Abstract
The increasing emergence of lead drugs for the resistance produced by the pathogenic strains and arrival of new diseases have initiated the need for searching novel metabolites with best anticancer and antimicrobial properties than the existing one. With this view, the investigation was conducted for the isolation, identification, and biological evaluation of potential endophytic fungi of Aegle marmelos, a medicinal tree used for more than three decades, for curing various disorders. A total of 169 endophytic fungal strains obtained from sampling and among those 67 were pigmented strains. Upon antagonistic screening, five endophytic fungal strains exhibited antagonistic potentiality by inhibiting the pathogens. These five potent strains were characterized at molecular level by sequencing the amplified internal transcribed spacer (ITS) 1 and ITS 4 regions of rDNA and they were grouped under order Pleosporales, Eurotiales, and Capnodiales. The metabolites from the respective strains were produced in fungal culturing media and extracted using polar solvents. Further, the extracts of five endophytes manifested antimicrobial activity against tested clinical pathogens and Alternaria alternata (FC39BY), Al. citrimacularis (FC8ABr), and Curvularia australiensis (FC2AP) exhibited significant antimicrobial profile against 9 of 12 tested pathogens, showing broad spectrum activity. The antioxidant levels of all the five endophytes revealed the highest activity at least concentrations, and major activity was unveiled by the members of order Pleosporales FC2AP and FC8ABr. This research explains the value of endophytic fungal extracts and its significance of antimicrobial and antioxidant properties.
Keywords: Antimicrobial, Antioxidants, Endophytes, Metabolite, Molecular characterization
The role of fungal species in the ecosystems have been understood by little sampling and lack of characterization of fungal diversity. Over the past three decades, the endophyte research has been popular by having knowledge of its ecology, life history and phylogeny. Although the term endophyte has been a controversial after its appearance, it has become synonymous with mutalism. Endophytes in woody plants are the poorly understood groups even though they are thought to be important in plant populations and communities. They play a major role in physiology of plant tissues and protect the plants from pests, nematodes, and insects and also provide resistance to the diseases caused by environmental factors [1]. These also help in nitrogen fixation and accelerate plant growth. Endophytic fungal research was expanded in recent years from cataloguing species to examining the nature of the endophyte or plant interaction with particular emphasis on the fungus of the medicinal plants in a way to develop or discover novel compounds [1].
People in Indian subcontinent have a long history for the usage of medicinal plants for curing several diseases. Western Ghats of India are found to be one of the hotspots, which comprises a diverse community of endophytic fungi residing in medicinal woody plants and it has been reported already. Endophytic fungi produce invaluable potential bioactive secondary metabolites which can be formulated as new drugs in biotechnological process and it can be a source for disease management [2]. The secondary metabolites are the intermediates formed during the metabolism and they are found to be the potential drugs. This study focuses on potential bioactive metabolites from endophytic fungal isolates from medicinal plant Aegle marmelos. A. marmelos (L.) Correa, is commonly known as bael and it belongs to the family Rutaceae. The parts of this plant such as root, bark, leaves, and fruit have been widely used in indigenous systems of Indian medicine like Ayurveda, Unani, and Siddha to treat various types of diseases like dysentery, diarrhoea and gastrointestinal problems [3]. The plant also has antidiabetic, antibacterial and antifungal effects. Due to the medicinal value of this host, we have selected it to isolate and identify the metabolites from endophytic mycoflora from A. marmelos around the regions of Western Ghats (India) and explore its bioactivities.
MATERIALS AND METHODS
Plant sample collection
Mature healthy, asymptomatic plant materials (bark, branches, leaves, and root) were collected by sampling different parts of the trees of A. marmelos growing randomly in the Western Ghats region (Nilgiris cluster, Tamil Nadu, India). The sampling was performed on five trees of A. marmelos collected from foot hills of Vellingiri and Marudhamalai (Coimbatore, TamilNadu, India [11.0183°N, 76.9725°E]). Bark samples were obtained by cutting tree bark at 150 cm above the ground level from a depth of 1~1.5 cm inwards with the help of sterile machete. Small discs of leaves (0.5 cm diameter) were cut using sterile pinch cutter. Root samples were obtained by digging the soil at least 1m away around the main trunk and 2 ft in depth. Fifteen samples were taken from each tree, five each from root, inner bark, inner branches and leaves. From each sample, 10 subsamples were prepared for further culturing to find out endophytic fungi. All the samples were collected in sterile polythene bags and brought to the laboratory in an icebox. Samples stored at 4℃ were used to isolate endophytic fungi within 24 hr of collection.
