Abstract
Purpose
To investigate the diagnostic performance of perfusion single-photon emission computed tomography/computed tomography (Q-SPECT/CT) in patients suspected to have pulmonary embolism (PE) but with indeterminate computed tomographic pulmonary angiography (CTPA) or planar ventilation/perfusion (V/Q) scans.
Methods
This retrospective study included two groups of patients. Group I consisted of 49 patients with nondiagnostic CTPA. These 49 patients underwent subsequent V/Q scans. Further Q-SPECTs were obtained in patients with indeterminate planar images and fused with existing CTPA. Group II consisted of 182 non-CTPA patients with indeterminate V/Q scans. These 182 patients underwent further Q-SPECT and separate noncontrast low-dose CT chest. Fusion Q-SPECT/CT scans were obtained through FDA-approved software and interpreted according to published criteria as positive, negative, or indeterminate for PE. Upon retrospective analyses, the final diagnosis was made using composite reference standards including all available clinical and imaging information for at least 6-month follow-up.
Results
In group I patients, 1 was positive, 24 were negative, and another 24 (49 %, 24/49) were indeterminate. In the subsequent 24 Q-SPECT/CTPAs, 4 were positive, 19 were negative, and 1 was indeterminate (4.2 %, 1/24). In group II patients, 9 (4.9 %, 9/182) were indeterminate, 33 were positive, and 140 were negative. The combined nondiagnostic rate for Q-SPECT/CT was only 4.9 % (10/206). There was six false-negative and one false-positive Q-SPECT/CT examinations. The sensitivity, specificity, and positive and negative predictive value of Q-SPECT/CT were 85.7 % (36/42), 99.4 % (153/154), 97.3 % (36/37) and 96.2 % (153/159), respectively.
Conclusions
Q-SPECT/CT improves the diagnostic rate with promising accuracy in diagnosing PE that yields a satisfactory clinical verdict, especially when the CTPA and planar V/Q scan are indeterminate.
Electronic supplementary material
The online version of this article (doi:10.1007/s13139-015-0359-8) contains supplementary material, which is available to authorized users.
Keywords: Q-SPECT/CT, V/Q, Pulmonary Embolism, CTPA
Background
Acute pulmonary embolism (PE) presents a clinical dilemma because of challenges inherent in diagnosis and its high attendant rates of morbidity and mortality. The annual incidence of PE has been reported to range between 23 and 69 cases per 100,000 people [1]. Case fatality rates vary widely depending on the severity of the disease; it can be as high as 90 % in patients with unrecognized massive PE, whereas it is reported as 2–10 % in treated patients [1, 2]. PE remains a leading cause of preventable in-hospital mortality, accounting for 100,000 to 200,000 deaths annually. It is estimated that 5–10 % of preventable hospital deaths are attributable to PE [3]. Given its broad spectrum of clinical presentations [4], and the inherent risks of anticoagulant therapy, the definitive diagnosis of PE is a priority to assure optimal healthcare. Imaging examinations are usually the decisive tests for PE diagnosis.
Computed tomographic pulmonary angiography (CTPA) provides direct visualization of occlusive or partially occlusive thrombus within the pulmonary arterial vasculature and is widely considered a first-line diagnostic test for PE [5, 6]. However, CTPA is contraindicated in patients with renal insufficiency, multiple myeloma, or allergy to intravenous (IV) contrast. Also, excessive patient motion and poor bolus enhancement can result in a mean of 6.4 % “indeterminate” examinations in one study [7] to as many as 8 % suboptimal and 5 % nondiagnostic examinations in other experience [8]. An alternative diagnostic test is radionuclide scintigraphic imaging of lung ventilation and perfusion (V/Q) [9, 10].
In the Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) study, among patients with images of adequate quality, CTPA had a sensitivity of 83 % and specificity of 96 % for the diagnosis of acute PE, with 6 % considered nondiagnostic [11]. The widely used PIOPED II criteria for interpretation of V/Q scans compare the size of a perfusion abnormality to the size of a radiographic or ventilation abnormality on planar images. The inherent limitation of such comparison, especially in patients with significant airway disease, renders 26.3 % of the V/Q scans nondiagnostic [12].
In patients with clinical suspicion of PE, but with indeterminate CTPA or planar V/Q results, the choice of imaging tests is limited. One rational strategy is to use the single-photon emission computed tomography (SPECT) imaging technique to improve both the sensitivity and specificity of planar V/Q scanning [13]. As an extension of the initial endeavor from one of our authors (YL) [14], we developed a software-based hybrid diagnostic modality combining perfusion SPECT (Q-SPECT) and CT at our institution to expand the armamentarium in diagnosing PE [15, 16]. The advent of commercially available multimodality imaging fusion software permits superimposition of Q-SPECT images with separate CT data, either from a noncontrast low-dose CT (LDCT) or CTPA, thereby taking advantage of the physiologic information obtained from SPECT functional imaging and anatomic detail from CT scans. We adopted the “MSKCC criteria” [14] into the interpretation of the software-generated Q-SPECT/CT data, aiming to minimize the nondiagnostic tests and increase diagnostic accuracy. This retrospective study is to investigate the diagnostic performance of such software-based Q-SPECT/CT in patients with nondiagnostic CTPA and indeterminate planar V/Q scans.
