Abstract
In recent years, the click reaction has found rapidly growing applications in the field of radiochemistry, ranging from a practical labeling method to molecular imaging of biomacromolecules. This present review details the development of highly reliable, powerful and selective click chemistry reactions for the rapid synthesis of new radiotracers for molecular imaging.
Keywords: Click reaction, 18F, CuAAC, Molecular imaging, Pretargeting
Introduction
Molecular imaging aims to noninvasively visualize, characterize and quantify biological processes at the cellular and molecular levels in vivo. Current biomedical science is rapidly adapting molecular imaging techniques to reveal the molecular processes of disease in living subjects. To this end, nuclear imaging, magnetic resonance imaging and near-infrared fluorescence imaging are emerging as key imaging modalities.
Nuclear imaging techniques are based on the radiolabeling of specific molecular probes using decaying radioisotopes. In biologically active probes, the emitted radiation can be detected by performing either positron emission tomography (PET) or single-photon computed tomography (SPECT) [1]. Both methods can provide insights into the specific biochemical processes of molecular probes and the pathophysiology of disease. Usually, the synthetic preparation of suitable radiolabeled probes has been developed and optimized using radiochemistry techniques. For the synthesis of novel radiotracers ranging from small organic and bioactive molecules to high-molecular-weight compounds such as peptides, proteins or oligonucleotides, most of the radiolabeling strategies focus on the radioactive prosthetic groups, which allow an effective and bioorthogonal conjugation reaction between bioactive molecules and radiolabeled building blocks.
Cu(I)-catalyzed formation of 1,2,3-triazole by Huisgen’s [2 + 3] cycloaddition of terminal alkynes and azides, commonly called “click chemistry,” was first described by Kolb et al. in 2001 [2]. The formation of 1,4-disubstituted 1,2,3-triazole is a particularly powerful ligation method with comparably favorable properties, including high and reproducible yields under mild aqueous conditions. Following the basic concepts of click chemistry, radiochemistry exploits the special advantages of this class of reactions for the introduction of radionuclides in small molecules as well as biomacromolecules. The present review addresses the recent applications of click chemistry reactions to the radiolabeling of biomolecules in the field of molecular imaging.
Copper Catalyzed Azide-Alkyne Cycloaddition (CuAAC)
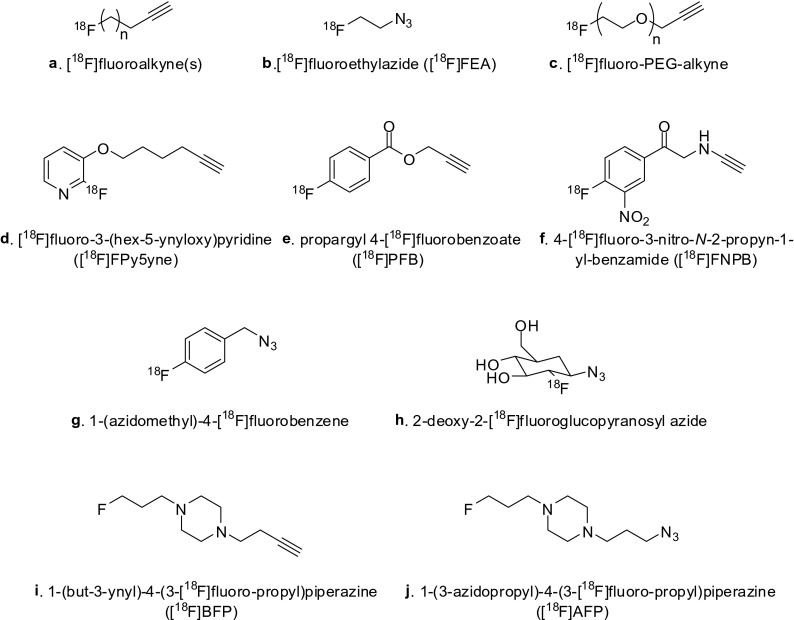
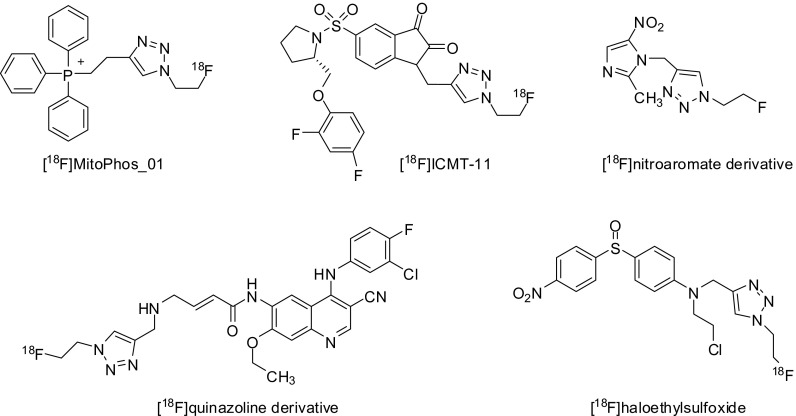
Cu(Ι)-catalyzed azide-alkyne cycloaddtion has become a useful tool in various fields of organic synthesis. Notably, this reaction has been rapidly applied in radiochemical procedures for the introduction of radioisotopes in small molecules as well as peptides. Figure 1 summarizes the diverse approaches to the synthesis of radiotracers using various 18F-labeled prosthetic groups. In 2006, Marik and Sutcliffe first described the preparation of 18F-labeled peptides using the CuAAC reaction. The Cu(I)-mediated cycloaddition reaction between [18F]fluoroalkynes (Fig. 1a) and various peptides produced 18F-labeled peptides in quantitative radiochemical yields (RCY, 54–99 %) and high purities [3]. Moreover, 18F-CuAAC offers a short radiolabeling time under mild conditions. Thus, 18F-CuAAC is a very attractive strategy for the design and synthesis of radiotracers for molecular imaging purposes. Ross and coworkers used 6-[18F]fluoro-1-hexyne to generate, by click reaction, a folic acid derivative as a promising target molecule in oncology [4]. 