Isolation of endophytic fungi
The samples were rinsed gently in running tap water to remove dusts and debris. The samples were surface sterilized by modified method of Dobranic et al. [4]. The samples were immersed in 70% ethanol for 5 sec, followed by 4% sodium hypochlorite for 90 sec and then rinsed in sterile distilled water for 10 sec. This was done to remove epiphytic mycelia and bacteria adhered to the bark. The excess moisture was blotted in a sterile filter paper. The surface sterilized segments were taken for dissection using a sterile blade. The outer bark was removed and the inner cortex was cut into 1.0 cm × 0.5 cm pieces; leaves were pinched into 5-mm diameter using pinch paper cutter and placed in petridishes containing potato dextrose agar (PDA), malt extract agar (MEA), and Sabouraud's dextrose agar (SDA) media supplemented with streptomycin (250 mg/L) and incubated at 28 ± 2℃ for 11~21 days in light/dark cycle. Tissues were observed for mycelial growth at an interval of 2 days and actively growing fungal mycelia was subcultured onto a fresh PDA/MEA/SDA media and stored in cryovials at -20℃.
Screening for potential strains
Isolated pigmented endophytic fungal strains were screened for antagonism by cross streak method (dual culture technique). The 4-day-old culture (pigmented isolates) was streaked on the centre of PDA, MEA, and SDA media and incubated for 48 hr at 28 ± 2℃. The clinical pathogens (procured from PSG Hospitals, Coimbatore, TN, India) which are facultative anaerobic bacteria falls under Gram positive and Gram negative bacteria were Staphylococcus aureus, S. epidermidis, Enterococcus faecalis, Shigella sp., and Escherichia coli, Pseudomonas aeruginosa, Klebsiella pnuemoniae, Salmonella typhi, and Proteus mirabilis, respectively, were taken for antagonist assessment. Similarly, the fungal pathogens (Ascomycota) such as Aspergillus niger and Candida albicans were also taken for this study. All these pathogens were selected on the basis of common infections (nosocomial infections, gastro intestinal infections, and urinary tract infections) caused in humans. The percentage of inhibition was calculated.
Identification and characterization of endophytic fungi
The potential fungal isolates were identified up to the genus level by observing the presence of conidial mycelium, spore mass colour, distinctive reverse colony colour, diffusible pigment, sporophore, and spore chain morphology. The strains were mounted on the sterile glass slides for staining with lactophenol cotton blue [5] and examined at 400× and 1,000× light microscopy. The spore morphology (aerial hyphae, substrate mycelia, and spore chain arrangement) was studied by growing the fungal strains in PDA, MEA, and SDA on cover slips and viewed at 1,000× under light microscopy after incubation for 4~7 days at 28 ± 2℃. Molecular analysis was done using amplification of ITS1 and ITS4 fragments of rDNA. 18s rRNA or rDNA sequence analysis was performed using specific polymerase chain reaction (PCR) primers to amplify rDNA fragments of endophytes that was used to validate the morphospecies of different groups of mycelia [6,7].
Production and extraction of pigments
The pigmented strains were inoculated in different media such as potato dextrose broth (PDB), Sabouraud dextrose broth (SDB), malt extract broth (MEB), and Czapek Dox broth (CDB) to determine the maximum pigmentation inducing media. The flasks were kept in stationary phase and shaking condition at 100 rpm for 11~15 days at 28 ± 2℃ incubation in light and dark cycle. After incubation, the total pigments were extracted from biomass and culture filtrate by solvent extraction method using polar and non-polar solvents. In this study, we have extracted biomass from 100mL of culture broth and the pigmented metabolites were extracted. The total pigments was evaluated using formula 1 {AbtsT=Absintra+Absextra [Absintra =Absextract×D, D = 50V, Yp/x= ΔAbtsT/Δx]; AbsT: Absorbance of extra-plus intracellular pigments (U); Absextra and Absintra: extracellular and intracellular absorbance (U); Absextract: absorbance in the extract of cell disruption (U); V: volume of the sample submitted to cell disruption for pigment extraction (mL); x: cell concentration (g/L); Yp/x: yield factor of pigments on cell growth (1/ULg)}. The total pigment extract of each strain was concentrated in rotary vacuum evaporator and it was taken for further bioactivities studies.
Assessment of antibacterial profile
Antimicrobial assessment for the concentrated crude biomass pigment extract and cell free culture filtrate pigment extract of each potential isolates were determined by well-diffusion method [8,9] against clinical pathogens S. aureus, S. epidermidis, E. faecalis, Shigella sp., E. coli, P. aeruginosa, K. pnuemoniae, S. typhi, and P. mirabilis on Muller-Hinton agar (MHA) media. Forty micrograms of aliquots of extracts were filled in the respective wells. The diameter of inhibition zone was measured after 24 hr of incubation at 37℃ for bacteria.
Assessment of antimycotic profile
This was determined by well diffusion technique using fungal clinical pathogens A. niger and C. albicans by swabbing on MHA plate and by filling 40 µg of aliquots of extracts in the respective wells. The plates were incubated for 48 hr at 28 ± 2℃ and then zone of inhibition was measured.
