Abstract
A compressive description of tropical milky white mushroom (Calocybe indica P&C var. APK2) is provided in this review. This mushroom variety was first identified in the eastern Indian state of West Bengal and can be cultivated on a wide variety of substrates, at a high temperature range (30~38℃). However, no commercial cultivation was made until 1998. Krishnamoorthy 1997 rediscovered the fungus from Tamil Nadu, India and standardized the commercial production techniques for the first time in the world. This edible mushroom has a long shelf life (5~7 days) compared to other commercially available counterparts. A comprehensive and critical review on physiological and nutritional requirements viz., pH, temperature, carbon to nitrogen ratio, best carbon source, best nitrogen source, growth period, growth promoters for mycelia biomass production; substrate preparation; spawn inoculation; different supplementation and casing requirements to increase the yield of mushrooms has been outlined. Innovative and inexpensive methods developed to commercially cultivate milky white mushrooms on different lignocellulosic biomass is also described in this review. The composition profiles of milky white mushroom, its mineral contents and non-enzymatic antioxidants are provided in comparison with button mushroom (Agaricus bisporus) and oyster mushroom (Pleurotus ostreatus). Antioxidant assay results using methanol extract of milky white mushroom has been provided along with the information about the compounds that are responsible for flavor profile both in fresh and dry mushrooms. Milky white mushroom extracts are known to have anti-hyperglycemic effect and anti-lipid peroxidation effect. The advantage of growing at elevated temperature creates newer avenues to explore milky white mushroom cultivation economically around the world, especially, in humid tropical and sub-tropical zones. Because of its incomparable productivity and shelf life to any other cultivated mushrooms in the world, milky white mushroom could play an important role in satisfying the growing market demands for edible mushrooms in the near future.
Keywords: Antioxidants, Flavor compounds, Medicinal value, Milky white mushrooms, Mineral composition, Neutraceuticals
INTRODUCTION TO MILKY WHITE MUSHROOMS
The world population is currently 7 billion and it is increasing at a faster rate. By the year 2050 the global population is expected to reach 9 billion and during 2100 it could be 20 billion [1]. Shortage of food and diminishing quality of human health will be growing concerns because of the population increase and urbanization, with a concomitant reduction in arable land. Converting lignocellulosic agricultural and forest residues into protein-rich mushrooms is one of the most economically viable and sustainable biotechnology processes to address world food demand, especially protein demand [2]. Consumption of edible fungi to fulfill human nutritional needs has been a common denominator in the history of mankind [3,4]. Since most of these edible mushrooms have favorable growth conditions at lower temperatures (< 25℃), creation of infrastructure for commercial cultivation, especially in warm humid tropical regions, is always expensive [5]. Identification and cultivation of warm-weather (30~38℃) varieties of edible mushrooms has been scientifically challenging. Milky white (Calocybe indica var. APK2) is one of such mushroom varieties (Fig. 1), where complete commercial production techniques have been standardized [6]. The first ever milky white mushroom variety (Calocybe indica P&C) var. APK2 was released from Tamil Nadu Agricultural University, Coimbatore, India during 1998. Over a decade, commercial production of this mushroom variety has assumed greater impetus in India, uplifting rural livelihood [6].
Fig. 1. Tropical milky white mushroom (Calocybe indica var. APK2).

The first report on wild occurrence of Calocybe indica P&C, commonly called "Dhuth chatta" (means "Milky white mushroom" originated from India). For several decades, people from West Bengal (Eastern Indian State) have collected these mushrooms and sold in local markets. Its milky white color and robust nature are appealing to consumers (Fig. 1) [7]. In nature, milky white mushrooms are seen grown on humus rich soil in agricultural fields or along the roadside in tropical and subtropical parts of India, especially in the plains of Tamil Nadu (South Indian State) and in Rajasthan (located in the western edge of India) [8]. These mushrooms grow every year between the months of May and August, which normally coincides with sufficient showers after a prolonged dry spell. C. indica is mainly a grassland species, saprophytic (organisms which obtain nutrients from dead organic matter) in nature and sometimes ectomycorrhizal (symbiotic relationship with root of some plants) with Cocos nucifera, Borassus flabellifer, Tamarindus indicus, and Peltophorum ferruginum. Detailed studies on preferential physiological and cultural requirements viz., pH, temperature, carbon and nitrogen source ratio (C : N), light and substrate requirement for growing C. Indica were reported [7,8,9]. Since this mushrooms is morphologically similar to Agaricus bisporus (button mushroom), it has been quite popular in southern Indian states and slowly getting popular in other countries (China, Malaysia, and Singapore). Small scale mushroom growers prefer to grow this tropical mushroom due to the following reasons: (1) ideally suited to warm humid climate (30~38℃; 80% to 85% humidity), (2) its longer shelf life without any refrigeration (can be stored up to 7 days at room temperature), (3) retains fresh look and does not turn brown or dark black like that of button mushrooms, (4) lesser contamination due to competitor molds and insects during crop production under controlled conditions, (5) infrastructure needed to grow this mushroom is very much affordable and cost of production is comparatively low, which means industrial production could be attractive, and (6) has a short crop cycle (7~8 wk) and good biological efficiency of 140% (140 kg fresh mushroom/100 kg dry substrate). Methods have been developed to cultivate this mushroom on commercial scale since late nineties [6]. This review mainly focuses on the various aspects of milky white mushroom cultivation (viz., growth conditions, casing requirements, yield on different substrates), proximate composition of milky white mushrooms, flavor producing ingredients, medicinal value of the mushroom and its future prospects with indications for certain researchable issues.