18F-click folate was synthesized by a convenient and efficient two-step radiosynthesis. For a more flexible 18F-labeling of peptides by click reaction, the labeling agent 2-[18F]fluoroethylazide ([18F]FEA, Fig. 1b) was developed by Glaser and Årstad [5]. Despite the difficult handling of volatile [18F]FEA, the convenient labeling step and easy transfer of [18F]FEA were successfully developed in an automated module [6]. Hence, [18F]FEA is certainly the most popular 18F-prosthetic group used in click chemistry for the preparation of various radiotracers (Fig. 2), e.g., [1-(2-[18F]fluoroethyl),1H[1, 2, 3]triazole 4-ethylene] triphenylphosphonium bromide ([18F]MitoPhos_01) [7] and [18F]ICMT-11 [8] (for apoptosis), [18F]quinazoline derivative (for EGFR activity) [9], [18F]FET-G-TOCA (for somatostatin receptor) [10], [18F]haloethylsulfoxides [11] and [18F]nitroaromates [12] (for hypoxia), [18F]RGD peptides (for the integrin ανβ3 receptor) [13] and [18F]AFETP (for brain tumor) [14]. Recently, Zhou et al. reported a facile purification of [18F]FEA using a C18 and Oasis®HLB cartridge via a simple CuAAC reaction, which was carried out on the HLB cartridge by loading the alkyne substrate and copper catalyst dissolved in DMF onto the cartridge [15]. This solid-phase extraction technique is safe, simple, reproducible in high yield and compatible with an automated [18F]FEA click reaction.
Fig. 1.
Structure of [18F]-prosthetic groups
Fig. 2.
Various radiotracers using 18F-FEA
As reported by Chi’s group, 18F-labeled PEGylated alkyne (Fig. 1c) shows suitable advantages for in vivo studies because of a longer circulation time and an enhanced blood clearance [16]. In terms of radiolabeling, the corresponding 18F-labeled PEGylated alkyne and azide are thermally stable and nearly nonvolatile at the reaction temperature under ambient pressure required by the one-pot, two-step synthetic protocol. An example, reported by Chen’s group, is the synthesis of [18F]fluoro-PEG containing RGD peptide via a CuAAC reaction[17].
18F-labeled aryl alkyne precursors are commonly used in radiochemistry because of their high lipophilicity and in vivo metabolic stability. Numerous aryl 18F-labeled alkynes or azides have been reported. In 2008, Inkster et al. first introduced a pyridine-based 18F-prosthetic group, [18F]fluoro-3-(hex-5-ynyloxy)pyridine ([18F]FPy5yne), for CuAAC reaction (Fig. 1d). Bioconjugation between [18F]FPy5yne and peptide(N3-(CH2)4-CO-Tyr-Lys-Arg-Ile-OH, BG142) via click reaction proceeded in 18.7 % RCY after 160 min overall reaction time [18]. As another application, [18F]FPy5yne was ligated to a 5′-azid-modified DNA sequence antisense to mdr1 mRNA in the presence of Cu(I)-stabilizing ligand tris(benzyltriazolymethyl)amine and 2,6-lutidine [19]. The 18F-labeled oligonucleotide was obtained in 24.6 % ± 0.5 % RCYs (decay corrected) after 276 min total synthesis time. This method represents the first synthesis of an 18F-labeled DNA analog using a CuAAC reaction.
A new 18F-labeled aryl precursor, propargyl 4-[18F]fluorobenzoate ([18F]PFB), was presented by Vaidyanathan’s group (Fig. 1e). Several model compounds containing an azide moiety such as benzyl azide, lysine derivatives and a transglutaminase-reactive peptide were labeled with [18F]PFB via click reaction [20]. Li et al. reported a new stable aromatic prosthetic group with a similar structure, 4-[18F]fluoro-3-nitro-N-2-propyn-1-yl-benzamide ([18F]FNPB, Fig. 1f), for the efficient labeling of cRGDfK and a D4 peptide [21].
In another approach, the Thonon group introduced an 18F-labeled aryl azide, namely, 1-(azidomethyl)-4-[18F]-fluorobenzene (Fig. 1g), which was used to label the 4-ethynyl-L-phenylalanine-containing peptide via click reaction [22]. The desired 18F-labeled peptide was prepared in quantitative RCY within 15 min. The same group reported that 1-(azidomethyl)-4-[18F]-fluorobenzene can also be used to label a double-stranded oligonucleotide (siRNA) in 15 ± 5 % of RCYs [23]. In order to enhance the in vivo behavior of the targeted macromolecules, such as blood clearance and stability, an [18F]fluorodeoxyglycosyl azide was designed and developed by Schibli’s group in 2012 [24].
