Abstract
Two white rot fungi, Ceriporia sp. ZLY-2010 (CER) and Stereum hirsutum (STH) were used as biocatalysts for the biotransformation of (-)-α-pinene. After 96 hr, CER converted the bicyclic monoterpene hydrocarbon (-)-α-pinene into α-terpineol (yield, 0.05 g/L), a monocyclic monoterpene alcohol, in addition to, other minor products. Using STH, verbenone was identified as the major biotransformed product, and minor products were myrtenol, camphor, and isopinocarveol. We did not observe any inhibitory effects of substrate or transformed products on mycelial growth of the fungi. The activities of fungal manganese-dependent peroxidase and laccase were monitored for 15 days to determine the enzymatic pathways related to the biotransformation of (-)-α-pinene. We concluded that a complex of enzymes, including intra- and extracellular enzymes, were involved in terpenoid biotransformation by white rot fungi.
Keywords: α-terpineol, Biotransformation, Ceriporia sp. ZLY-2010, Stereum hirsutum, (-)-α-pinene
Essential oils are predominantly composed of terpene groups, resulting in a large and structurally diverse family of natural products [1]. Terpene hydrocarbons ((C5H8)n) are biochemically synthesized by the coupling of active isoprene units. Monoterpenoids are flavor and fragrance compounds that possess pleasant odors [2], and are produced by branched chain C-10 hydrocarbons formed from two isoprene units. In general, monoterpenoids are extracted from herbs and higher plants. However, the isolation of these compounds is difficult and expensive because of their purity; therefore monoterpenoids are recovered at low concentrations. In addition, the supply of plant materials can become limited because of seasonal variations and diseases. Although many synthetic organic compounds are available at low prices, consumers prefer natural compounds. Therefore, biotransformation has been investigated as a means of producing large quantities of natural products. There has been an increased focus on the biotransformation of terpene because it can be converted by biocatalysts under mild reaction conditions to yield products that are considered, "natural" [3]. The reactions involved can modify the structures of biologically active compounds with high stereo-selectivity [4]. Enzymes, cell extracts, whole bacteria cell, cyanobacteria, yeast, microalgae, fungi, and plants have been used as biocatalysts for biotransformation [3].
The use of whole cells as biocatalysts is considerable cheaper and simpler than using isolated enzymes. Many microorganisms have been used as biocatalysts of terpene in previous studies, revealing a number of issues that need to be overcome.These include the toxicity of terpene, and low product yields due to the substrate specificity of enzymes. White rot fungi are basidiomycetes; they colonize wood and are able to produce volatile compounds [5]. These particular fungi can completely degrade lignin, a polymer of p-hydroxycinnamyl alcohols, and metabolize the resulting phenolic monomer into an aromatic compound [6]. The extra-cellular ligninolytic enzymes of white rot fungi, such as lignin peroxidase (LiP, EC 1.11.1.14) and manganese-dependent peroxidase (MnP, EC 1.11.1.13), and laccase (EC 1.10.3.2) are the main enzymes involved in lignin depolymerization [7]. Lignin degrading enzymes of white rot fungi are highly nonspecific, and are able to utilize a wide range of substrates. Laccase can catalyze the oxidation of a wide range of compounds, including phenols, polyphenols, aroma amines, and nonphenolic substituents [8]. It has been postulated that fungal laccase can be used for the allylic oxidation of valencene, a bicyclic sesquiterpene, to nootkatone [9]. Cell-free enzymatic generation of sesquiterpene has been proposed, using isolated fungal laccase or LiP [10]. Fungal oxidases such as LiP and MnP are highly regarded as biocatalysts because of their high redox potentials [11].
The microbial transformation of monoterpene hydrocarbons has been conducted as early as 1960 [12]. The biotransformation of easily available monoterpene hydrocarbons is used during the processing of fragrances for the cosmetic and food industries [3]. Monoterpene hydrocarbons such as (+)-limonene, (-)-α-pinene, and (-)-β-pinene, are inexpensive monoterpenes that are commonly used as substrates for chemical synthesis [13]. In particular, (-)-α-pinene is the major constituent of turpentine oil, which is derived from the wood and leaves of most conifers at a relatively low price. We used two white rot fungi, Ceripora sp. ZLY-2010 and Stereum hirsutum, for the biotransformation of, (-)-α-pinene in valuable terpenoids.
