Abstract
Objective
Although surgical resection is used to treat meningeal hemangiopericytoma (MHPC), there is a high risk of subsequent recurrence. This study investigated factors associated with treatment outcomes and recurrence in patients who had undergone surgical resection of intracranial MHPC.
Methods
Fifteen patients underwent surgical treatments performed by one senior neurosurgeon between 1997 and 2013. Clinical data, radiologic images, surgical outcomes, recurrence, and other relevant characteristics were reviewed and analyzed.
Results
Fifteen patients were included in the analysis, 12 (80%) of whom had tumors in the supratentorial region, and 3 (20%) of whom had tumors in the infratentorial region. Complete resection was achieved in all 15 patients, and 3 (20%) patients were administered radiosurgery and conventional radiotherapy after surgery as adjuvant radiotherapy. Three patients developed recurrence, 2 of whom had not received adjuvant radiotherapy. In 1 of the patients who had not received adjuvant radiotherapy, recurrence developed at the original tumor site, 81 months after surgery. The other 2 recurrences occurred at other sites, 78 and 41 months after surgery. The 5- and 10-year overall survival rates were 88.3%, while the 5- and 10-year recurrence-free survival rates were 83% and 52%, respectively. Additionally the mean Ki-67 index differed significantly between patients who did and did not develop recurrence (43% vs. 14%; p=0.001).
Conclusion
Because of the high risk of MHPC recurrence, MHPC tumors should be completely resected, whenever feasible. However, even when complete resection is achieved, adjuvant radiotherapy might be necessary to prevent recurrence.
Keywords: Meningeal hemangiopericytoma, Recurrence, Radiation therapy, Extraneural metastasis, Complete resection
INTRODUCTION
Meningeal hemangiopericytoma (MHPC) was first reported by Stout and Murray in 194228,32). MHPC tumors arise from Zimmermann's pericytes around capillaries and post-capillary venules. Begg and Garret have reported several similarities between MHPC and angioblastic meningioma, and have additionally used immunohistochemistry and genetic analyses to demonstrate the ways in which MHPC differs from meningioma5,16). Although MHPC was initially considered to be a transformation of meningioma, it was ultimately recognized as a distinct pathological entity with clinical behaviors, immune cell features, and ultrastructural features that differ from meningiomas7,18,22,26). In 1993, the World Health Organization recognized MHPC as a distinct clinicopathological entity, based on MHPC's tendencies toward recurrence and extraneural metastasis, its distinct clinical behavior, and its immunohistochemical, ultrastructural, and genetic characteristics28). MHPC accounts for 2.5% of meningeal tumors and 1% of intracranial tumors2,9,15,17). They tend to recur even after macroscopic total resection, with local recurrence rates as high as 91%. A significant number of patients with MHPC live as long as 15 years and beyond after the initial surgery, developing second and third local recurrences as well as distant metastasis, thus mandating vigilant long-term follow-up26). In this study, we explored treatment outcomes, recurrence, and clinicopathological characteristics in patients with surgically treated MHPC.
MATERIALS AND METHODS
Patients
This study included 15 patients who underwent surgical resections performed by one senior neurosurgeon between 1997 and 2013. Clinicopathological characteristics, such as age, sex, tumor location, tumor size, extent of surgical resection, adjuvant radiotherapy, recurrence, and survival were retrospectively collected from medical records. Prior to surgery, the researchers explained to the patients that their medical records would be used for research. Our analysis was limited to the patients who provided informed consent. Postoperative progress was assessed using the patients' medical records and telephone contact. The extent of tumor resection was identified from surgical records and postoperative imaging. Complete resection (CR) refers to cases in which the tumors were not visible to the naked eye and could not be seen in postoperative imaging. The average time between disease diagnosis and the presentation of symptoms was 11 months. Headaches were the most common symptom. We analyzed characteristics such as age, sex, tumor size and location, extent of resection, status of adjuvant radiotherapy, and the histological grade of the recurrence.
Statistical analysis
All statistical analyses were performed using SPSS (version 16.0, IBM SPSS Inc., Chicago, IL, USA). The findings are presented as mean values±standard deviations (SDs). Survival and recurrence rates were estimated using the Kaplan-Meier method. The relationships between various factors and recurrence times were assessed using the log-rank test. p-values<0.05 were considered statistically significant.
RESULTS
The records of 15 patients were analyzed. Ten (67%) of the patients were men. The patient's ages ranged from 33 to 76 years (mean 47.2 years). Twelve (80%) patients had tumors in the supratentorial region and 3 (20%) patients had tumors in the infratentorial region. One patient had received transcatheter arterial chemoembolization and adjuvant radiotherapy for hemangiopericytoma of the liver prior to the diagnosis of intracranial MHPC. In this case, the tumor had already spread to the lungs and spine, as identified using a preoperative positron emission tomography scan.