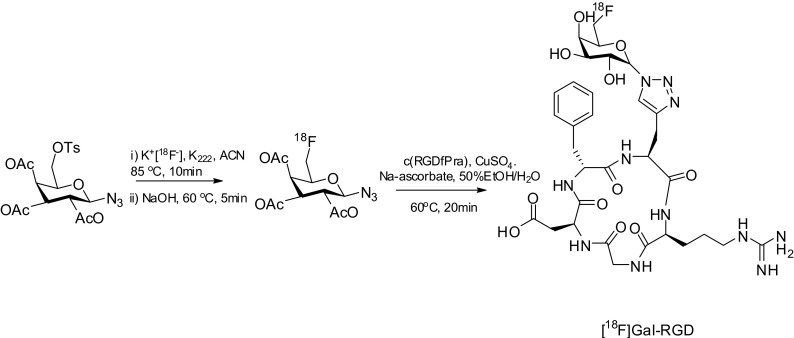
The reaction between the 18F-labeled 2-deoxy-2-fluoroglucopyranosyl azide (Fig. 1h) and folate alkyne proceeded in 5–25 % RCY. The 18F-labeled folate derivative containing a glucopyranosyl moiety showed an increasing tumor accumulation in ex vivo biodistribution as well as PET imaging studies. Recently, the Prante group prepared various 18F-labeled glycosyl azide derivatives and developed a click chemistry-based method for the [18F]fluoroglycosylation of alkyne-bearing RGD-peptides targeting the integrin receptor [25]. In animal PET imaging using U87MG tumor-bearing nude mice, 6-deoxy-6-[18F]fluoro-D-galactopyranosyl azide and 6′-deoxy-6′-[18F]fluoro-β-maltosyl azide were the most attractive among glycosyl derivatives containing monosaccharide or disaccharide units. Notably, 6′-deoxy-6′-[18F]fluoro-β-maltosyl containing RGD peptide revealed uptake and retention in the U87MG tumor comparable to those of [18F]galacto-RGD (Fig. 3). Through their favorable biodistribution and in vivo tissue clearance, a click reaction-based glycopeptide method would provide useful viable alternative radiotracers for PET imaging.
Fig. 3.
Radiosynthesis of 18F-galacto-RGD peptide using the click reaction
Piperazine-based alkyne 1-(but-3-ynyl)-4-(3-[18F]fluoropropyl)piperazine ([18F]BFP, Fig. 1i) and azide 1-(3-azidopropyl)-4-(3-[18F]fluoropropyl)piperazine ([18F]AFP, Fig. 1j), with beneficial properties such as high hydrophilicity and medium molar mass, were developed by Pretze and Mamat [26]. Both show advantageous properties such as desriable logP/D, solubility and in vitro stability. In addition, because spiro compounds allow a convenient separation using silica gel cartridges, the corresponding automated synthesis based on the CuAAC reaction is available.
Unlike previous studies, Mindt et al. developed an application of the Cu-catalyzed cycloaddition reaction for radiometal chemistry [27]. They described a click-to-chelate methodology that allows the successful labeling of peptides with 99mTc(CO)3 generating click-based constructs with improved in vivo stability (Fig. 4) [28].
Fig. 4.
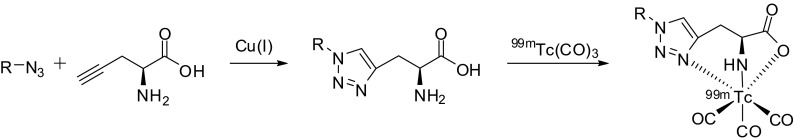
Click-to-chelate for tricarbonyl 99mTc-labeling
Copper-Free Azide-Alkyne Cycloaddition
The CuAAC reaction is one of the most attractive synthetic routes; however, the obvious limitation of this method is the cytotoxicity of copper. Because of safety concerns regarding the contamination of radiolabeled compounds with traces of copper, the CuAAC reaction is not suitable for the development of in vivo pretargeting methodologies.
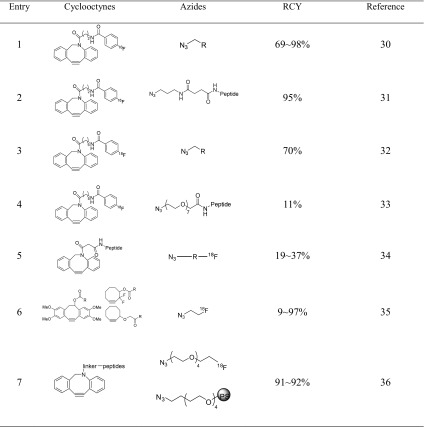
In recent years, the Bertozzi group pioneered the copper-free click (CFC) reaction, which was referred to as strain-promoted azide-alkyne cycloaddition (SPAAC) [29]. Numerous research groups have employed this reaction for the incorporating of radioisotopes into biomolecules. In 2011, Bouvet et al. described the copper-free click reaction for 18F-labeled azadibenzocyclooctyne (ADIBO) and azides such as 4-azidoaniline, 6-azido-6-deoxyglucose and azido-geldanamycin (Table 1, entry 1). The SPAAC reaction, carried out in different solvents in the presence of low concentrations of various azides, affords the desired product in good yields, can be used to label molecules or peptides with fluorine-18 and has great potential for in vivo pretargeting applications [30]. The Arumugam group synthesized a radiolabeled peptide via a CFC reaction using [18F]ADIBO (Table 1, entry 2) [31]. Similarly, Carpenter et al. described the conversion of a bifunctional ADIBO amine to the 18F-labeled cyclooctyne with >70 % RCY at 37 °C within 1 h (Table 1, entry 3) [32]. For imaging application, Hausner et al. prepared a radiolabeled αvβ6 integrin-targeting peptide (A20FMDV2) via a strain-promoted click reaction using 18F-labeled azadibenzocyclooctyne and the peptide azide (Table 1, entry 4) [33]. The [18F]FBA-C6-ADIBO-based prosthetic group was reported to not interfere with the αvβ6-binding in in vitro experiments, but was found to affect the pharmacokinetics because of its size and lipophilic nature. In view of the undesirable in vivo results, the cyclooctyne moiety should be modified to focus particularly on rapid chemical techniques and in vivo compatibility.