DPPH radical scavenging activity
The antioxidants present in fungal crude pigment extracts were aliquoted into different concentrations (10~200 µg) to determine the ability of extract for scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals using the method of Yildirim et al. [10]. DPPH solution (1 mM DPPH radical solution in 95% ethanol) was added to the crude pigment extracts and made upto 1 mL, vortexed well, and then incubated for 30 min in dark chamber at room temperature (RT). After incubation, the samples were poured into microfuge tubes and centrifuged for 5 min at 13,500 rpm at RT. The absorbance of each sample at λ = 517 nm was measured and 1 mL of 95% EtOH/MeOH was used as a control, and ascorbic acid was used as reference compounds. The antioxidant activity is given as percent (%). DPPH scavenging assay was calculated using the formula: [(control absorbance - extract absorbance)/(control absorbance) × 100].
Reducing power assay
Total reducing power was determined as described by Oyaizu [11]. One millilitre of sample solution at different concentrations was mixed with 2.5 mL of phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50℃ for 20 min; 2.5 mL of 10% trichloroacetic acid was added to the mixture and centrifuged at 3,000 ×g for 10 min. The supernatant (5 mL) was mixed with 1 mL of ferric chloride (0.1%), and the absorbance was measured at 700 nm in a spectrophotometer. Increased absorbance of the reaction mixture indicated increased reducing power.
Metal chelating activity
The metal chelating activity was analysed by the method of Dinis et al. [12] with slight modification. The reaction was performed in HEPES buffer (20mM) at pH 7.2. Various concentrations (10~200 µg) of samples were mixed with a solution of 12.5 µM ferrous sulphate solution. To initiate the reaction, 75 µm ferrozine was added and the mixture was shaken vigorously and incubated for 20 min at RT. After incubation, the absorbance was measured at 562 nm. Ascorbic acid was used as the reference compound and the percentage chelating capacity was calculated as: % chelating activity = [(A0 - A1)/A0] × 100, where, A0 = absorbance of the blank; A1 = absorbance of the sample.
RESULTS
Isolation, screening, and identification of endophytic fungi
A total of 169 strains belonging to different taxa were obtained from 300 segments (15 from each root, inner stem, inner branch, and leaves) of 5 trees and from that 67 were pigmented fungi. Of these 67 pigmented endophytic fungi, 27 strains of A. marmelos were isolated from Marudhamalai hills and 40 strains were obtained from Vellingiri hills and this proved that this hill prevails to be diverse in nature. Each fungus was named in the order of fungus, sample location, sample number, strain number, and strain mat colour. The isolated strains belonged to Ascomycota, Basidiomycota, and Dueteromycota.
Pigmented fungal isolates were screened for antagonistic property against facultative aerobic bacteria and among that 67 fungal isolates, maximum of 32 strains showed moderate activity. Major activities against maximum pathogens were observed in five fungal isolates (FC39BY, FC2AP, FC8ABr, FC75ABr, and FC30AGr). The inhibition zone was measured in centimeters in terms of diameter.