PHYSIOLOGY AND CULTURAL REQUIREMENTS
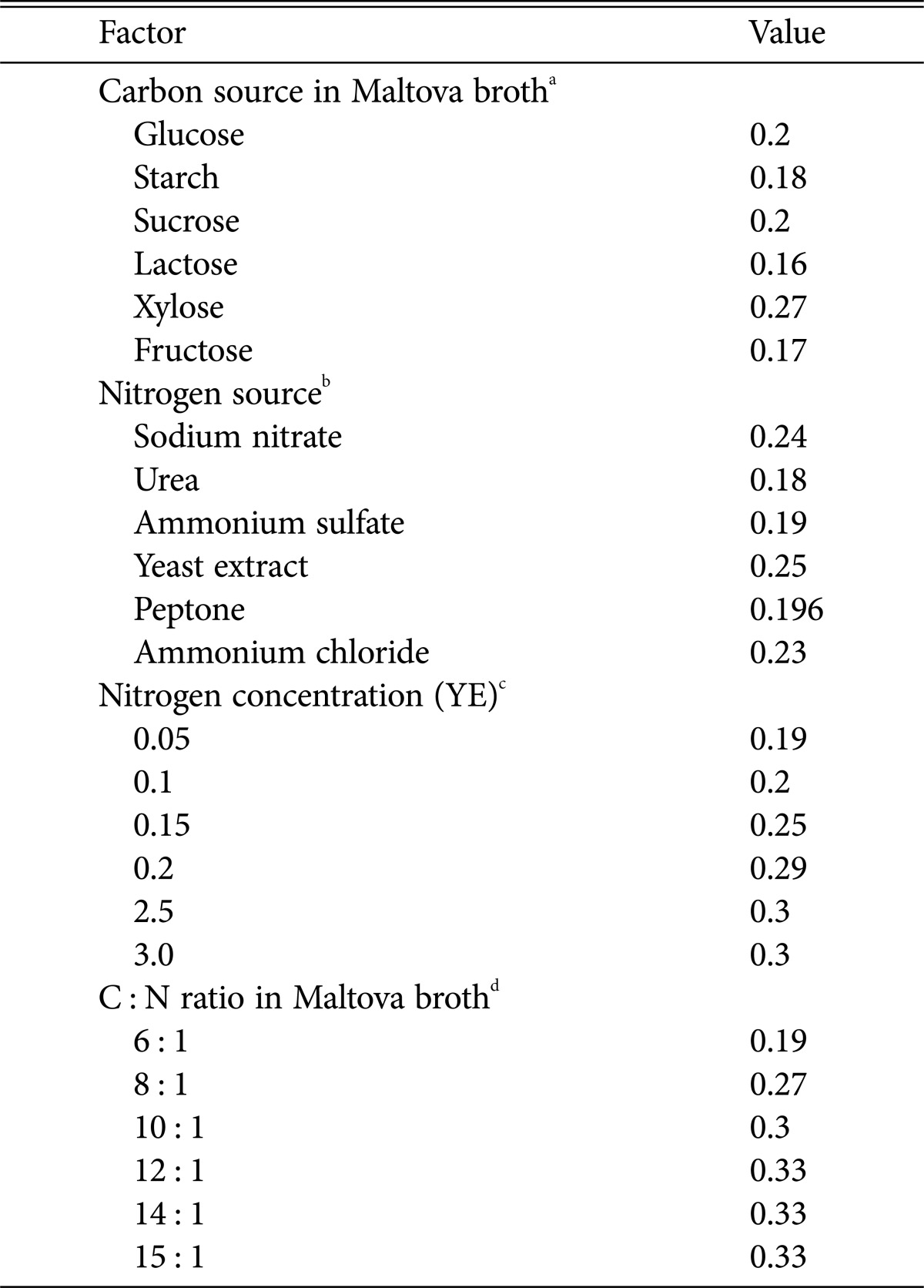
In general, mycelia growth profile and sporophore production of any cultivated mushroom is a function of time, temperature, pH, available C : N ratio, light, CO2 and O2 requirements during morphogenesis. Considerable attention has been given by various authors to understanding the optimal physiological and culture requirements of C. indica for mycelia growth, tissue culture, spawn production and cultivation [6,9,10,11]. The majority of the results indicate that the time required for maximum mycelia growth in culture media like potato dextrose agar or malt extract agar is 8 to 10 days. The pH requirement has been reported to have a wide optimal range, between 5.5 and 8.5. The optimum temperature for mycelia growth and mushroom production has been reported to be around 30~35℃ (Fig. 2). At temperatures below 25℃ or above 38℃ did not support the growth of C. indica. Cumulative results on growing mycelia are summarized in Table 1. As far as the mycelia production in liquid broth is concerned, potato dextrose broth yielded the maximum of 0.22 g in 100 mL of broth, while malt extract broth produced 0.19 g per 100 mL in 8 days at pH 6 when incubated at 30℃ [11]. Among the various water soluble carbon sources studied, including glucose, starch, sucrose, lactose, xylose, fructose and maltose, xylose gave the best results (0.27 g of biomass/100 mL). Similarly, among the different nitrogen sources tested (NaNO3, Urea, (NH4)2SO4, yeast extract, peptone, NH4Cl), yeast extract was the most beneficial to mycelial growth when combined with xylose (0.25 g of mycelial mat/100 mL). The optimum nitrogen concentration with yeast extract as nitrogen source was found to be 2.5% (0.32 g/100 mL); and the optimum C : N ratio that favored the maximum growth was found to be 12 : 1 (0.35 g/100 mL) (Table 1). Light intensity, duration and wavelength are the important components of sporophore initiation. This effect is known as tropic effect. It has long been known that low light intensity, or absence of light, may result in sporophores of curious shape, often with elongated stripes and poor pileus development. Purkayastha and Chandra [12] reported that cultures of C. inidica kept continuously in dark, showed no initiation of fruit bodies. But, when exposed to diffused light, elongation of stipe to a considerable extent occurred. Low light intensity of 800 lux and below favored spawn run. But, increased mushroom yield was obtained only at higher light intensity of about 1,600 lux (455 g per bed containing 500 g of paddy straw). According to the availability of light, the size of the mushroom also varied. At higher light intensity, the stipe length was significantly reduced, whereas pileus width increased substantially.
Fig. 2. Effect of physical factors on the growth of Calocybe indica mycelia growth in two different media (maltova broth [MB] and potato dextrose broth [PDB]). A, Effect of temperature on mycelia growth; B, Effect of pH on mycelia growth and mycelia growth as a function of time.
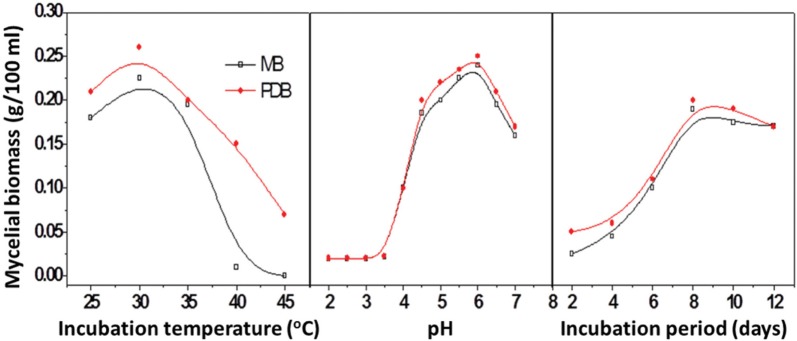
Table 1. Effect of carbon, nitrogen, concentration of nitrogen source and carbon to nitrogen ratio on the mycelia growth of Calocybe indica [16].
aCarbon source: Six synthetic carbon sources namely, glucose (control), starch, sucrose, lactose, xylose, fructose were used. Their equivalent quantities were replaced with glucose of the Maltova broth (glucose, 10.0; Maltova, 10.0; NaNO3, 1.5; KH2PO4, 0.5; MgSO4 · 7H2O, 0.2; KCl, 0.1; FeSO4 · 7H2O, 0.02). All ingredients are given in g/L.
bNitrogen source: Four different nitrogen sources replaced sodium nitrate (control) of maltova broth on the basis of equivalent quantities.
cNitrogen concentration: Six concentrations of yeast extract (YE) and sodium nitrate (best N sources), i.e., 0.05%, 0.1%, 0.15%, 0.20%, 0.25%, and 0.30% were tried using Maltova broth with 1% xylose.
dC : N ratio: the medium was prepared with different C : N ratios, 6 : 1 to 14 : 1, using yeast extract at 2.5 g (best concentration from previous experiment) and different amounts of xylose.
Spawn and spawning
Sorghum or wheat grains were found to be the best substrates for C. indica spawn production [12,13]. During preparation of the spawn culture, these substrates are half cooked in water for about 30 min and the excess water is usually drained before the grains are slightly air-dried and mixed thoroughly with 2 wt% calcium carbonate [14]. This wet substrate is then transferred to autoclavable polypropylene bags (usually 30 × 12 cm), which should filled up to 75% volume and sterilized at 1.42 kg/cm2 pressure for 2 hr. After cooling to ambient temperature, the bags should be aseptically inoculated with the mushroom mycelia, closed and incubated at 30℃. After 15 to 20 days of incubation, complete colonization of the substrate by the mushroom mycelia should be observed, meaning that they can be used for culture bed inoculation [15,16]. The age of spawn is an important factor that influences the flushing pattern and yield of milky white mushroom. An interesting study developed by Pani [17], who prepared spawns with wheat grains and stored for different periods (14~60 days), revealed that the best milky white mushroom yields were obtained using 21-day-old spawn. Prolonged storage of spawn reduced the productivity and total yield. Studies conducted on the amount of grain spawn to wet substrate by various authors revealed that 2% spawning is good for the best spawn run and crop production. Any further increase in the inoculum showed only marginal improvements in mushroom yield. Layer spawning reduced the colonization time in the substrate (15 days) as compared to through spawning (more than 20 days).
