Abstract
Culture filtrates of six different edible mushroom species were screened for antimicrobial activity against tomato wilt bacteria Ralstonia solanacearum B3. Hericium erinaceus, Lentinula edodes (Sanjo 701), Grifola frondosa, and Hypsizygus marmoreus showed antibacterial activity against the bacteria. Water, n-butanol, and ethyl acetate extracts of spent mushroom substrate (SMS) of H. erinaceus exhibited high antibacterial activity against different phytopathogenic bacteria: Pectobacterium carotovorum subsp. carotovorum, Agrobacterium tumefaciens, R. solanacearum, Xanthomonas oryzae pv. oryzae, X. campestris pv. campestris, X. axonopodis pv. vesicatoria, X. axonopodis pv. citiri, and X. axonopodis pv. glycine. Quantitative real-time PCR revealed that water extracts of SMS (WESMS) of H. erinaceus induced expressions of plant defense genes encoding β-1,3-glucanase (GluA) and pathogenesis-related protein-1a (PR-1a), associated with systemic acquired resistance. Furthermore, WESMS also suppressed tomato wilt disease caused by R. solanacearum by 85% in seedlings and promoted growth (height, leaf number, and fresh weight of the root and shoot) of tomato plants. These findings suggest the WESMS of H. erinaceus has the potential to suppress bacterial wilt disease of tomato through multiple effects including antibacterial activity, plant growth promotion, and defense gene induction.
Keywords: Antibacterial activity, Bacterial wilt disease of tomato, Defense genes, Spent mushroom substrate, Suppression
The global cultivation of edible mushrooms is attributed to their nutritional, medicinal, ecological, and economical value. In Korea, the production of edible mushrooms was estimated to be 614,224 tons a year [1]. Mushrooms belong to class Basidiomycetes and produce a large number of biologically active compounds that exhibit antibacterial, antifungal and antiviral, cytotoxic, or hallucinogenic activity [2,3,4]. Previous screening studies on 317 isolates representing 204 species of basidiomycetes evaluated their antimicrobial activity against a range of human pathogens including bacterial and fungal species [3]. Several bioactive compounds such as antibiotics, pigments, antioxidants, antihypertensive agents, antitumor agents, and biosurfactants have been extracted from mycelial leachate and fruiting bodies of mushrooms and have been characterized.
The culture types of fungi have been classified into solid-state fermentation (SSF) and submerged fermentation (SmF) based primarily on the type of substrate they use [5,6]. SSF utilizes solid substrates such as bran, bagasse, and paper pulp and is best suited for fermentation of fungi to obtain high yield of certain bioactive compounds. Recent advances have also suggested that SSF produces more stable and greater quantity of antibiotics than that obtained by SmF [6]. Spent mushroom substrate (SMS) is the post-harvest substrate and retains a variety of bioactive substances such as extracellular enzymes, antibiotics, secondary metabolites, and carbohydrates produced during mycelium and fruiting-body formation [7]. Approximately 5 kg of substrate is estimated to produce 1 kg of mushroom, and consequently about 2.5 million tons of SMS is annually generated as agricultural waste in Korea. Different SMSs have been used for production of value-added products such as decolorization [1] and lignocellulytic enzymes [8], bioconversion into organic fertilizer [9], and for use as animal feed supplements [7]. Mushrooms are known to naturally produce low- and high molecular weight, compounds against gram-negative and -positive bacteria [4].
Current researches have focused mainly on fungal therapeutics for human pathogens rather than control of plant diseases. Phytopathogenic bacteria have resulted in tremendous economic loss of different crops. One such soil borne bacterium is Ralstonia solanacearum, which causes bacterial wilt in tomato, resulting in severe yield and quality reductions (about 20~25% yearly) in Korea. The bacterium enters plant roots through wounds and colonizes the vascular tissues of the stem, leading to wilt and decay of the infected tissues [10,11]. Agrochemicals, copper, and antibiotics are being used as pesticides to control these bacterial disease, but are expensive and detrimental to the environment because of their effect on groundwater, air pollution, human health, and the ecosystem. Hence, nonchemical (biological) control of plant pathogens has received much attention worldwide. SMS derived from edible mushrooms are eco-friendly and can be used for suppression of plant pathogenic bacteria as it not harmful to humans. Water extract of SMS from mushroom species have been used to suppress plant diseases such as Pythium dampening-off, apple scab, cucumber anthracnose caused by fungal pathogens [12,13].