Materials and Methods
Patient Selection
This institutional review board-approved retrospective study included all patients undergoing radionuclide perfusion imaging between December 2011, coinciding with the inception of Q-SPECT imaging at our institution, and June 2013. Patients were categorized into two groups. Group I included patients who underwent scintigraphic perfusion imaging within 7 days of a nondiagnostic CTPA study. Group II included patients either having perfusion imaging as a result of contraindication to CTPA or as a result of clinician preference. All patients had follow-up for at least 6 months after imaging.
Imaging Protocols
CT Pulmonary Angiography
All scans were obtained by using a GE CT scanner (GE Healthcare, Wisconsin) with 16 or 64 detector rows. Patients were scanned in the supine position with acquisition parameters at 120 kVp and 299–700 mAs. A standard collimation of 16 × 0.75 mm was used, with a gantry rotation speed of 0.5 s and a pitch factor of 1.15. Patients received IV injections of 80 ml Omnipaque-350 contrast at 3.5-4.5 ml/s via IV access, followed by a 40-ml saline flush. Individual contrast optimization was achieved by using a 20-ml test bolus in the right ventricle with a trigger level of 100 HU. An additional delay of 7 s was added before image acquisition in every examination.
For further post-processing, thin-section reconstruction was performed with a 1.25-mm section thickness and smooth reconstruction kernel. In cases where CTPA proved nondiagnostic, and clinical suspicion remained to warrant further imaging, radionuclide V/Q scans were then performed.
Planar V/Q Scan and Software-Generated Q-SPECT/CT
Planar images were recorded with large field-of-view dual detector SPECT cameras (Skylight, Philips Medical Systems, or GE Infinia Hawkeye 4 SPECT/CT) using parallel-hole high-resolution low-energy collimators, with an energy window of 20 % at a centerline of 140 keV. A dose of 3.0 mCi Tc-99 m macro-aggregated albumin was injected intravenously with the patient supine. Planar images were recorded for 5 min each in the anterior, posterior, and anterior and posterior oblique views. In a few patients, usually according to the attending physician’s choice during the scan time, planar ventilation (V) images were obtained with the patient breathing 20 mCi Xe-133 through the Xenon delivery system and detectors projected at the position best showing the perfusion defects. The detector energy window was reset at 20 % at a centerline of 80 keV. Single-breath, equilibrium, and washout images are obtained.
In cases where a definitive diagnostic interpretation of very low probability or high probability by modified PIOPED II standards [12] could not be rendered on the basis of planar V/Q or planar Q imaging alone, patients underwent additional Q-SPECT imaging and a separate noncontrast LDCT chest acquired on a separate 8-, 16-, or 64-slice GE CT scanner (GE Healthcare, Wisconsin) at 120 kVp, 15–30 mAs, as the CT component of the GE Infinia Hawkeye 4 SPECT/CT scanner is not suitable for lung images (with a maximum current of 4 mAs). Q-SPECTs of the chest were acquired on the same camera for planar images at 20 s/stop, with 3° steps, in a 128 × 128 matrix, and reconstructed with an iterative ordered subset expectation maximization (OSEM) algorithm. Hybrid SPECT/CT fusion images were obtained using the FDA-approved multimodality imaging software GE Xeleris 2 (GE Healthcare, Madison, WI) or MIM Software (MIM Software Inc., Cleaveland, OH) to fuse the Q-SPECT images with CT data, i.e., the “nondiagnostic” CTPA or separate noncontrast LDCT chest images.
Image Interpretation and Final Diagnosis (Table 1)
Table 1.
V/Q scan and Q SPECT/CT scan interpretative criteria for PE
| Modified PIOPED II12 17 | Perfusion only PISA-PED18 19 | MSKCC Q SPECT/CT14 20 21 |
|---|---|---|
| PE present (include high probability V/Q scans) a. ≥2 large mismatched (V/Q or Q/CXR) segmental perfusion defects b. 1 large and 2 moderate segmental defects c. ≥4 moderate segmental defects |
PE present: ≥1 wedge-shaped Q defects | PE present ≥1 wedge-shaped ≥ 50 % segmental peripheral defect(visually reduced by > 70 % compared with normally perfused lung) a. Without corresponding CT image abnormality and clearly seen in all three orthogonal planes b. Corresponding to the equivocal pulmonary arterial filling defects on a recent CTPA |
| Nondiagnositc (include low and intermediate probability V/Q scans) All other findings |
Indeterminate: All other findings |
Indeterminate: All other findings |
| PE absent: (includes normal and very low probability V/Q scans) a. No defects present on the perfusion scan b. <3 small perfusion defects with a normal CXR c. Nonsegmental perfusion defect d: ≥2 matched (V:Q) defects, regionally normal CT or CXR e. Solitary triple matched (V:Q:CXR) defect (<1 segment) in mid or upper lung f. Stripe sign‡ g. Solitary large pleural effusion |
PE absent: a. Non-wedge-shaped Q defects b. Near normal or normal Q c. Contour defect caused by heart, mediastinum, or diaphragm |
PE absent: a. Normal perfusion b. Nonperipheral, nonsegmental perfusion defects c. Perfusion defects < 50 % of an involved segment d. Perfusion defects corresponding to CT abnormalities such as radiation fibrosis, pleural effusion, emphysematous bullae, infectious consolidation, or solid tumor mass, etc. |
Planar V/Q images were interpreted in accordance with the modified PIOPED II criteria [17, 12]; planar Q only scans were interpreted with the PISA-PED criteria [18, 19]. The software-generated fusion Q-SPECT/CT images were deemed qualified for interpretation when anatomic markers (heart silhouette, aortic arch, bilateral hilum) were matched between SPECT and CT data, and the SPECT lung margins were all projected within the CT lung borders. The Q-SPECT/CT images were interpreted using the previously published “MSKCC Q-SPECT/CT criteria” [20, 14, 21], where PE is indicated by at least one wedge-shaped peripheral defect estimated as ≥50 % of a pulmonary segment [22] without corresponding CT image abnormality and clearly seen in all three orthogonal planes. Any perfusion defects corresponding to CT abnormalities (Fig. 1) (such as radiation fibrosis, pleural effusion, emphysematous bullae, pneumonia, or solid tumor mass, etc.) are deemed negative for PE.