SUBSTRATES AND SUPPLEMENTS
A variety of substrates were tested for the cultivation of C. indica [12,18], but, with limited success. These authors tried to induce fruit bodies in a number of growth media, including soil-sand, soil-sand-maize meal and soil-sandpulse powder. In soil-sand-meal, primordial fungus appeared in 5~6 wk, but another 2~3 wk were required to have a matured well differentiated fruiting body (stipe 4.2 cm long; pileus 4.0 cm dia. and fresh weight 6.11 g). Later attempts were made to develop suitable, low cost synthetic compost for the production of more fruit bodies. By 1981, it had become possible to grow C. indica on unsterilized, paddy straw-maize or wheat bran substrate [19]. Purkayastha [8] used chopped rice straw, pre-soaked for 18 to 24 hr in water and put in hot water for 2~3 hr. This substrate was filled in trays and seeded with spawn. Doshi and his group evaluated wheat straw, maize stalks, sorghum stalks, maize meal, rice meal, sorghum meal, and wheat bran as basal substrates for the production of C. indica [20,21]. The results indicated that wheat straw was the best substrate for fruit body production. Addition of different supplements to the substrate also influences the spawn run, days for pinning, number of pinhead initiation, flushing pattern and overall mushroom yield. Krishnamoorthy [22] concluded that substrates like paddy straw and sorghum stalks were colonized more quickly by the milky white mushroom fungus compared to black gram hay, soybean hay, maize stalks and finger millet straw [6]. The study also indicated that substrates like coconut coir pith compost, paddy straw compost and saw dust did not favor the growth of C. indica. In addition, supplementation of paddy straw with neem (Azadirachta indica) cake, black gram husk and red gram husk, followed by cotton seed cake (5 wt% of the wet weight of the substrate), significantly improved the yield of milky white mushrooms (300~380 g increase over control). Interestingly, the average weight of individual sporophores (spore-producing hyphae, which may be loosely arranged) was always found to be high when the beds were supplemented with black gram husk, red gram husk and neem cake. While, minimum weight of individual mushrooms was recorded in the sporophores harvested from beds that were supplemented with wheat bran.
Several other lignocellulosic agricultural residues like sunflower stalks and hulled head, jackfruit rind, cotton stalks, waste cotton, sugar cane bagasse, jute waste, corn cobs, groundnut hulls, coffee/tea waste have also been tried by many workers with limited success [23,24,25,26]. In a separate study, Kumar et al. [27] evaluated 11 different supplements viz., wheat bran, soybean flour, pigeon pea powder, green gram powder, cotton cake, mustard cake, neem cake and lentil powder. Alam et al. [28] have used 30% maize powder to supplement paddy straw substrate in order to increase mushroom yields. More promisingly, supplements like soybean and cotton seed cake gave the highest absolute mushroom yields (64.8% and 59.2% increased biological efficiency over control).
Milky white mushroom production
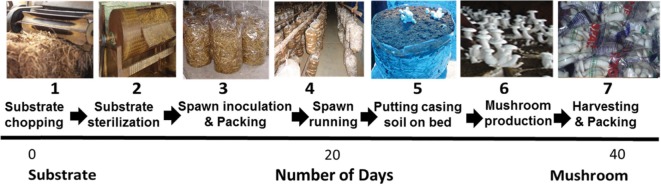
Currently, the milky white mushroom farming is done manually. The cultivation process is labor intensive and fairly energy demanding. The crop production process involves six different steps viz., (1) spawn production, (2) substrate pretreatment, (3) mushroom bed preparation, (4) cropping room maintenance during spawn run and mushroom production, (5) harvesting and packaging, and (6) management of spent mushroom substrate (Fig. 3). Farmers normally use milled paddy straw as substrate (2~4 cm) The milled straw is soaked in water for 4~5 hr, prior to hot water (80℃) or steaming treatment for 45~60 min. After pre-treatment, the materials are shade dried to get appropriate moisture condition (60~70%) before bed preparation. Polypropylene bags (60 × 30 cm) are normally used as containers for bed production and layer spawning with grain spawn is typically the adopted technique. For spawn run, the bags are kept in clean rooms maintained at 25~30℃ and 80~85% humidity for 15~20 days. At this stage, steam-treated casing soil is applied on half-cut beds to a depth of 1.5~2.0 cm. The beds are then transferred to cropping rooms (polythene sheet covered rooms) maintained at 30~35℃, with humidity of higher than 80%. Sufficient natural light should be made available inside the cropping room. Pin heads will appear on the casing surface within a week and the mushrooms attain harvesting maturity in a couple of days. The first flush of mushrooms will normally appear within 24~30 days of bed preparation. Over a period of 40~45 days, mushrooms could be harvested in three to four flushes [6].
Fig. 3. Different processing steps involved in milky mushroom production.
Several cases of less organized cultivation in thatched houses or under tarpaulin roofs surrounded by brick walls have also been reported with limited success [29]. These sheds are not properly insulated and the growth requirements, including temperature and relative humidity, could fluctuate depending on the external environment. A one-year study on the yield of milky white mushrooms in such sheds, using rice straw as substrate, showed the best performance during the months of May and June (peak summer season in South India) [27]. Temperature ranges of less than 25℃, degenerated the mushroom growth. Other hindrances for milky mushroom production include over-matured spawn, insect infestations, contaminant fungi and bacteria due to unhygienic conditions and poor farm maintenance.
Casing requirements
Casing is an important agronomic practice in the cultivation of any humicolous mushroom (that grow on soil) and milky white mushroom is not an exception. Casing triggers off the change from vegetative to reproductive phase. Compact casing interfaces impede the diffusion of harmful metabolic gases on mushroom bed surface. Thus accumulation of high concentrations of carbon dioxide in the soil during fructification usually results in yield depression [30]. Smerdon defined clearly the qualities of casing soil and said that it should have a high water holding capacity, besides retaining a good air space ratio to facilitate gaseous exchange [31]. They also concluded that the pH of such soil must be neutral to alkaline. Singh et al. [32] concluded that steam sterilized casing soil produced better yield than the chemically treated with formalin or using heat sterilization.
Casing was found to be an absolute requirement for proper fructification in C. indica by several workers. Purkayastha [8] used loamy soil or garden soil and sand (1 : 1) mixed thoroughly with calcium carbonate at 12% level (pH 7) on milky white mushroom beds. Krishnamoorthy et al. [6,10] have concluded that partially steamed clay loam soil (pH 8.4) generates maximum yields and a higher number of buttons than other media, including peat soil, sand, biogas slurry, farm yard manure and coir pith compost. In sandy soil and farm yard manure, the fungus took more than 10 days for the production of pinheads and attained harvesting maturity after 10.6 and 9.2 days, respectively. In clay loam soil and peat, the buttons appeared almost 2 days earlier when compared to all other casing media tried. Interestingly, the clay loam soil had the quality to absorb moisture quickly and release it slowly. In this soil, less water was needed to maintain the required moisture level and a delay in spraying did not lead to the total drying of bed surface. Using vermin-compost as casing substrate was also reported with limited success. In addition to its composition, pH, EC, water holding capacity, porosity and bulk density of casing mixture are some of the important factors to be considered while selecting substrates for casing [34].
Role of growth regulators in crop production
Several growth regulators like indole acetic acid, indole-3-butryic acid, gibberllic acid (GA), and kinetin were tested for their effect on the sporophore size and yield of milky white mushroom [35]. The results clearly indicated that GA at 40 ppm increased the yield of sporophores (Table 2). The average weight of individual mushrooms was found to be high when GA and kinetin were sprayed. Pileus (cap of mushroom) diameter and its weight were found to be slightly higher in the above treatments, but none of the growth regulators showed a significant influence on the stipe length and its weight. Pani has also reported that spraying GA at 50 ppm at the time of pinhead formation had greatly influenced the size of sporophores and yield of milky white mushrooms (82% as compared to 68% in control) [35].
Table 2. List of growth regulators and their structure that increase milky white mycelia and mushroom yield [15,33].