Mycelial leachate of Lentinula edodes (Shiitake) was applied as biological control of plant disease, to suppress the bacterial wilt of tomato [14]. SMS is believed to possess multiple beneficial functions. Recently, it was reported that autoclaved water extract from SMS of the edible mushroom, Lyophyllum decastes (hatakeshimeji) could induce expression of systemic acquired resistance (SAR) related defense genes in plants [15]. H. erinaceus has been known to have anti-microbial activity on bacteria infecting humans [16]; however, there has been no report of its activity for bacterial disease control in plants.
Our study aims to investigate antibacterial activity of H. erinaceus SMS against phytopathogenic bacteria and evaluate the role of this extract in improving plant defense and growth. Further, the water extracts from SMS of H. erinaceus were also tested for their ability to suppress tomato bacterial wilt disease caused by R. solanacearum.
MATERIALS AND METHODS
Mushroom species and bacterial strains
Edible mushroom species Hericium erinaceus Noru 2, Pleurotus eryngii, Lentinula edodes, Flammulina velutipes, Grifola frondosa, Daedaleopsis tricolor, Pleurotus ostreatus, Pycnoporus coccineus, and Hypsizygus marmoreus were obtained from the Mushroom Research Institute (Gwangju, Korea). These edible mushrooms were maintained on potato dextrose agar (PDA; Difco, Sparks, MD, USA). The plant pathogenic bacteria used in this study, namely, Xanthomona scampestris pv. campestris (black rot on Chinese cabbage), Ralstonia solanacearum (bacterial wilt on tomato), Agrobacterium tumefaciens 208 (crown gall), Pectobacterium carotovorum subsp. carotovorum (soft rot), Xanthomonas oryzae pv. oryzae KACC10859 (bacterial blight on rice), X. axonopodis pv. citri KACC 10443, Pseudomonas tolaasii KACC10365, and X. axonopodis pv. glycines KACC 11144 were obtained from Korean Agricultural Type Collection (KACC) and National Academy of Agricultural Science (NAAS), Rural Development Administration (RDA), Wanju. Escherichia coli DH5 was obtained from a laboratory collection.
Preparation of culture filtrates of edible mushrooms
Mycelia of edible mushrooms (listed in Table 1) were grown on PDA (Difco). Mycelial blocks (5 mm × 5 mm) were cut out from the media, inoculated in 100mL of sterile potato dextrose broth (PDB) in a 500-mL Erlenmeyer flask, and incubated at 24℃ for 28 days with shaking at 120 rpm. The culture fluid was harvested by filtration through a Whatman No. 1 filter paper and a 0.22-µm filter (Millipore, Billerica, MA, USA).
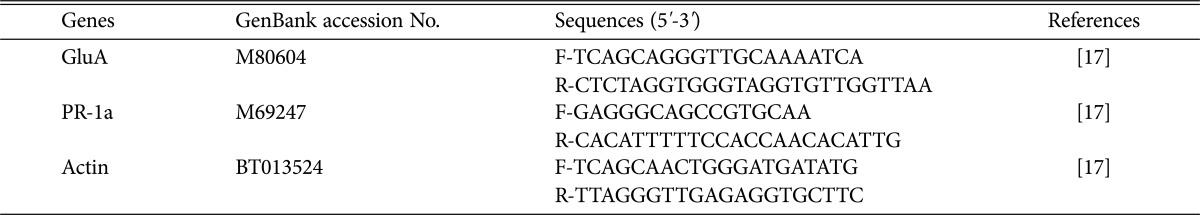
Table 1. Primer used for the quantitative real-time RT-PCR analysis of defense related tomato genes.
RT-PCR, reverse transcriptase polymerase chain reaction.