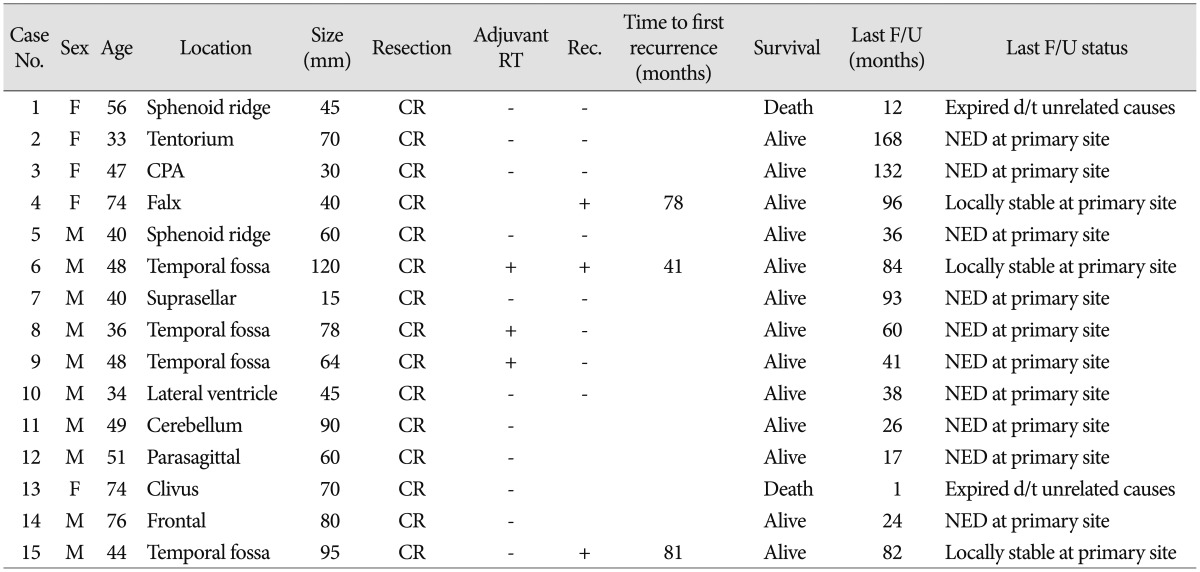
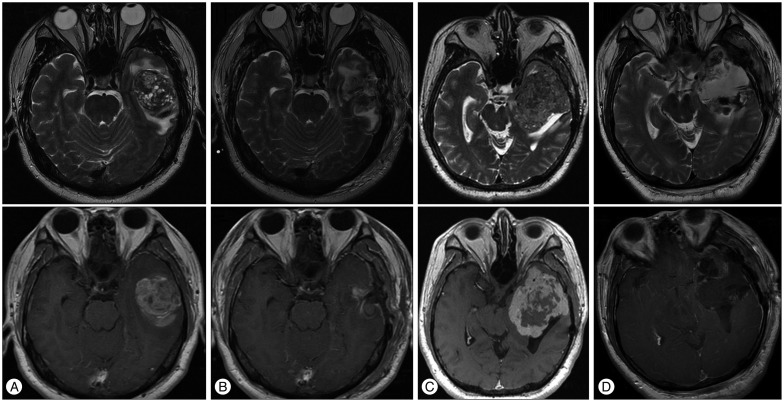
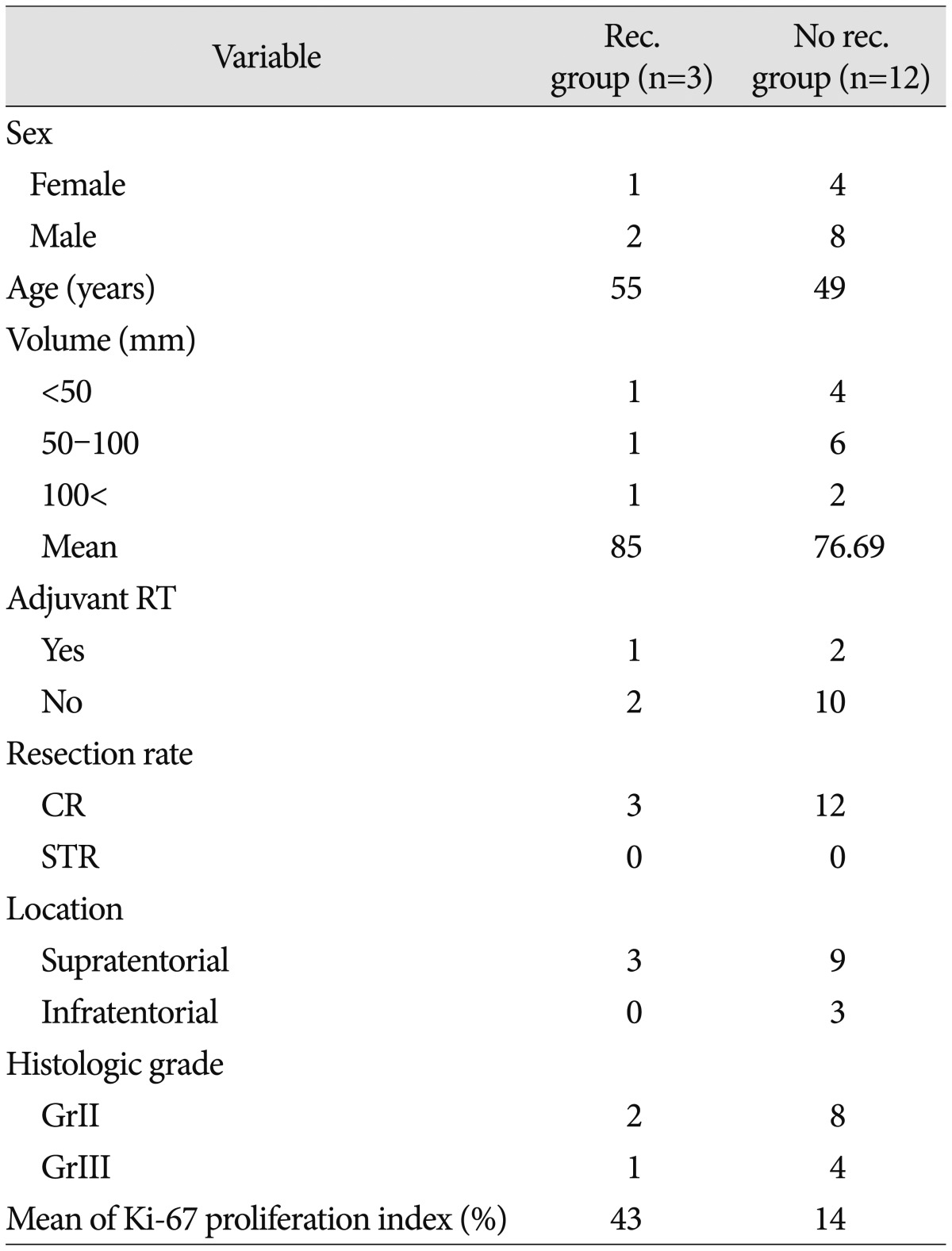
All 15 patients underwent CR including the dura of origin of tumor. Three (20%) patients received post-surgical adjuvant radiotherapy without any chemotherapy. We recommended adjuvant radiotherapy for five patient of histologic grade III. But two patients refused adjuvant therapy. Two (13%) patients with tumors that were at least 100 mm in size had received tumor embolization before surgery. Patient characteristics, such as the extent of tumor resection, adjuvant radiotherapy, recurrence, time to recurrence, and status at last follow-up, are summarized in Table 1. The average duration of follow-up was 53 months. Recurrence occurred in 3 (20%) patients : 1 patient (Case No. 15) developed recurrence at the original site (Fig. 1) and 2 patients (Case Nos. 4, 6) developed recurrences at other sites. Recurrence occurred in 1 patient who had received radiosurgery and 2 patients who had not received adjuvant radiotherapy. We used IMRT (Brainlab, Munich, Germany) for conventional radiotherapy. There was no significant relationship between recurrence and adjuvant radiotherapy (p=0.3) (Fig. 2).
Table 1. Patient characteristics in 15 case of intracranial hemangiopericytomas.
No. : number, F : female, M : male, RT : radiotherapy, Rec : recurrence, F/U : follow up, d/t : due to, CPA : cerebellopontine angle, CR : complete resection, NED : no evidence of disease
Fig. 1. A : Preoperative magnetic resonance (MR) images show a temporal fossa meningeal hemangiopericytoma in Case No. 15. B : Immediate postoperative MR images show that the tumor was completely resected. C : MR images taken 81 months later show tumor recurrence. The patient underwent tumor embolization prior to removal. D : Immediate postoperative MR images show complete resection of the tumor.
Fig. 2. Kaplan-Meier analysis of recurrence-free survival rates in patients who were and were not treated with adjuvant radiotherapy after complete resection in the first surgery. There was no significant difference between these groups (p=0.3).