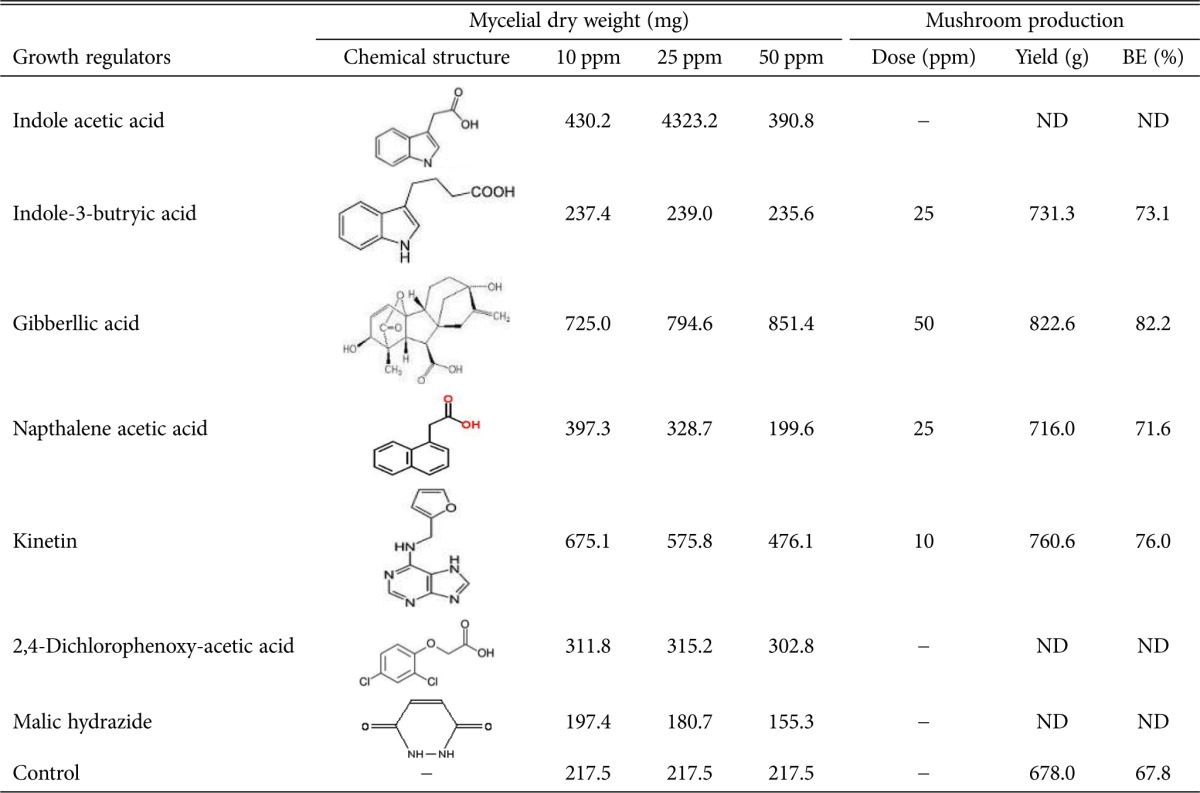
BE, Biological Efficiency; ND, not detected.
CHEMICAL COMPOSITION AND NUTRITIONAL VALUE
The six major constituents of mushrooms are water, proteins, carbohydrates, dietary fiber, fat, and ash [33]. The moisture content of mushrooms is usually determined by drying at 105℃ in a hot air oven overnight to a constant weight. The difference in weight before and after drying is expressed in terms of percentage. The protein content is determined by Kjeldahl method and the lipids are estimated by Twisselman method using extractive solvent like diethyl ether [36]. The lipids in mushrooms include free fatty acids, mono-, di-, and triglycerides, sterols, sterol esters and phospholipids. The sporophore samples are incubated in a muffle furnace at 500℃ to estimate the ash content which normally contains potassium and phosphorous. Total carbohydrate content in a given mushroom sample is calculated using the formula, 100 - moisture (%) - protein (%) - crude fat (%) - ash (%) and expressed as g/100 g of fresh or dry sample [37]. According to Crisan and Sands, the energy content in mushrooms is influenced by the composition of crude protein, fat and carbohydrates whose conversion factors are 2.62, 8.37, and 3.50 kcal/g of the individual components, respectively [38]. These conversion factors are slightly lower than the actual conversion factors used for other food ingredients because they are estimated as crude components.
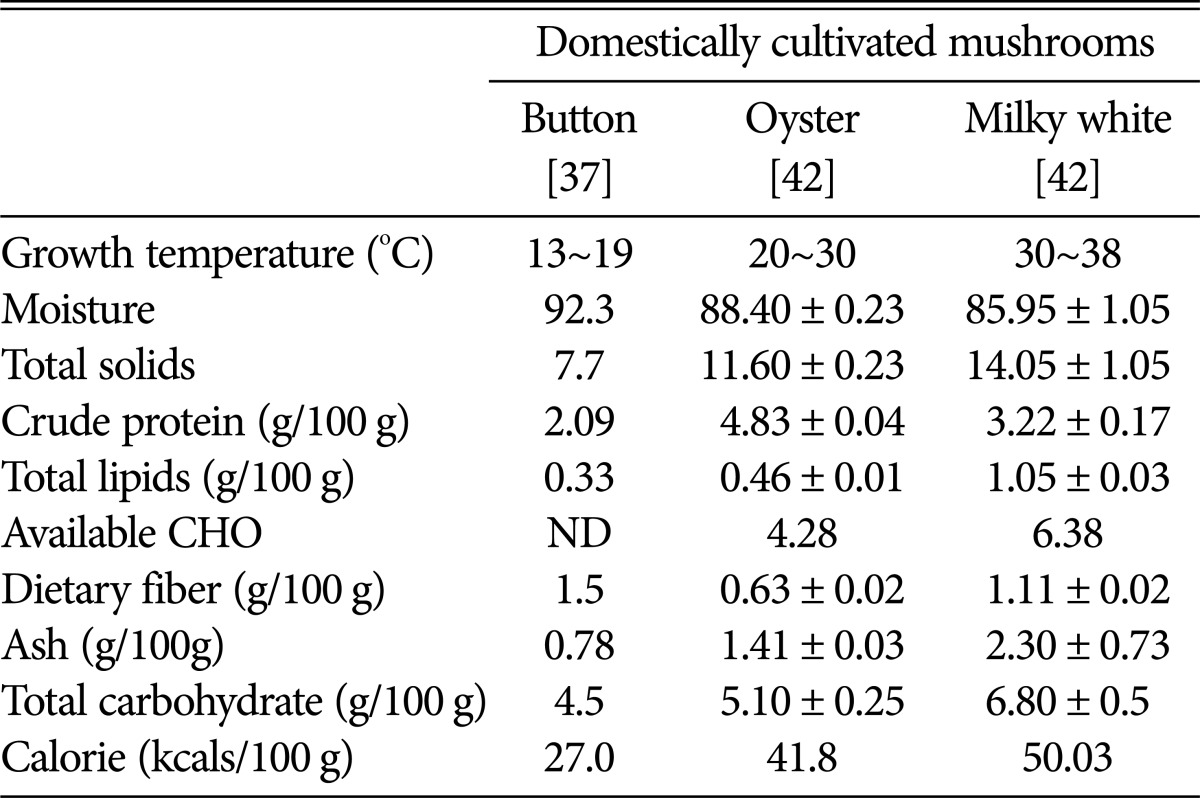
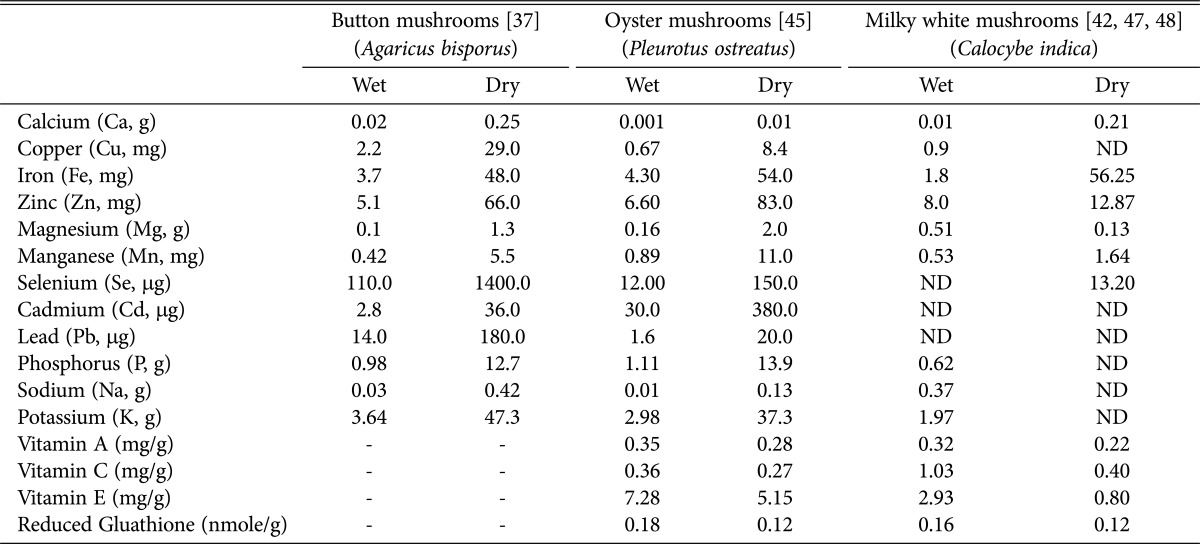
Sivaprakasam and Doshi et al. [38,39], recorded 20.2% protein from the caps of milky white mushroom (on dry weight basis). Krishnamoorthy et al. [6] reported 32.2% protein (dry weight basis) in a medium sized milky mushroom. Interestingly, among the three mushrooms viz., button, oyster, and milky white mushrooms, the latter has been reported by several authors to contain more protein [6]. Milky white mushroom also has lower moisture content (85.6%) and increased fiber content (61.1% on dry weight basis). Total carbohydrates and crude fat were also found to be more abundant in milky mushrooms (59.9% and 0.67%, respectively) when compared to oyster mushroom varieties (P. eous and P. sajor caju). These differences could be due to variations in mushroom culture conditions, time of sampling and substrates used. Saranya et al. [40] have reported that the type of substrates and supplements used for mushroom cultivation had greatly influenced the proximate composition (carbohydrate, protein, fat, fiber, ash content, and moisture content) including antioxidants. Based on the availability of soluble sugars. These authors also reported increased levels of calcium, phosphorus and iron in the milky white mushroom. Similarly, total lipids, carbohydrates and ash contents in milky white mushroom were also found to be comparatively higher, ultimately increasing its total calorific value (50.03 kcal/100 g). The dietary fiber (fungal cell wall components mostly, chitin-N-acetyl-glycosamine units) and protein contents of milky white mushrooms are higher than button mushroom, but lower than oyster mushroom (Table 3). It has been reported that chitosan from C. indica sporophores ranged from 2.5% to 2.9% on dry weight basis [41]. In case of P. florida, an oyster mushroom species, it ranged from 2.0% to 2.3%. The FTIR spectrum of the fungal chitosan obtained from C. indica, P. florida and shrimp chitosan exhibited eight major peaks at the ranges of 3,000~3,500/cm, 3,426/cm, 2,885/cm, 1,650/cm, 1,589/cm, 1,400~1,650/cm, 1,326/cm, 1,080/cm and revealed a strong structural similarity among them. The beta-glycans present in dietary fibers of mushrooms are reported to have stimulatory effect on immune system with anti-mutagenic, anticancer and antitumor activities [37]. Mushrooms are good sources of minerals (Ca, K, Mg, Na, and P), trace elements (Cu, Fe, Mn, and Zn) and sometimes, toxic heavy metals (Cd and Pb) as compared to vegetables [43]. The mineral components of milky white mushrooms [44,45], as reported in the literature are given in Table 4.