Water extract of SMS (WESMS)
Post-harvest substrates from bag-cultivated mushroom species were provided by MushArt farm in the Gyeonggi-do region, Republic of Korea. The SMS (300 g) was mixed with 900 mL distilled water by shaking at 150 rpm for 2 hr. The mixture was then filtered through two layers of Miracloth (Calbiochem, La Jolla, CA, USA). The filtrate was centrifuged for 10 min at 10,000 rpm and the supernatant was used as the water extract of SMS.
Antibacterial assay
Each aliquot (50 µL) of SMS extracts or culture filtrate from each edible mushroom species was loaded on filter paper disks (5 mm in diameter) and the filter paper was placed on nutrient agar overlaid with soft agar (0.8%) mixed with bacterial cells (5 × 106 cells). Filter paper disks soaked in sterile PDB or water extract of pre-inoculation substrate (PIS) were used as controls. The plates were incubated at 28℃ for 3 days and the zone of inhibition with exception of the paper disk were evaluated. To study the antibacterial spectrum of solvent extracts from SMS of H. erinaceus, 5 × 105 cells of each phytopathogenic bacteria were used.
Antibacterial solvent extraction from SMS of H. erinaceus
Methanol solution (70%) was mixed with SMS (500 g) of H. erinaceus, homogenized 1,200 rpm for 2min, filtered through two layers of Miracloth (Calbiochem) and the filterate was centrifuged for 10 min at 10,000 rpm. The supernatant was evaporated under reduced pressure to remove methanol and the resulting residue was extracted with n-hexane. Subsequently the resulting water layer residue was serially extracted with chloroform, ethyl acetate and nbutanol to separate different antibacterial compounds. Each solvent extract was concentrated in a rotary evaporator, dried at room temperature, dissolved in 70% methanol and adjusted to a final concentration of 150 mg/L.
Bacterial wilt disease assay on tomato
Seedlings (25 days old) of tomato cultivar, Superdoterang with four to five fully expanded leaves grown in pot (9 cm) were used for this experiment. The tomato seedlings were irrigated with WESMS (50 mL) of H. erinaceus. Three days later, marginal roots of the seedlings were wounded with scissors and drenched in the bacterial suspension (5 × 106 cells) of R. solanacearum B3. After 6~14 days of inoculation, disease index was evaluated on a scale of 0~5; with 0, no visible disease symptoms; 1~4, 1~25%, 26~50%, 51~70% disease symptoms; 4, 71~90% of plant wilt; and 5, complete plant wilt.
Gene expression analysis using quantitative real-time PCR (qRT-PCR)
Expression patterns of the defense response genes encoding β-1,3-glucanase (GluA) and pathogenesis-related protein-1a (PR-1a) in tomato [17] were analyzed using qRT-PCR. Each primer set used in this study to amplify GluA and PR-1a are listed in Table 1. For RNA extraction, tomato seedlings grown in greenhouse for four weeks were treated with (50 mL) and roots and leaves were collected at 0, 24, 48, and 72 hr post-treatment. Samples were snap frozen in liquid nitrogen and stored at 80℃ until required. Total