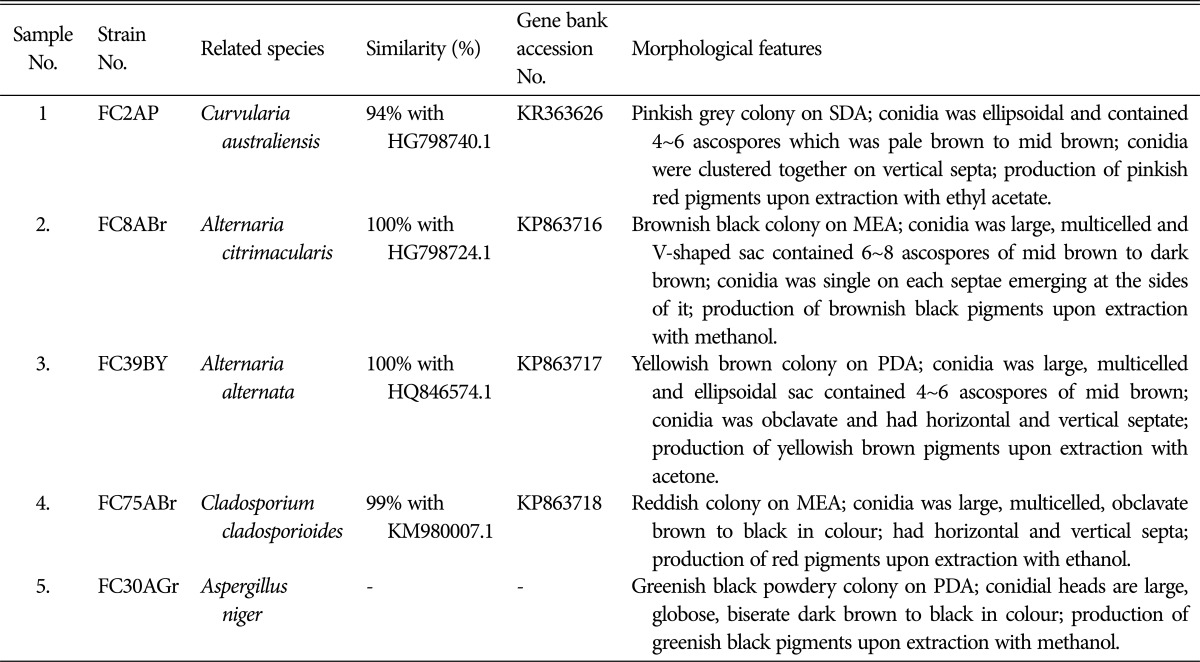
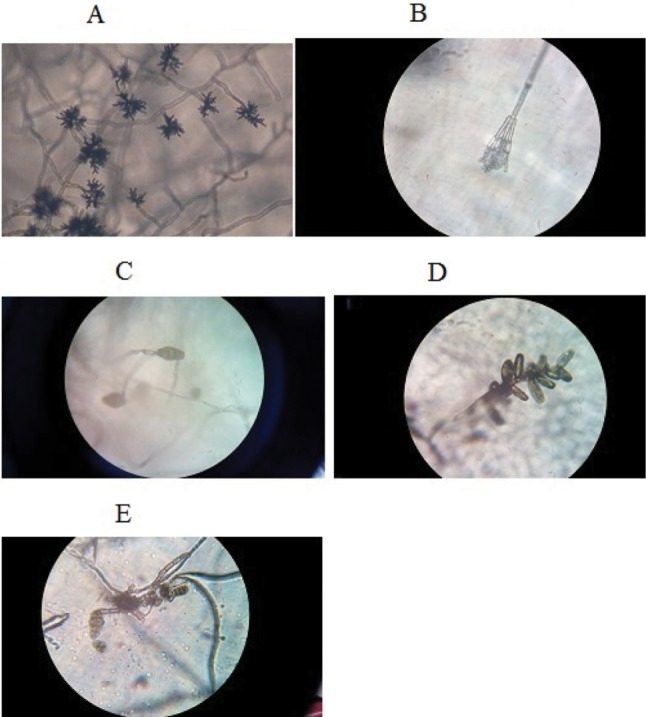
Five potential fungal strains were examined for morphological and molecular characteristics. The spore morphology of each isolate differed according to their phylum and class. The conidia of the isolates FC75ABr and FC8ABr were large, multicelled having ellipsoidal or V-shaped sac which contained 4~8 ascospores which were similar to strain FC2AP containing 4~6 ascopores in the sac and the sac was pale brown to mid brown in colour. These three strains belonged to class Dothideomycetes. FC39BY conidia were large multicelled obclavate and it had a horizontal septa. The conidia of FC30AGr appeared as biseriate with philiades borne on brown septate. The characteristics of the five fungal strains are displayed in Table 1 and Fig. 1.
Table 1. Characteristics of potential isolates.
SDA, Sabouraud's dextrose agar; MEA, malt extract agar; PDA, potato dextrose agar.
Fig. 1. Spore morphology of endophytic fungi from Aegle marmelos. A, Cladosporium cladosporioides FC75A in MEA; B, Aspergillus niger FC30AGr in PDA; C, Alternaria alternata FC39BY in PDA; D, Curvularia australiensis FC2AP in SDA; E, Al. citrimacularis FC8ABr in MEA. The figures depicted were captured in 400× under light microscopy. MEA, malt extract agar; PDA, potato dextrose agar; SDA, Sabouraud's dextrose agar.
The internal transcribed spacer (ITS) rDNA region for the isolates were amplified, sequenced and compared with the sequence of organisms represented in NCBI database gene bank BLAST tool search and by using this we constructed the phylogenetic tree. The strains were C. australiensis (FC2AP), Al. citrimacularis (FC8ABr), Al. alternata (FC39BY), Cladosporium cladosporioides (FC75ABr), and As. niger (FC30AGr). The sequences were submitted in gene bank and the accession number was obtained (Table 1).
Production and extraction of secondary metabolites
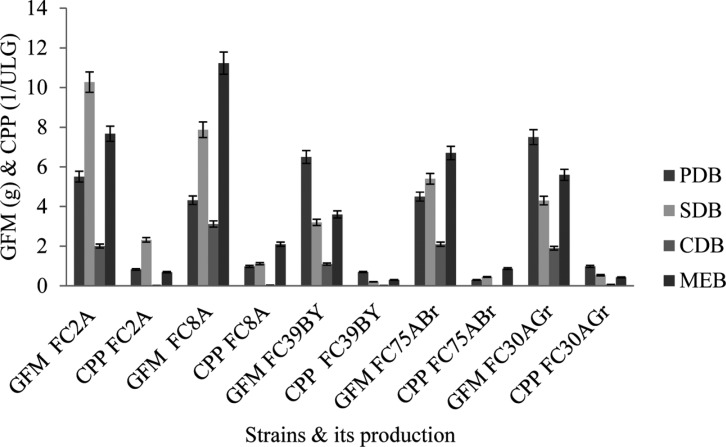
The mass production of crude secondary metabolites from each isolated strains were carried out in selected media after summarizing the evaluation of maximum secondary metabolites production in different media (PDB, SDB, MEB, and CDB) using formula 1. The foremost things noted for mass production was the cultivation time, water content which were the best factors for biosynthesis of metabolites in fermentation technique. During early stage of cultivation, the fungal strains produced white and short mycelium and then it developed into fungal mat. All the five strains did not utilize the CDB media components for their secondary metabolite production. On one hand FC8ABr and FC75ABr produced maximum metabolites in MEB, and on the other hand PDB induced the maximum secondary metabolites production from FC39BY and FC30AGr. FC2AP was found to grow well and produced highest units of metabolites in SDB (Fig. 2). The crude metabolites from each isolates were harvested and extracted using different polar solvents. The crude secondary metabolites from FC2AP yielded 0.83/ULg in ethyl acetate whereas FC8ABr and FC30AGr produced 0.98/ULg separately using methanol. About 0.7/ULg and 0.3/ULg of metabolites were yielded by FC39BY and FC75ABr by utilizing acetone and ethanol, respectively.
