Abstract
Background
Recent policy clarifications by the Centers for Medicare and Medicaid Services have changed access to outpatient dialysis care at end stage renal disease (ESRD) facilities for individuals with acute kidney injury in the United States. Tools to predict “ESRD” and “acute” status in terms of kidney function recovery among patients who previously initiated dialysis in the hospital could help inform patient management decisions.
Study Design
Historical cohort study
Setting & Participants
Incident hemodialysis patients in the Mayo Clinic Health System who initiated in-hospital RRT and continued outpatient dialysis following hospital dismissal (2006 to 2009)
Predictor
Baseline estimated glomerular filtration rate (eGFR), sepsis/surgery acute tubular necrosis (ATN), heart failure, intensive care unit, and dialysis access.
Outcomes
Kidney function recovery defined as sufficient kidney function for outpatient hemodialysis discontinuation.
Results
Cohort consisted of 281 patients with mean age 64 years, 63% men, 45% heart failure, and baseline eGFR ≥30 mL/min/1.73m2 in 46%. Over a median 8 months, 52 (19%) recovered, most (94%) within 6 months. Higher baseline eGFR (Hazard Ratio 1.27 per 10 ml/min/1.73m2; 95% CI 1.16–1.39; p<0.001), ATN from sepsis or surgery (HR 3.34; CI 1.83- 6.24; p<0.001), and heart failure (HR 0.40; CI 0.19–0.78, p=0.007) were independent predictors of recovery within 6 months while first RRT in the intensive care unit and a catheter dialysis access were not. There was a positive interaction between absence of heart failure and eGFR≥30 ml/min/1.73m2 for predicting kidney function recovery (p<0.001).
Limitations
Sample size.
Conclusions
Kidney function recovery in the outpatient hemodialysis unit following in-hospital RRT initiation is not rare. As expected, higher baseline eGFR is an important determinant of recovery. However, patients with heart failure are less likely to recover even with higher baseline eGFR. Consideration of these factors at hospital discharge informs decisions on “ESRD” status designation and long-term hemodialysis care.
Keywords: acute kidney injury, heart failure, chronic kidney disease, hospitalization, risk factors, renal recovery
Introduction
Acute kidney injury (AKI) in hospitalized patients has become increasingly common with reported prevalences of 3% to 20% 1–3 and occurs at an even higher frequency within the intensive care unit (ICU) population, 22% to 67%4,5. Patients with severe AKI requiring initiation of renal replacement therapy (RRT) have the highest in-hospital mortality rate ranging from 45% to 70%6–10. Among survivors of this high-risk event, as many as 13% to 32% require dialysis at the time of hospital discharge7,11–13. Limited data are available regarding recovery of sufficient kidney function to allow discontinuation of dialysis following hospital discharge in patients with severe AKI14 yet patients initiating in-hospital RRT comprise a significant proportion, 39% to 64%, of the incident dialysis population each year 15–17.
Kidney function recovery from perceived end stage renal disease (ESRD), following 30 or more days of dialysis, has been studied in a heterogenous manner18–26. From these investigations, the occurrence is rare (1–5%) but a recent study in Medicare patients by Mohan et al26 reported rates above 5% and suggested an increasing recovery rate over time. The reason for this observation is unclear but may reflect improved patient survival after severe AKI episodes, changes in practice patterns such as timing of RRT initiation, or an overall change in patient case-mix. Among incident hemodialysis patients, a substantial proportion initiate RRT in the hospital, primarily due to 1) severe AKI episodes of varying etiologies or 2) unprepared or suboptimal dialysis starts for advanced renal failure27. In such patients faced with a potentially lifelong illness, concern over the possibility of recovery is paramount. This concern is shared by dialysis providers facing the difficult task of determining the prognosis for kidney function recovery, balancing timely kidney transplantation referral, and avoiding more permanent dialysis access placement in patients who will eventually recover.
In July 2012, the Centers for Medicare and Medicaid Services (CMS) clarified policy on coverage for outpatient dialysis services provided to AKI patients in the United States28,29. This clarification prohibited ESRD facilities from furnishing acute dialysis to hospital outpatients, restricting dialysis care to continued treatment in the hospital or locations qualifying for provider-based departments of the hospital. As such, these locations may not be convenient or readily accessible to patients and caregivers. Subsequently, hospital nephrologists indirectly receive added pressure to categorize renal failure events at hospital discharge as either “ESRD” or “AKI.” Unfortunately, readily available clinical prediction tools for kidney function recovery in the outpatient setting are lacking. Gaining an understanding of potential predictors of recovery following hospital discharge may further aid in early clinical decision making in the care of incident hemodialysis patients. In this study, we examined the likelihood of recovery of sufficient kidney function to discontinue outpatient hemodialysis and predictors of such recovery among incident hemodialysis patients who initiated in-hospital RRT.
Methods
Patient selection
The Mayo Clinic Health System provides a comprehensive integrated health care network in an area with 395,000 residents in Southeast Minnesota, Northern Iowa, and Southwest Wisconsin. Mayo Clinic Dialysis Services (MCDS) provides all hemodialysis in the Mayo Clinic Health System through eight community-based outpatient hemodialysis facilities and is staffed solely by Mayo Clinic nephrologists who also provide the inpatient hemodialysis care. All adults (age ≥18 years; n=470) in the Mayo Clinic Health System initiating outpatient in-center hemodialysis from January 1, 2006 through December 31, 2009 with Minnesota Research Authorization were identified. Only patients whose RRT initiation occurred in the hospital just prior to transitioning to outpatient in-center hemodialysis were included in this study (n=281). Long term acute care facilities were not utilized and following hospital discharge all patients transitioned directly to outpatient in-center hemodialysis within 1–3 days. Patients receiving home dialysis therapies as their first treatment (peritoneal or hemodialysis) were not included in this study. The primary outcome was recovery of sufficient kidney function to completely discontinue outpatient hemodialysis. Patients were followed for recovery through December 08, 2010. The Mayo Clinic Institutional Review Board approved this study.
Data collection
Baseline characteristics, comorbidities, and laboratory tests were collected through review of the electronic medical records. The Charlson Comorbidity Index score, consisting of 19 comorbid conditions, was obtained by a previously validated automatic note search strategy (automated digital algorithm)30. Charts were reviewed to determine the cause of kidney failure, baseline kidney function, dialysis access, dialysis location, and duration of hospital stay. Baseline kidney function (n=253) was determined from the last available stable serum creatinine within 1 year prior to hospitalization or lowest inpatient creatinine prior to renal failure event if outpatient creatinine values were unavailable. Heart failure included the diagnoses of congestive or systolic heart failure, diastolic heart failure, or cardiomyopathy based on manual review of medical records at the time of hospitalization. For patients hospitalized and/or receiving medical care at a non-Mayo institution at time of RRT initiation, outside records were reviewed to obtain baseline serum creatinine values. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI creatinine based equation31.