MATERIALS AND METHODS
Substrates and microorganisms
(-)-α-Pinene and α-terpineol were purchased from Sigma-Aldrich (Seoul, Korea) at purities greater than 95% and 98%, respectively. Stereum hirsutum KFRI 234 (STH), Ceriporia sp. ZLY-2010 isolate M183 (CER), Trametes versicolor, and Phanerochaete chrysosporium KCTC 6728 were provided by the Korea Forest Research Institute and Korean Collection for Type Culture. The strains were pre-inoculated in potato dextrose agar medium in sterile Petri dishes at 28℃. After fully growing to the edge of the Petri dishes for 7 days, the fungal mycelia were directly homogenized.
Medium for biotransformation
Biotransformation was performed in aqueous system to obtain a high recovery rate of both of transformed products and biocatalysts, after the reaction. Shallow stationary culture (SSC) medium (1% glucose, 0.02% ammonium tartrate, 0.2% KH2PO4, 0.01% trace element solution, 0.05% MgSO4 · 7H2O, 0.01% CaCl2 · 2H2O) was used as previously described in Kirk et al. [14].
Enzymatic assays
Protein assay
Protein concentration was measured by the Bradford method [15] using the protein assay reagent (coomasie brilliant blue solution). Bovine serum albumin was used as a standard. Protein concentration was spectrophotometrically monitored by light absorbance at 595 nm.
Determination of MnP, laccase, and LiP activities
MnP activity was determined by following the oxidation of 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) as a substrate at 420 nm for 30 min [16]. The reaction mixture for MnP was prepared using 0.8 mL of 0.2M lactate buffer (pH 4.5) with 50 µL of ABTS (0.8 g/mL). Thirty-three microliters of 6 mM MnSO4 was mixed with 100 µL of the culture medium. Then, 17 µL of 0.1mM H2O2 was injected. The absorbance at 420 nm was measured after 30 min. Laccase activity was spectrophotometrically measured using ABTS as a substrate [17]. The reaction mixture contained 0.85 mL of 0.2M lactate buffer (pH 4.5), 50 µL of ABTS, and 100 µL of extracellular enzyme solutions to make total volume of 1 mL [17]. The absorbance of the mixture was monitored at 420 nm after 3 min of incubation at room temperature. LiP activity was measured by the oxidation of veratryl alcohol at 310 nm. Supernatant was incubated with 0.54mM H2O2 and 0.4 mM veratryl alcohol in 0.1M sodium tartrate at 37℃. Activity was expressed as the moles of veratryl alcohol oxidized in 10 sec [18].
Biotransformation
Mycelia, which were separated from the agar medium using platinum wire, were mixed with distilled water and then they were homogenized into suspension by homogenizer. One milliliter of homogenized mycelium samples (cell dry weight, 5mg/mL) were inoculated in 50 mL of SSC medium in 250-mL Erlenmeyer flask. The mycelia were pre-incubated in a stationary incubator at 28℃ for 3 days, during which germination of the spore and mycelia growth took place, to avoid toxic effects of the substrate to the whole cell. After 72 hr, biotransformation was initiated by directly adding 0.5 g/L of (-)-α-pinene into the culture flasks under sterile conditions. Then, the flasks were sealed with a silicon tube and placed on a shaking incubation at 26℃. Every 24 hr, Supernatant solution of the cultures was separated from the mycelia by centrifugation at 15,000 rpm for 15 min. The supernatant layer was used for enzyme activity assays and qualitative analysis. Finally, the mycelia were filtrated using a glass filter with a pore size of 1G3 to measure the biomass. Also, we established two different controls, free-substrate culture (only homogenized mycelium suspension) and free-mycelia culture.
Biotransformation by culture supernatant or by lignindegrading enzymes
Homogenized fungal suspensions were grown in 500mL of medium (SSC) in 1-L Erlenmeyer flasks. After 7 days, the mycelia were removed by filtration using Whatman No. 42 filter papers. The filtrates (50 mL) were transferred to 250-mL flasks containing 25 mg of substrate, and then placed in a stationary incubator at 26℃. Two enzymes, laccase (Sigma-Aldrich) purified from Trametes versicolor and peroxidase (Sigma-Aldrich) from horseradish, were purchased from Sigma-Aldrich. Experiment was performed in 10 mL reaction tube with 0.1M sodium tartarate buffer solution at pH 4.5. Laccase and horseradish peroxidase were added to make a final concentration 4U/mL and (-)-α-pinene to a concentration of 0.3mg/mL. Reaction mixture was incubated at room temperature for 6 days.