Table 3. Composition of fresh milky white mushroom and comparison with two other domestically cultivated mushrooms (button and oyster).
Table 4. Comparison of mineral and non-enzymatic reducing compounds composition milky white mushroom with two other widely popular mushrooms (white button and oyster) both in fresh and dry form (/100 g).
ND, not detected.
NON-ENZYMATIC ANTIOXIDANT
Several chronic diseases like rheumatoid arthritis, cirrhosis and life threatening diseases like cancer are caused due to reactive oxygen species and free radicals. Enzymes like superoxide dismutase, catalase and chemicals compounds like vitamin E, C, polyphenols, carotenoids, and glutathione play important role in neutralizing free radicals. Mushrooms are a good source of some of these biologically active compounds that protect the human body against several chronic and degenerative diseases. Most of the mushrooms are rich in vitamins and minerals, particularly, B complex vitamins (thiamine, riboflavin, pridoxine, pantotene acid, nicotinic acid, nicotinamide, folic acid, and cobalamin); as well as ergosterol and biotin, vitamin A in fresh and dry milky white mushrooms have been reported to be 0.35 mg and 0.275mg per g respectively (Table 4) [46]. Water soluble vitamin C (a free radical scavenger and a well-known antioxidant and inhibitor of lipid peroxidation [LPO]) has been reported in fresh and dry milky white mushrooms (1.03 and 0.4mg/100 g, respectively) [42]. Similarly, vitamin E (tocopherol), an antioxidant that protects membranes, lipids and lipoproteins [43] has also been reported in fresh and dry milky white mushroom samples (2.8 mg/g and 0.80 mg/g, respectively). The most abundant non-protein thiol (organic chemical compounds similar to the alcohols and phenols but containing a sulfur atom in place of the oxygen atom) in animal cells is glutathione. Glutathione exist is both reduced (GSH) and oxidized (GSSG) state. GSH will be able to donate a reducing equivalent (H+ + e-) to other unstable molecules, such as reactive oxygen species and at high concentration they self-react and form GSSG. GSH can be regenerated from GSSG by the enzyme glutathione reductase [47]. The GSH is essential for protein and DNA synthesis, regulation of enzyme activities and protection against free radicals [42]. Presence of GSH in fresh and dry milky white mushroom samples were found to be 0.15 and 0.025 nmole/g respectively. The vitamin C content in milky white mushroom was higher than oyster mushroom, which contains higher levels of vitamin E [42,48]. In general, fresh mushrooms contain more antioxidants compared to dried samples.
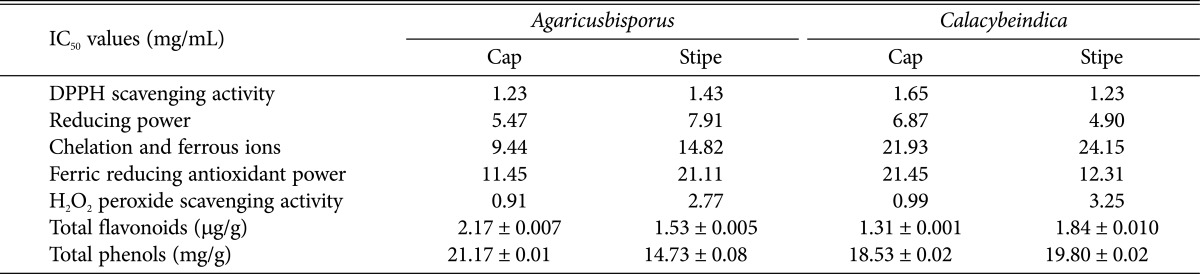
Mahfuz and co-workers used methanolic extract of commercially cultivated mushrooms to quantify the amount of antioxidants [49]. Some other interesting in vitro antioxidant studies include (1) 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity; (2) ferrous ion chelating activity; (3) reducing power using potassium fericyanide and ferric chloride mixture and measuring absorbance at 700 nm; (4) reduction of ferric tripyridyltriazene (Fe3+-TPTZ) complex to ferrous (Fe2+) ion determined by measuring absorbance at 595 nm; (5) hydrogen peroxide scavenging activity using luminescence spectrophotometer; (6) estimation of phenolic contents; and (7) estimation of flavonoids. Similarly, methanol extracts of cap and stem of C. indica have been reported [50] for the presence of antioxidants. Comparative estimates of antioxidant properties of methanolic extracts of A. bisporus and C. indica are presented in Table 5. Mirunalini et al. [48] and Babu and Rao [50], have reported in vitro antioxidant activities of C. indica extracts. The results showed that the DPPH scavenging activity, reducing power, chelation, and hydrogen peroxide scavenging activity were higher in C. indica when compared to Agaricus bisporus. Interestingly, the stipe of C. indica exhibited more chelation, hydrogen peroxide scavenging activity, flavonoid and total phenolic contents as compared to its cap.