Table 1.
Copper-free azide-alkyne cycloaddition
In 2011, the Feringa group reported the rapid radiolabeling of bombesin with fluorine-18 by using the SPAAC reaction [34]. This straightforward protocol was performed by simple stirring of various 18F-labeled azides and the modified bombesin analog for 10–15 min at room temperature (Table 1, entry 5). As a result, 18F-labeled bombesin peptides with different properties are readily accessible from the same modified bombesin peptide, thus allowing for rapid modification and fine-tuning. In addition, the optimal lipophilicity for the target binding site and metabolic in vivo clearance can be achieved.
The Carroll group demonstrated, through in vitro stability measurements and small animal PET images of a BALB/c nude mouse, that 2-[18F]fluoroethylazide is advantageous for pretargeting with a SPAAC reagent [35]. In addition, they optimized the SPAAC reaction with various cyclooctynes and proposed that these reactions represent an attractive strategy for application-specific tuning in pretargeting systems (Table 1, entry 6).
The Kim group developed a labeling protocol based on the SPAAC reaction followed by a chemo-orthogonal purification reaction with an azide-bound resin [36]. This process provides a rapid and selective isolation method without the need for HPLC purification and formulation (Table 1, entry 7). Using this simple solid-phase extraction with the azide-bound resin, called ADIBO scavenger resin, 18F-labeled ADIBOT (azadibenzocyclooctatriazole) RGD dimer peptide was successfully synthesized in high RCY via the SPAAC reaction between 18F-labeled PEG-azide and RGD-ADIBO peptide without HPLC purification in high RCY [37].
Diels-Alder Cycloaddition
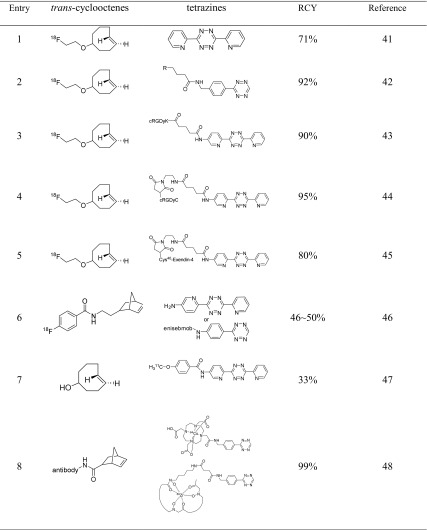
Although the SPAAC reaction in the presence of ADIBO eliminates the risk of cytotoxic copper in the final formulation, the hydrophobicity and the relatively slow in vivo compatibility of ADIBO can be problematic for pretargeting systems [38, 39]. Recently, a new click ligation, i.e., the inverse electron demand [4 + 2] Diels-Alder (IEDDA) cycloaddition between a 1,2,4,5-tetrazine and strained alkene dienophile, was reported as an alternative SPAAC reaction [40]. The IEDDA reaction is selective, high-yielding, clean, biocompatible and bioorthogonal. In 2011, Li et al. reported the first application of the IEDDA ligation in radiochemistry using an 18F-labeled trans-cyclooctene (18F-TCO) [41]. The synthesis of 18F-TCO was performed under relatively mild radiolabeling conditions to afford the desired product in yields of up to ~70 %; next a rapid, selective, and clean IEDDA ligation was performed without the need for any catalyst (Table 2, entry 1).
Table 2.
Diels-Alder cycloaddition
The Reiner group reported the IEDDA reaction between TCO and tetrazine for the preparation of 18F-AZD2281, a poly-ADP-ribose-polymerase1 (PARP1) PET imaging agent (Table 2, entry 2) [42]. In this work, in order to avoid lengthy HPLC purifications, TCP-decorated scavenger beads (4 mol equiv. of TCO) were used to remove unreacted AZD2281-tetrazine. Subsequent magnetic removal of the beads provided 18F-AZD2281 in high RCY (92 ± 0.4 %). This TCO/terazine IEDDA reaction has several advantages such as extremely fast kinetics at room temperature, independency of catalysts and high selectivity. In addition, the IEDDA-derived small-molecule imaging agent, 18F-AZD2281, was shown to effectively target PARP1 expression in vivo in murine models of ovarian and pancreatic cancer.
In a similar approach, Sevaraj et al. reported an IEDDA-generated 18F-labeled RGD peptide [43]. The modified RGD precursor containing 3,6-di-(2-pyridyl)-s-tetrazine was used for IEDDA ligation (Table 2, entry 3). The resulting 18F-RGD peptide proved to be effective for the imaging of integrin αvβ3 expression in in vivo models.
As an extended application of IEDDA click reactions in radiochemistry, Liu et al. reported an efficient method for the 18F-labeling of cysteine containing peptides and proteins based on the click reaction with tetrazinyl-maleimide and subsequent 18F-labeling through terazine-trans-cyclooctene ligation (Table 2, entry 4) [44]. 18F-labeled RGD peptide and VEGF protein were obtained within 5 min in 95 and 75 % RCY, respectively. Moreover, Wu’s group used 18F-tetrazine trans-cycloctene (TTCO) ligation for the development of an 18F-labeled exendin-4 targeting the glucagonlike peptide-1 receptor (GLP-1R) (Table 2, entry 5). The resulting 18F-TTCO-Cys40-exendin-4 shows specific binding to GLP-1R and provides a method to noninvasively image transplanted islets through small animal PET studies [45]. With the aim to increase the in vivo stability of the 18F-TCO moiety, Knight et al. used an 18F-labeled norbornene derivative, which was synthesized by acylation reaction of the amine-functionalized norbornene precursor with the known 18F-labeled prosthetic group, [18F]fluorobenzoate ([18F]SFB) (Table 2, entry 6) [46]. The 18F-labeled bombesin peptide, obtained through a rapid and high-yielding IEDDA click reaction, showed high bioavailability and metabolic stability with approximately 90 % of the compound remaining intact up to 30 min postinjection in normal BALB/c mice.