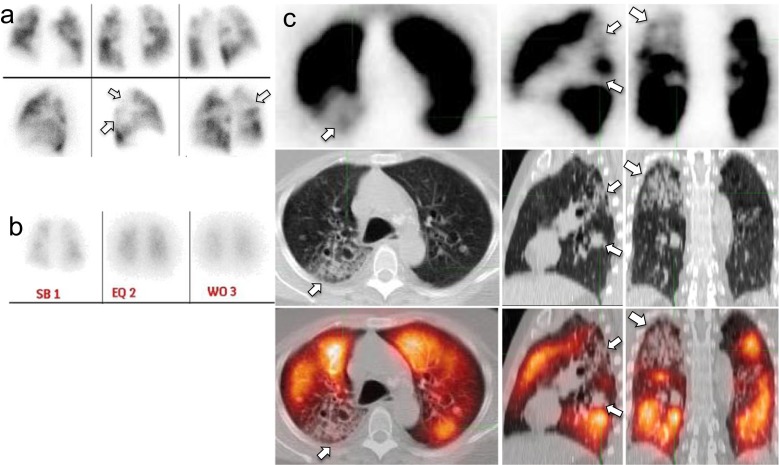
Fig. 1.
Nondiagnostic V/Q scan interpreted as negative for PE on Q-SPECT/CT. Mismatched perfusion defects (arrows, panel a: planar Q scan; panel b: posterior planar V scan with Xenon, which was performed after the planar Q scan) would be read as intermediate probability for PE according to the PIOPED II criteria, but correlate with branching ground-glass opacities on Q-SPECT/CT images (arrows, panel b, top row: Q-SPECT; mid row: noncontrast LDCT; lower row: fusion Q-SPECT/CT), consistent with inflammatory/infectious change, thus interpreted as negative for PE
At the first round of revisiting all the planar and Q-SPECT/CT images, all readers were blinded to the medical records and other imaging results such as D-dimer results, available lower extremity Doppler ultrasound, and follow-up pulmonary embolism imaging tests (i.e., planar Q and V/Q, Q-SPECT/CT and CTPA). All planar Q and V/Q scans were re-interpreted independently by a board eligible radiology resident (NK) and a chest radiologist (WM). In discrepancy cases, board-certified nuclear medicine physicians (MJB, TA and YL) were brought in as referee. The noncontrast LDCT chest and CTPA studies were interpreted independently by board-eligible radiology residents (NK and NM) and board-certified body-imaging radiologists (KX and WM). After finishing a training set of Q-SPECT/CT studies using published data [20, 14, 21, 15, 16], a board-certified nuclear medicine physician (MJB) and a double-boarded nuclear medicine and radiology physician (TA), both having more than 25 years of experience in interpreting traditional V/Q scans, and a board-eligible radiology resident (NK) reinterpreted the Q-SPECT/CT images independently. A fourth reader with 5 years of experience in interpreting Q-SPECT/CT scans (YL) was the final arbiter of the Q-SPECT/CT studies.
Upon further analysis, demographic information for all patients was recorded. For all patients, Wells scores [23] were tabulated (supplemental Table 1). All charts were reviewed, and at least 6 months of clinical and imaging follow-up were tracked to determine the presence or absence of PE. The final diagnosis of PE was determined by consensus of the pulmonologist (RM) and all imaging physicians using a composite of all clinical information, including clinical symptoms and presentation, physical examination, ECG, D-dimer levels, and all available initial and at least 6-month follow-up imaging tests such as lower-extremity Doppler ultrasound, planar Q and V/Q, Q-SPECT/CT and CTPA.
Statistical Analysis
The characteristics of the study population were expressed as the mean ± standard deviation. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated as the evaluation of diagnostic performance. Statistical analyses were performed with SPSS, version 19.0 (IBM) software.
Results
In group I (CTPA group) of this study (Fig. 2), of a total of 759 CTPA studies performed at our institution between December 2011 and June 2013, 49 patients had initial indeterminate or nondiagnostic CTPA. The 49 patients had an average age of 44 years (range 18–83 years). A total of 32 of 49 (64 %) patients were female (Table 2). Group II (non-CTPA arm) of the study included patients who underwent Q-SPECT scintigraphic imaging without concurrent CTPA. Of the 447 patients who underwent initial planar V/Q or planar Q scans during the study period, a total of 182 patients (40.7 %) were considered at low and intermediate probability based on the modified PIOPED II criteria and underwent subsequent Q-SPECT and LDCT imaging (Fig. 3). These 182 patients had an average age of 55 years (range 20-91 years), and 121 (66.4 %) were female. Upon retrospective Wells score calculation (Table 2), patients in the CTPA arm considered negative for PE had an average Wells score of 2.25 ± 1.68. Comparatively, those interpreted as positive for PE had Wells scores averaging 6.0 ± 1.90. In the non-CTPA arm, patients with negative examinations had an average Wells score of 1.67 ± 1.38, while the positive group had a mean Wells score of 5.67 ± 2.29. The indeterminate group had an average Wells score of 2.06 ± 1.65. Radionuclide imaging was chosen as the primary modality for PE diagnosis for the non-CTPA group (182 patients with different indications) (Table 3).