Table 5. Various antioxidant assay results using methanol extract [47,49].
DPPH, 2,2-diphenyl-1-picrylhydrazyl.
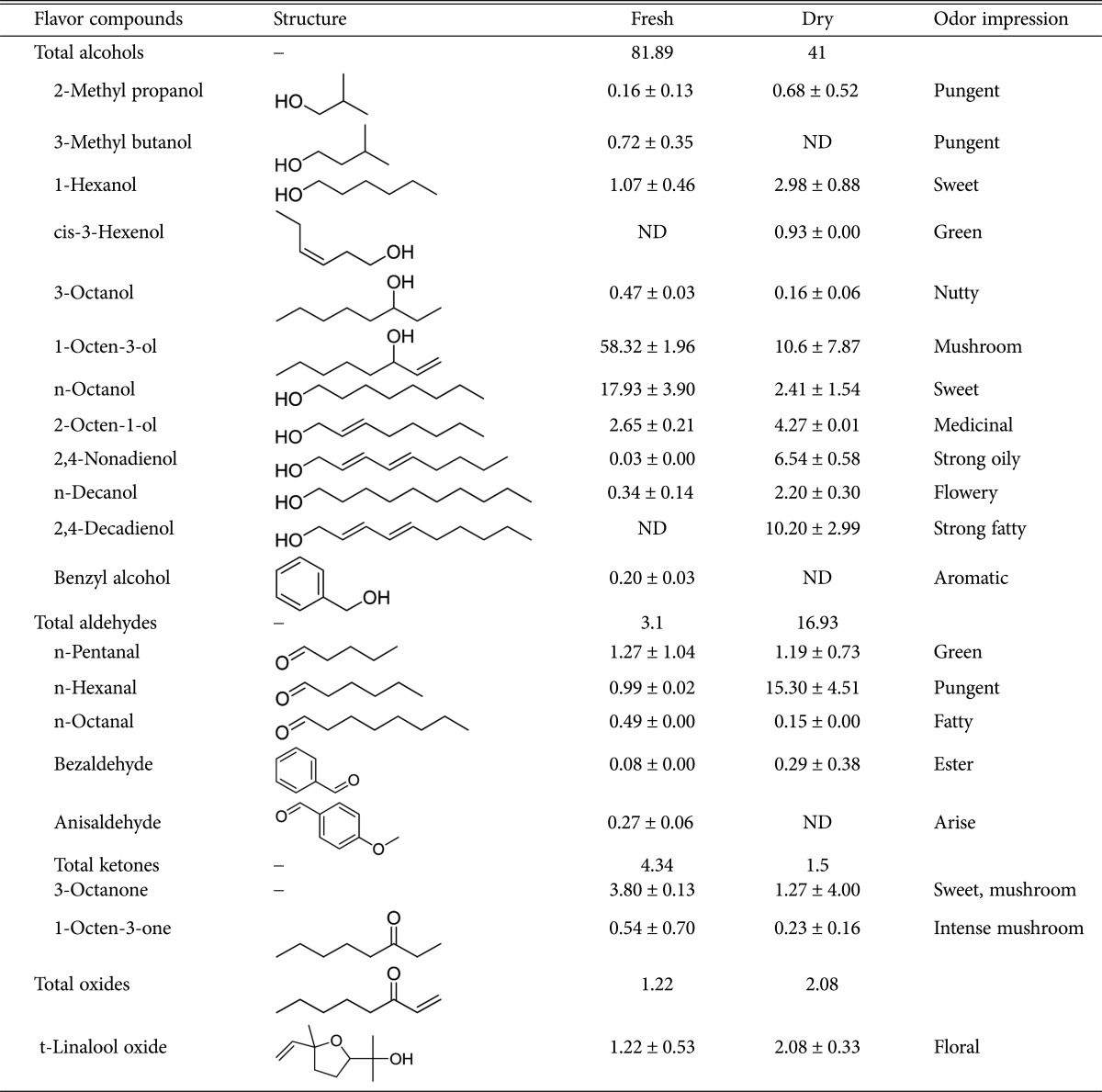
VOLATILE AND FLAVOUR COMPOUNDS
Mushrooms are known to produce a wide range of volatile and flavor compounds with a distinctive profiles that vary according to species, variety and sometimes cultural conditions [52,53]. The flavor profile also changes when mushrooms are dried, primarily due to the high level of oxidation [54]. Usually, these compounds are extracted using a combination of polar and non-polar solvents including water and diethyl ether. Apart from this, vacuum distillation, nitrogen flow conveyance, capillary gas chromatography and use of carbon tetrachloride are also followed routinely and the concentration of the flavor compounds significantly change depending upon the extraction method [53]. Most of these flavor compounds include alcohols, aldehydes, ketones and oxides [55,56,57]. In C. indica, a total of 20 compounds have been identified as listed [58] in Table 6. Two of the most abundant compounds present in fresh C. indica sporophores include eight carbon containing volatiles like 1-octen-3-ol (58.3%) and n-octanol (17.9%) of the total volatile fractions. The concentration of these compounds decreased to 10.6% and 2.4% respectively after drying [58]. In addition, eight carbon volatiles including 1-octen-3-one, 3-octanone and 3-octanol have been also reported in several mushrooms, which are normally produced due to the enzymatic interaction of hydro-peroxide lyase utilizing fatty acids, more specifically linoleic acid as precursor. Noticeably, benzyl alcohol and n-Hexanal present in trace amounts in fresh mushrooms, increased to about 10.2% and 15.3%, respectively, after drying. Also, during the process of drying, total alcohol content decreased from 81.9% to 41%, while the concentration of aldehydes increased from 3.1% to 16.9%. In addition, drying decreased the total ketone levels from 4.3% to 1.5% and increased the oxide concentration from 1.2% to 2.1%. The number of unknown compounds is substantially higher in dried mushrooms as compared to fresh ones.
Table 6. Volatile flavoring compounds present in milky white mushrooms [51].
ND, not detected.
OTHER MEDICINAL PROPERTIES
Nutritional quality of mushroom is influenced by the substrate used, organic supplementation and other additive effect [51,59]. Medicinal mushrooms are known to be abundant source of nutraceuticals which could decrease/reverse the progression of several diseases. One such disease is diabetes mellitus, which is otherwise characterized as hyperglycemia associated with insulin deficiency. Complication of this disease includes hypertension, atherosclerosis, microcirculatory disorder and changes in large and small blood vessels. Along with medicinal herbs, mushrooms are believed to play an important role in treating diabetic patients without any harmful side effect. Both cold and hot water extracts of milky white mushroom powder was tested for anti-hyperglycemic effect on diabetes induced rats (using streptozotocin) [60]. The rats were orally given with mushroom extract for 45 days and tested for insulin and glycosylated hemoglobin levels. The results indicated normal values at the end of treatment. In addition, positive effect on hematological parameters including increase in lymphocytes, platelets and red blood cell (RBC) counts were observed. The life span of treated rats was also found to be increased. LPO inhibition is a process that deteriorates polyunsaturated lipids to release several toxic compounds through oxygen free radicals, which are otherwise known to induce cellular damage [61]. In an interesting study, Selvi et al. [61] used two different membrane model systems viz., goat liver homogenate and RBC ghost, which have different lipid compositions; and C. indica mushroom extract to evaluate LPO inhibition. A higher level of LPO inhibition (71.3%) was observed for RBC ghost when compared to goat liver homogenate (59%).