Unlike previous works, Herth et al. presented the use of 11C-labeled tetrazine for the TTCO ligation (Table 2, entry 7). Although this methodology underlines the disadvantage of the short half-life of carbon-11, the first 11C-labeled tetrazine was successfully prepared in 33 % RCY and the final click ligation proceeded rapidly (within 20 s) [47].
In antibody-based PET bioconjugates, positron-emitting radiometals (e.g., 64Cu, 86Y, and 89Zr) offer significant advantages such as high image quality, desirable radioactive half-lives and a simple radiolabeling method. In 2011, the Lewis group reported the use of the IEDDA reaction for the synthesis of PET radiometal-based agents [48]. In this work, the antibody trastuzumab was first covalently coupled to norbornene and then reacted with tetrazines bearing the chelators 1,4,7,10-tetraazacyclo-dodecane-1,4,7,10-tetraacetic acid (DOTA) or desferrioxamine (DFO) and subsequently radiometalated with 64Cu or 89Zr, respectively (Table 2, entry 8). 64Cu- and 89Zr-trastuzumab proved to be nearly identical in terms of the stability (>96 %), number of chelates per antibody (2.2 ± 0.3 for DOTA/mAb and 2.3 ± 0.4 for DFO/mAb), high specific activity (>2.9 mCi/mg) and in vitro immunoreactivity (>93 % in all cases). In vivo PET images revealed significant, selective and specific uptake of 64Cu- and 89Zr-trastuzumab in HER2-positive BT-474 xenografts.
Application of the Click Reaction to Pretargeting Strategies
Because of its very fast kinetics, the IEDDA reaction holds promise as an ideal methodology for in vivo pretargeting applications. To this end, pretargeted imaging and therapy were designed to avoid the high radiation exposure due to the slow pharmacokinetics of radioimmunoconjugates and high background doses by decoupling the targeting vector from the radioisotope at the time of injection.
The pretargeting approach consists of two steps. First, target-specific molecules or immunoconjugates are injected and bind to the target site. Next, radiolabeled compounds are added, which selectively conjugate with the pretreated molecules on the target site. This method presents several advantages, including superior image contrast, possible use of short-lived PET radionuclides and a decrease in the radiation doses to the nontarget [49]. Therefore, the pretargeting imaging and therapy require a rapid and accurate chemical reaction in in vivo models.
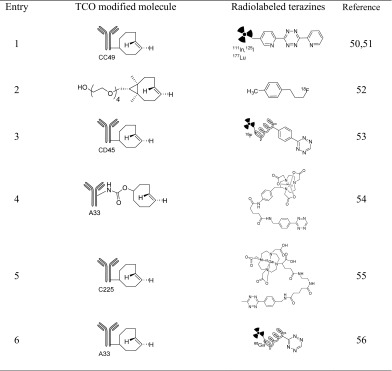
In 2010, Rossin et al. reported a tumor pretargeting strategy using the IEEDA reaction between a TCO-modified mAb and radiolabeled tetrazine [50]. In this work, an axially linked TCO-oxymethylbenzamide tagged CC49 antibody and 111In-labeled tetrazine were used (Table 3, entry 1). 111In-labeled tetrazine was injected 24 h after the treatment with CC49-TCO (100 μg). This system reduced the amount of free circulating CC49-TCO, while significantly increasing the tumor-to-nontumor ratios in tumor-bearing mice. Robillard’s group described a pretargeted radioimmunotherapy using the IEEDA reaction between a 177Lu-labeled tetrazine probe and TCO-tagged antibody, and they obtained SPECT/CT images in carcinoma xenografts [51].
Table 3.
TCO-modified antibodies and radiolabeled tetrazines used for pretargeting strategies
Using direct [18F]fluorination and subsequent IEEDA reactions, Denk et al. developed an 18F-labeled tetrazine with fast and reliable pharmacokinetics [52]. In this work, the water-soluble 18F-labeled tetrazine proved to be suitable for bioothogonal PET imaging and in vivo detection of dienophile-tagged molecules (Table 3, entry 2). With the aim of developing a predictable method for efficient in vivo click reactions, Devaraj et al. designed pharmacokinetically optimized reactants, namely polymer-modified tetrazines (PMT) with dextran scaffolds, which proved to be a key enabler for pretargeting imaging [53]. Using fluorescent PMT for cellular resolution and 18F-labeled PMT for in vivo imaging combining TCO-anti-CD45 monoclonal antibodies, cancer cell epitopes were easily conjugated in vivo (Table 3, entry 3).
The Lewis group reported a methodology for pretargeted PET imaging based on the bioorthogonal IEEDA reaction between the TCO-modified huA33 antibody and a 64Cu-labeled NOTA-modified tetrazine (Table 3, entry 4) [54].
In in vivo experiments, SW1222 xenografts were injected with TCO-huA33 and accumulation of the antibody in the tumor was allowed over 24 h. Subsequently, mice were injected with the 64Cu-labeled NOTA-modified tetrazine. Interestingly, although huA33 directly labeled with either 64Cu or 89Zr showned higher tumor uptake than the pretargeted huA33, the pretargeting system yielded comparable images and significantly enhanced tumor-to-muscle ratios.