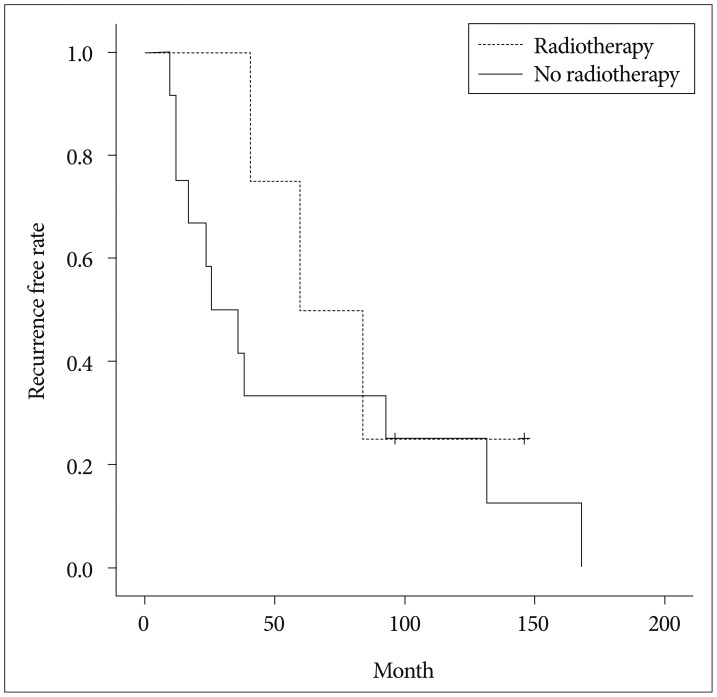
Among the patients who developed recurrence during follow-up, the average time to the first recurrence was 66.7 months. The 10-year recurrence-free survival (RFS) rate was 80%, whereas the median RFS was 146 months. The mean Ki-67 index differed significantly between patients who did and did not develop recurrence (43% vs. 14%; p=0.001) (Fig. 3). Comparison of 2 groups in terms of age, sex, tumor size, location, adjuvant radiotherapy status, histological grade, the Ki-67 index are summarized in Table 2. The relationship between recurrence and the resection rate could not be assessed because CR was performed in all patients.
Fig. 3. The Ki-67 index differed significantly between patients who did and did not develop recurrence (p=0.001).
Table 2. Baseline Characteristics of 15 cases of intracranial hemangiopericytomas.
Rec : recurrence, RT : radiotherapy, CR : complete resection, STR : subtotal resection, Gr : grade
Three (20%) patients received adjuvant radiotherapy. One of them underwent radiosurgery (18 Gy at the 90% isodose line)for the single lesion. The others underwent conventional radiotherapy (50 Gy in 25 fractions) because of tumors at the multiple brain sites. Although the patient who received radiosurgery experienced temporal recurrence within 3 years, neither of the patient who received conventional radiotherapy experienced recurrence.
We investigated recurrence status according to histological grade and found that 2 (67%) of the patients with recurrence had grade II disease, and 1 (33%) of the patients with recurrence had grade III disease. Among patients without recurrence, 8 (67%) had grade II disease and 4 (33%) had grade III disease. Accordingly, recurrence rates did not differ significantly according to histological grade (p=0.931)33).
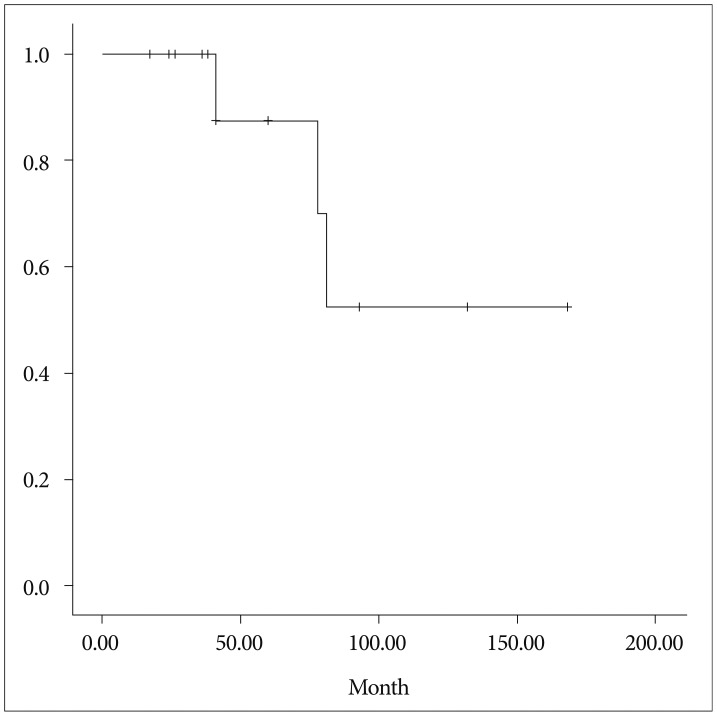
The 5- and 10-year overall survival rate was 88.3%, while the 5- and 10-year recurrence-free survival rates were 83% and 52%, respectively (Fig. 4). Two patients died of respiratory problems during the follow-up period : 1 patient with metastatic lung tumors died of acute respiratory distress syndrome in 12 months after surgery, and the other patient died of pneumonia 1 month after surgery.