Fig. 2. Pigment inducing media for five endophytic fungal strains from Aegle marmelos. PDB, potato dextrose broth; SDB, Sabouraud's dextrose broth; CDB, Czapek Dox broth; MEB, malt extract broth; GFM, growth of fungal mat in grams; CPP, crude pigmented metabolite production in grams.
Assessment of antimicrobial activity
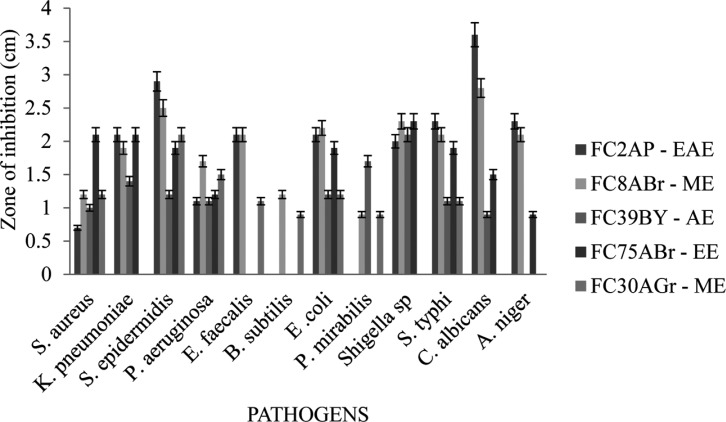
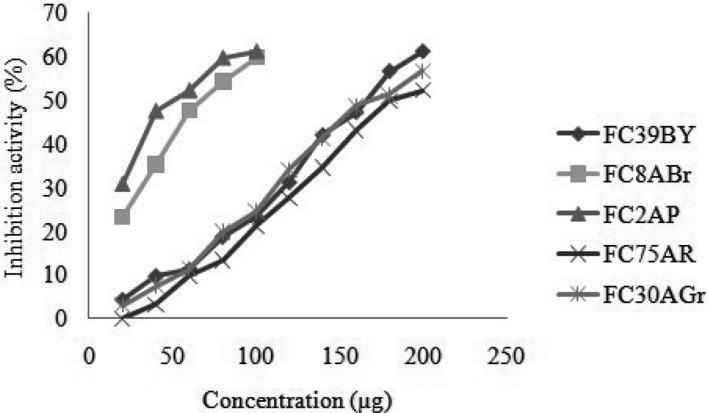
During resistance development in human pathogens, adverse effects of chemically synthesized drugs and emergence of new diseases needs urgent demands for new natural antimicrobial agents which have no/low impact to human health and environment. The results in Fig. 3 displayed the antimicrobial activity for the five potential strains against stated pathogens. FC2AP and FC8ABr extracts belonging to the order Pleosporales showed similar activity for all 12 pathogens, whereas FC2AP exhibited higher activity against C. albicans and S. epidermidis and did not reveal any activity against B. subtilis and P. mirabilis. Crude metabolties extracts of FC8ABr, FC39BY, and FC75ABr showed maximum activity against S. aureus, K. pnuemoniae, P. aeruginosa, E. coli, Shigella sp., and S. typhi. FC30AGr extract inhibited the growth of S. epidermidis and P. aeruginosa by forming maximum zone. Among all these five strains FC2AP, FC8ABr, and FC75ABr exhibited highest activity against maximum of ten pathogens.
Fig. 3. Antimicrobial activity of crude extracts from five endophytic fungal strains. EAE, ethyl acetate extract; ME, methanol extract; AE, acetone extract; EE, ethanol extract.
