Abstract
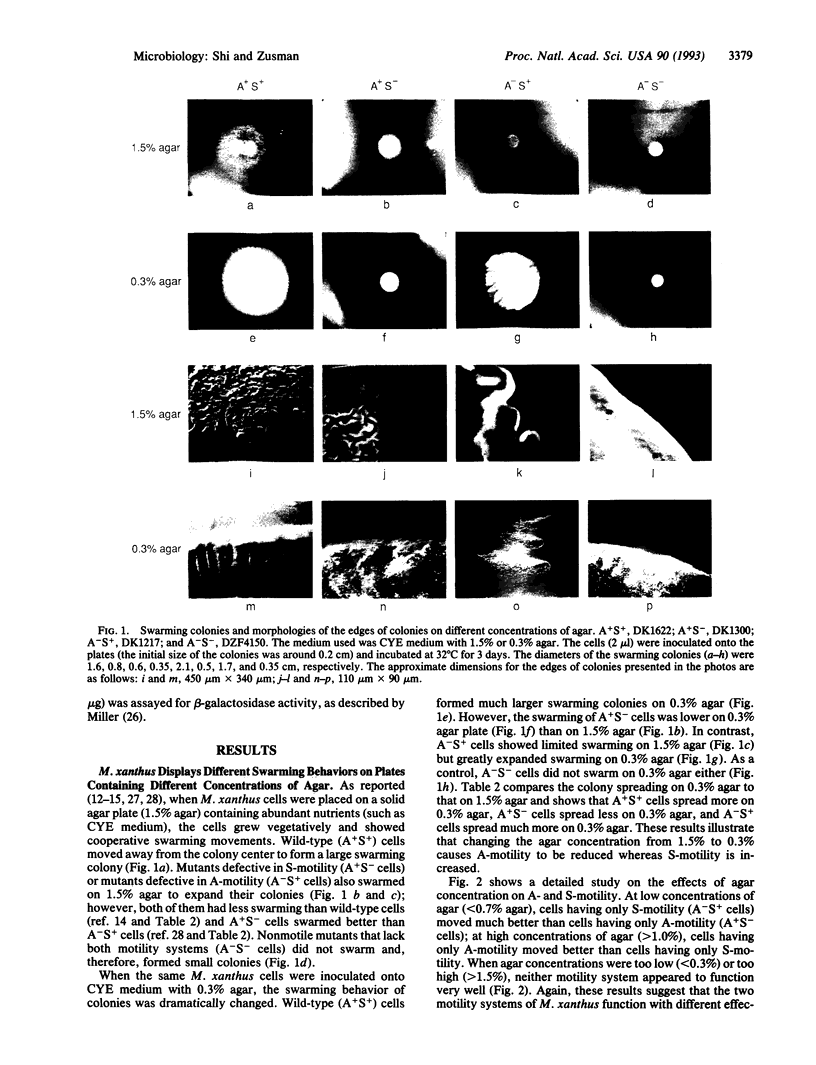
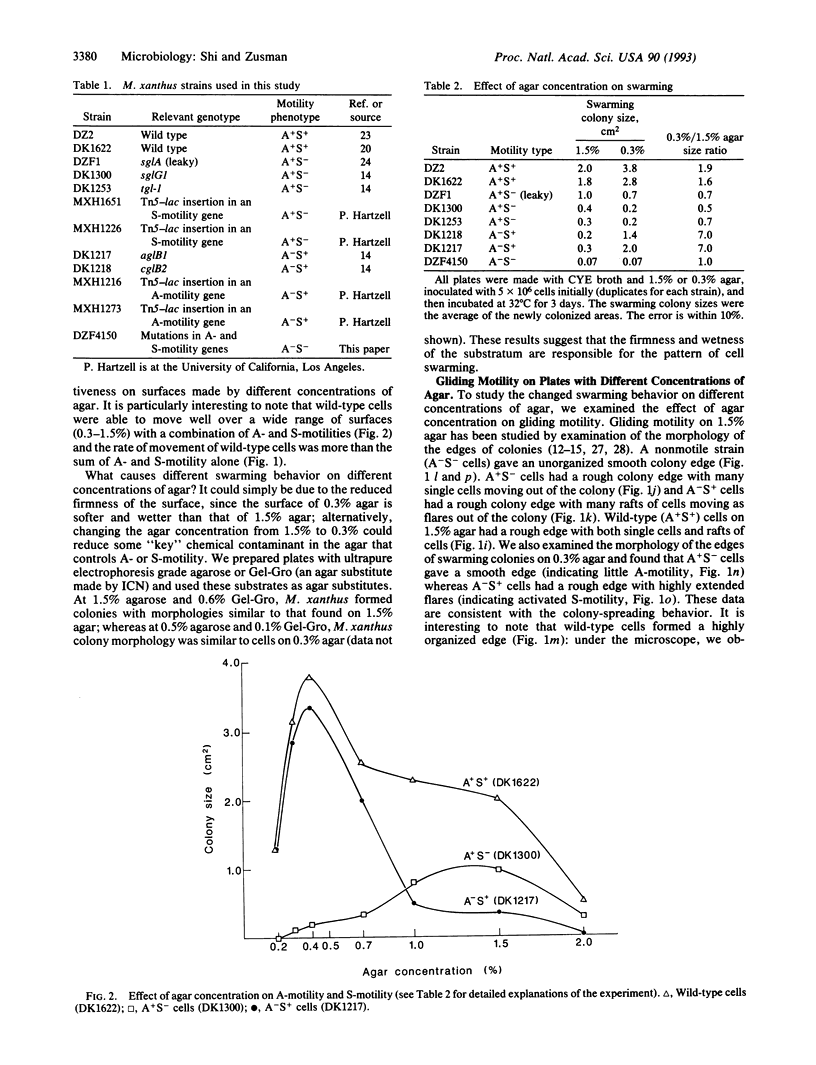
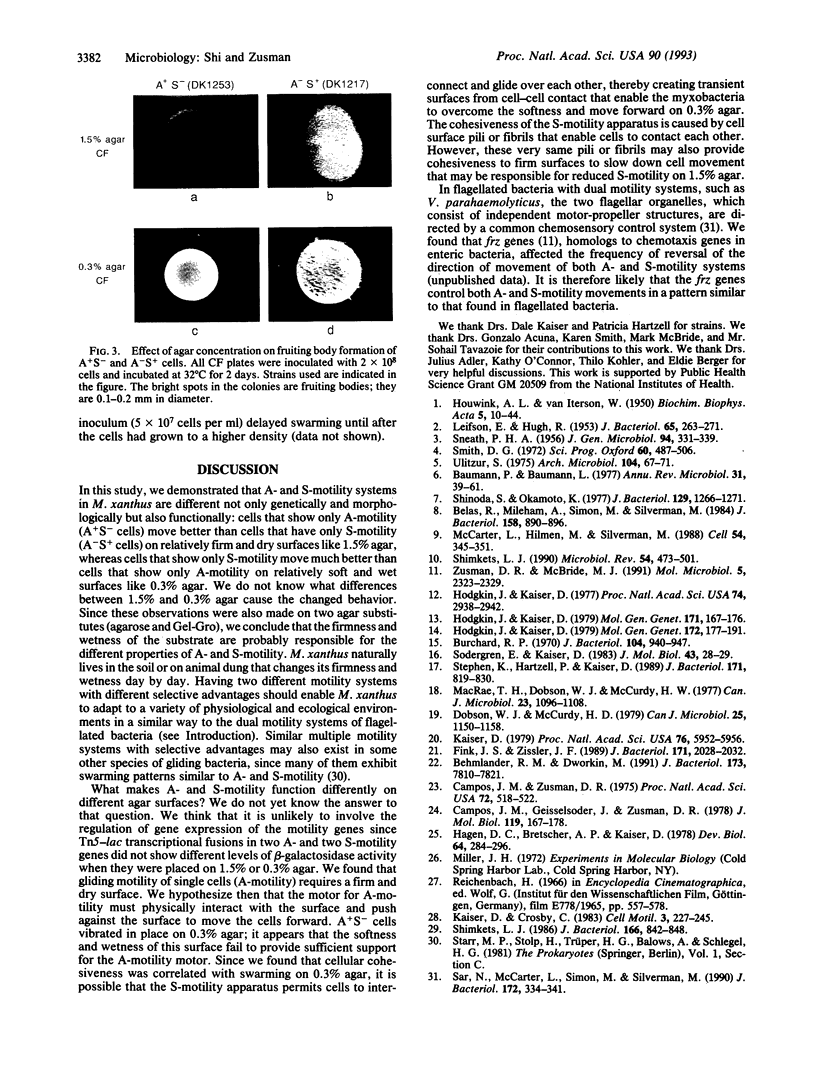
Myxococcus xanthus, a bacterium that forms fruiting bodies, moves by gliding motility utilizing dual motility systems that differ both genetically and morphologically [system A, having at least 21 genetic loci and moving mainly single cells, and system S, having at least 10 genetic loci and moving groups (rafts) of cells] [Hodgkin, J. & Kaiser, D. (1979) Mol. Gen. Genet. 172, 177-191]. In this study, we found that A- and S-gliding-motility systems have different selective advantages on surfaces containing different concentrations of agar. We observed that colonies of A+S- cells (A-motile cells) swarmed better than A-S+ cells (S-motile cells) on relatively firm and dry surfaces (e.g., 1.5% agar). In contrast, colonies of A-S+ cells swarmed much better than A+S- cells on soft and wet surfaces (e.g., 0.3% agar). Individual A-motile cells moved at a rate of 2-4 microns/min on 1.5% agar but they barely moved on 0.3% agar (< 0.5 microns/min); in contrast S-motile cells moved 3-5 times faster on 0.3% agar than on 1.5% agar. Wild-type cells with both A- and S-motility systems were able to move well over a wide range of surfaces. These results suggest that dual motility systems enable the myxobacteria to adapt to a variety of physiological and ecological environments and show similarities in function to the dual motility systems of flagellated bacteria such as Vibrio spp.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Baumann L. Biology of the marine enterobacteria: genera Beneckea and Photobacterium. Annu Rev Microbiol. 1977;31:39–61. doi: 10.1146/annurev.mi.31.100177.000351. [DOI] [PubMed] [Google Scholar]