Analysis
Qualitative analysis of the transformation product
For the qualitative analysis, purge and trap gas chromatographymass spectrometry (GC-MS) was carried out by full scanning the products in water base medium. Solvent extraction could be limited due to inaccurate prediction of products. The purge and trap is an applicable technique for both solid and liquid samples. It is used as a routine technique for volatile compounds because it requires only small amount of sample, and does not require organic solvent and extraction procedure [19]. The volatile compounds were extracted with an automatic purge and trap sample concentrator (Hewlett Packard 5890 series Plus; Hewlett Packard, Wilmington, DE, USA) with a Tenax trap (Hewlett Packard). The samples placed in the 40 mL vial were purged under the following condition: a 40mL/min flow of ultra-pure helium was used as the purge gas, and purging was performed at 80℃ for 20 min, which was controlled by a thermal sleeve. The concentrated compounds in the non-cold trap were desorbed at 220℃ for 2 min; the valves were kept at 180℃ and transfer line at 120℃. After desorption, the trap was washed by purging at 220℃ for 40 min. The Purge and Trap Sample Concentrator 4460A (OI Analytical, College Station, TX, USA) was connected to an HP 6890 Series GC System (Agilent, Wilmington, DE, USA) and 5973 Mass Selective Detector. Separation of volatile compounds was performed on an HP VOC capillary column (60 m × 0.25 mm inside diameter). The separation conditions were as follows: the carrier gas was helium at constant flow of 1 mL/min; the split ratio was 5 : 1; and the injection temperature was at 200℃. Oven temperature programming was as follows: 40℃ for 7 min, which was then increased at a rate of 5℃/min to 250℃. The detector was operated in scan mode from 19 to 250 m/z. Peak identification was based upon mass spectrometry (MS) spectra comparison with the HP Wiley 275 library and with the spectra of injected standards.
Quantitative analysis of α-terpineol
Calibration was carried out by using external standard of α-terpineol (Sigma-Aldrich) which was purchased from Sigma-Aldrich Korea. The quantitative analysis of α-terpineol was performed with an Agilent model 6890A gas chromatography equipped with a split injector and FID detector. The stationary phase was a DB-5 column (30m×0.25mm, inside diameter) and carrier gas was ultra-pure helium at flow rate of 1 mL/min. The working conditions were as follows: injector temperature at 250℃ and detector temperature at 280℃. The oven temperature was increased from 70℃ to 280℃ at 5℃/min, with an initial holding time 5 min and a final holding time of 10 min. The detector was operated in scan mode from 19 to 250 m/z. Peak identification was based upon MS spectra comparison with the HP Wiley 275 library and with spectra of injected standards.