Patients were divided into four groups based on baseline eGFR available within 1 year prior to the kidney failure episode that precipitated hemodialysis during hospitalization: acute kidney injury (AKI), acute kidney injury on chronic kidney disease (AoCKD), chronic kidney disease stage 5 (CKD5), and acuity unknown. AKI was defined as loss of baseline normal renal function (eGFR ≥60 ml/min/1.73m2) requiring dialysis. AoCKD was defined as loss of renal function precipitating initiation of dialysis in patients with impaired renal function (eGFR ≥15 and <60 ml/min/1.73m2) at baseline. CKD5 was defined as loss of baseline advanced renal disease (eGFR <15 ml/min/1.73m2) requiring initiation of dialysis in-hospital. Acuity unknown consisted of patients with no known baseline creatinine to calculate baseline eGFR. In addition to eGFR cutoffs, clinical and pathological causes of acute and chronic kidney injury were defined for each patient based on kidney biopsy (performed prior to or at time of hospitalization), supportive laboratory testing, and clinical judgement at time of kidney failure episode. Acute clinical and pathological etiologies included: infection/sepsis-induced AKI, postoperative AKI, glomerulonephritis/tubulointerstitial disease (GN/TIN), drugs, and other/unknown. For the purpose of this study, infection/sepsis and postoperative AKI were later combined to encompass a diagnosis of acute tubular necrosis (ATN). Sepsis/postoperative ATN classification was further supported by the common presence of urinary renal tubular epithelial cells, muddy brown casts or granular casts, though renal biopsy confirmation was not usually performed. Chronic clinical and pathological etiologies included: diabetes mellitus (DM), hypertension, GN/TIN, polycystic kidney disease, refractory acute tubular necrosis, failing kidney transplant, and other/unknown. First dialysis access was categorized as arteriovenous fistula, arteriovenous graft, or central venous catheter. Catheters were further classified as temporary (non-tunneled, non-cuffed) or tunneled (cuffed).
For each incident patient, recovery events were collected during follow up. Kidney function recovery was defined as the development of sufficient kidney function allowing for complete discontinuation of outpatient hemodialysis.
Statistical analysis
Continuous variables were reported as mean ± standard deviation or median with inter-quartile ranges (IQRs) for non-normally distributed variables. Categorical variables were expressed as count (percent). Comparison of proportions between groups was made using the Chi square test. Recovery rate was estimated using the Kaplan-Meier method. Since there were very few recovery events after 6 months, Cox models were developed to predict the risk of kidney function recovery within 6 months of starting outpatient hemodialysis. Multivariable hazard regression models for kidney function recovery considered only variables that were statistically significant in the univariate model and readily available to the practicing clinician. Kaplan-Meier survival curves (plotted for failure) were generated to characterize the timing of recovery by level of eGFR and presence of heart failure. Subjects were censored at time of transfer to a non-MCDS dialysis facility, study period end (December 08, 2010), or at death. Patients who discontinued in-center hemodialysis due to kidney transplantation or transitioned from in-center to home dialysis therapies (peritoneal or hemo- dialysis) were assigned maximum follow up time under the assumption that they did not recover kidney function. A subgroup analysis was also performed restricted to patients with a baseline eGFR>15 ml/min/1.73m2 (n=225). The purpose of this analysis was to exclude patients with CKD stage 5 since they are often deemed “ESRD” and unlikely to recover kidney function. A sensitivity analysis was also conducted assigning the maximum follow-up time to patients who died (n=97) as these patients may not have recovered kidney function had they lived. Statistical analyses were performed with JMP 9.0 (SAS Institute Inc, Cary, NC). .0
Results
From January 2006 to December 2009, there were 470 new patients who started outpatient hemodialysis in the MCDS. Our study was limited to the 281 (60%) patients who initiated and continued RRT in the hospital prior to transitioning to outpatient hemodialysis. The mean follow up of hospital starters in this study was 15±16 months (median 8; IQR 2, 26). Baseline characteristics and recovery events by baseline kidney function subgroups are shown in Table 1. Mean age overall was 64±16 years (median 66; Interquartile range (IQR): 54, 77), 63% were men, 89% were Caucasian, 49% had DM, and 45% had heart failure. A Charlson comorbidity score was ≥8 in 49%. Baseline serum creatinine was available in 253 patients (90%) and median eGFR was 26 mL/min/1.73m2 (IQR: 16, 48). Baseline eGFR was ≥30 mL/min/1.73m2 in 43% with known eGFR.
Table 1.
Comparisons of baseline demographics, comorbid conditions, and kidney function recovery by baseline kidney function among incident hemodialysis patients with in-hospital initiation of renal replacement therapy.
| Baseline demographics, comorbid conditions, and kidney function | ||||||
|---|---|---|---|---|---|---|
| All (n=281) |
Acute Kidney Injury (n=42) |
Acute on CKD (n=155) |
CKD stage 5 (n=56) |
Acuity Unknown (n=28) |
||
| Age, years | Mean ±SD | 64 ± 16 | 59 ± 16 | 66 ± 14 | 68 ± 16 | 55 ± 19 |
| Median (IQR) | 66 (54,77) | 61 (46, 73) | 68 (58,77) | 72 (57,81) | 55 (43,71) | |
| Age ≥75 | 81 (29%) | 9 (21%) | 44 (28%) | 23 (41%) | 5 (18%) | |
| Age 70 to <75 | 36 (13%) | 3 (7%) | 24 (15%) | 7 (12.5%) | 2 (7%) | |
| Age 65 to <70 | 35 (12%) | 4 (10%) | 24 (15%) | 6 (11%) | 1 (4%) | |
| Age 60 to <65 | 26 (9%) | 6 (14%) | 15 (10%) | 3 (5%) | 2 (7%) | |
| Age 55 to <60 | 30 (11%) | 1 (2%) | 18 (12%) | 7 (12.5%) | 4 (14%) | |
| Age 50 to <55 | 26 (9%) | 5 (12%) | 14 (9%) | 2 (4%) | 5 (18%) | |
| Age 45 to <50 | 14 (5%) | 4 (10%) | 5 (3%) | 4 (7%) | 1 (4%) | |
| Age 40 to <45 | 14 (5%) | 5 (12%) | 5 (3%) | 1 (2%) | 3 (11%) | |
| Age <40 | 19 (7%) | 5 (12%) | 6 (4%) | 3 (5%) | 5 (18%) | |
| Gender | ||||||
| Male | 176 (63%) | 28 (67%) | 95 (61%) | 34 (61%) | 19 (68%) | |