Using 68Ga-labeled cetuximab, pretargeted EGFR PET imaging was performed by the Aboagye group in 2014 [55]. The Devaraj group also reported 68Ga chelating bioorthogonal tetrazine polymers for the IEDDA reaction with pretargeted TCO-A33 antibody [56].
In summary, the present review details the current attempts in the development of highly reliable, selective and rapid ligation methodologies for the synthesis of radiotracers through click chemistry reactions. Their application to in vivo imaging and therapy was reported to be very effective and allow the potential use of radiotracers in pretargeting strategies. Thus, the click reaction is expected to serve as a convenient radiolabeling methodology and a point of entry for a wide range of molecular imaging techniques.
Acknowledgments
This study was supported by grants from the Korean Research Foundation (NRF-2014R1A2A2A01007980) and Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) (HI14C1072-010014, HI12C0035-030015), funded by the Ministry of Health & Welfare, Republic of Korea.
Conflict of Interest
Ji Young Choi and Byung Chul Lee declare that they have no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors. This manuscript has not been published before, is not under consideration for publication anywhere else and has been approved by all coauthors.
References
- 1.Mamat C, Ramenda T, Wuest FR. Recent applications of click chemistry for the synthesis of radiotracers for molecular imaging. Mini Rev Org Chem. 2009;6:21–34. doi: 10.2174/157019309787316148. [DOI] [Google Scholar]
- 2.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Marik J, Sutcliffe JL. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006;47:6681–6684. doi: 10.1016/j.tetlet.2006.06.176. [DOI] [Google Scholar]
- 4.Ross TL, Honer M, Lam PYH, Mindt TL, Groehn V, Schibli R, et al. Fluorine-18 click radiosynthesis and preclinical evaluation of a new 18F-labeled folic acid derivative. Bioconjug Chem. 2008;19:2462–2470. doi: 10.1021/bc800356r. [DOI] [PubMed] [Google Scholar]
- 5.Glaser M, Årstad E. “Click labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjug Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 6.Kobus D, Giesen Y, Ullrich R, Backes H, Neumaier B. A fully automated two-step synthesis of an 18F-labelled tyrosine kinase inhibitor for EGFR kinase activity imaging in tumors. Appl Radiat Isot. 2009;67:1977–1984. doi: 10.1016/j.apradiso.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Haslop A, Gee A, Plisson C, Long N. Fully automated radiosynthesis of [1-(2-[18F]fluoroethyl),1H[1,2,3]triazole 4-ethylene] triphenylphosphonium bromide as a potential positron emission tomography tracer for imaging apoptosis. J Label Compd Radiopharm. 2013;56:313–316. doi: 10.1002/jlcr.3024. [DOI] [PubMed] [Google Scholar]
- 8.Smith G, Glaser M, Perumal M, Nguyen QD, Shan B, Årstad E, et al. Design, synthesis, and biological characterization of a caspase 3/7 selective isatin labeled with 2-[18F]fluoroethylazide. J Med Chem. 2008;51:8057–8067. doi: 10.1021/jm801107u. [DOI] [PubMed] [Google Scholar]
- 9.Pisaneschi F, Nguyen QD, Shamsaei E, Glaser M, Robins E, Kaliszczak M, et al. Development of a new epidermal growth factor receptor positron emission tomography imaging agent based on the 3-cyanoquinoline core: synthesis and biological evaluation. Bioorg Med Chem. 2010;18:6634–6645. doi: 10.1016/j.bmc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Iddon L, Leyton J, Indrevoll B, Glaser M, Robins EG, George AJT, et al. Synthesis and in vitro evaluation of [18F]fluoroethyl triazole labelled [Tyr3]octreotate analogues using click chemistry. Bioorg Med Chem Lett. 2011;21:3122–3127. doi: 10.1016/j.bmcl.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Laurens E, Yeoh SD, Rigopoulos A, Cao D, Cartwright GA, O’Keefe GJ, et al. Radiolabelling and evaluation of novel haloethylsulfoxides as PET imaging agents for tumor hypoxia. Nucl Med Biol. 2012;39:871–882. doi: 10.1016/j.nucmedbio.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Bejot R, Carroll L, Bhakoo K, Declerck J, Gouverneur V. A fluorous and click approach for screening potential PET probes: evaluation of potential hypoxia biomarkers. Bioorg Med Chem. 2012;20:324–329. doi: 10.1016/j.bmc.2011.10.084. [DOI] [PubMed] [Google Scholar]
- 13.Glaser M, Solbakken M, Turton DR, Pettitt R, Barnett J, Arukwe J, et al. Methods for 18F-labeling of RGD peptides: comparison of aminooxy [18F]fluorobenzaldehyde condensation with ‘click labeling’ using 2-[18F]fluoroethylazide, and S-alkylation with [18F]fluoropropanethiol. Amino Acids. 2009;37:717–724. doi: 10.1007/s00726-008-0200-0. [DOI] [PubMed] [Google Scholar]
- 14.McConathy J, Zhou D, Shockley SE, Jones LA, Griffin EA, Lee H, et al. Click synthesis and biologic evaluation of (R)- and (S)-2-amino-3-[1-(2- [18F]fluoroethyl)-1H-[1,2,3]triazol-4-yl]propanoic acid for brain tumor imaging with positron emission tomography. Mol Imaging. 2010;9:329–342. [PubMed] [Google Scholar]