RNA was isolated using TRIzol-Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and treated with DNase (Qiagen, Valencia, CA, USA). One microgram of DNA-free RNA was used for first-strand cDNA synthesis using the SuperScript III First-strand Synthesis system for RT-PCR (Invitrogen) according to the manufacturer's instructions. High-throughput qRT-PCR reactions were performed in a LightCycler 96 System (Roche, Mannheim, Germany) using SYBR Green 1 (Roche). Reaction parameters were set as follows: 10 min for polymerase enzyme activation, followed by 40 cycles of 95℃ for 15 sec and 57℃ for 1 min. Actin was used as a reference gene and tomato plants were used as the no treatment controls (NTC). The fold increase in the expression of each gene was calculated after normalization using the formula:
RESULTS AND DISCUSSION
Antibacterial activity of mushroom species
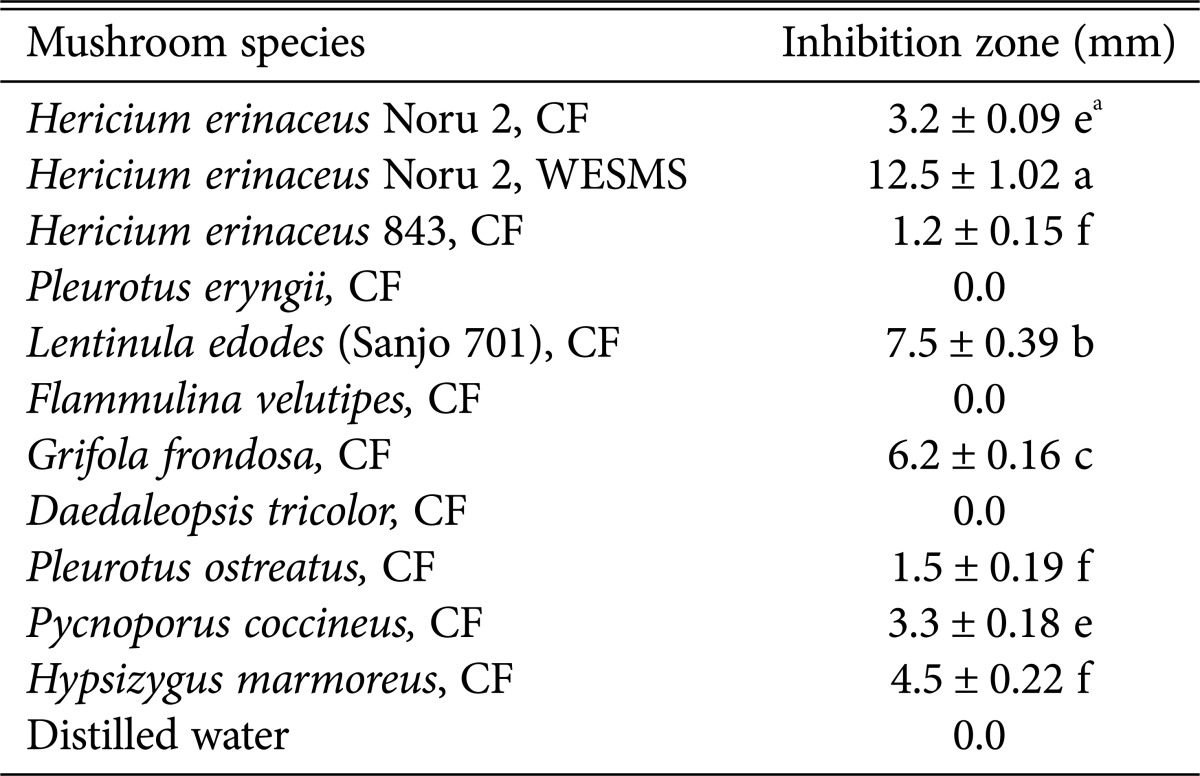
The paper disk assay was performed to analyze the antibacterial activity of SMS from mushroom species. Culture filtrates and WESMS from different edible mushroom species was concentrated thrice for use in the assay. Culture filtrates of Hericium erinaceus, Lentinula edodes (Sanjo 701), Grifola frondosa, and Hypsizygus marmoreus showed antibacterial activity against R. solanacearum B3 forming clear zones of inhibition (Table 2). However, culture filtrate of Pleurotus eryngii, Flammulina velutipes, and Pleurotus ostreatus did not exhibit this property. The water extracts of different edible mushrooms showed different degrees of growth inhibition of tomato bacterial wilt pathogen, R. solanacearum B3. WESMS of H. erinaceus formed a larger inhibition zone (12.5 mm) than that of its culture filtrate (3.2 mm) of its culture. Studies on edible and wild type mushrooms, such as Agaricus bisporus, Lentinula edodes, and Auricularia auricula, and many Pleurotus species have focused primarily on the antagonistic effects against human pathogenic bacteria and fungi [3,4]. Fruiting bodies of edible mushrooms and mycelia have been the main source of clinical effects against these pathogens. Recently, mycelial cultures of certain mushroom species have been implicated in the suppression of phytopathogenic fungi and bacteria [18]. In practice, however, the use of mushroom culture filtrates in the field is limited owing to the need for expensive equipment for mass culturing and labor-intensive aseptic culturing processes. Though SMS from edible mushrooms such as Lentinus edodes, Lyophyllum decastes, P. eryngii, and P. columbines have been used to suppress fungal and bacterial diseases [12,13], most previous investigations using SMS focused on the biological control of fungal plant diseases than bacterial. Interestingly, the mycelial leachate of L. edode suppressed the growth of several plant pathogenic bacteria [14]. Although WESMS of H. erinaceus have been shown to exhibit antibacterial activity against human bacterial pathogens [16], this is the first report of its growth inhibition effect on a plant pathogenic bacterium.