| Female | 105 (37%) | 14 (33%) | 60 (39%) | 22 (39%) | 9 (32%) | |
| Caucasian race | 249 (89%) | 38 (90%) | 142 (92%) | 49 (88%) | 20 (71%) | |
| Diabetes mellitus | 137 (49%) | 10 (24%) | 85 (55%) | 28 (50%) | 14 (50%) | |
| Coronary artery disease | 122 (43%) | 10 (24%) | 74 (48%) | 30 (54%) | 8 (29%) | |
| Heart failure | 127 (45%) | 12 (29%) | 76 (49%) | 31 (55%) | 8 (29%) | |
| Prior outpatient nephrology evaluation (n=276) |
133 (48%) | 4 (10%) | 79 (52%) | 46 (82%) | 4 (15%) | |
| Charlson comorbidity index score (n=272) | ||||||
| Mean ±SD | 7 ± 4 | 5 ± 3 | 8 ± 3 | 9 ± 4 | 4 ± 3 | |
| Median (IQR) | 7 (4,10) | 4 (3,7) | 8 (6,10) | 8 (6,11) | 4 (2,7) | |
| Charlson score ≥8 | 133 (49%) | 9 (21%) | 87 (58%) | 33 (60%) | 4 (15%) | |
| First dialysis location in hospital | ||||||
| Intensive care unit | 99 (35%) | 22 (52%) | 57 (37%) | 10 (18%) | 10 (36%) | |
| Non-Intensive care unit | 176 (63%) | 18 (43%) | 95 (61%) | 46 (82%) | 17 (61%) | |
| Outside hospital | 6 (2%) | 2 (5%) | 3 (2%) | 0 (0%) | 1 (3%) | |
| First dialysis access utilized | ||||||
| Non-cuffed, temporary catheter | 150 (53%) | 36 (86%) | 88 (57%) | 14 (25%) | 12 (43%) | |
| Cuffed tunneled catheter | 110 (39%) | 6 (14%) | 61 (39%) | 30 (54%) | 13 (46%) | |
| Arteriovenous fistula/graft | 21 (7%) | 0 (0%) | 6 (4%) | 12 (21%) | 3 (11%) | |
| Hospital duration, days | ||||||
| Total (Median, IQR) | 9 (5,17) | 20 (10,31) | 11 (6,17) | 5 (4,9) | 7 (3,13) | |
| Prior to RRT | 2 (1,6) | 6 (1,11) | 2 (1,6) | 1 (1,3) | 1 (0,3) | |
| Following RRT | 6 (3,12) | 12 (6,23) | 7 (3,12) | 4 (2,7) | 4 (3,8) | |
| Baseline kidney function | n= 253 | n= 42 | n= 155 | n= 56 | n= 0 | |
| Creatinine, mg/dL | ||||||
| Mean ±SD | 2.6 ± 1.6 | 0.9 ± 0.2 | 2.2 ± 0.8 | 4.9 ± 1.3 | - - | |
| Median (IQR) | 2.2 (1.4,3.5) | 0.9 (0.8,1.1) | 2.1 (1.5,2.8) | 4.6 (3.9,5.7) | - - | |
| eGFR, ml/min/1.73m2 | ||||||
| Mean ±SD | 37 ± 29 | 85 ± 32 | 32 ± 13 | 11 ± 2 | - - | |
| Median (IQR) | 27 (17,52) | 77 (66,93) | 28 (21,42) | 12 (10,13) | - - | |
| eGFR ≥60 | 42 (15%) | 42 (100%) | 0 (0%) | 0 (0%) | - - | |
| eGFR <60 and ≥45 | 28 (10%) | 0 (0%) | 28 (18%) | 0 (0%) | - - | |
| eGFR <45 and ≥30 | 40 (14%) | 0 (0%) | 40 (26%) | 0 (0%) | - - | |
| eGFR <30 and ≥15 | 87 (31%) | 0 (0%) | 87 (56%) | 0 (0%) | - - | |
| eGFR<15 | 56 (20%) | 0 (0%) | 0 (0%) | 56 (100%) | - - | |
| eGFR unknown | 28 (10%) | - - | - - | - - | 28 (100%) | |
| Kidney function recovery during follow-up | n= 281 | n= 42 | n= 155 | n= 56 | n= 28 | |
| 6 months | 49 (17%) | 21 (50%) | 26 (17%) | 0 (0%) | 2 (7%) | |
| Overall | 52 (19%) | 22 (52%) | 27 (17%) | 0 (0%) | 3 (11%) | |
Baseline kidney function classifications include: Acute kidney injury (baseline eGFR ≥60 mL/min/1.73m2); Acute on chronic kidney disease (baseline eGFR≥15 and <60); Chronic kidney disease stage 5 (baseline eGFR <15); Acuity unknown (baseline eGFR unknown within 12 months prior to dialysis initiation). CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate. Values expressed as Mean± Standard deviation, Median (IQR: interquartile range), or Number (%) unless otherwise specified. Charlson score: Carlson comorbidity index score; ICU: intensive care unit; RRT: renal replacement therapy; AVF/G: arteriovenous fistula or graft; eGFR: estimated glomerular filtration rate calculated from the CKD-Epi equation.
Within baseline kidney function subgroups, the distribution of patients included AKI (15%), AoCKD (55%), CKD5 (20%), and Acuity unknown (10%). The AKI group was generally younger and had less comorbidity, more ICU starts, and longer hospital stays. Besides classification by baseline eGFR, the acute and chronic clinical and pathological etiologies of kidney failure as obtained from chart review are shown in Table 2. Acute causes precipitating dialysis initiation were found in 211 (75%) patients while chronic cases of kidney failure were identified in 221 (79%). Overall, the most common acute etiologies were infection/sepsis or a complication of surgery, and together these comprised the category of sepsis/postoperative ATN (32% of entire cohort studied). The most common causes of chronic injury were diabetes, glomerulonephritis/tubulointerstitial nephritis, and hypertension. A kidney biopsy was performed either at the time of hospitalization or historically in 71 (25%) patients.
Table 2.
Comparisons of clinical and pathological kidney failure etiologies by baseline kidney function classification among incident hemodialysis patients with in-hospital renal replacement therapy initiation.
| Clinical and pathological diagnoses of kidney failure by baseline kidney function classification* | ||||||
|---|---|---|---|---|---|---|
| All (n=281) |
Acute Kidney Injury (n=42) |
Acute on CKD (n=155) |
CKD stage 5 (n=56) |
Acuity Unknown (n=28) |
||
| Acute clinical and pathological etiologies# | ||||||
| Infection/Sepsis | 56 (20%) | 13 (31%) | 39 (25%) | 2 (4%) | 2 (7%) | |
| Postoperative | 33 (12%) | 8 (19%) | 22 (14%) | 3 (4%) | 0 (0%) | |
| Glomerulonephritis/Tubulointerstitial nephritis | 30 (11%) | 7 (17%) | 17 (11%) | 2 (4%) | 4 (14%) | |
| Drugs | 16 (6%) | 5 (12%) | 8 (5%) | 2 (4%) | 1 (4%) | |
| Other/Unknown | 76 (27%) | 9 (21%) | 48 (31%) | 9 (16%) | 10 (36%) | |
| None | 70 (25%) | 0 (0%) | 21 (14%) | 38 (68%) | 11 (39%) | |
| Chronic clinical and pathological etiologies# | ||||||
| Diabetes | 77 (27%) | 2 (5%) | 46 (30%) | 23 (41%) | 6 (21%) | |
| Hypertension | 21 (7%) | 0 (0%) | 6 (4%) | 10 (18%) | 5 (18%) | |
| Glomerulonephritis/Tubulointerstitial nephritis | 37 (13%) | 2 (5%) | 22 (14%) | 10 (18%) | 3 (11%) | |
| Polycystic kidney disease | 5 (2%) | 0 (0%) | 2 (1%) | 3 (5%) | 0 (0%) | |
| Refractory acute tubular necrosis | 5 (2%) | 2 (5%) | 3 (2%) | 0 (0%) | 0 (0%) | |
| Failing kidney transplant | 19 (7%) | 0 (0%) | 16 (10%) | 2 (4%) | 1 (4%) | |
| Other/Unknown | 57 (20%) | 3 (7%) | 40 (26%) | 8 (14%) | 6 (21%) | |
| None | 60 (21%) | 33 (79%) | 20 (13%) | 0 (0%) | 7 (25%) | |
Baseline kidney function classifications include: Acute kidney injury (baseline eGFR ≥60 mL/min/1.73m2); Acute on chronic kidney disease (baseline eGFR≥15 and <60); Chronic kidney disease stage 5 (baseline eGFR <15); Acuity unknown (baseline eGFR unknown). CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate calculated from the CKD-Epi equation.