RESULTS AND DISCUSSION
Biotransformation of (-)-α-pinene
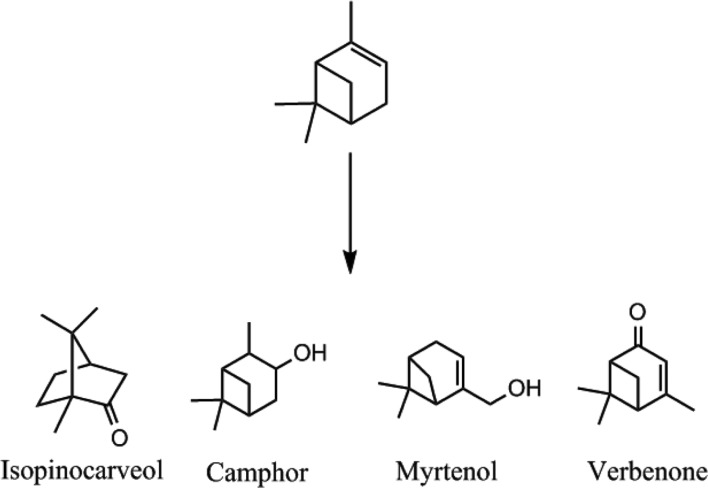
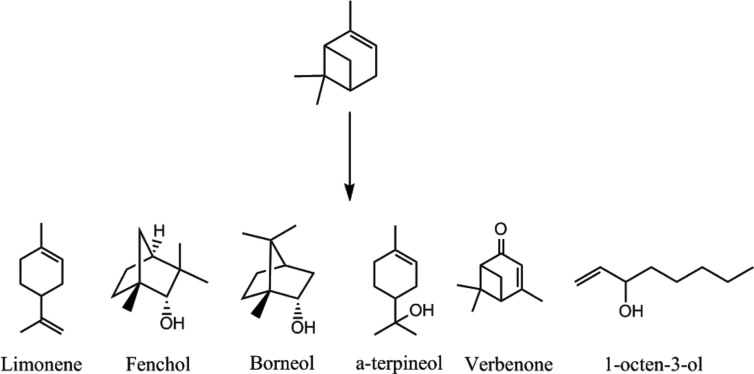
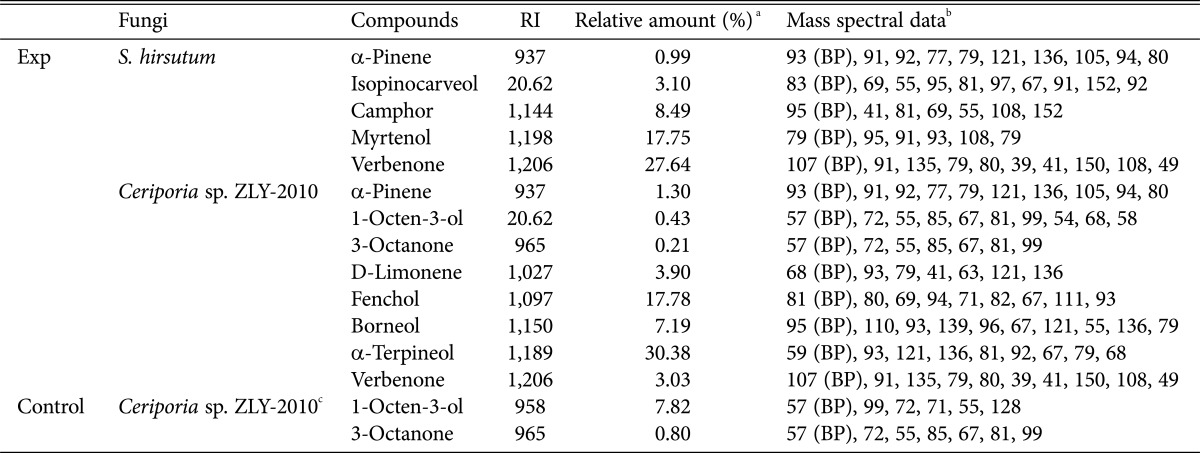
The biotransformation of (-)-α-pinene by fungi was conducted for 4 days following the addition of substrates to 3-day-old cultures. Qualitative analysis of products was conducted by purge and trap GC-MS. We identified a number of transformed products for CER and STH (Figs. 1 and 2). STH transformed (-)-α-pinene into verbenone (27.64%), with minor products such as myrtenol (17.75%), camphor (8.49%), and isopinocarveol (3.10%), relatively (Table 1). The transformed products had a bicyclic structure, similar to the substrate (Fig. 1). Verbenone, verbenol, myrtenol, and isopinocarveol were previously described as major biotransformation products for Hormaonema sp. [20], basidiomycetes fungi [21], and Aspergillus niger [12]. Prema and Bhattacharyya [12] suggested that (-)-α-pinene has to undergo two reactions, hydroxylation to produce verbenol and dehydrogenation to form verbenone. Therefore, STH can transform (-)-α-pinene to verbenol through microbial hydroxylations which is difficult to accomplish using chemical methods [22]. CER transformed (-)-α-pinene into α-terpineol (30.38%), at a concentration of 0.05 g/L, with minor products such as limonene (3.90%), fenchol (17.78%), borneol (7.19%), 1-octen-3-ol (0.43%), 3-octanone (0.21%), and verbenone (3.03%) also identified (Table 1).
Fig. 1. Structures of biotransformed products of (-)-α-pinene by Stereum hirsutum at day 4.
Fig. 2. Structures of biotransformed products of (-)-α-pinene by Ceripora sp. ZLY-2010 at day 4.
Table 1. Transformed products from (-)-α-pinene by Stereum hirsutum and Ceriporia sp. ZLY-2010.
RI, retention index.
aPercentage of peak area is the ratio of each peak area to total peak area on the basis of total intensity count values of gas chromatography-mass spectrometry purge and trap analysis.
bMajor fragmentation ions, base peak (BP) and other ions in decreasing order of relative abundance.
cCompounds of secondary metabolites from Ceriporia sp. ZLY-2010 without substrate.
We observed a high concentration of α-terpineol for 4-days, indicating no further production of this compound, or metabolism, during the biotransformation of α-pinene. α-Terpineol, a monocyclic monoterpene alcohol [23] which is found in many essential oils but can only be produced in, small quantities. The annual global consumption of artificial α-terpineol flavors is estimated at 13,000 kg, placing it among the top 30 commonly used flavor compounds [24].