Table 2. Antibacterial activity of cultural extracts from different edible mushroom species.
All data is abstained from four replicates.
CF, cultural filtrate; WESMS, water extract spent mushroom substrate.
aThe different letters are significantly different (p < 0.05) according to Duncan's multiple range test.
The mushroom substrate uses lignocellulosic wastes such as corncobs, sugarcane wastes, cottonseed hull, cotton and beet pulp, sawdust supplement and rice bran similar to that used for SSF. SSF is the best fermentation technique for fungi [5], since the substrates are utilized very slowly and steadily through long fermentation periods and bioactive compounds with high quantity are released in the substrate. It is known that SSF produces higher quantities of certain bioactive compounds and more stable antibiotics compared to SmF. Therefore, higher antibacterial activity in SMS of H. marmoreus than that of its culture filtrate in this study may be attributed to the analogous characteristics of SMS and SSF.
Antibacterial spectrum of solvent extracts from SMS of H. erinaceus
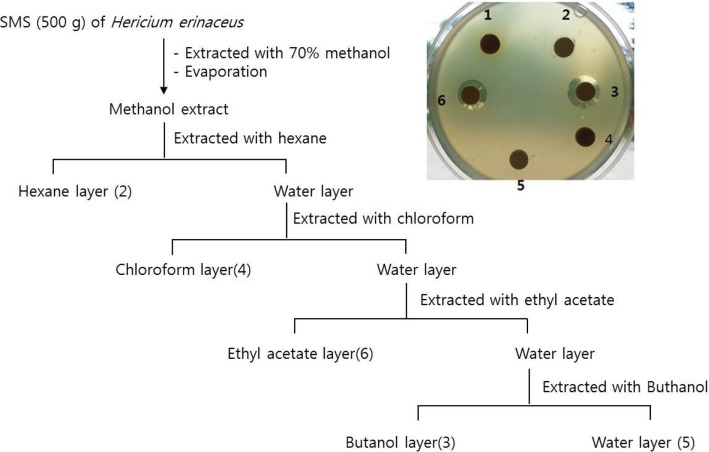
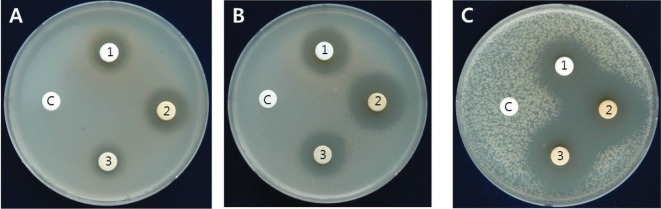
In order to know antibacterial property in H. erinaceus SMS, 70% methanol extraction solution from the SMS was used for extracting antibacterial fractions with the procedure depicted in Fig. 1. To start with, the solution was extracted with hexane; however, antibacterial activity exhibited in water fraction and not in hexane fraction. The extraction from the water layer was done with chloroform, ethyl acetate and n-butanol. As shown in Fig. 1, out of different solvent extracts, ethyl acetate and n-butanol extracts showed antibacterial activity against the bacterial cells, forming inhibition zone; whereas the remnant water fractions did not show antibacterial activity. Generally, it has been known hexane is used to extract lipophilic compounds, while ethyl acetate and n-butanol is useful for extracting non-polar compounds [19]. Actually, the dried ethyl acetate and n-butanol fractions were dissolved in absolute methanol, but not in water. Thus, it is assumed that the nonpolar compounds in the WESMS of H. erinaceus play critical role in antibacterial function. Furthermore, antibacterial spectrum of WESMS, ethyl acetate and butanol extract was tested on nine plant pathogenic bacteria that caused major bacterial diseases on different crops in Korea (Table 3, Fig. 2). The extracts exhibited growth inhibition zones ranging from 2.4 to 26.5 mm on the bacterial cells, especially, strongly inhibiting the growth of Agrobacterium tumefaciens 208 and Xanthomonas campestris pv. campestris, Xanthomonas axonopodis pv. vesicatoria, X. axonopodis pv. citri, and X. axonopodis pv. glycines forming inhibition zones greater than 14 mm.