Patients could have either acute, chronic, or both (acute and chronic) clinical and pathological etiologies for kidney injury.
Kidney function recovery
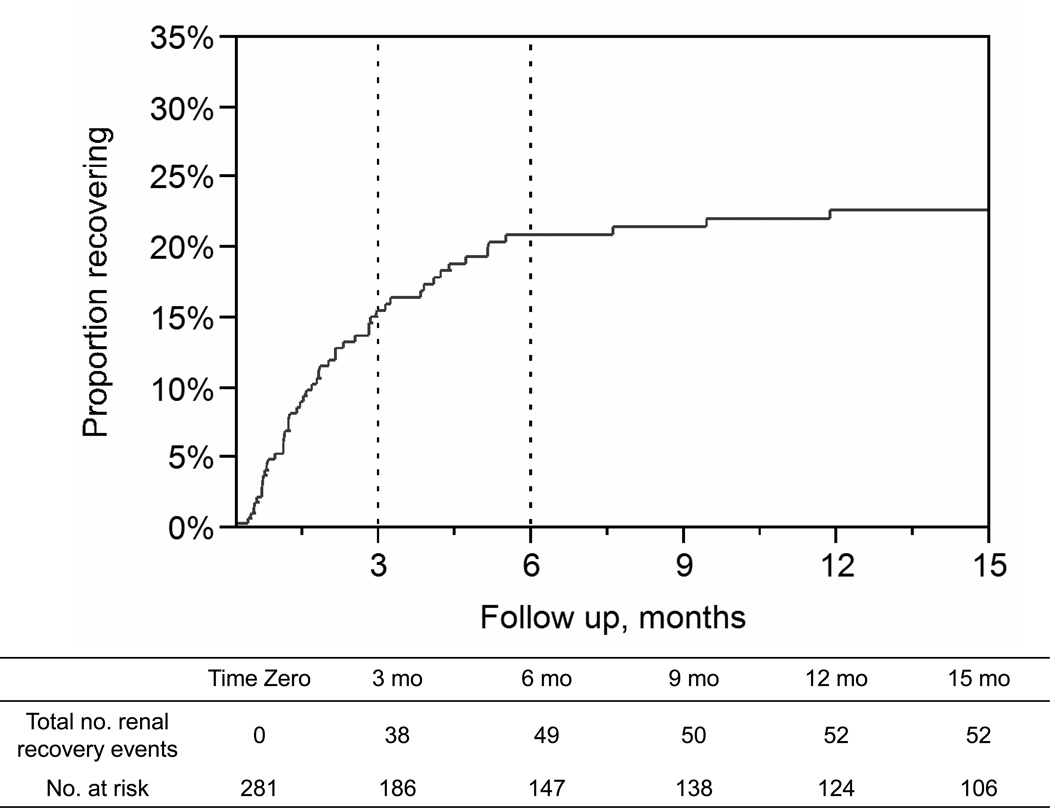
A total of 52 patients recovered. At 6 months, the cumulative recovery rate was 21%, Figure 1. Most recovery (73%) occurred within the first 3 months of RRT initiation (n=38). Thereafter, 11 patients (21%) recovered between 3- to 6-months and only 3 (6%) beyond 6 months. Notably, the last recovery event occurred at 12 months.
Figure 1.
Outpatient kidney function recovery events following in-hospital initiation of renal replacement therapy.
Kidney function recovery by 6 months
The majority (94%, n=49) of recovery events occurred within 6 months. Notably, 52% of patients with AKI recovered kidney function within 6 months while no patients with CKD5 recovered kidney function. Table 3 shows the association between baseline characteristics and kidney function recovery by 6 months. On univariate analysis, factors predictive of 6-month recovery were absence of heart failure, lack of prior outpatient nephrology evaluation within 1 year, lower Charlson comorbidity index score, ICU initiation of RRT, later calendar year (2007–2009 vs. 2006), catheter dialysis access, AKI (eGFR-based) subgroup, sepsis/postoperative ATN, and higher baseline eGFR. Multivariable Cox models intentionally considered clinically relevant and readily available variables to practitioners: ICU initiation, catheter dialysis access, heart failure, sepsis/postoperative ATN, and baseline eGFR. In multivariable analysis only 3 variables (higher baseline eGFR, sepsis/postoperative ATN, and heart failure) were independent predictors of recovery.
Table 3.
Association between kidney function recovery and baseline features.
| Unadjusted associations between baseline characteristics and Kidney function recovery at 6 months | |||||
|---|---|---|---|---|---|
| Entire Cohort (n=281) | Subgroup excluding CKD5 (n=225) |
||||
| Predictors of Kidney function recovery at 6 months* |
Hazard Ratio (95% CI) |
p | Hazard Ratio (95% CI) |
p | |
| Age, per 5 year | 0.93 (0.85–1.01) | 0.1 | 0.94 (0.87–1.03) | 0.2 | |
| Female gender | 1.43 (0.80–2.50) | 0.2 | 1.39 (0.78–2.44) | 0.3 | |
| Caucasian race | 2.19 (0.80–9.02) | 0.1 | 2.11 (0.78–8.67) | 0.2 | |
| Diabetes mellitus | 0.64 (0.36–1.12) | 0.1 | 0.58 (0.33–1.03) | 0.06 | |
| Coronary artery disease | 0.67 (0.37–1.18) | 0.2 | 0.70 (0.39–1.24) | 0.2 | |
| Heart failure | 0.36 (0.19–0.67) | <0.001 | 0.39 (0.20–0.71) | 0.002 | |
| Prior outpatient nephrology evaluation | 0.21 (0.09–0.41) | <0.001 | 0.31 (0.14–0.61) | 0.004 | |
| Charlson comorbidity score | |||||
| Charlson score, per 1 point | 0.84 (0.77–0.91) | <0.001 | 0.85 (0.78–0.93) | <0.001 | |
| Charlson score ≥8 | 0.37 (0.19–0.68) | <0.001 | 0.41 (0.21–0.75) | 0.004 | |
| Hospital | |||||
| ICU vs. non-ICU first dialysis location | 1.96 (1.11–3.44) | 0.02 | 1.59 (0.90–2.79) | 0.1 | |
| Time between admission and RRT start, per 1 day | 1.02 (0.98–1.06) | 0.3 | 1.01 (0.96–1.05) | 0.8 | |
| Time between RRT start and discharge, per 1 day | 0.99 (0.98–1.01) | 0.8 | 0.99 (0.98–1.00) | 0.4 | |
| Length of hospital stay, per 1 day | 1.00 (0.99–1.01) | 0.9 | 1.00 (0.98–1.00) | 0.5 | |
| Incident HD year | Incident HD year 2006 | 1.0 (REFERENT) | 1.0 (REFERENT) | ||
| Incident HD year 2007 | 2.87 (1.15–8.12) | 0.02 | 3.11 (1.25–8.79) | 0.01 | |
| Incident HD year 2008 | 3.45 (1.43–9.57) | 0.005 | 3.20 (1.33–8.86) | 0.009 | |
| Incident HD year 2009 | 2.64 (1.02–7.58) | 0.04 | 2.91 (1.13–8.37) | 0.03 | |
| First Dialysis access | |||||
| Catheters vs. Arteriovenous fistula/graft | 4.95 (1.08–87.55) | 0.03 | 2.76 (0.61–48.93) | 0.2 | |
| Arteriovenous fistula/graft | 1.0 (REFERENT) | 1.0 (REFERENT) | |||
| Cuffed tunneled catheter | 1.33 (0.23–25.07) | 0.8 | 0.85 (0.15–16.11) | 0.9 | |
| Non-cuffed, temporary catheter | 8.22 (1.79–145.75) | 0.003 | 4.11 (0.90–72.91) | 0.08 | |
| Renal failure type subgroups | |||||
| Acute kidney injury | 1.0 (REFERENT) | 1.0 (REFERENT) | |||
| Acute on chronic kidney disease | 0.25 (0.14–0.45) | <0.001 | 0.25 (0.14–0.45) | <0.001 | |
| Chronic kidney disease stage 5 | 0.00 (0.00–0.04) | <0.001 | Not included | ||
| Acuity unknown | 0.09 (0.01–0.31) | <0.001 | 0.09 (0.01–0.31) | <0.001 | |
| Sepsis/Postoperative ATN vs. other AKI & non-AKI | 3.33 (1.90–5.93) | <0.001 | 2.57 (1.46–4.58) | 0.001 | |