- Behmlander R. M., Dworkin M. Extracellular fibrils and contact-mediated cell interactions in Myxococcus xanthus. J Bacteriol. 1991 Dec;173(24):7810–7820. doi: 10.1128/jb.173.24.7810-7820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Mileham A., Simon M., Silverman M. Transposon mutagenesis of marine Vibrio spp. J Bacteriol. 1984 Jun;158(3):890–896. doi: 10.1128/jb.158.3.890-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P. Gliding motility mutants of Myxococcus xanthus. J Bacteriol. 1970 Nov;104(2):940–947. doi: 10.1128/jb.104.2.940-947.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. M., Geisselsoder J., Zusman D. R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Campos J. M., Zusman D. R. Regulation of development in Myxococcus xanthus: effect of 3':5'-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. M., Zissler J. F. Characterization of lipopolysaccharide from Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989 Apr;171(4):2028–2032. doi: 10.1128/jb.171.4.2028-2032.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUWINK A. L., van ITERSON W. Electron microscopical observations on bacterial cytology; a study on flagellation. Biochim Biophys Acta. 1950 Mar;5(1):10–44. doi: 10.1016/0006-3002(50)90144-2. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIFSON E., HUGH R. Variation in shape and arrangement of bacterial flagella. J Bacteriol. 1953 Mar;65(3):263–271. doi: 10.1128/jb.65.3.263-271.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae T. H., Dobson W. J., McCurdy H. D. Fimbriation in gliding bacteria. Can J Microbiol. 1977 Aug;23(8):1096–1108. doi: 10.1139/m77-165. [DOI] [PubMed] [Google Scholar]
- McCarter L., Hilmen M., Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988 Jul 29;54(3):345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- Sar N., McCarter L., Simon M., Silverman M. Chemotactic control of the two flagellar systems of Vibrio parahaemolyticus. J Bacteriol. 1990 Jan;172(1):334–341. doi: 10.1128/jb.172.1.334-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986 Jun;166(3):842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990 Dec;54(4):473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Okamoto K. Formation and function of Vibrio parahaemolyticus lateral flagella. J Bacteriol. 1977 Mar;129(3):1266–1271. doi: 10.1128/jb.129.3.1266-1271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens K., Hartzell P., Kaiser D. Gliding motility in Myxococcus xanthus: mgl locus, RNA, and predicted protein products. J Bacteriol. 1989 Feb;171(2):819–830. doi: 10.1128/jb.171.2.819-830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzer S. The mechanism of swarming of Vibrio alginolyticus. Arch Microbiol. 1975 Jun 20;104(1):67–71. doi: 10.1007/BF00447301. [DOI] [PubMed] [Google Scholar]
- Zusman D. R., McBride M. J. Sensory transduction in the gliding bacterium Myxococcus xanthus. Mol Microbiol. 1991 Oct;5(10):2323–2329. doi: 10.1111/j.1365-2958.1991.tb02077.x. [DOI] [PubMed] [Google Scholar]