Limonene has been used as a starting substrate for biotransformation into α-terpineol, because both compounds have a monocyclic structure, unlike (-)-α-pinene (Fig. 2) [25,26,27]. Certain bacterial species, such as Penicillium digitatum [28] and Escherichia coli [29] possess hydratase, which is able to hydrate the C=C double bond of limonene. Bicyclic α-pinene can be converted into monocyclic compounds, such as limonene and sobrerol through ring-opening reaction [30,31,32]. It is possible that CER enzyme might conduct ring-opening and hydration reactions during the biotransformation of (-)-α-pinene.
The analysis of free substrate culture was conducted because white rot fungi are capable of producing diverse volatile flavors in young fruiting bodies [33]. We identified 1-octen-3-ol and 3-octanone as metabolites in CER (Table 1). Volatile compounds, such as 1-octen-3-ol, that are produced by basidiomycetes can attract predators of fungi, insects and pests, or act as a defense mechanism [34]. The formation of volatile compounds is unique to fungi, and likely involves a specific fungal pathway. These compounds are formed via the enzyme catalyzed oxidation of polyunsaturated fatty acids such as linoleic acid, by a lipoxygenase and hydroperoxide lyase [35,36].
Fungal cell dry weight
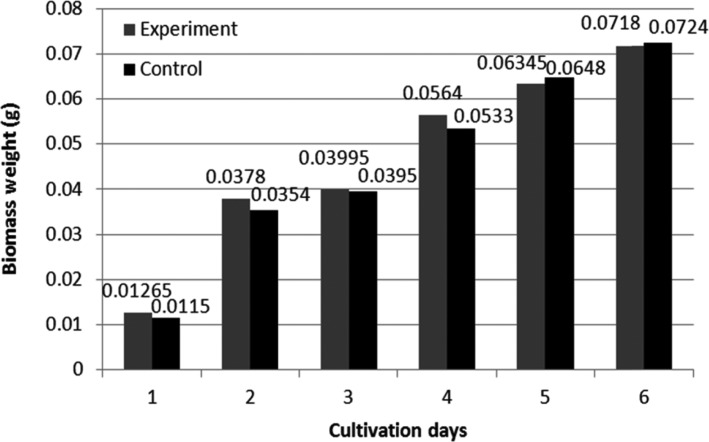
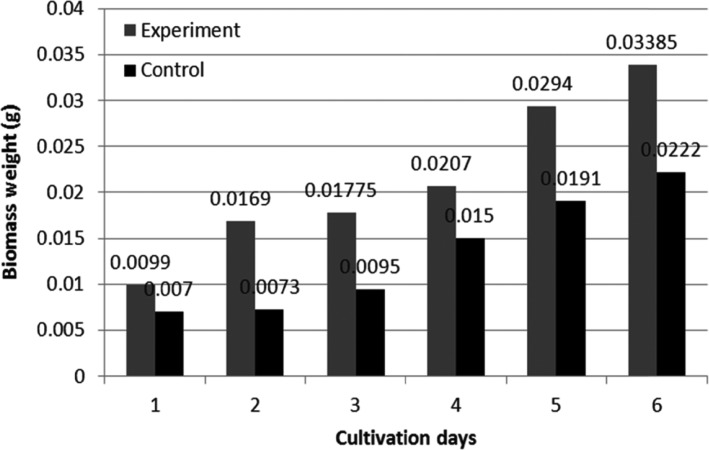
Before biotransformation, the toxic effects of (-)-α-pinene on fungal mycelia were investigated. It was previously reported that the addition of terpenes to cell cultures inhibits the growth of mycelia, with high concentrations of monoterpenes resulting in the lysis of some bacteria (Bacillus sp.) and Saccharomyces cerevisiae [37,38]. However, CER biomass was increased by 0.5mg after the addition of substrate. We observed considerable increases in STH and CER mycelium weight over 6 days, compared with that for controls (Figs. 3 and 4). The inhibitory effects of terpene upon the growth of microorganisms are one of the main obstacles in successful and efficient biotransformation. The growth of fungal mycelia was no longer observed when the concentration of terpene was greater than 0.1% (w/v).
Fig. 3. Mycelial growth of Stereum hirsutum during 6 cultivation days.
Fig. 4. Mycelial growth of Ceripora sp. ZLY-2010 during 6 cultivation days.