| Acute and chronic pathology subgroups | |||||
| Sepsis/Postoperative ATN pathology | 1.0 (REFERENT) | 1.0 (REFERENT) | |||
| Non-Sepsis/Postoperative ATN pathology | 0.51 (0.28–0.89) | 0.02 | 0.53 (0.30–0.92) | 0.03 | |
| Chronic pathology only | 0.00 (0.00–0.07) | <0.001 | 0.0 (0.00–0.00) | <0.001 | |
| GFR Measurements: n= 253 | n= 197 | ||||
| eGFR, per 10 ml/min/1.73m2 | 1.30 (1.21–1.40) | <0.001 | 1.24 (1.15–1.34) | <0.001 | |
| eGFR ≥30 ml/min/1.73m2 | 7.50 (3.78–16.56) | <0.001 | 4.40 (2.22–9.71) | <0.001 | |
|
Multivariable-adjusted#
associations between baseline characteristics and Kidney function recovery at 6 months | |||||
|
Predictors of Kidney function recovery at 6 months with acute and acute on chronic |
Hazard Ratio (95% CI) |
p |
Hazard Ratio (95% CI) |
p | |
| Variable (n=253) | (n=197) | ||||
| ICU location of first dialysis | 0.83 (0.44–1.56) | 0.6 | - | - | |
| First dialysis access was a catheter | 1.74 (0.36–31.34) | 0.6 | - | - | |
| Heart Failure | 0.40 (0.19–0.78) | 0.007 | 0.38 (0.19–0.74) | 0.004 | |
| Sepsis/Postoperative ATN pathology | 3.34 (1.83–6.24) | <0.001 | 2.78 (1.54–5.13) | <0.001 | |
| eGFR, per 10 ml/min/1.73m2 | 1.27 (1.16–1.39) | <0.001 | 1.21 (1.11–1.32) | <0.001 | |
Subgroup analysis excluded CKD5 patients (n=56) with baseline eGFR<15 mL/min/1.73m2;
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate calculated from the CKD-Epi equation; Charlson score: Charlson comorbidity index score; AKI: acute kidney injury; HD: hemodialysis; ICU: intensive care unit; RRT: renal replacement therapy.
Sepsis/Postoperative ATN pathology includes all patients (AKI or Acute on Chronic injury) with sepsis or postoperative clinical or pathological etiologies.
Adjusted for each other variable in the model, excluding patients with unknown baseline eGFR in the subgroup analysis.
In a subgroup analysis (n=225) that excluded patients with CKD5, ICU initiation and catheter access were no longer predictors of recovery in the unadjusted models. In multivariable analysis, heart failure, sepsis/postoperative ATN, and eGFR continued to be independent predictors of 6-month recovery (Table 3). As a sensitivity analysis, we assigned the full 6 months follow-up for all patients who died (n=97), but this did not meaningfully change the multivariable analysis findings (Hazard Ratio (HR) =0.39 for heart failure, HR=2.85 for sepsis/postoperative ATN, and HR=1.22 for eGFR per 10 ml/min/1.73 m2).
Baseline eGFR
Baseline eGFR was an important determinant of 6-month recovery. In the unadjusted model, patients with an eGFR ≥30 mL/min/1.73m2 had a 7.5-fold higher likelihood of recovery. Following adjustment for ICU initiation, catheter access, sepsis/postoperative ATN, and heart failure this relationship was preserved [HR=5.86; p<0.001]. The relationship between baseline eGFR, heart failure, and 6-month recovery is illustrated in Table 4. With eGFR <30 mL/min/1.73m2 as the reference group, eGFR 30–44 mL/min/1.73m2 trended toward a higher likelihood of recovery (HR=2.63; p=0.09). However, patients with eGFR 45–59 mL/min/1.73m2 and eGFR ≥60 mL/min/1.73m2 had a significantly higher likelihood of recovery (HR=8.19 and HR=9.45; p<0.001 for both).
Table 4.
Association between kidney function recovery, estimated glomerular filtration rate (eGFR), and heart failure (HF) at baseline.
| Adjusted association between eGFR, HF, and kidney function recovery in the entire cohort | |||||
|---|---|---|---|---|---|
| eGFR subgroups* | eGFR & HF subgroups | ||||
| Variable# | Hazard Ratio (95% CI) |
p | Variable## | Hazard Ratio€ (95% CI) |
p |
| eGFR ≥60 | 9.45 (4.05–23.97) | <0.001 | eGFR ≥30 & no-HF | 8.00 (3.60–20.54) | <0.001 |
| eGFR <60 and ≥45 | 8.19 (3.13–22.17) | <0.001 | eGFR ≥30 & HF | 1.02 (0.32–3.20) | 0.9 |
| eGFR <45 and ≥30 | 2.63 (0.86–7.58) | 0.09 | eGFR<30 & no-HF | 0.33 (0.05–1.37) | 0.1 |
| eGFR <30 | 1.00 (REFERENT) | eGFR<30 & HF | 1.0 (REFERENT) | ||
eGFR: estimated glomerular filtration rate in mL/min/1.73m calculated from the CKD-Epi equation; HF: heart failure. Patients without baseline eGFR (Acuity unknown group, n=28) were excluded from the analysis.
Adjusted for ICU location, first dialysis access catheter, heart failure, sepsis/postoperative ATN.
Adjusted for ICU location, first dialysis access catheter, sepsis/postoperative ATN.
There was an interaction of heart failure with eGFR≥30 in predicting kidney function recovery (p<0.001 in adjusted and unadjusted models).