DPPH radical scavenging activity and reducing power of the fungal extracts
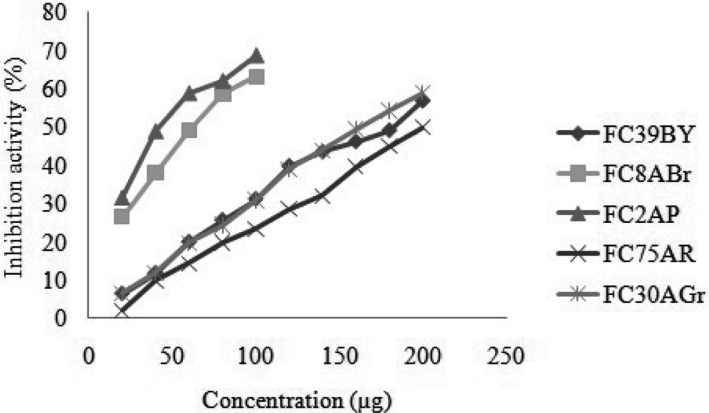
The free radical scavenging activities of fungal strains were determined by using ascorbic acid as standard. The absorbance of DPPH radical decreases by the antioxidant due to the scavenging mechanism so the radical by hydrogen donation. It was noted by its colour change from purple to yellow. The strains FC39BY, FC8ABr, FC2AP, FC75ABr, and FC30AGr showed 50% inhibition at a concentration of 174 µg, 62 µg, 43 µg, 200 µg, and 161 µg, respectively. This proved that the fungal extracts had the hydrogen donating capabilities at stipulated concentration and act as an antioxidant. Among the five extracts, FC2AP and FC8ABr were found to be acting as antioxidants, at least IC50 value (Fig. 4).
Fig. 4. 2,2-Diphenyl-1-picrylhydrazyl radical scavenging assay for five potential endophytic fungi from Aegle marmelos. Crude metabolites in different concentration (10~200 µg/µL) make up to 1mL concentration for the test.
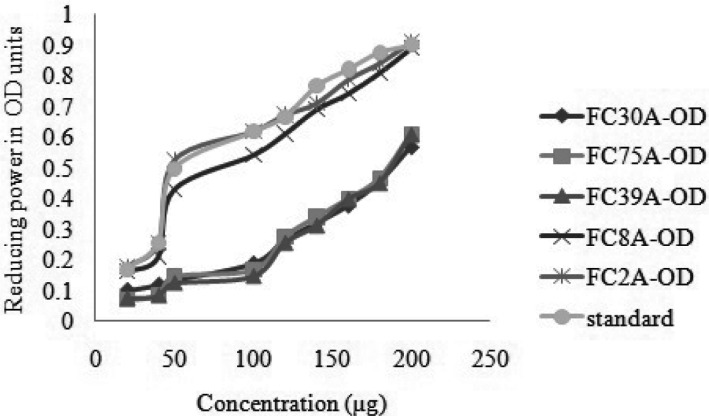
The reducing capacity of five different fungal extracts was determined by increasing Optical Density units (Fig. 5). On comparing with five fungal crude metabolite extracts, FC2AP had highest reductive power ability even at least concentration. During the reaction, the antioxidant present in the fungal extracts convert Fe3+ to Fe2+ and generally they were associated with the presence of reductones by breaking the free radical chain on donation of hydrogen.
Fig. 5. Reducing power assay for five potential endophytic fungi from Aegle marmelos. Crude metabolites in different concentration (10~200 µg/µL) make up to 1mL concentration for the test.
Metal chelating activity of the fungal extracts
The fungal extracts interfered with the formation of ferrous and ferrozine complex, indicating the presence of chelating activity by the antioxidants which leads to the capturing of ferrous ion before ferrozine can quantitatively form complexes with Fe2+. Therefore, the levels of this Fe2+ in cells should be monitored and controlled carefully as the increased levels leads to cell damage. As all the five fungal extract were assessed to interrupt the complex formation at specific levels. The interruption activity at 50% of concentrations for FC39BY, FC8ABr, FC2AP, FC75ABr, and FC30AGr were 156 µg, 66 µg, 50 µg, 181 µg, and 178 µg, respectively (Fig. 6). So, the extracts of these strains were well determined to be best antioxidants by the inhibition of red coloured ferrozine complex at lower concentrations.
Fig. 6. Metal chelating assay for five potential endophytic fungi from Aeglemarmelos. Crude metabolites in different concentration (10~200 µg/µL) make up to 1mL concentration for the test.
DISCUSSION
In searching for novel molecules, the endophytic fungi represent an important genetic resource [13]. There is a greater opportunity to find new and interesting endophytic metabolites by considering the myriad of plants in the world [14]. Several investigations were made with the endophytic fungal isolation from medicinal plants but non-invasive plants are overlooked for this study despite their uses in some traditional medicines and the uniqueness of their survival features. There are several reports solely on ecological and diversity role with biological elucidation. The research has been carried out on endophytic fungal isolation from the traditional medicinal tree (Aegle marmelos) which was considered as a religious and a sacred tree in India. In this research, prominent 67 pigmented endophytic fungi were isolated which included the members of Ascomycotina and Deuteromycotina isolated from the regions of the Western Ghats (Hotspot area). Reports of Gond et al. [2] exhibited that about 79 fungal strains were isolated from A. marmelos containing prevalent members of ascomycotina than deuteromycotina. The pigmented fungi were aimed for this research as they may contain more antioxidant properties than non-pigmented strains through the fragmentary reports already stated. In this investigation, five potential fungal isolates were screened through antagonism and this revealed that the Western Ghats were more prevalent and rich with the diversity of bioactive metabolites/compounds. The endophytic fungal strains that exhibited characteristic colony morphology and microscopic features such as hyphal and spore arrangements were putatively identified at genus level which was similar to the investigation of Larone [15]. The endophytes which could not be easily identified through these morphological features were subjected to sequencing of ITS1 and ITS4, as molecular identification and the sequences were aligned with the databases from NCBI to obtain the species level and closely related species for the working strain. The results showed that the characterized fungal strains belonged to Ascomycetes.