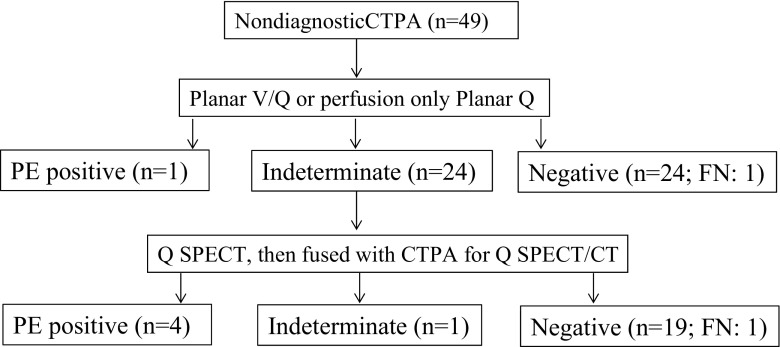
Fig. 2.
Flow diagram of the workup and results for patients with nondiagnostic CTPA. FN: false negative
Table 2.
Patient characteristics
| Variables | Sex | Age (years) | Wells scores (mean ± SD) | ||||
|---|---|---|---|---|---|---|---|
| Female (%) | Male (%) | Age range | Mean ± SD | Positive PE (n) | Negative PE (n) | Indeterminate PE (n) | |
| Patients with nondiagnostic CTPA (n = 49) | 32 (64 %) | 17 (36 %) | 18–83 | 44.3 ± 16.9 | 6.0 ± 1.90 (5) | 2.25 ± 1.68 (43) | 2.5 (1) |
| Patients with nondiagnostic planar V/Q (n = 182) | 121 (66.4 %) | 61 (33.6 %) | 20–91 | 55.0 ± 18.3 | 5.67 ± 2.29 (33) | 1.67 ± 1.38 (140) | 2.06 ± 1.65 (9) |
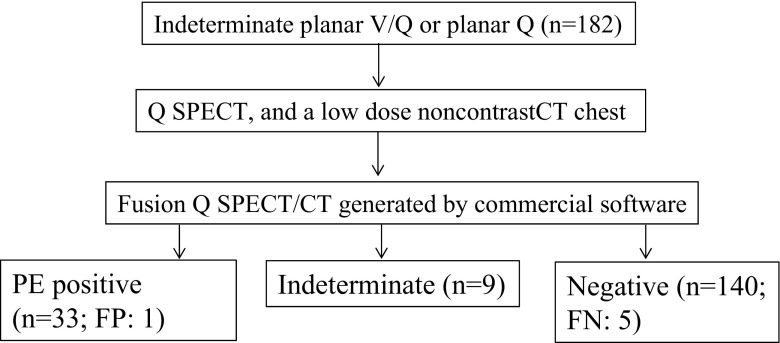
Fig. 3.
Flow diagram of the workup and results for patients with nondiagnostic/indeterminate planar V/Q scan or planar Q scan. FP: false positive; FN: false negative
Table 3.
Indications for choosing radionuclide imaging as the primary modality for PE diagnosis
| Indications | Patients: N (%) |
|---|---|
| Acute or chronic renal insufficiency | 112 (112/182, 61.5 %) |
| Borderline renal function (GFR between 60 and 80 ml/min) | 23 (23/182, 12.6 %) |
| Radiation exposure reduction | 10 (10/182, 5.5 %) |
| Allergy to iodinated contrast medium | 9 (9/182, 4.9 %) |
| Referring physicians preference | 7 (7/182, 3.8 %) |
| Intravenous contrast administration for an unrelated examination within 24 h | 6 (6/182, 3.3 %) |
| Renal transplant patient | 6 (6/182, 3.3 %) |
| Poor intravenous access | 3(3/182, 1.6 %) |
| Contrast infiltration precluding CTPA | 2(2/182, 1.1 %) |
| Active lupus nephritis exacerbations | 2(2/182, 1.1 %) |
| Proteinuria with normal creatinine | 1(1/182, 0.55 %) |
| Technical malfunction with the CT scanner | 1(1/182, 0.55 %) |
| Total | 182 (100 %) |
Of the 49 patients within the CTPA group, 25 underwent planar imaging with a definitive diagnosis (1 was interpreted as high probability; the remaining 24 had no perfusion defects and were considered negative examinations). Upon retrospective review, one patient was a false negative as a 1-month follow-up CTPA showed a small subsegmental PE. In the remaining 24 of the 49 patients (49 %, 24/49), as perfusion abnormalities were seen on planar images and results were considered indeterminate, further Q-SPECT images were obtained and fused with existing CTPA. Utilizing the trinary interpretation structure, only 1 of the 24 (4 %, 1/24) examinations was considered indeterminate, while 4 of 24 (16.7 %) were positive, and 19/24 (79.1 %) were negative. Upon retrospective review, there was one false-negative Q-SPECT/CTPA due to a small nonocclusive subsegmental PE (Fig. 4).