Heart failure and baseline eGFR
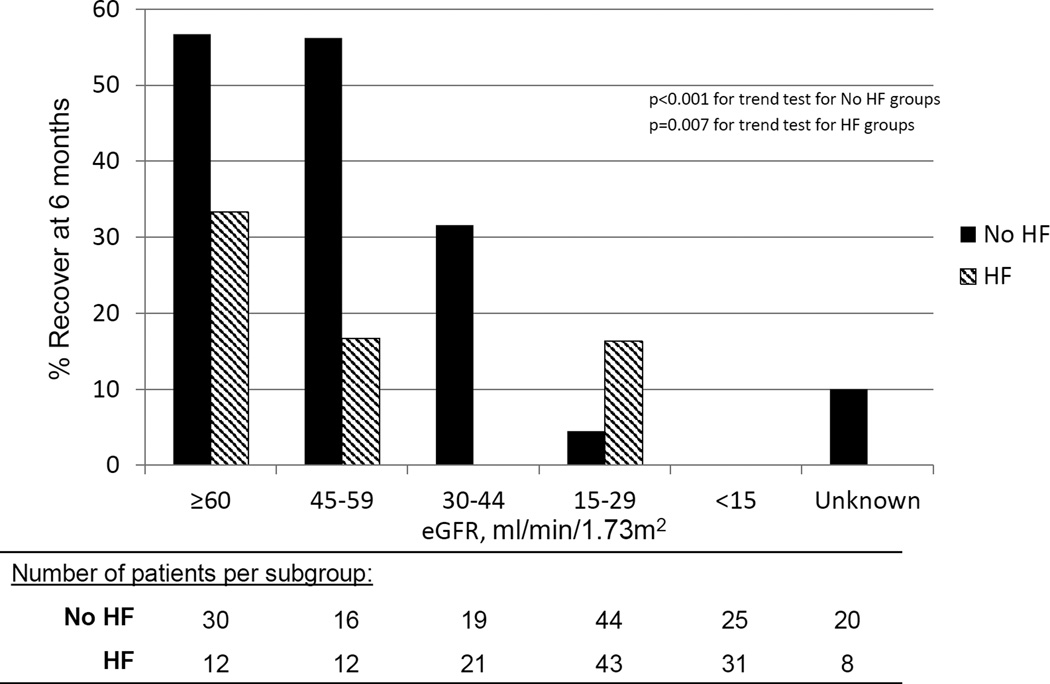
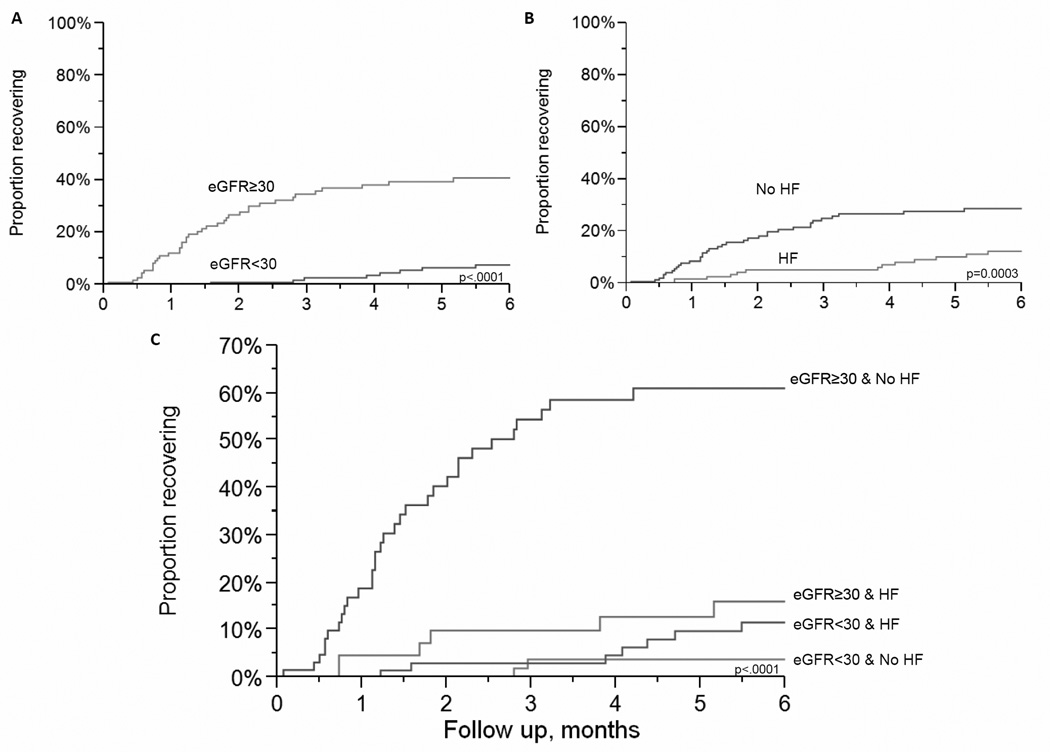
To determine the predictive utility of two important and readily available clinical variables for providers, the interaction of heart failure and baseline eGFR was also explored. In Table 4 a baseline eGFR ≥30 mL/min/1.73m2 and no history of heart failure was associated with a higher likelihood of recovery compared to patients with eGFR<30 mL/min/1.73m2 and a history of heart failure (HR=8.00; p<0.001). Among patients with heart failure, the probability of recovery at 6 months for an eGFR<30 mL/min/1.73m2 was 9% and eGFR ≥30 mL/min/1.73m2 was 13%. Among patients without heart failure, the probability of recovery for an eGFR<30 mL/min/1.73m2 was 3% and for an eGFR ≥30 mL/min/1.73m2 was 49%. Those with higher baseline eGFR subcategories were most likely to recover in either heart failure or non-heart failure group, Figure 2. However, heart failure appeared to be an effect modifier of the relationship between eGFR≥30 and kidney function recovery (p<0.001 test for interaction in adjusted and unadjusted models). In particular, eGFR≥30 mL/min/1.73m2 was a weaker predictor of recovery in patients with heart failure than in patients without heart failure, Figure 3.
Figure 2.
Outpatient kidney function recovery at 6 months stratified by baseline estimated glomerular filtration rate (eGFR) and heart failure (HF). A. Patients without HF (n=154). B. Patients with HF (n=127).
Figure 3.
Outpatient kidney function recovery stratified by estimated glomerular filtration rate (eGFR) and heart failure (HF). A. Stratified by eGFR<30 or ≥30 mL/min/1.73m2. B. Stratified by HF status. C. Stratified by eGFR <30 or ≥30 mL/min/1.73m2 and HF status.
Other Patient Outcomes
Over a mean study period of 15±16 months, 227 (81%) of 281 incident cohort patients discontinued outpatient in-center dialysis at MCDS. Fifty-two of 227 (23%) recovered kidney function. Other reasons for discontinuation of outpatient in-center dialysis included: death (43%), transfer to a non-MCDS dialysis center (24%), kidney transplantation (8%), and transfer to home dialysis therapies (2%).
Discussion
Among incident outpatient in-center hemodialysis patients directly transitioning from RRT that was started during a preceding hospitalization, kidney function recovery was not uncommon. Despite a high prevalence of comorbid conditions, we found a cumulative recovery rate of 21% at 6 months. Recovery most often occurred within the first 3 months of RRT start. However, the 3- to 6-month period remained an important time frame for further recovery events. Predictors associated with recovery within 6-months were ICU initiation, ATN in the setting of sepsis or surgery, higher baseline eGFR, later time period, lower Charlson comorbidity score, catheter as first dialysis access, lack of prior outpatient nephrology evaluation within 1 year, and absence of heart failure. Baseline eGFR was a strong and independent predictor of recovery. However, the association was modified by the presence of heart failure. Taken together, these data fill an important knowledge gap and provide a working platform from which providers may estimate the likelihood of recovery, plan scheduled monitoring for recovery when transitioning patients at hospital discharge, and arrange permanent access placement or transplantation referrals in those who are unlikely to recover.