Fig. 4. Analysis of the recurrence-free survival in our study. The 5- and 10-year recurrence-free survival rates were 83% and 52%, respectively.
DISCUSSION
Because MHPC carries high risks of recurrence and spread to other sites, precise identification of the tumor's features is necessary to provide appropriate treatment and accurate assessments of prognosis4,5,8,9,12,14,15,22,25). Long-term studies have shown that MHPC tends to recur in brain and spinal meninges, but not at the original site4,5,7,8,15,16,22).
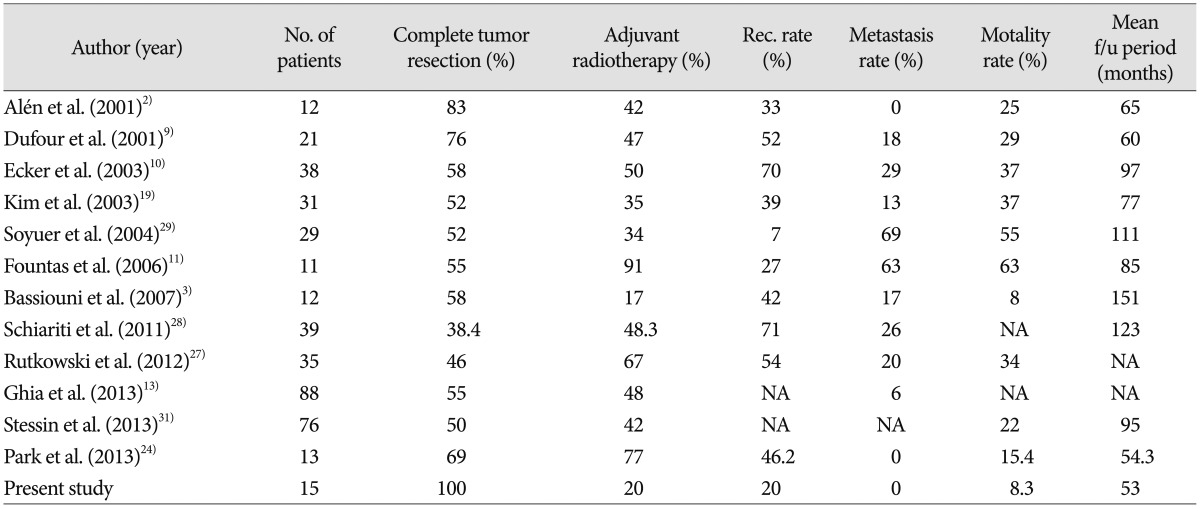
Surgical resection has an important effect on recurrence and prognosis3,11,12,15,24,28). Galanis et al.12) and Guthrie et al.15) have emphasized the importance of achieving CR during the first surgery to reduce the risk of recurrence and prolong survival. They have also suggested that aggressive surgical therapy plays an important role in the success or failure of treatment. In a study of MHPC, Schiaritis et al.28) reported RFS rates of 77% after CR and 3% after incomplete resection. They stated that the time to recurrence after CR was 63 months longer than the time to recurrence after incomplete resection. In the current study, all patients received CR and the recurrence rate was 20%. This value is substantially lower than has been observed in other studies that included cases of incomplete resection. Accordingly, the results of the current study also tend to suggest that CR poses a lower risk of recurrence than incomplete resection. A literature review of recent studies on meningeal hemangiopericytomas showed an average CR rate of 57.7% (Table 3).
Table 3. Literature review of recent studies of meningeal hemangiopericytomas.
No : number, Rec : recurrence, F/U : follow up, NA : not available
Adjuvant radiotherapy has been reported to reduce recurrence rates and extend survival2,4,10,23,30). In 1976, Lal et al.21) demonstrated that adjuvant radiotherapy was efficacious for MHPC. Guthrie et al.15) reported a 52% recurrence rate in patients treated with adjuvantradiotherapy, as compared with an 86% rate in those who did not receive adjuvantradiotherapy. Further, the average times to recurrence were 75 and 34 months for patients who did and did not receive adjuvantradiotherapy15). In Guthrie et al.'15)s study, adjuvant radiotherapy was performed in 4 patients with surgical findings of an unclear boundary between the tumor and the normal brain tissue; biopsy results showed where the normal brain tissue had been invaded by tumor cells in the boundary area. In 1 of the 3 patients who developed recurrence, recurrence occurred in the boundary of the area where the tumor had originated. It is logical to suggest that adjuvant radiotherapy is helpful when incomplete resection is performed; however, even in cases of CR, adjuvant radiotherapy may be helpful when the boundary area of the tumor is not clear.