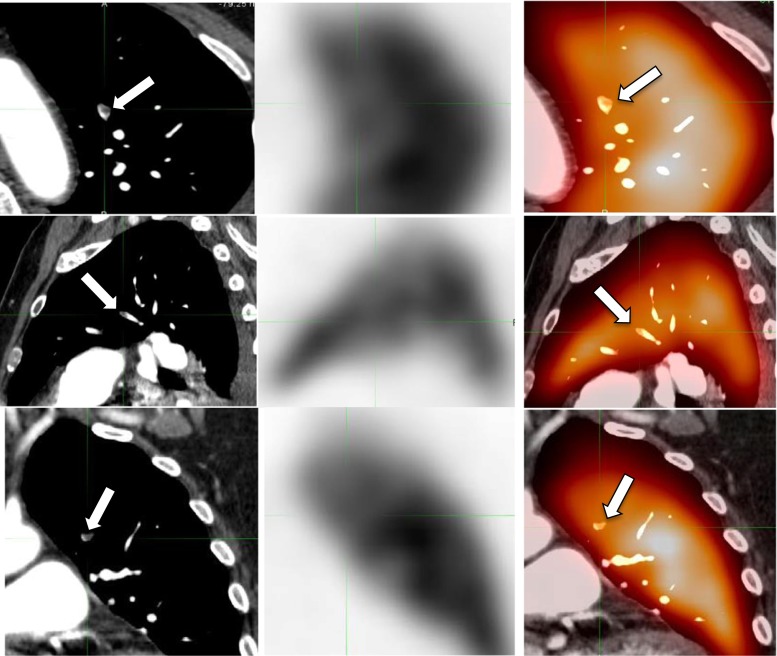
Fig. 4.
False-negative Q-SPECT/CT. A small subsegmental left upper lobe PE confirmed on the second reading of the CTPA (arrows, left column), but without a corresponding perfusion defect on Q-SPECT (mid column) and Q-SPECT/CT (arrows, right column)
In the non-CTPA group of 182 patients with initial indeterminate planar V/Q or planar Q, who underwent subsequent Q-SPECT and LDCT scanning, definitive interpretation was rendered in all but nine cases, yielding an indeterminate rate of approximately 4.9 % (9/182). A total of 33 patients were positive for PE (18 %, 33/182). Upon retrospective review, 1 of these 33 patients was deemed false positive because of air trapping and pleural effusions (Fig. 5). Among the 140 patients (77 %, 140/182) interpreted as negative, 5 were false negative retrospectively. One patient developed DVT in 3 months. Another four patients were deemed false negative as Q-SPECT/CT failed to detect nonocclusive PE diagnosed on a follow-up CTPA.
Fig. 5.
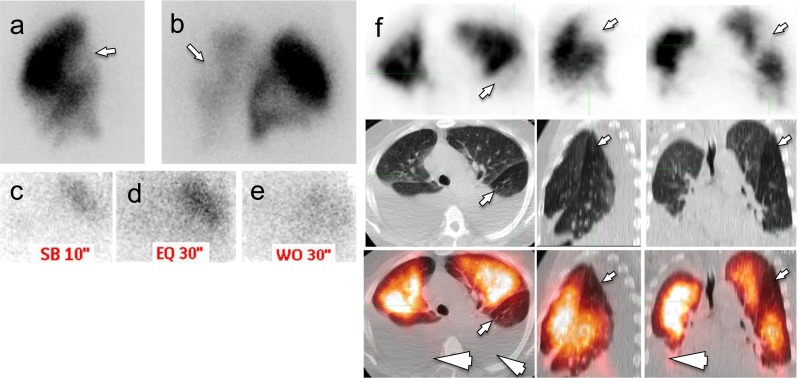
False-positive Q SPECT/CT. Nondiagnostic planar V/Q scan showed perfusion defects in the left lung (arrows, a right lateral planar Q scan; b right posterior oblique planar Q scan; limited planar V scan with Xenon on right posterior oblique view after planar Q scan; c inspiration phase; d equilibrium phase; e wash-out phase). On Q-SPECT/CT images (panel f, top row: Q-SPECT; mid row: noncontrast LDCT; lower row: fusion Q-PECT/CT), the perfusion defects (small arrows) appeared without a corresponding CT abnormality and was read as positive for PE. Additional bilateral pleural perfusions were also noted (arrowheads). A CTPA obtained 3 days later showed a patent pulmonary artery branch in this region. The case is deemed false positive for Q-SPECT/CT
While pooling the data from the two groups, the sensitivity, specificity, and positive and negative predictive value of Q-SPECT/CT (including Q-SPECT/CTPA and Q-SPECT/LDCT) in these two patient cohorts were 85.7 % (36/42), 99.4 % (153/154), 97.3 % (36/37), and 96.2 % (153/159), respectively. The indeterminate rate of Q-SPECT/CT was 4.9 % (10/206).