Enzymatic activities
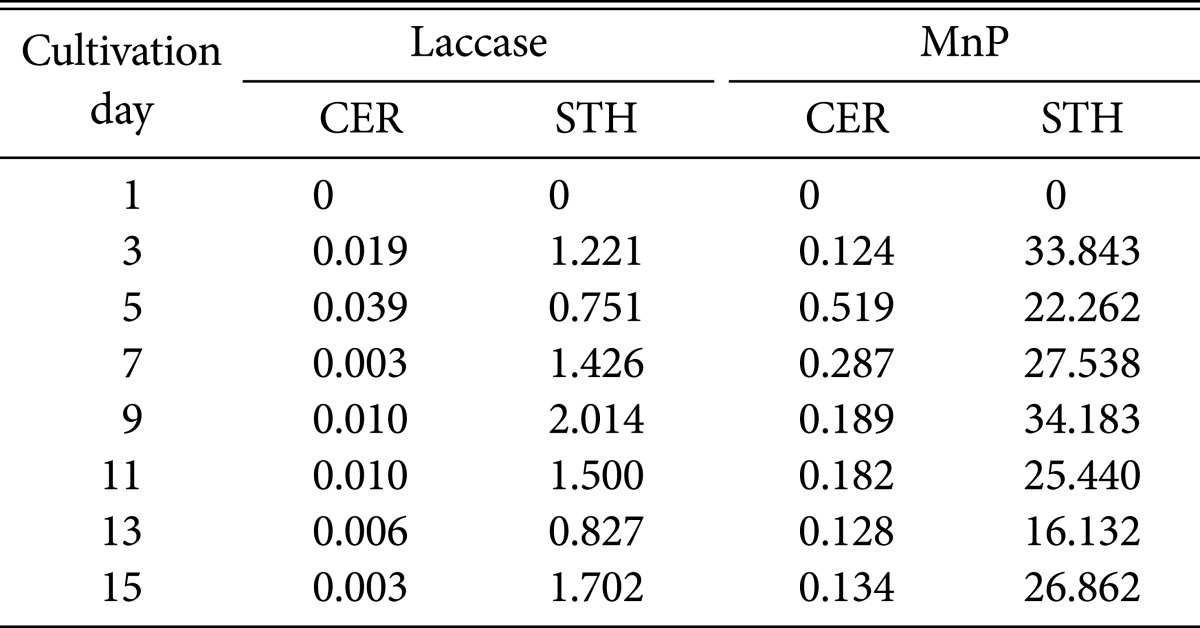
The two fungal strains we investigate exhibited different laccase and MnP activities (Table 2). We observed high laccase and MnP activites on days 3~9 for STH, with enzyme activities peaking at day 9 (laccase, 2.01 units/mg; MnP, 34.18 units/mg). We observed low MnP and laccase activities (0.01 and 0.51 unit/mg) during the cultivation of CER. We were unable to determine LiP activity for either STH or CER in the current study. The production of LiP has been reported for Phanerochaete chrysosporium [7], Phlebia radiata [39], Trametes versicolor [40], Panus tigrinus [39], and Chrysosporium pruinosum [41]. It was initially assumed that ligninolytic enzymes such as MnP, laccase, and LiP, play major roles in the biotransformation of (-)-α-pinene. However, there was no significant relationship between biotransformation ability and enzyme systems in STH and CER. Therefore, it is necessary to determine wheter laccases and peroxidases of other white rot fungi (P. chrysosporium and T. versicolor), and cell free extracts undergo biotransformation. The ligninolytic properties of P. chrysosporium and T. versicolor allow for these microorganisms to be commonly used models in lignin biodegradation studies [42]. According to our results, it can be inferred that there is no significant correlation between ligninolytic enzymes and the biotransforming abilities of CER; however cell free CER culture medium was able to transform (-)-α-pinene to α-terpineol at a concentration of 0.01 g/L. In future work, we will conduct transcriptome analyses to predict the involvement of enzymes in the biotransformation of (-)-α-pinene.
Table 2. Laccase and MnP activities (units/mg) two fungi, CER and STH, for 15 days.
MnP, manganese-dependent peroxidase; CER, Ceripora sp. ZLY-2010; STH, Stereum hirsutum.
Conclusions
CER and STH were able to transform (-)-α-pinene into oxygenated monoterpenoids. In particular, CER was able to convert (-)-α-pinene into the useful terpenoid, α-terpineol. We concluded that biotransformation of terpenoids by white rot fungi could be applied in the production of valuable compounds for the flavor and fragrance industries. However, to efficiently use and develop the enzymes of white rot fungi for biotransformation, it is necessary to understand the roles for these enzymes. Extracelluar and intracellular enzyme systems, including specific ligninolytic enzymes, are likely involved in biotransformation. The enzymatic mechanism of ring-opening and hydration by CER require elucidation, while the broad substrate specificities of this fungus require characterization for the improved production of aromatic compounds. Approach based gene levels might support the catalytic function of biocatalyst. Therefore, profiling of transcript of P. brumalis has being performed to understand catalytic function on biotransformation process.