- 15.Zhou D, Chu W, Peng X, McConathy J, Mach RH, Katzenellenbogen JA. Facile purification and click labeling with 2-[18F]fluoroethyl azide using solid phase extraction cartridges. Tetrahedron Lett. 2015;56:952–954. doi: 10.1016/j.tetlet.2014.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirion U, Kim HJ, Lee JH, Seo JW, Lee BS, Lee SJ, et al. An efficient F-18 labeling method for PET study: Huisgen 1,3-dipolar cycloaddition of bioactive substances and F-18-labeled compounds. Tetrahedron Lett. 2007;48:3953–3957. doi: 10.1016/j.tetlet.2007.04.048. [DOI] [Google Scholar]
- 17.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Click chemistry for 18F-labeling of RGD peptides and microPET imaging of tumor integrin αvβ3 expression. Bioconjug Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inkster JAH, Guérin B, Ruth TJ, Adam MJ. Radiosynthesis and bioconjugation of [18F]FPy5yne, a prosthetic group for the 18F labeling of bioactive peptides. J Label Compd Radiopharm. 2008;51:444–452. doi: 10.1002/jlcr.1561. [DOI] [Google Scholar]
- 19.Inkster JAH, Adam MJ, Storr T, Ruth TJ. Labeling of an antisense oligonucleotide with [18F]FPy5yne. Nucleosides Nucleotides Nucleic Acids. 2009;28:1131–1143. doi: 10.1080/15257770903400691. [DOI] [PubMed] [Google Scholar]
- 20.Vaidyanathan G, White BJ, Zalutsky MR. Propargyl 4-[18F]fluorobenzoate: a putatively more stable prosthetic group for the fluorine-18 labeling of biomolecules via click chemistry. Curr Radiopharm. 2009;2:63–74. doi: 10.2174/1874471010902010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Liu Y, Zhang L, Xu Y. One-step radiosynthesis of 4-[18F]flouro-3-nitro-N-2-propyn-1-yl-benzamide ([18F]FNPB): a new stable aromatic porosthetic group for efficient labeling of peptides with fluorine-18. J Label Compd Radiopharm. 2012;55:229–234. doi: 10.1002/jlcr.2931. [DOI] [Google Scholar]
- 22.Thonon D, Kech C, Paris J, Lemaire C, Luxen A. New strategy for the preparation of clickable peptides and labeling with 1-(azidomethyl)-4-[18F]-fluorobenzene for PET. Bioconjug Chem. 2009;20:817–823. doi: 10.1021/bc800544p. [DOI] [PubMed] [Google Scholar]
- 23.Mercier F, Paris J, Kaisin G, Thonon D, Flagothier J, Teller N, et al. General method for labeling siRNA by click chemistry with fluorine-18 for the purpose of PET imaging. Bioconjug Chem. 2011;22:108–114. doi: 10.1021/bc100263y. [DOI] [PubMed] [Google Scholar]
- 24.Fischer CR, Müller C, Reber J, Müller A, Krämer SD, Ametamey SM, et al. [18F]Fluoro-deoxy-glucose folate: a novel PET radiotracer with improved in vivo properties for folate receptor targeting. Bioconjug Chem. 2012;23:805–813. doi: 10.1021/bc200660z. [DOI] [PubMed] [Google Scholar]
- 25.Maschauer S, Haubner R, Kuwert T, Prante O. 18F-Glyco-RGD peptides for PET imaging of integrin expression: efficient radiosynthesis by click chemistry and modulation of biodistribution by glycosylation. Mol Pharm. 2014;11:505–515. doi: 10.1021/mp4004817. [DOI] [PubMed] [Google Scholar]
- 26.Pretze M, Mamat C. Automated preparation of [18F]AFP and [18F]BFP: two novel bifunctional 18F-labeling building blocks for Huisgen-click. J Fluor Chem. 2013;150:25–35. doi: 10.1016/j.jfluchem.2013.02.028. [DOI] [Google Scholar]
- 27.Zeng D, Zeglis BM, Lewis JS, Anderson CJ. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J Nucl Med. 2013;54:829–832. doi: 10.2967/jnumed.112.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mindt TL, Müller C, Melis M, Jong MD, Schibli R. “Click-to-chelate”: in vitro and in vivo comparison of a 99mTc(CO)3-labeled N(τ)-histidine folate derivative with its isostructural, clicked 1,2,3-triazole analogue. Bioconjug Chem. 2008;19:1689–1695. doi: 10.1021/bc800183r. [DOI] [PubMed] [Google Scholar]
- 29.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J Am Chem Soc. 2008;130(34):11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouvet V, Wuest M, Wuest F. Copper-free click chemistry with the short-lived positron emitter fluorine-18. Org Biomol Chem. 2011;9:7393–7399. doi: 10.1039/c1ob06034a. [DOI] [PubMed] [Google Scholar]
- 31.Arumugam S, Chin J, Schirrmacher R, Popik VV, Kostikov AP. [18F]Azadibenzocyclooctyne ([18F]ADIBO): a biocompatible radioactive labeling synthon for peptides using catalyst free [3 + 2] cycloaddition. Bioorg Med Chem Lett. 2011;21:6987–6991. doi: 10.1016/j.bmcl.2011.09.126. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter RD, Hausner SH, Sutcliffe JL. Copper-free cClick for PET: rapid 1,3-dipolar cycloadditions with a fluorine-18 cyclooctyne. ACS Med Chem Lett. 2011;2:885–889. doi: 10.1021/ml200187j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausner SH, Carpenter RD, Bauer N, Sutcliffe JL. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl Med Biol. 2013;40:233–239. doi: 10.1016/j.nucmedbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Campbell-Verduyn LS, Mirfeizi L, Schoonen AK, Dierckx RA, Elsinga PH, Feringa BL. Strain-promoted copper-free “Click” chemistry for 18F radiolabeling of bombesin. Angew Chem Int Ed. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- 35.Evans HL, Slade RL, Carroll L, Smith G, Nguyen QD, Iddon L, et al. Copper-free click—a promising tool for pre-targeted PET imaging. Chem Commun. 2012;48:991–993. doi: 10.1039/C1CC16220A. [DOI] [PubMed] [Google Scholar]