Discussion
Seeking an effective means of diagnosing PE is both desirable and an important measure in improving patient outcomes. Although CTPA has a higher proportion of definitive diagnostic results and offers the ability to diagnose alternative causes of chest pain, with high sensitivity (96–100 %) as well as a specificity of 86–89 % [24], it carries certain important limitations. For patients with impaired renal function or contraindications to iodine-containing IV contrast, CTPA is not indicated. Furthermore, a small but significant proportion of examinations is considered indeterminate by the interpreting radiologist. It has been reported that approximately 6 % of studies are considered nondiagnostic [11]. Frequently, this occurs because of motion artifacts, poor image quality, or inadequate contrast enhancement of the pulmonary vasculature [8, 7]. Our institutional nondiagnostic CTPA rate is 6.5 % (49/759), similar to what has been reported [11]. On the other hand, current techniques may render CTPA as too sensitive, resulting in overdiagnosis and overtreatment of PE, i.e., finding clinically unimportant PE and exposing patients to potential harm from unnecessary treatment [25–27]. False negative Q-SPECT/CT cases in our studies echoed this argument, as there was no functional consequences of PE, i.e., perfusion defect, in small nonocclusive subsegmental PE (Fig. 4). Obviously, this observation would need verification in large samples, preferably through a multicenter prospective trial to define what would be clinically significant PE.
Furthermore, there is increasing concern regarding the high radiation dose from CTPA (average 15 mSv) versus an average dose of 2.2 mSv in a typical V/Q scan in the USA [28]. Conventional CTPA has a 20–40 times greater dose to the female breast than the V/Q scan (typically 10–70 mGy for CTPA vs. <1.5 mGy for V/Q to the breast) [29]. The typical radiation doses encountered by patients who receive two to three CT scans during their lifetime cause significantly increased cancer risk over the course of their lifetimes, and this risk is increased in younger populations [30]. Utilizing the models based on the National Research Council's “Biological Effects of Ionizing Radiation” report, it has been estimated that 29,000 future cancers could be related to CT scans performed in a given year [31]. Moreover, there is higher risk in patients who undergo recurrent CT scanning [32]. In pregnant patients, although the V/Q scan delivers a higher dose to the fetus than CTPA, there is typically only low, negligible fetal exposure from either study (<1 mGy), and the difference between CTPA and V/Q scans is small when compared with that to the maternal breast. Thus, V/Q study has an advantage over CTPA in this group [33]. Compared to an average dose of 15 mSv in CTPA, the total radiation exposure from a Q-SPECT plus a noncontrast LDCT scan is approximately 3.5 mSv, which is only 1.5 mSv more than a perfusion scan alone and only 1.2 mSv more than a V/Q scan combined with a chest radiograph [14, 28].
For patients with contraindications to CTPA, or nondiagnostic/indeterminate CTPA results while clinical suspicion remains high, the alternative test would be nuclear medicine V/Q studies. Unfortunately, the relatively high rate of indeterminate (low and intermediate probability) V/Q scans limits the clinical utility of scintigraphic imaging, which circumvents the aforementioned limitations of CTPA.
In cases with indeterminate planar V/Q images, our study demonstrates an improved diagnostic rate with Q-SPECT/LDCT compared to planar V/Q. While over 40 % of planar V/Q examinations in our cohort had indeterminate interpretations based on the PIOPED II criteria, only 4.9 % (9/182) of Q-SPECT/LDCT scans were considered indeterminate. In software-generated Q-SPECT/CTPA studies, the nondiagnostic rate was only 4.2 % (1/24). With improved coregistration of anatomic details and physiologic information, fused Q-SPECT/CT can provide superior delineation of perfusion abnormalities and their spatial relationship with underlying thoracic disease. We posit that the superiority of this correlation relative to planar images with radiographs produces more reliable diagnostic information for clinicians grappling with potential PE.
Via commercially available, FDA-approved multimodality imaging fusion software, Q-SPECT images can be fused with CT images from either a CTPA study (in cases where CTPA had proven nondiagnostic) or with a noncontrast LDCT performed specifically for fusion purposes (in patients who had equivocal V/Q scans). With the combination of functional perfusion images and anatomic CT images, specific perfusion abnormalities can be compared to and analyzed for concomitant abnormalities of the lung parenchyma, airways, and pleural spaces. The study is interpreted under the presumption that diminished perfusion that cannot be explained by corresponding pleural, parenchymal, or airway disease represents a pulmonary embolus. This modality has the promise of substantially reducing the overall radiation dosimetry for examinations conducted with the intention of diagnosing clinically suspected PE. Our data and those from the Memorial Sloan-Kettering Cancer Center (MSKCC) [14] suggest that this method of Q-SPECT/CT scanning reduces the radiation dose by a factor of almost 10.
Although Q-SPECT/CT has been used in diagnosing PE in several institutions, there are no definitive Q-SPECT/CT interpretation criteria for PE. In combination with the published MSKCC data [14], we propose the “MSKCC-UIC Q-SPECT/CT criteria” for PE diagnosis. This study is in accordance with the published MSKCC data, which validate the Q-SPECT/CT criteria with a diagnostic advantage over planar V/Q scan. Our data indicate that the “MSKCC-UIC Q-SPECT/CT criteria” can be used to interpret software-generated Q-SPECT/CT images through fusion of Q-SPECT with separately acquired CTPA and/or noncontrast LDCT. This is especially advantageous when CTPA and routine V/Q scans are indeterminate/nondiagnostic.