References
- 1.Weiss EA. Essential oil crops. Wallingford: CAB International; 1997. [Google Scholar]
- 2.Van der Werf MJ, de Bont JA, Leak DJ. Opportunities in microbial biotransformation of monoterpenes. In: Berger RG, Babel W, Blanch HW, Cooney CL, Enfors SO, Eriksson EL, Fiechter A, Klibanov AM, Mattiasson B, Primrose SB, et al., editors. Biotechnology of aroma compounds. Berlin: Springer-Verlag; 1997. pp. 147–177. [Google Scholar]
- 3.De Carvalho CC, Da Fonseca MM. Biotransformation of terpenes. Biotechnol Adv. 2006;24:134–142. doi: 10.1016/j.biotechadv.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Abraham B, Onken JG, Berger RG. Strategies toward an efficient biotechnology of aromas; Proceedings of the 5th Wartburg Aroma Symposium, Flavour perception-aroma evaluation; 1997 Mar 17-20; Eisenach, Germany. Potsdam: Universitat Potsdam, Bergholz-Rehbrucke; 1997. pp. 357–373. [Google Scholar]
- 5.Abraham BG, Berger RG. Higher fungi for generating aroma components through novel biotechnologies. J Agric Food Chem. 1994;42:2344–2348. [Google Scholar]
- 6.Kirk TK. Effects of microorganisms on lignin. Annu Rev Phytopathol. 1971;9:185–210. [Google Scholar]
- 7.Kirk TK, Farrell RL. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 8.Tzialla AA, Taha AA, Kalogeris E, Stamatis H. Improving the catalytic performance of fungal laccases in monoterpenebased reaction systems. Biotechnol Lett. 2009;31:1451–1456. doi: 10.1007/s10529-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 9.Christenson PA, Labuda IM, Rongmin H, inventors. European patent 1083233. Production of natural flavours by laccase catalysis. 2001 Mar 14;
- 10.Haider K. Biochemie des Bodens. Stuttgart: Enke; 1996. [Google Scholar]
- 11.Burton SG. Oxidizing enzymes as biocatalysts. Trends Biotechnol. 2003;21:543–549. doi: 10.1016/j.tibtech.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Prema BR, Bhattacharyya PK. Microbiological transformation of terpenes. II. Transformation of α-pinene. Appl Microbiol. 1962;10:524–528. doi: 10.1128/am.10.6.524-528.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohloff G. Scent and fragrances: the fascination of odors and their chemical perspectives. Berlin: Springer-Verlag; 1994. [Google Scholar]
- 14.Kirk TK, Croan S, Tien M, Murtagh KE, Farrell RL. Production of multiple ligninases by Phanerochaete chrysosporium: effect of selected growth conditions and use of a mutant strain. Enzyme Microb Technol. 1986;8:27–32. [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Ürek RÖ, Pazarlioğlu NK. Purification and partial characterization of manganese peroxidase from immobilized Phanerochaete chrysosporium. Process Biochem. 2004;39:2061–2068. [Google Scholar]
- 17.Shin KS, Lee YJ. Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch Biochem Biophys. 2000;384:109–115. doi: 10.1006/abbi.2000.2083. [DOI] [PubMed] [Google Scholar]
- 18.Tien M, Kirk TK. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161:238–249. [Google Scholar]
- 19.Bosset JO, Gauch R, Mariaca R, Klein B. Comparison of various sample treatments for the analysis of volatile compounds by GC-MS: application to the Swiss Emmental cheese. Mitt Geb Lebensmittelunters Hyg. 1995;86:672–698. [Google Scholar]
- 20.Van Dyk MS, Van Rensburg E, Moleleki N. Hydroxylation of (+) limonene, (−) α-pinene and (−) β-pinene by a Hormonema sp. Biotechnol Lett. 1998;20:431–436. [Google Scholar]
- 21.Busmann D, Berger RG. Conversion of myrcene by submerged cultured basidiomycetes. J Biotechnol. 1994;37:39–43. [Google Scholar]
- 22.Holland HL. Organic synthesis with oxidative enzymes. Weinheim: Wiley-VCH; 1992. [Google Scholar]
- 23.Tan Q, Day DF. Bioconversion of limonene to α-terpineol by immobilized Penicillium digitatum. Appl Microbiol Biotechnol. 1998;49:96–101. [Google Scholar]