- 36.Sachin K, Jadhav VH, Kim EM, Kim HL, Lee SB, Jeong HJ, et al. F-18 labeling protocol of peptides based on chemically orthogonal strain-promoted cycloaddition under physiologically friendly reaction conditions. Bioconjug Chem. 2012;23:1680–1686. doi: 10.1021/bc3002425. [DOI] [PubMed] [Google Scholar]
- 37.Kim HL, Sachin K, Jeong HJ, Choi W, Lee HS, Kim DW. F-18 labeled RGD probes based on bioorthogonal strain-promoted click reaction for PET imaging. ACS Med Chem Lett. 2015;6:402–407. doi: 10.1021/ml500464f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van den Bosch SM, Rossin R, Verkerk PR, Hoeve W, Janssen HM, Lub J, et al. Evaluation of strained alkynes for Cu-free click reaction in live mice. Nucl Med Biol. 2013;40:415–423. doi: 10.1016/j.nucmedbio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Reiner T, Zeglis BM. The inverse electron demand Diels–Alder click reaction in radiochemistry. J Label Compd Radiopharm. 2014;57:285–290. doi: 10.1002/jlcr.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackman ML, Royzen M, Fox JM. Tetrazine Ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J Am Chem Soc. 2008;130:13518–135189. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, et al. Tetrazine–trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem Commun. 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew Chem Int Ed. 2011;50:1922–1925. doi: 10.1002/anie.201006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvaraj R, Liub S, Hassink M, Huang CW, Yap LP, Park R, et al. Tetrazine-tans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg Med Chem Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, Hassink M, Selvaraj R, Yap LP, Park R, Wang H, et al. Efficient 18F labeling of cysteine containing peptides and proteins using the tetrazine-trans-cyclooctene ligation. Mol Imaging. 2013;12:121–128. [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, et al. Development and evaluation of 18F-TTCO-Cys40-Exendin-4: a PET probe for imaging transplanted islets. J Nucl Med. 2013;54:244–251. doi: 10.2967/jnumed.112.109694. [DOI] [PubMed] [Google Scholar]
- 46.Knight JC, Richter S, Wuest M, Way JD, Wuest F. Synthesis and evaluation of an 18F-labelled norbornene derivative for copper-free click chemistry reactions. Org Biomol Chem. 2013;11:3817–3825. doi: 10.1039/c3ob40548f. [DOI] [PubMed] [Google Scholar]
- 47.Herth MM, Andersen VL, Lehel S, Madsen J, Knudsen GM, Kristensen JL. Development of a 11C-labeled tetrazine for rapid tetrazine–trans-cyclooctene ligation. Chem Commun. 2013;49:3805–3807. doi: 10.1039/c3cc41027g. [DOI] [PubMed] [Google Scholar]
- 48.Zeglis BM, Mohindra P, Weissmann GI, Divilov V, Hilderbrand SA, Weissleder R, et al. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjug Chem. 2011;22:2048–2059. doi: 10.1021/bc200288d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossin R, Robillard MS. Pretargeted imaging using bioorthogonal chemistry in mice. Curr Opin Chem Biol. 2014;21:161–169. doi: 10.1016/j.cbpa.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, et al. In vivo chemistry for pretargeted tumor imaging in live mice. Angew Chem Int Ed. 2010;49:3375–3378. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 51.Rossin R, Duijnhoven SMJ, Läppchen T, van den Bosch SM, Robillard MS. Trans-Cyclooctene tag with improved properties for tumor pretargeting with the Diels–Alder reaction. Mol Pharm. 2014;11:3090–3096. doi: 10.1021/mp500275a. [DOI] [PubMed] [Google Scholar]
- 52.Denk C, Svatunek D, Filip T, Wanek T, Lumpi D, Frçhlich J, et al. Development of a 18F-labeled tetrazine with favorable pharmacokinetics for bioorthogonal PET imaging. Angew Chem Int Ed. 2014;53:9655–9659. doi: 10.1002/anie.201404277. [DOI] [PubMed] [Google Scholar]
- 53.Devaraj NK, Thurber GM, Keliher EJ, Marinelli B, Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. PNAS. 2012;109:4762–4767. doi: 10.1073/pnas.1113466109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, et al. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J Nucl Med. 2013;54:1389–1396. doi: 10.2967/jnumed.112.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans HL, Nguyen QD, Carroll LS, Kaliszczak M, Twyman FJ, Spivey AC, et al. A bioorthogonal 68Ga-labelling strategy for rapid in vivo imaging. Chem Commun. 2014;50:9557–9560. doi: 10.1039/C4CC03903C. [DOI] [PubMed] [Google Scholar]
- 56.Nichols B, Qin Z, Yang J, Vera DR, Devaraj NK. 68Ga chelating bioorthogonal tetrazine polymers for the multistep labeling of cancer biomarkers. Chem Commun. 2014;50:5215–5217. doi: 10.1039/c3cc49530b. [DOI] [PMC free article] [PubMed] [Google Scholar]