Some authors have reported that V/Q SPECT or V/Q SPECT/CT is superior to Q SPECT/CT alone in diagnosing PE [34, 35]. In some scenarios, adding V SPECT would be helpful for more accurate diagnosis: for example, asthma exacerbations, hypoxic vasoconstrictive responses to reduced ventilation and some obstructive airway diseases can show perfusion defects without CT abnormalities and may cause a false-positive diagnosis of PE on Q SPECT/CT. In extreme cases, acute PE causing pulmonary infarction might be diagnosed as false negative on Q SPECT/CT because of the corresponding CT abnormality with perfusion defects. Unfortunately, the ideal V-SPECT imaging tracer Kr-81 m needs a cost-prohibitive generator and thus is not available to the majority of hospitals in the US. Tc-99 m DTPA, as the most widely used for V-SPECT imaging in the US, often settles to a large degree in the central airways, especially in patients with poor respiratory efforts, resulting with nondiagnostic quality images in the patients where V-SPECT is most needed. This situation may change once smaller labeled particles such as Tc-99 m Technegas become available in the US. Nonetheless, cautious investigation of the medical history and physical examination helps to make the correct diagnosis on Q SPECT/CT images. Our data based on the 6-month follow-ups showed that Q SPECT/CT had a satisfying clinical outcome with a minimal nondiagnostic rate in the setting of initial indeterminate CTPA and planar V/Q studies.
An intrinsic limitation of this retrospective study was the lack of a clear gold standard. Reassuringly, the Wells scores of the patients deemed to have negative scans were, on average, less than 3, which denotes a relatively low risk for PE, and studies interpreted as positive, on average, would be categorized as moderate to high risk for PE. Another limitation related to this retrospective study is that we did not have corresponding Q-SPECT/CT results for all the patients with definitive positives or negatives for the PE diagnosis based on planar V/Q scans, such as the 25 patients with initially nondiagnostic CTPA but with subsequent definitive planar V/Q results (Fig. 2), and 1 of the 24 patients negative on the planar scan was deemed as false negative upon the 1-month follow-up CTPA. It is imaginable that patients could have different results for PE diagnosis through Q-SPECT/CT compared to the results of the planar scan. Challenges in widespread adoption of Q-SPECT/CT as a primary modality in clinical use remain. Validating the Q-SPECT/CT model for a PE diagnosis through a multicenter prospective trial is a prerequisite to its prevalent clinical application.
Conclusion
In conclusion, our clinical experience utilizing software-generated Q-SPECT/CT has demonstrated an improved diagnostic rate with promising accuracy in diagnosing PE, yielding a satisfactory clinical verdict, especially when CTPA and planar V/Q are nondiagnostic or indeterminate.
Electronic supplementary material
(DOCX 86 kb)
Compliance with Ethical Standards
Conflict of Interest
Nishant Kumar, Karen Xie, Winnie Mar, Thomas M. Anderson, Benjamin Carney, Nikhil Mehta, Roberto Machado, Michael J. Blend, and Yang Lu declare that they have no conflict of interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived at the approval of the IRB for this retrospective study.
The manuscript has not been published before and is not under consideration for publication anywhere else, and it has been approved by all coauthors.
References
- 1.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/S0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 2.Laporte S, Mismetti P, Decousus H, Uresandi F, Otero R, Lobo JL, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–1716. doi: 10.1161/CIRCULATIONAHA.107.726232. [DOI] [PubMed] [Google Scholar]
- 3.Park B, Messina L, Dargon P, Huang W, Ciocca R, Anderson FA. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136:983–990. doi: 10.1378/chest.136.4_MeetingAbstracts.88S-b. [DOI] [PubMed] [Google Scholar]
- 4.Courtney DM, Kline JA, Kabrhel C, Moore CL, Smithline HA, Nordenholz KE, et al. Clinical features from the history and physical examination that predict the presence or absence of pulmonary embolism in symptomatic emergency department patients: results of a prospective, multicenter study. Ann Emerg Med. 2010;55:307–315 e1. doi: 10.1016/j.annemergmed.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomgarden DC, Rosen MP. Newer diagnostic modalities for pulmonary embolism. Pulmonary angiography using CT and MR imaging compared with conventional angiography. Emerg Med Clin North Am. 2001;19:975–994. doi: 10.1016/S0733-8627(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 6.Mayo JR, Remy-Jardin M, Muller NL, Remy J, Worsley DF, Hossein-Foucher C, et al. Pulmonary embolism: prospective comparison of spiral CT with ventilation-perfusion scintigraphy. Radiology. 1997;205:447–452. doi: 10.1148/radiology.205.2.9356627. [DOI] [PubMed] [Google Scholar]
- 7.Jones SE, Wittram C. The indeterminate CT pulmonary angiogram: imaging characteristics and patient clinical outcome. Radiology. 2005;237:329–337. doi: 10.1148/radiol.2371041520. [DOI] [PubMed] [Google Scholar]
- 8.Abujudeh HH, Kaewlai R, Farsad K, Orr E, Gilman M, Shepard JA. Computed tomography pulmonary angiography: an assessment of the radiology report. Acad Radiol. 2009;16:1309–1315. doi: 10.1016/j.acra.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 9.McNeil BJ, Holman BL, Adelstein SJ. The scintigraphic definition of pulmonary embolism. JAMA. 1974;227:753–756. doi: 10.1001/jama.1974.03230200011002. [DOI] [PubMed] [Google Scholar]