Care of the incident dialysis population can be challenging. Approximately 50%–65% of our incident outpatient hemodialysis patients had first initiated in-hospital RRT 16. This experience is common across the U.S. and other regions15,17,27,32. Based on USRDS reporting, 32% of incident ESRD patients in 2011 initiated RRT without prior nephrology care32. Among our cohort, only 48% of incident patients initiating RRT in the hospital had been under the care of a nephrologist within 12 months prior to dialysis initiation. Many of these patients have normal baseline eGFR, hence there may have been no prior reason for nephrology referral before the hospitalization. For individuals with evidence of CKD, early nephrology referral is routinely promoted given the survival benefits of advanced planning, education, and permanent access creation for long-term dialysis33. Even among those with early nephrology referral, AoCKD frequently leads to unplanned hospital RRT starts as illustrated by O’Hare et al. In the 2 year period before dialysis initiation in 5,606 U.S. veterans, there were heterogenous patterns of kidney function loss enhanced by AoCKD contributing to suboptimal hospital starts15. An AKI episode occurred during hospitalization in 53% and 64% of the total veteran cohort initiated in-hospital RRT. At the time of hospital discharge, patients carry additional burdens of physical debility, infection or wound management, re-hospitalization risk, and/or loss of independence, especially among elderly patients16,34,35. As such, the complexity of new outpatient hemodialysis patients who started RRT in the hospital is often overwhelming for both patients and the providers who manage them
During the transition from hospital discharge to outpatient hemodialysis, the possibility of recovery remains a significant concern, and hope, for patients and their families. One important area for improvement in communication may be through early discussions regarding the potential for recovery of kidney function. By understanding the predictors of recovery in hospital starters, providers can more appropriately identify which patients should be more closely monitored for kidney function recovery and minimize unnecessary dialysis and healthcare costs or patient harm. In our study, we identified several variables which were predictive of recovery. Over half of the patients with AKI (defined as eGFR ≥60 mL/min/1.73m2) recovered kidney function while no patients with eGFR <15 mL/min/1.73m2 were free of dialysis support by study end. Patients who had an eGFR <15 mL/min/1.73m2 and only chronic pathology at baseline have little to no chance of recovery since there is no reversible acute component to their kidney failure.
In multivariable analysis, higher baseline eGFR, ATN from sepsis or surgery, and absence of heart failure were independent predictors of recovery. Similar to other studies, those with normal baseline kidney function and less comorbidity represent a group more likely to recover36–39. In an attempt to provide specific cut points regarding recovery within 6 months, we found that patients with a baseline eGFR ≥30 mL/min/1.73m2 had a 6-fold higher likelihood of recovery that was independent of other predictors (heart failure and ATN following sepsis or surgery). Comorbid conditions contribute to the prediction of poorer outcomes; however, calculation of Charlson scores can be cumbersome in busy day-to-day practice. Therefore, we chose to evaluate heart failure in combination with eGFR as two readily available clinical factors. In our study, patients with no heart failure and with eGFR ≥30 mL/min/1.73m2 had a much higher likelihood of recovery than with either factor alone. Overall, heart failure predicted a lower risk of recovery in the outpatient setting. This finding is relatively intuitive given the interrelationship of acute and chronic cardiorenal pathophysiology, difficulties in volume management, and potential for hemodialysis-induced myocardial injury43–45. Once heart failure patients require RRT, they are much less likely to recover kidney function even with a higher baseline eGFR.
This study has several potential limitations. First, the sample size may have been too small to detect all the characteristics that predict kidney function recovery. The predominantly white population limits the generalizability of the results to other groups. Nonetheless the integrative practice allowed for population-based estimates of kidney function recovery for the Midwest population which has been shown to be reasonably similar to the general U.S. population46. Second, baseline kidney function was often determined by a single serum creatinine measurement in the previous 12 months and in some cases (10%) was not even available. Although less optimal, we believe this to be consistent with the realities of clinical practice wherein baseline serum creatinine data are not always available. In addition, we did not assess recovery in peritoneal or home hemo- dialysis patients in whom the likelihood and predictors of recovery may differ from in-center hemodialysis patients. Third, we did not study patients who started RRT in the hospital but either died or had kidney function recovery prior to outpatient hemodialysis. The subset of new kidney failure patients in the hospital who survive their hospitalization but need to continue dialysis as an outpatient is a population of particular clinical interest that deserves separate study. Lastly, we did not have cardiac physiologic data (e.g., echocardiograms) in all patients which may have led to under-reporting of heart failure.
In conclusion, given that AKI has a likelihood of kidney function recovery not present with “true” ESRD26, identification of new outpatient hemodialysis patients who started RRT in the hospital and who may recover kidney function is important. In particular, higher baseline eGFR is a potent predictor of recovery in the absence of heart failure. Since we lack biomarkers to distinguish acute reversible from chronic irreversible renal injury47,48 uncertainty in the designation of “end stage renal disease” in such patients should be recognized not only by patients and providers but also by payers. Close monitoring for kidney function recovery is warranted. Conversely, in patients with low baseline kidney function (eGFR<30 mL/min/1.73m2) or heart failure, psychosocial support, education regarding alternative RRT modalities including transplantation and home dialysis modalities, and early more permanent dialysis access placement should be pursued.
Acknowledgements
This project was supported by a Mary Kathryn and Michael B. Panitch Career Development Award for L.J.H. and Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional thanks to Eric Bergstralh for statistical support. Study content was presented in abstract form at Kidney Week 2011 in Philadelphia, Pennsylvania.
Footnotes
Disclosure
The authors have no conflict of interest to disclose.
Contributions: research idea and study design: LJH, SC, ADR, SMN, AWW, BT, JTM, JRG, RCA, JJD; data acquisition: LJH, SC, SMN, AWW; data analysis/interpretation: LJH, ADR, SMN, SC, AWW, BT, JTM, JRG, RCA, JJD; statistical analysis: LJH, ADR; supervision or mentorship: ADR, SMN, AWW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. LJH takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Contributor Information
LaTonya J. Hickson, Email: hickson.latonya@mayo.edu.
Sanjay Chaudhary, Email: sanjay1995@gmail.com.
Amy W. Williams, Email: williams.amy@mayo.edu.
John J. Dillon, Email: dillon.john@mayo.edu.
Suzanne M. Norby, Email: norby.suzanne@mayo.edu.
James R. Gregoire, Email: jgregoire@mayo.edu.
Robert C. Albright, Jr, Email: albright.robert@mayo.edu.
James T. McCarthy, Email: mccarthy.james@mayo.edu.
Bjoerg Thorsteinsdottir, Email: thorsteinsdottir.Bjorg@mayo.edu.
Andrew D. Rule, Email: rule.andrew@mayo.edu.