CONCLUSIONS
Worldwide mushroom production technology has been emerging as a multibillion dollar industry [3,4,62]. Current world mushroom production is about 30.2 million tons and (worth $ 2,800 billion). Mushroom production in India for the year 2013 is roughly 40 thousand tons contributing approximately 1% of total world production [5]. Milky white mushrooms are highly suitable for commercial production in humid tropical and subtropical regions of the world where, the average temperature falls between 25℃ and 35℃ throughout the year [63]. Apart from India, several regions in Africa, North America, South America, Middle East, South East Asia, and Australia where the cost of labor is also comparatively cheaper are suitable for growing milky white mushrooms. In the tropical region, infrastructure required (cooling and heating) to grow milky white mushroom is comparatively less expensive when compared to button mushroom production. Since milky white mushroom resembles button mushroom in several aspects, with higher shelf life, increased productivity, appealing milky white color, it will have greater stake hold in the world market. The mushrooms are robust and flexible for production in varied sizes from a small button (average weight, 35~40 g) to large caps depending upon consumer demand, which is normally not possible in any other cultivated mushrooms around the world. The demand statistics, indicate a 3~5% increase in world mushroom consumption every year; and milky white mushroom cultivator could explore this opportunity to establish newer markets in developed countries like US and Europe where, the demand for mushrooms is quite high [7]. Industrialization of the new concept by mechanization and controlled environment production will be required to further reduce the cost of production and increase profit margins to mushroom producers. Mass production of mushrooms will pay way to produce processed mushrooms (dry, canned, pickled, and fried) will increase the shelf life that could be marketed in different regions of the world.
ACKNOWLEDGEMENTS
We would like to thank the Department of Chemical Engineering and Material Science, Michigan State University and Tamil Nadu Agricultural University, Coimbatore, India for their support in publishing this review. The authors would like to thank Dr. Suresh Waghmode, University of Pune, India for helping to draft some of the figures and table of this manuscript. The authors like to thank Dr. Dushyanthi Hoole and Dr. Leonardo da Costa Sousa for revising the manuscript.
References
- 1.Livi-Bacci M. A concise history of world population. 5th ed. Malden (MA): Wiley-Blackwell; 2012. [Google Scholar]
- 2.Hawksworth DL. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res. 1991;95:641–655. [Google Scholar]
- 3.Chang ST. The world mushroom industry: trends and technological development. Int J Med Mushrooms. 2006;8:297–314. [Google Scholar]
- 4.Wakchaure GC. Production and marketing of mushrooms: global and national scenario. In: Singh M, Vijay B, Kamal S, Wakchaure GC, editors. Mushrooms: cultivation, marketing and consumption. Solan (HP): Directorate of Mushroom Research; 2010. pp. 15–22. [Google Scholar]
- 5.Thakur MP, Singh HK. Advances in the cultivation technology of tropical mushrooms in India. JNKVV Res J. 2014;48:120–135. [Google Scholar]
- 6.Krishnamoorthy AS, Muthuswamy MT, Nakkeeran S. Technique for commercial production of milky mushroom Calocybe indica P&C. Indian J Mushrooms. 2000;18:19–23. [Google Scholar]
- 7.Vikineswary S, Chang ST. Edible and medicinal mushrooms for sub-health intervention and prevention of lifestyle diseases. Tech Monitor. 2013:33–43. [Google Scholar]
- 8.Purkayastha RP. Cultivation of Calocybe indica (P&C) Indian J Mushrooms. 1984-1985:10–17. [Google Scholar]
- 9.Doshi A, Sidana N, Chakravarti BP. Cultivation of summer mushroom Calocybe indica (P&C) in Rajasthan. Mushroom Sci. 1989;12:395–400. [Google Scholar]
- 10.Krishnamoorthy AS. Studies on cultivation of milky mushroom (Calocybe indica P&C) Coimbatore: Tamil Nadu Agriculture University; 1995. [Google Scholar]
- 11.Trivedi A, Sharma SS, Doshi A. Cultivation of Calocybe indica under semi-arid conditions. In: Nair MC, editor. Indian mushrooms. Proceedings of the National Symposium on Mushroom. Vellanikkara: Kerala Agricultural University; 1991. pp. 166–169. [Google Scholar]
- 12.Purkayastha RP, Chandra AA. A new technique for in vitro production of Calocybe indica as edible mushroom from India. Mushroom J. 1976;40:112–113. [Google Scholar]
- 13.Krishnamoorthy AS, Muthuswamy M. Yield performance of Calocybe indica (P&C) on different substrates. Mushroom Res. 1997;6:29–32. [Google Scholar]
- 14.Theradimani M, Meena B, Krishnamoorthy AS. Innovative techniques for improvement of sporophore size and yield of milky mushroom (Calocybe indica) Mushroom Res. 2001;10:23–26. [Google Scholar]
- 15.Phutela UG, Phutela RP. Effect of physical and chemical factors on growth of Calocybe indica (P & C) Int J Adv Life Sci. 2012;2:8–16. [Google Scholar]
- 16.Pandey M, Lakhanpal TN, Tewari RP. Studies on spawn production of Calocybe indica. Indian J Mushrooms. 2000;18:15–18. [Google Scholar]
- 17.Pani BK. Effect of age and quantity of spawn on milky mushroom production. Asian J Exp Biol Sci. 2011;2:769–771. [Google Scholar]
- 18.Purkayastha RP, Nayak D. A new method of cultivation of Calocybe indica: an edible mushroom. Taiwan Mushrooms. 1979;3:14–18. [Google Scholar]
- 19.Purkayastha RP, Nayak D. Analysis of protein patterns of an edible mushroom by gel-electrophoresis and its amino acid composition. J Food Sci Technol. 1981;18:89–91. [Google Scholar]
- 20.Doshi A, Sharma SS, Trivedi A. A promising edible mushroom for the tropics Calocybe indica P. & C. Mushroom Info. 1993;86:14–22. [Google Scholar]
- 21.Doshi A, Sharma M. Prospects of cultivation of specialty mushrooms in Rajasthan, India. Mushroom Biol Biotechnol. 2007;213:293. [Google Scholar]
- 22.Krishnamoorthy AS. Commercial prospects of milky mushroom (Cotocybe indica) on tropical plains of India. In: Upadhyay RC, Singh SK, Rai RD, editors. Current vistas in mushroom biology and production. Solan (HP): Mushroom Society of India; 2003. pp. 131–135. [Google Scholar]
- 23.Amin R, Khair A, Alam N, Lee TS. Effect of different substrates and casing materials on the growth and yield of Calocybe indica. Mycobiology. 2010;38:97–101. doi: 10.4489/MYCO.2010.38.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhakara MN, Mallesha BD. Comparative study on growth and yield performance of milky mushroom (Calocybe indicia) on different substrates. Mysore J Agric Sci. 2010;44:89–91. [Google Scholar]
- 25.Sharma SK, Lall AM. Non-enzymatic antioxidant expression and nutritional composition of Calocybe indica under different organic supplementations. J Cell Tissue Res. 2013;13:3541–3544. [Google Scholar]
- 26.Singh M, Singh AK, Gautam RK. Screening of substrates for growth and yield of Calocybe indica. Indian Phytopathol. 2009;62:109–111. [Google Scholar]