- 10.Gottschalk A, Stein PD, Goodman LR, Sostman HD. Overview of prospective investigation of pulmonary embolism diagnosis II. Semin Nucl Med. 2002;32:173–182. doi: 10.1053/snuc.2002.124177. [DOI] [PubMed] [Google Scholar]
- 11.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 12.Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology. 2008;246:941–946. doi: 10.1148/radiol.2463070270. [DOI] [PubMed] [Google Scholar]
- 13.Reinartz P, Wildberger JE, Schaefer W, Nowak B, Mahnken AH, Buell U. Tomographic imaging in the diagnosis of pulmonary embolism: a comparison between V/Q lung scintigraphy in SPECT technique and multislice spiral CT. J Nucl Med : Off Publ Soc Nucl Med. 2004;45:1501–1508. [PubMed] [Google Scholar]
- 14.Lu Y, Lorenzoni A, Fox JJ, Rademaker J, Vander Els N, Grewal RK, et al. Noncontrast perfusion single-photon emission CT/CT scanning: a new test for the expedited, high-accuracy diagnosis of acute pulmonary embolism. Chest. 2014;145:1079–1088. doi: 10.1378/chest.13-2090. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Xie KL, Carney B, Atueyi U, Blend MJ. Successful implementation of software fusion of perfusion SPECT and low dose noncontrast CT with a simple trinary interpretation strategy as an alternative to CT Pulmonary Angiography in diagnosis of PE. Eur J Nucl Med Mol Imaging. 2012;39:S288. [Google Scholar]
- 16.Mehta N, Xie KL, Bressler M, Blend M. lu y. Improved diagnostic ACCURACY OF PE with a novel “hybrid imaging” method: transforming ugly duckling into swan. Clin Nucl Med. 2012;37:1202. [Google Scholar]
- 17.Stein PD, Woodard PK, Weg JG, Wakefield TW, Tapson VF, Sostman HD, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Radiology. 2007;242:15–21. doi: 10.1148/radiol.2421060971. [DOI] [PubMed] [Google Scholar]
- 18.Miniati M, Pistolesi M, Marini C, Di Ricco G, Formichi B, Prediletto R, et al. Value of perfusion lung scan in the diagnosis of pulmonary embolism: results of the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISA-PED) Am J Respir Crit Care Med. 1996;154:1387–1393. doi: 10.1164/ajrccm.154.5.8912753. [DOI] [PubMed] [Google Scholar]
- 19.Miniati M, Sostman HD, Gottschalk A, Monti S, Pistolesi M. Perfusion lung scintigraphy for the diagnosis of pulmonary embolism: a reappraisal and review of the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis methods. Semin Nucl Med. 2008;38:450–461. doi: 10.1053/j.semnuclmed.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Schoder H. Comparison of non-contrast perfusion SPECT-CT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism in patients with contraindications for CT angiography. SNM annual meeting 2010; Salt Lake City, UT: J Nucl Med; 2010. p. 39
- 21.Lu Y, Fox JJ. Acute pulmonary embolism detected by perfusion SPECT/CT masquerading as an intermediate probability planar V/Q scan. Clin Nucl Med. 2010;35:941–943. doi: 10.1097/RLU.0b013e3181f9de54. [DOI] [PubMed] [Google Scholar]
- 22.Howarth DM, Booker JA, Voutnis DD. Diagnosis of pulmonary embolus using ventilation/perfusion lung scintigraphy: more than 0.5 segment of ventilation/perfusion mismatch is sufficient. Intern Med J. 2006;36:281–288. doi: 10.1111/j.1445-5994.2006.01070.x. [DOI] [PubMed] [Google Scholar]
- 23.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 24.Sadigh G, Kelly AM, Cronin P. Challenges, controversies, and hot topics in pulmonary embolism imaging. AJR Am J Roentgenol. 2011;196:497–515. doi: 10.2214/AJR.10.5830. [DOI] [PubMed] [Google Scholar]
- 25.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheh SH, Bellin E, Freeman KD, Haramati LB. Pulmonary embolism diagnosis and mortality with pulmonary CT angiography versus ventilation-perfusion scintigraphy: evidence of overdiagnosis with CT? AJR Am J Roentgenol. 2012;198:1340–1345. doi: 10.2214/AJR.11.6426. [DOI] [PubMed] [Google Scholar]
- 27.Schissler AJ, Rozenshtein A, Kulon ME, Pearson GD, Green RA, Stetson PD, et al. CT pulmonary angiography: increasingly diagnosing less severe pulmonary emboli. PLoS ONE. 2013;8:e65669. doi: 10.1371/journal.pone.0065669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 29.Schembri GP, Miller AE, Smart R. Radiation dosimetry and safety issues in the investigation of pulmonary embolism. Semin Nucl Med. 2010;40:442–454. doi: 10.1053/j.semnuclmed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 31.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175–184. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 33.Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline—Evaluation of Suspected Pulmonary Embolism in Pregnancy. Radiology. 2012;262:635–646. doi: 10.1148/radiol.11114045. [DOI] [PubMed] [Google Scholar]
- 34.Gutte H, Mortensen J, Jensen CV, Johnbeck CB, von der Recke P, Petersen CL, et al. Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. J Nucl Med : Off Publ Soc Nucl Med. 2009;50:1987–1992. doi: 10.2967/jnumed.108.061606. [DOI] [PubMed] [Google Scholar]
- 35.Palmowski K, Oltmanns U, Kreuter M, Mottaghy FM, Palmowski M, Behrendt FF. Diagnosis of pulmonary embolism: conventional ventilation/perfusion SPECT is superior to the combination of perfusion SPECT and nonenhanced CT. Respir Int Rev Thorac Dis. 2014;88:291–297. doi: 10.1159/000365817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 86 kb)