Radiosurgery is an effective treatment for recurrent MHPC with well-separated, small tumors. Bastin and Mehta4) reported that the tumor was effectively reduced by a single fraction of stereotactic radiosurgery for recurrent MHPC. Galanis et al.12) performed radiosurgery on 17 tumors in 7 patients who had recurrent tumors or persistent tumors, even after they had received conventional fractionated radiotherapy with 50 to 60 Gy. Guthrie et al.15) demonstrated that recurrence rates reduced with increasing levels of radiation, and found no recurrence in patients who had received more than 50 Gy of radiation. Dufour et al.9) reported recurrences in 2 patients who had received less than 30 Gy of radiation, but no recurrence in patients who had received doses of 50 and 64 Gy. In our study, recurrence was observed in 1 patient who had received radiosurgery with 18 Gy, but no recurrence was observed in either of the 2 patients who received conventional radiotherapy with more than 50 Gy. Even though we did not observe a statistically significant difference in recurrence rates, we consider a dose of at least 50 Gy to be both helpful and within the range of tolerable doses.
Several clinicians have performed adjuvant radiotherapy before surgery for cases of MHPC that are associated with elevated surgical risk. Carella et al.6) presented the hemangiopericytic variant of angioblastic meningioma, which had better responses to adjuvant radiotherapy than normal meningioma. In an analysis of 5 patients who had undergone adjuvant radiotherapy before surgery, Uemura et al.35) reported that tumors were easily removed without substantial bleeding and, further, that many tumor cells were pathologically reduced after adjuvant radiotherapy. However, it is difficult to obtain histological findings before surgery, and it is necessary to fully consider adjuvant radiotherapy after surgery. Preoperative adjuvant radiotherapy should be performed only when surgical treatment is predicted to be difficult. Vuorinen et al.34) suggested that the Ki-67 index was not correlated with clinical outcomes, even though survival times tended to be longer among patients with a Ki-67 index <5%. In our study, the Ki-67 index was significantly higher in patients who developed recurrence, as compared with patients who did not develop recurrence (43% vs. 14%; p<0.001).
Most recurrences have occurred at the original site of MHPC. Galanis et al.12) reported recurrence in 32 of 34 cases, including 19 patients with recurrence at the original site, 11 with recurrence at both the original site and a distant site, and 2 with leptomeningeal seeding at the site of the original tumor, but no recurrence. In our study, 1 patient had a recurrent tumor at the original site, and 2 patients had recurrent tumors at different sites. However, leptomeningeal seeding was not observed. MHPC also tends to recur in at sites that are distant from the nervous system. Extraneural metastasis simultaneously occurs in the bones, lungs, kidneys, pancreas, adrenal glands, and liver. Galanis et al.12) showed that metastasis is most common in the bones and liver, whereas Brunori et al.5) reported more frequent metastasis in the lungs, bones, soft tissues, and liver. The current study includes 1 case in which extraneural metastasis had already occurred in the lungs, bones, and liver at the time of diagnosis with intracranial MHPC. However, none of the other patients had extraneural metastasis after surgery.
Follow-up observations are important after surgery for MHPC. Bastin and Mehta4) reported a 5-year recurrence rate of ≤33% and Brunori et al.5) reported that the average time to the first recurrence was 84 months. Guthrie et al.15) stated that the 10-year recurrence rate after surgery was 76% and Goellner et al.14) reported that the 15-year recurrence rate was 76%. Schiariti et al.28) reported that the time to recurrence after the first surgery averaged 80 months.
In this study, The 5- and 10-year overall survival rate were 91.7% and 88.3%, respectively, while the 5- and 10-year recurrence-free survival rates were 83% and 52%, respectively. Recurrence after surgery has been described as the last stage in the natural history of HPC4,5,14,15,17). Therefore, it may be necessary to continue follow-up observations for as long as is possible. The duration of observational follow-up has an important effect on the selection of treatment methods because a long duration of observational follow-up allows an effective method, such as radiosurgery, to be selected for small recurrent tumors (in addition to surgical treatment), before the tumor has had the opportunity to grow large.
