessful, as no arterial blood was aspirated. The acute development of abdominal pain during the ED stay and noted clinical findings of presumed distal limb ischemia with a confirmation of common femoral artery occlusion by bedside sonography allowed for the development of a presumptive diagnosis of a proximal and likely aortic thrombus, which was rapidly confirmed by CTA. Interestingly, the simultaneous discovery of a common femoral vein DVT was incidental, and the likely source of pulmonary embolism that was also later also seen on CTA. While ultimately this patient expired, possibly due to a delay in presentation to the emergency department, this case demonstrates the importance of rapid diagnosis of this time-sensitive condition and the ease and feasibility of point-of-care ultrasound in diagnosing peripheral arterial occlusion. Additionally, to the authors’ best knowledge, while rarely noted on CTA or MRA, no previous reports of a concurrent discovery of both arterial and venous thrombosis in the same limb have been noted on point-of-care ultrasound to date [16–18].
Acute limb ischemia is a time-sensitive, limb-threatening disease entity that if missed or not treated promptly can lead to death. Physicians must be able to suspect limb ischemia early, based on history and clinical examination findings. Point-of-care ultrasound may help to diagnose and increase the suspicion for a more proximal thrombus, such as in the iliacs or aorta, in the appropriate clinical scenario, despite a history of anticoagulation. This case demonstrates the ease and importance of detecting a femoral artery occlusion, using point-of-care ultrasound, in an unstable patient presenting with signs and symptoms of acute limb ischemia.
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest in regards to any of the authors listed.
Ethical Approval
All procedures performed involving the patient included in this case report were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and it’s later amendments or comparable ethical standards.
Informed consent
The patient is deceased and unable to provide consent. Attempts made to contact next of kin, however, were unsuccessful.
References
- 1.Verma H, Meda N, Vora S, George RK, Tripathi RK. Contemporary management of symptomatic primary aortic mural thrombus. J Vasc Surg. 2014;60:1524–1534. doi: 10.1016/j.jvs.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 2.Fayad ZY, Seeman E, Fahoum B, Briggs M, Tortolani A, D’Ayala M. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27:282–290. doi: 10.1016/j.avsg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Machleder HI, Takiff H, Lois JF, Holburt E. Aortic mural thrombus: an occult source of arterial thromboembolism. J Vasc Surg. 1986;4:473–478. doi: 10.1016/0741-5214(86)90383-6. [DOI] [PubMed] [Google Scholar]
- 4.Reber PU, Patel AG, Stauffer E, Muller MF, Do DD, Kniemeyer HW. Mural aortic thrombi: an important cause of peripheral embolization. J Vasc Surg. 1999;30:1084–1089. doi: 10.1016/S0741-5214(99)70047-9. [DOI] [PubMed] [Google Scholar]
- 5.Gagliardi JM, Batt M, Khodja RH, Le bas P. Mural thrombus of the aorta. Ann Vasc Surg. 1988;2:201–204. doi: 10.1016/S0890-5096(07)60001-6. [DOI] [PubMed] [Google Scholar]
- 6.De Rango P. Mural thrombus of thoracic aorta: few solutions and more queries. Eur J Vasc Endovasc Surg. 2011;41:458–459. doi: 10.1016/j.ejvs.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Williams GM, Harrington D, Burdick J, White RI. Mural thrombus of the aorta: an important, frequently neglected cause of large peripheral emboli. Ann Surg. 1981;194:737–744. doi: 10.1097/00000658-198112000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker GT. Acute limb ischemia. Tech Vasc Interventional Rad. 2009;12:117–129. doi: 10.1053/j.tvir.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Giovanni N, Daniela M, Giovanni M, Maria Teresa O, Silvia S, Davide F, Domenico Giuseppe T (2011) Endovascular treatment of thoracic aortic floating thrombus in patients presenting with acute lower limb ischemia. Int J Vasc Med 2011:604362 [DOI] [PMC free article] [PubMed]
- 10.Iuliano L, Misuraca M, Varroni A, Raponi M, Massucci M, Pagnanelli A, Cimino G, Bertoletti G. Multiple thromboembolism with multiple causes in a 69-year-old woman: a case report. J Med Case Rep. 2011;5:186. doi: 10.1186/1752-1947-5-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brearley S. Acute leg ischaemia. BMJ. 2013;346:f2681. doi: 10.1136/bmj.f2681. [DOI] [PubMed] [Google Scholar]
- 12.Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 13.Yassin MMI, Harkin DW, Barros D’Sa AAB, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26(1):115–121. doi: 10.1007/s00268-001-0169-2. [DOI] [PubMed] [Google Scholar]
- 14.Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334:1257. doi: 10.1136/bmj.39217.473275.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJW. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301:415–424. doi: 10.1001/jama.301.4.415. [DOI] [PubMed] [Google Scholar]
- 16.Dixon T, Panda M, Desbiens N. The simltenous occurance of deep vein thrombosis and pulmonary and arterial embolization. J Gen Intern Med. 2007;22(7):1040–1041. doi: 10.1007/s11606-007-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abunnaja S, Clyde M, Cuviello A, Brenes RA, Tripodi G. Concomitant deep venous thrombosis, femoral artery thrombosis, and pulmonary embolism after air travel. Case Rep Vasc Med. 2014;2014:174147. doi: 10.1155/2014/174147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadizade DR, Kukuk GM, Fahlenkamp UL, et al. Simultaneous MR arteriography and venography with blood pool contrast agent detects deep venous thrombosis in suspected arterial disease. Am J Roentgenol. 2012;198(5):1188–1195. doi: 10.2214/AJR.11.7306. [DOI] [PubMed] [Google Scholar]