Majorly, three species were dominated by class dothideomycetes and it included C. australiensis (FC2AP), Al. alternata (FC39BY), and A. citrimacularis (FC8ABr). These isolates were very potent, which was evident by the production of antimicrobial and antioxidant agents. There were differences between the fungal strains in the functional characteristics with respect to their ability in the production of pigmented secondary metabolites [16]. Our investigation implies that the members of dothideomycetes especially the order Pleosporales showed a higher functional versatility, and the fungi belonging to this group produce a wide range of biomolecules with bioactivity such as antimicrobial agents and the same was also reported in a research by Bhagat et al. [17]. The strains FC2AP and FC8ABr belonging to the order Pleosporales exhibited highest antimicrobial activities against clinical pathogens. In order to induce the highest production of antimicrobial and antioxidant metabolites, the study had designed by employing different media and it was found that the metabolite production increased when amended with dextrose, this piece of work was also observed in the investigation by Padhi and Tayung [18] and they investigated the different media for increased metabolite production by the endophytic fungi. The metabolite production increased with the incubation time as it is a crucial parameter in metabolite production. Maximum metabolite production was observed to be 9~10 days in most of the fungi [19], but in this study we observed the optimum incubation period range for all the five strains to be 11~21 days approximately. Maximum metabolites were produced by members in order Pleosporales and Eurotiales. A report from Strobel et al. [20] stated that the fungus Gliocladium roseum produced volatile antimicrobial metabolites after 18th day of incubation. Similarly, in our investigation FC8ABr strain produced coloured volatile metabolite after 18th day incubation and it showed the maximum inhibition against tested pathogens.
The metabolites are found to contain antioxidant properties and hereby antioxidant is defined as a biomolecule which donates hydrogen to the free radicals, causes damage to cells and tissues in humans and animals. In order to prevent this, it is essential for us to intake the antioxidant at specific levels in diet and hence, the microbial secondary metabolites are likely to have antioxidant properties where these can be modulated for medicinal purposes. The endophytic fungal extracts on antioxidant evaluation exhibited highest antioxidant activity even at least concentrations, especially isolates belonging to order Pleosporales unveiled highest antioxidant levels at lower concentration than other isolates and this is similar to the reports of Bhagat et al. [17]. The extracts were able to reduce the stable radical DPPH to the yellow coloured diphenylpicryl hydrazine. The scavenging activity with DPPH radical was found to increase with an increase in concentrations. Extracts of Al. alternata FC39BY manifested antioxidant activity at 50% which is similar to the earlier reports. Also these results coincide with the investigation of Silva et al. [21] that Al. alternata extract represented a moderate antioxidant activity when compared to antimicrobial testing where the concentration of these two testing differed in activity. Earlier reports suggested that the members of order Pleosporales explored anticancer properties and in the same way our investigation also substantiated by the results obtained in antioxidant assessment for FC2AP and FC8ABr. Comparing DPPH radical scavenging activity with the metal chelating activity, the concentrations of fungal extracts were found to evince higher activity at lower concentrations for the second. It was reported that formation of r-bonds with the metal by chelating agents, which are found to be very effective because the redox potential was reduced as they are secondary antioxidants and thereby stabilizing the oxidized form of the metal ion [22]. Though the Cu2+ and Fe2+ are necessary for the organism's transport, protection against oxidative stress, cell growth and development, they can also catalyze hydroxyl radical formation via Fenton's reaction [23]. Extracts of potent fungal isolates are antioxidants and they inhibit free ions by chelating effects and thus helped in controlling the levels of Cu2+ and Fe2+ without causing any damage to the cell. With respect to this, isolates of Pleosporales (FC2AP and FC8ABr) had high chelating capacity which could easily trap the free radicals at minimal concentration.
In conclusion, the endophytic fungal strains isolated from ethnomedicinal plants were explicating antioxidant components and scavenging effects at different levels. From the results obtained, it is evident that the potent strains of this research have the prospective to be exploited as sources of novel bioactive metabolites, which may exhibit anticancer activity at lower concentrations as they revealed high antioxidant activities. Further, this research will focus on biomolecule purification and understanding the chemical nature of members in order Pleosporales for their anticancer evaluation through in vivo studies.