Moreover, it is important to identify metastatic status during follow-up because extraneural metastasis frequently occurs in MHPC. In retrospective analyses, Stout et al.32) and Adegbite et al.1) demonstrated rates of extraneural metastases of 12% and 57%, respectively. Treatment methods, surgical outcomes, and many other factors may lead to differences in the rate of extra-neural metastasis. The duration of observational follow-up may also lead to differences. Guthrie et al.15) presented 5-, 10-, and 15-year metastasis rates of 13%, 33%, and 64%, respectively. Koyama et al.20) reported that metastases occurred an average of 8 years after initial treatment, and have tracked patients for 16 years.
CONCLUSION
MHPC is an aggressive tumor with a high risk of recurrence. To prevent recurrence, CR must be followed by adjuvant radiotherapy and periodic follow-up that is commensurate with the degree of surgical resection.
References
- 1.Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 2.Alén JF, Lobato RD, Gómez PA, Boto GR, Lagares A, Ramos A, et al. Intracranial hemangiopericytoma : study of 12 cases. Acta Neurochir (Wien) 2001;143:575–586. doi: 10.1007/s007010170062. [DOI] [PubMed] [Google Scholar]
- 3.Bassiouni H, Asgari S, Hübschen U, König HJ, Stolke D. Intracranial hemangiopericytoma : treatment outcomes in a consecutive series. Zentralbl Neurochir. 2007;68:111–118. doi: 10.1055/s-2007-981674. [DOI] [PubMed] [Google Scholar]
- 4.Bastin KT, Mehta MP. Meningeal hemangiopericytoma : defining the role for radiation therapy. J Neurooncol. 1992;14:277–287. doi: 10.1007/BF00172604. [DOI] [PubMed] [Google Scholar]
- 5.Brunori A, Delitala A, Oddi G, Chiappetta F. Recent experience in the management of meningeal hemangiopericytomas. Tumori. 1997;83:856–861. doi: 10.1177/030089169708300516. [DOI] [PubMed] [Google Scholar]
- 6.Carella RJ, Ransohoff J, Newall J. Role of radiation therapy in the management of meningioma. Neurosurgery. 1982;10:332–339. doi: 10.1227/00006123-198203000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Coffey RJ, Cascino TL, Shaw EG. Radiosurgical treatment of recurrent hemangiopericytomas of the meninges : preliminary results. J Neurosurg. 1993;78:903–908. doi: 10.3171/jns.1993.78.6.0903. [DOI] [PubMed] [Google Scholar]
- 8.Craven JP, Quigley TM, Bolen JW, Raker EJ. Current management and clinical outcome of hemangiopericytomas. Am J Surg. 1992;163:490–493. doi: 10.1016/0002-9610(92)90394-7. [DOI] [PubMed] [Google Scholar]
- 9.Dufour H, Métellus P, Fuentes S, Murracciole X, Régis J, Figarella-Branger D, et al. Meningeal hemangiopericytoma : a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001;48:756–762. discussion 762-763. doi: 10.1097/00006123-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Ecker RD, Marsh WR, Pollock BE, Kurtkaya-Yapicier O, McClelland R, Scheithauer BW, et al. Hemangiopericytoma in the central nervous system : treatment, pathological features, and long-term follow up in 38 patients. J Neurosurg. 2003;98:1182–1187. doi: 10.3171/jns.2003.98.6.1182. [DOI] [PubMed] [Google Scholar]
- 11.Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS, et al. Management of intracranial meningeal hemangiopericytomas : outcome and experience. Neurosurg Rev. 2006;29:145–153. doi: 10.1007/s10143-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 12.Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82:1915–1920. [PubMed] [Google Scholar]
- 13.Ghia AJ, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE, Brown PD. Intracranial hemangiopericytoma and the role of radiation therapy : a population based analysis. Neurosurgery. 2013;72:203–209. doi: 10.1227/NEU.0b013e31827b9e68. [DOI] [PubMed] [Google Scholar]
- 14.Goellner JR, Laws ER, Jr, Soule EH, Okazaki H. Hemangiopericytoma of the meninges. Mayo Clinic experience. Am J Clin Pathol. 1978;70:375–380. doi: 10.1093/ajcp/70.3.375. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma : histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. [PubMed] [Google Scholar]
- 16.Hara M, Aoyagi M, Nagashima G, Wakimoto H, Mikami T, Yamamoto S, et al. Recurrence in meningeal hemangiopericytomas. Surg Neurol. 1998;50:586–591. doi: 10.1016/s0090-3019(98)00043-3. [DOI] [PubMed] [Google Scholar]