References
- 1.Fang Y, Ding X, Zhong Y, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30(2):120–126. doi: 10.1159/000319972. [DOI] [PubMed] [Google Scholar]
- 2.Lafrance JP, Miller DR. Defining acute kidney injury in database studies: the effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis. 2010 Oct;56(4):651–660. doi: 10.1053/j.ajkd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006 Jul;34(7):1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10(3):R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009 Sep;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 6.Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008 Jul 3;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005 Aug 17;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002 Sep;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JT. Prognosis of patients with acute renal failure in the intensive-care unit: a tale of two eras. Mayo Clin Proc. 1996 Feb;71(2):117–126. doi: 10.4065/71.2.117. [DOI] [PubMed] [Google Scholar]
- 10.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009 Jun;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001 Oct;29(10):1910–1915. doi: 10.1097/00003246-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagshaw SM, Uchino S, Kellum JA, et al. Association between renal replacement therapy in critically ill patients with severe acute kidney injury and mortality. J Crit Care. 2013 Dec;28(6):1011–1018. doi: 10.1016/j.jcrc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008 Aug;52(2):262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 15.O'Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012 Apr;59(4):513–522. doi: 10.1053/j.ajkd.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoonover KL, Hickson LJ, Norby SM, et al. Risk Factors for Hospitalization Among Older, Incident Hemodialysis Patients. Nephrology (Carlton) 2013 Jul 12; doi: 10.1111/nep.12129. [DOI] [PubMed] [Google Scholar]
- 17.Mendelssohn DC, Curtis B, Yeates K, et al. Suboptimal initiation of dialysis with and without early referral to a nephrologist. Nephrol Dial Transplant. 2011 Sep;26(9):2959–2965. doi: 10.1093/ndt/gfq843. [DOI] [PubMed] [Google Scholar]
- 18.Spurney RF, Fulkerson WJ, Schwab SJ. Acute renal failure in critically ill patients: prognosis for recovery of kidney function after prolonged dialysis support. Crit Care Med. 1991 Jan;19(1):8–11. doi: 10.1097/00003246-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Fehrman-Ekholm I, Bergenhag AC, Heimburger O, Schon S. Recovery of renal function after one-year of dialysis treatment: case report and registry data. Int J Nephrol. 2010;2010:817836. doi: 10.4061/2010/817836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekkarie MA, Port FK, Wolfe RA, et al. Recovery from end-stage renal disease. Am J Kidney Dis. 1990 Jan;15(1):61–65. doi: 10.1016/s0272-6386(12)80593-2. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui S, Norbury M, Robertson S, Almond A, Isles C. Recovery of renal function after 90 d on dialysis: implications for transplantation in patients with potentially reversible causes of renal failure. Clin Transplant. 2008 Mar-Apr;22(2):136–140. doi: 10.1111/j.1399-0012.2007.00755.x. [DOI] [PubMed] [Google Scholar]
- 22.Rottembourg J. Residual renal function and recovery of renal function in patients treated by CAPD. Kidney Int Suppl. 1993 Feb;40:S106–S110. [PubMed] [Google Scholar]
- 23.Macdonald JA, McDonald SP, Hawley CM, et al. Recovery of renal function in end-stage renal failure--comparison between peritoneal dialysis and haemodialysis. Nephrol Dial Transplant. 2009 Sep;24(9):2825–2831. doi: 10.1093/ndt/gfp216. [DOI] [PubMed] [Google Scholar]
- 24.Craven AM, Hawley CM, McDonald SP, Rosman JB, Brown FG, Johnson DW. Predictors of renal recovery in Australian and New Zealand end-stage renal failure patients treated with peritoneal dialysis. Perit Dial Int. 2007 Mar-Apr;27(2):184–191. [PubMed] [Google Scholar]
- 25.Pichette V, Querin S, Desmeules M, Ethier J, Copleston P. Renal function recovery in end-stage renal disease. Am J Kidney Dis. 1993 Sep;22(3):398–402. doi: 10.1016/s0272-6386(12)70142-7. [DOI] [PubMed] [Google Scholar]
- 26.Mohan S, Huff E, Wish J, Lilly M, Chen SC, McClellan WM. Recovery of Renal Function among ESRD Patients in the US Medicare Program. PLoS One. 2013;8(12):e83447. doi: 10.1371/journal.pone.0083447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong SP, Kreuter W, O'Hare AM. Healthcare Intensity at Initiation of Chronic Dialysis among Older Adults. J Am Soc Nephrol. 2013 Nov 21; doi: 10.1681/ASN.2013050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Services CfMaM. [Accessed 03/15/2014];Hospital Dialysis Services for Patients with and without End Stage Renal Disease (ESRD). Medicare Learning Network: MM7762 for CR7762. 2011 http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM7762.pdf.
- 29.Services CfMaM. [Accessed 07/18/2012];Can Certified ESRD Facilities Furnish Acute Dialysis to Hospital Outpatients? [Question and answer document for CR7762] 2012 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/Acute-Dialysis-Site.pdf.
- 30.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012 Sep;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, Maryland: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 33.Slinin Y, Guo H, Gilbertson DT, et al. Meeting KDOQI guideline goals at hemodialysis initiation and survival during the first year. Clin J Am Soc Nephrol. 2010 Sep;5(9):1574–1581. doi: 10.2215/CJN.01320210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorsteinsdottir B, Swetz KM, Tilburt JC. Dialysis in the frail elderly--a current ethical problem, an impending ethical crisis. J Gen Intern Med. 2013 Nov;28(11):1511–1516. doi: 10.1007/s11606-013-2494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams AW. Older Adults with CKD and Acute Kidney Failure: Do We Know Enough for Critical Shared Decision Making? J Am Soc Nephrol. 2013 Nov 21; doi: 10.1681/ASN.2013090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of Kidney Function After Acute Kidney Injury in the Elderly: A Systematic Review and Meta-analysis. American Journal of Kidney Diseases. 2008;52(2):262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011 Dec;80(11):1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 38.Bagshaw SM. Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care. 2006 Dec;12(6):544–550. doi: 10.1097/01.ccx.0000247444.63758.0b. [DOI] [PubMed] [Google Scholar]
- 39.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009 May;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dandamudi S, Chen HH. Evolving treatment strategies for management of cardiorenal syndrome. Curr Treat Options Cardiovasc Med. 2011 Dec;13(6):556–569. doi: 10.1007/s11936-011-0148-3. [DOI] [PubMed] [Google Scholar]
- 41.Martin FL, McKie PM, Cataliotti A, et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol. 2012 Jan 15;302(2):R292–R299. doi: 10.1152/ajpregu.00194.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronco C, Maisel A. Volume overload and cardiorenal syndromes. Congest Heart Fail. 2010 Jul;16(Suppl 1) doi: 10.1111/j.1751-7133.2010.00176.x. Si-iv; quiz Svi. [DOI] [PubMed] [Google Scholar]
- 43.Bongartz LG, Braam B, Gaillard CA, et al. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol. 2012 Nov 1;303(9):F1253–F1263. doi: 10.1152/ajprenal.00392.2012. [DOI] [PubMed] [Google Scholar]
- 44.Liang KV, Pike F, Argyropoulos C, et al. Heart failure severity scoring system and medical- and health-related quality-of-life outcomes: the HEMO study. Am J Kidney Dis. 2011 Jul;58(1):84–92. doi: 10.1053/j.ajkd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009 Dec;4(12):1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012 Feb;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srisawat N, Wen X, Lee M, et al. Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol. 2011 Aug;6(8):1815–1823. doi: 10.2215/CJN.11261210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013 Feb 6;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]