References
- 1.Ding X, Liu K, Deng B, Chen W, Li W, Liu F. Isolation and characterization of endophytic fungi from Camptotheca acuminata. World J Microbiol Biotechnol. 2013;29:1831–1838. doi: 10.1007/s11274-013-1345-x. [DOI] [PubMed] [Google Scholar]
- 2.Gond SK, Verma VC, Kumar A, Kumar V, Kharwar RN. Study of endophytic fungal communities from different parts of Aegle marmelos Correae (Rutaceae) from Varanasi (India) World J Microbiol Biotechnol. 2007;23:1371–1375. [Google Scholar]
- 3.Rani P, Vasisht K, Khullar N. Bio-autography: an efficient method to check the in vitro antimicrobial activity of Aegle marmelos against enteric pathogens. IIOAB J. 2013;4:4–9. [Google Scholar]
- 4.Dobranic JK, Johnson JA, Alikhan QR. Isolation of endophytic fungi from eastern larch (Larix laricina) leaves from New Brunswick, Canada. Can J Microbiol. 1995;41:194–198. [Google Scholar]
- 5.Parija SC, Prabhakar PK. Evaluation of lacto-phenol cotton blue for wet mount preparation of feces. J Clin Microbiol. 1995;33:1019–1021. doi: 10.1128/jcm.33.4.1019-1021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doss RP, Welty RE. A polymerase chain reaction-based procedure for detection of Acremonium coenophialum in tall fescue. Phytopathology. 1995;85:913–917. [Google Scholar]
- 7.Lacap DC, Hyde KD, Liew EC. An evaluation of the fungal 'morphotype' concept based on ribosomal DNA sequences. Fungal Divers. 2003;12:53–66. [Google Scholar]
- 8.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 9.Barry AL, Garcia F, Thrupp LD. An improved single-disk method for testing the antibiotic susceptibility of rapidly-growing pathogens. Am J Clin Pathol. 1970;53:149–158. doi: 10.1093/ajcp/53.2.149. [DOI] [PubMed] [Google Scholar]
- 10.Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 11.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–315. [Google Scholar]
- 12.Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 13.Guimarães DO, Borges WS, Kawano CY, Riebeiro PH, Goldman GH, Nomizo A, Thiemann OH, Oliva G, Lopes NP, Pupo MT. Biological activities from extracts of endophytic fungi isolated from Viguiera arenaria and Tithonia diversifolia. FEMS Immunol Med Microbiol. 2008;52:134–144. doi: 10.1111/j.1574-695X.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 14.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larone DH. Medically important fungi: a guide to identification. 4th ed. Washington, DC: ASM Press; 2002. [Google Scholar]
- 16.Peláez F, Collado J, Arenal F, Basilio A, Cabello A, Díez Matas MT, García JB, González del Val A, González V, Gorrochatgui J, et al. Endophytic fungi from plants living on gypsum soils as a source of secondary metabolites with antimicrobial activity. Mycol Res. 1998;102:755–761. [Google Scholar]
- 17.Bhagat J, Kaur A, Sharma M, Saxena AK, Chadha BS. Molecular and functional characterization of endophytic fungi from traditional medicinal plants. World J Microbiol Biotechnol. 2012;28:963–971. doi: 10.1007/s11274-011-0894-0. [DOI] [PubMed] [Google Scholar]
- 18.Padhi S, Tayung K. Antimicrobial activity and molecular characterization of an endophytic fungus, Quambalaria sp. isolated from Ipomoea carnea. Ann Microbiol. 2013;63:793–800. [Google Scholar]
- 19.Gogoi DK, Boruah HP, Saikia R, Bora TC. Optimization of process parameters for improved production of bioactive metabolite by a novel endophytic fungus Fusarium sp. DF2 isolated from Taxus wallichiana of North East India. World J Microbiol Biotechnol. 2008;24:79–87. [Google Scholar]
- 20.Strobel GA, Knighton B, Kluck K, Ren Y, Livinghouse T, Griffin M, Spakowicz D, Sears J. The production of mycodiesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072) Microbiology. 2008;154:3319–3328. doi: 10.1099/mic.0.2008/022186-0. [DOI] [PubMed] [Google Scholar]
- 21.Silva GH, Teles HL, Trevisan HC, Bolzani VD, Young MC, Pfenning LH, Eberlin MN, Haddad R, Costo-Neto CM, Araujo AR. New bioactive metabolites produced by Phomopsis cassiae, an endophytic fungus in Cassia spectabilis. J Braz Chem Soc. 2005;16:1463–1466. [Google Scholar]
- 22.Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJ, editor. Food antioxidants. Amsterdam: Springer; 1990. pp. 1–18. [Google Scholar]
- 23.Campos EG, Jesuino RS, Dantas AD, Brígido MD, Felipe MS. Oxidative stress response in Paracoccidioides brasiliensis. Genet Mol Res. 2005;4:409–429. [PubMed] [Google Scholar]