- 17.Jääskeläinen J, Servo A, Haltia M, Wahlström T, Valtonen S. Intracranial hemangiopericytoma : radiology, surgery, radiotherapy, and outcome in 21 patients. Surg Neurol. 1985;23:227–236. doi: 10.1016/0090-3019(85)90087-4. [DOI] [PubMed] [Google Scholar]
- 18.Joseph JT, Lisle DK, Jacoby LB, Paulus W, Barone R, Cohen ML, et al. NF2 gene analysis distinguishes hemangiopericytoma from meningioma. Am J Pathol. 1995;147:1450–1455. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Jung HW, Kim YS, Kim CJ, Hwang SK, Paek SH, et al. Meningeal hemangiopericytomas : long-term outcome and biological behavior. Surg Neurol. 2003;59:47–53. discussion 53-54. doi: 10.1016/s0090-3019(02)00917-5. [DOI] [PubMed] [Google Scholar]
- 20.Koyama H, Harada A, Nakao A, Nonami T, Kurokawa T, Kaneko T, et al. Intracranial hemangiopericytoma with metastasis to the pancreas. Case report and literature review. J Clin Gastroenterol. 1997;25:706–708. doi: 10.1097/00004836-199712000-00040. [DOI] [PubMed] [Google Scholar]
- 21.Lal H, Sanyal B, Pant GC, Rastogi BL, Khanna NN, Udupa KN. Hemangiopericytoma : report of three cases regarding role of radiation therapy. AJR Am J Roentgenol. 1976;126:887–891. doi: 10.2214/ajr.126.4.887. [DOI] [PubMed] [Google Scholar]
- 22.Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE. Hemangiopericytoma of the central nervous system : a review of 94 cases. Hum Pathol. 1991;22:84–91. doi: 10.1016/0046-8177(91)90067-y. [DOI] [PubMed] [Google Scholar]
- 23.Mira JG, Chu FC, Fortner JG. The role of radiotherapy in the management of malignant hemangiopericytoma : report of eleven new cases and review of the literature. Cancer. 1977;39:1254–1259. doi: 10.1002/1097-0142(197703)39:3<1254::aid-cncr2820390335>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Park BJ, Kim YI, Hong YK, Jeun SS, Lee KS, Lee YS. Clinical analysis of intracranial hemangiopericytoma. J Korean Neurosurg Soc. 2013;54:309–316. doi: 10.3340/jkns.2013.54.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitkethly DT, Hardman JM, Kempe LG, Earle KM. Angioblastic meningiomas; clinicopathologic study of 81 cases. J Neurosurg. 1970;32:539–544. doi: 10.3171/jns.1970.32.5.0539. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey HJ. Fine structure of hemangiopericytoma and hemangio-endothelioma. Cancer. 1966;19:2005–2018. doi: 10.1002/1097-0142(196612)19:12<2005::aid-cncr2820191227>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, et al. Intracranial hemangiopericytoma : clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012;118:1628–1636. doi: 10.1002/cncr.26411. [DOI] [PubMed] [Google Scholar]
- 28.Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma : long-term outcome revisited. Clinical article. J Neurosurg. 2011;114:747–755. doi: 10.3171/2010.6.JNS091660. [DOI] [PubMed] [Google Scholar]
- 29.Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma : the role of radiotherapy : report of 29 cases and review of the literature. Cancer. 2004;100:1491–1497. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 30.Staples JJ, Robinson RA, Wen BC, Hussey DH. Hemangiopericytoma--the role of radiotherapy. Int J Radiat Oncol Biol Phys. 1990;19:445–451. doi: 10.1016/0360-3016(90)90556-y. [DOI] [PubMed] [Google Scholar]
- 31.Stessin AM, Sison C, Nieto J, Raifu M, Li B. The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma-experience from the SEER database. Int J Radiat Oncol Biol Phys. 2013;85:784–790. doi: 10.1016/j.ijrobp.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Stout AP, Murray MR. Hemangiopericytoma : a vascular tumor featuring Zimmermann's pericytes. Ann Surg. 1942;116:26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundaram C, Uppin SG, Uppin MS, Rekha JS, Panigrahi MK, Purohit AK, et al. A clinicopathological and immunohistochemical study of central nervous system hemangiopericytomas. J Clin Neurosci. 2010;17:469–472. doi: 10.1016/j.jocn.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Vuorinen V, Sallinen P, Haapasalo H, Visakorpi T, Kallio M, Jääskeläinen J. Outcome of 31 intracranial haemangiopericytomas : poor predictive value of cell proliferation indices. Acta Neurochir (Wien) 1996;138:1399–1408. doi: 10.1007/BF01411118. [DOI] [PubMed] [Google Scholar]
- 35.Uemura S, Kuratsu J, Hamada J, Yoshioka S, Kochi M, Ushio Y, et al. Effect of radiation therapy against intracranial hemangiopericytoma. Neurol Med Chir (Tokyo) 1992;32:328–332. doi: 10.2176/nmc.32.328. [DOI] [PubMed] [Google Scholar]