- 27.Kumar R, Singh G, Mishra P, Singh R. Effect of different organic supplements and casing mixtures on yield of two strains of milky mushroom (Calocybe indica) Indian Phytopathol. 2012;65:399–403. [Google Scholar]
- 28.Alam N, Amin R, Khair A, Lee TS. Influence of different supplements on the commercial cultivation of milky white mushroom. Mycobiology. 2010;38:184–188. doi: 10.4489/MYCO.2010.38.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raja RE, Ganesh P. Environmental factors influence on yield variation of Indian white summer mushroom (Calocybe indica) Int J Pharm Biol Arch. 2012;3:1544–1546. [Google Scholar]
- 30.MacCanna C. Spawned casing. Mushroom J. 1983;129:329–333. [Google Scholar]
- 31.Smerdon M. Thoughts on casing. Mushroom J. 1983;124:193–194. [Google Scholar]
- 32.Singh RN, Bhandari TP, Kanaujia JP. Effect of different casing media on the yield of button mushroom. Indian Phytopathol. 2007;38:502–506. [Google Scholar]
- 33.Reis FS, Barros L, Martins A, Ferreira IC. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms an inter-species comparative study. Food Chem Toxicol. 2012;50:191–197. doi: 10.1016/j.fct.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Yadav RS. Use of vermin products in the cultivation of milky mushroom (Calocybe indica) [dissertation] Dharwad: College of Agriculture; 2006. [Google Scholar]
- 35.Pani BK. Influence of some growth regulators on biomass production and sporophore yield of milky mushroom (Calocybe indica) Asian J Exp Biol Sci. 2011;2:328–331. [Google Scholar]
- 36.Mattila P, Salo-Väänänen P, Könkö K, Aro H, Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem. 2002;50:6419–6422. doi: 10.1021/jf020608m. [DOI] [PubMed] [Google Scholar]
- 37.Crisan EV, Sands A. Nutritional value. In: Chang ST, Hayes WA, editors. The biology and cultivation of edible mushrooms. New York: Academic Press; 1978. pp. 137–165. [Google Scholar]
- 38.Sivaprakasam K, Balasubramanian T, Sadasivam S, Shanmugam N. Nutritive values of sporophores of Calocybe indica. Mushroom Newslett Trop. 1986;6:14–15. [Google Scholar]
- 39.Doshi A, Munot JF, Chakravarti BP. Nutritionnel status of an edible mushroom Calocybe indica P.&C. Indian J Mycol Pathol. 1988;18:3301–3302. [Google Scholar]
- 40.Saranya V, Madhanraj P, Panneerselvam A. Cultivation, composting, biochemical and molecular characterization of Calocybe indica (C and A) Asian J Pharm Res. 2011;1:55–57. [Google Scholar]
- 41.Ragul M. Exploration of antimicrobial potentials of fungal chitosan and secondary metabolites against soil borne plant pathogens [dissertation] Coimbatore: Tamil Nadu Agricultural University; 2013. [Google Scholar]
- 42.Selvi S, Devi PU, Suja S, Murugan S, Chinnaswamy P. Comparison of non-enzymic antioxidant status of fresh and dried form of Pleurotus florida and Calocybe indica. Pak J Nutr. 2007;6:468–471. [Google Scholar]
- 43.Mattila P, Suonpää K, Piironen V. Functional properties of edible mushrooms. Nutrition. 2000;16:694–696. doi: 10.1016/s0899-9007(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 44.Mattila P, Könkö K, Eurola M, Pihlava JM, Astola J, Vahteristo L, Hietaniemi V, Kumpulainen J, Valtonen M, Piironen V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J Agric Food Chem. 2001;49:2343–2348. doi: 10.1021/jf001525d. [DOI] [PubMed] [Google Scholar]
- 45.Zahid MK, Barua S, Haque SM. Proximate composition and mineral content of selected edible mushroom varieties of Bangladesh. Bangladesh J Nutr. 2010;22-23:61–68. [Google Scholar]
- 46.Alam N, Amin R, Khan A, Ara I, Shim MJ, Lee MW, Lee TS. Nutritional analysis of cultivated mushrooms in Bangladesh: Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology. 2008;36:228–232. doi: 10.4489/MYCO.2008.36.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Bakel MM, Printzen G, Wermuth B, Weismann UN. Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am J Clin Nutr. 2000;72:976–981. doi: 10.1093/ajcn/72.4.976. [DOI] [PubMed] [Google Scholar]
- 48.Mirunalini S, Dhamodharan G, Deepalakshmi K. Antioxidant potential and current cultivation aspects of an edible milky mushroom-Calocybe indica. Int J Pharm Pharm Sci. 2012;4:137–143. [Google Scholar]
- 49.Elmastas M, Isildak O, Turkekul I, Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal. 2007;20:337–345. [Google Scholar]
- 50.Babu DR, Rao GN. Antioxidant properties and electrochemical behavior of cultivated commercial Indian edible mushrooms. J Food Sci Technol. 2013;50:301–308. doi: 10.1007/s13197-011-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma SK, Lall AM, Sharma M, Reishi M. Response of organic supplementation on yield and nutritional parameters of Calocybe indica. Vegetos. 2013;26:36–39. [Google Scholar]
- 52.Rapior S, Cavalié S, Andary C, Pélissier Y, Marion C, Bessière JM. Investigation of some volatile components of seven fresh wild mushrooms (Basidomycetes) J Essent Oil Res. 1996;8:199–201. [Google Scholar]
- 53.Rapior S, Marion C, Pélissier Y, Bessière JM. Volatile composition of fourteen species of fresh wild mushrooms (Boletales) J Essent Oil Res. 1997;9:231–234. [Google Scholar]
- 54.Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev. 2012;26:73–83. [Google Scholar]
- 55.Beltran-Garcia MJ, Estarron-Espinosa M, Ogura T. Volatile compounds secreted by the oyster mushroom (Pleurotus ostreatus) and their antibacterial activities. J Agric Food Chem. 1997;45:4049–4052. [Google Scholar]
- 56.Jennings W, Shibamoto T. Qualitative analysis of flavor and fragrance volatiles by glass capillary gas chromatography. London: Academic Press; 1980. [Google Scholar]
- 57.Picardi SM, Issenberg P. Volatile constituents of mushrooms (Agaricus bisporus): changes which occur during heating. J Agric Food Chem. 1973;21:959–962. [Google Scholar]
- 58.Chandravadana MV, Vekateshwarlu G, Babu CS, Roy TK, Shivashankara KS, Pandey M, Tewari RP, Selvaraj Y. Volatile flavour components of dry milky mushrooms (Calocybe indica) Flavour Fragr J. 2005;20:715–717. [Google Scholar]
- 59.Sohliya DS, Gogoi R, Puzari KC, Sarmah DK, Chelleng A. Effect of steaming and gibberellic acid on growth, yield and nutritional quality of Calocybe indica. Indian Phytopathol. 2011;64:506–514. [Google Scholar]
- 60.Rajeswari P, Krishnakumari S. Potent antihyperglycaemic activity of Calocybe indica in streptozotocin induced diabetic rats antihyperglycemic activity of Calocybe indica. Int J Pharm Pharm Sci. 2013;5:512–515. [Google Scholar]
- 61.Selvi S, Umadevi P, Suja S, Sridhar K, Chinnaswamy P. Inhibition of in vitro lipid peroxidation (LPO) evoked by Calocybe indica (milky mushroom) Anc Sci Life. 2006;26:42–45. [PMC free article] [PubMed] [Google Scholar]
- 62.Chakravarty B. Trends in mushroom cultivation and breeding. Aust J Agric Eng. 2011;2:102–109. [Google Scholar]
- 63.Navathe S, Borkar PG, Kadam JJ. Cultivation of Calocybe indica (P & C) in Konkan region of Maharashtra, India. World J Agric Res. 2014;2:187–191. [Google Scholar]