- 24.Welsh FW, Murray WD, Williams RE, Katz I. Microbiological and enzymatic production of flavor and fragrance chemicals. Crit Rev Biotechnol. 1989;9:105–169. [Google Scholar]
- 25.Maróstica MR, Jr, Pastore GM. Production of R-(+)-α-terpineol by the biotransformation of limonene from orange essential oil, using cassava waste water as medium. Food Chem. 2007;101:345–350. [Google Scholar]
- 26.Tan Q, Day DF, Cadwallader KR. Bioconversion of (R)-(+)-limonene by P. digitatum (NRRL 1202) Process Biochem. 1998;33:29–37. [Google Scholar]
- 27.Kraidman G, Mukherjee BB, Hill ID. Conversion of D-limonene into an optically active isomer of α-terpineol by a Cladosporium species. J Bacteriol. 1969;63:12–18. [Google Scholar]
- 28.Hamada H, Kondo Y, Ishihara K, Nakajima N, Hamada H, Kurihara R, Hirata T. Stereoselective biotransformation of limonene and limonene oxide by cyanobacterium, Synechococcus sp. PCC 7942. J Biosci Bioeng. 2003;96:581–584. doi: 10.1016/S1389-1723(04)70154-1. [DOI] [PubMed] [Google Scholar]
- 29.Savithiry N, Cheong TK, Oriel P. Production of α-terpineol from Escherichia coli cells expressing thermostable limonene hydratase. Appl Biochem Biotechnol. 1997;63-65:213–220. doi: 10.1007/978-1-4612-2312-2_20. [DOI] [PubMed] [Google Scholar]
- 30.Wright SJ, Caunt P, Carter D, Baker PB. Microbial oxidation of alpha-pinene by Serratia marcescens. Appl Microbiol Biotechnol. 1986;23:224–227. [Google Scholar]
- 31.Shukla OP, Bhattacharyya PK. Microbiological transformation of terpenes. Part XI. Pathways of degradation of α- and β-pinenes in a soil pseudomonad (PL strain) Indian J Biochem. 1968;5:92–101. [PubMed] [Google Scholar]
- 32.Narushima H, Omori T, Minoda Y. Microbial transformation of α-pinene. Eur J Appl Microbiol Biotechnol. 1982;16:174–178. [Google Scholar]
- 33.Fäldt J, Jonsell M, Nordlander G, Borg-Karlson AK. Volatiles of bracket fungi Fomitopsis pinicola and Fomes fomentarius and their functions as insect attractants. J Chem Ecol. 1999;25:567–590. [Google Scholar]
- 34.Mau JL, Chyau CC, Li JY, Tseng YH. Flavor compounds in straw mushrooms Volvariella volvacea harvested at different stages of maturity. J Agric Food Chem. 1997;45:4726–4729. [Google Scholar]
- 35.Tressl R, Bahri D, Engel KH. Formation of eight-carbon and ten-carbon components in mushrooms (Agaricus campestris) J Agric Food Chem. 1982;30:89–93. [Google Scholar]
- 36.Husson F, Thomas M, Kermasha S, Belin JM. Effect of linoleic acid induction on the production of 1-octen-3-ol by the lipoxygenase and hydroperoxide lyase activities of Penicillium camemberti. J Mol Catal B Enzym. 2002;19-20:363–369. [Google Scholar]
- 37.Andrews RE, Parks LW, Spence KD. Some effects of Douglas fir terpenes on certain microorganisms. Appl Environ Microbiol. 1980;40:301–304. doi: 10.1128/aem.40.2.301-304.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikkema J, De Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantelinen A, Hatakka A, Viikari L. Production of lignin peroxidase and laccase by Phlebia radiata. Appl Microbiol Biotechnol. 1989;31:234–239. [Google Scholar]
- 40.Jönsson L, Johansson T, Sjöström K, Nyman PO. Purification of ligninase isozymes from the white-rot fungus Trametes versicolor. Acta Chem Scand Ser B. 1987;41:766–769. [Google Scholar]
- 41.Waldner R, Leisola MS, Fiechter A. Comparison of ligninolytic activities of selected white-rot fungi. Appl Microbiol Biotechnol. 1988;29:400–407. [Google Scholar]
- 42.Jäger A, Croan S, Kirk TK. Production of ligninases and degradation of lignin in agitated submerged cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985;50:1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]