Fig. 1. Antibacterial activity on Ralstonia solanacearum B3 cells of layers of spent mushroom substrate (SMS) extracted by different solvents. Numbers on extraction layers of scheme are in accordance with those of image.
Table 3. Antibiotic activity of different extracts from spent mushroom substrate of Hericium erinaceus.
All data was abstained from four replicates.
WESMS, water extract spent mushroom substrate.
aThe different letters are significantly different (p < 0.05) according to Duncan's multiple range test.
Fig. 2. Antibacterial activity of different extracts from spent mushroom substrate of Hericium erinaceus on Ralstonia solanacearum Biovar 3 (A), Agrobacterium tumefaciens A208 (B), and X. campestris pv. glycines KACC 10491 (C), with water extract (1); ethyl acetate extract (2) (150 mg/L); butanol extract (3) (150 mg/L); and negative control treated with water (c).
In previous studies, culture filtrates of 27 edible mushrooms were screened for antimicrobial activity against plant pathogens [18], and Agrocybe cylindracea, Grifola frondosa, and Lentinula edodes showed varying zones of inhibition against different bacterial species. In addition, the mycelial leachate of L. edodes has also been reported to contain substances that suppressed the growth of plant pathogens, such as Pseudomonas syringae pv. glycinea, P. syringae pv. tabaci, Xanthomonas campestris pv. glycines, Erwinia amylovora, and Curtobacterium flaccumfaciens pv. flaccumfaciens [14].
Effect of WESMS on expression of defense genes in H. erinaceus
Gene expression analysis using real-time PCR showed that the expressions of the PR-1a and GluA genes were significantly enhanced in WESMS-treated tomato plants compared to water-treated control plants at 72 hr (Fig. 3). Both these genes are related to SAR, which can be induced by a variety of agents, including necrotizing pathogens and certain chemicals and are regulated by a salicylic acid-dependent process [20]. In contrast, induced systemic resistance (ISR) is caused because of colonization of plant roots by certain strains of plant growth-promoting rhizobacteria and is mediated by a jasmonate- and ethylene-sensitive pathway. Treatment of plants with agents such as cell wall fragments, plant extracts, and synthetic chemicals, can induce resistance to subsequent pathogen attack both locally and systemically [21]. In tomato, defense genes related to SAR and ISR are activated by acibenzolar-S-methyl [22], β-aminobutyric acid [23], and extracellular polysaccharide of R. solanacearum [17].
Fig. 3. Real-time PCR analysis of β-1,3-glucanase (A) and pathogenesis-related protein-1a (B) gene expressions in tomato plants treated with water extract from spent mushroom substrate (WESMS). The total RNA was extracted from tomato leaves at 0 and 72 hr after treatment with water and WESMS and subjected to quantitative real-time PCR. Each value is the average of three replications and the bar indicates the standard deviation. Striped and black bars indicate gene expressions of β-1,3-glucanase and pathogenesis-related protein-1a, respectively, treated with WESMS. White bars indicate those treated with water. Asterisks indicate significant differences compared with the results after distilled water (DW) treatment (t-test, p < 0.05).
Several studies have reported the use of elicitors from the cell walls of pathogens and non-pathogens to induce resistance in plants [17]. Chitins and glucans are key skeletal polysaccharides of fungal cell walls, and in general, have been recognized as elicitors of a plant defense response for many years [24,25]. In mushrooms, elicitors from fruiting body of Shiitake (Lentinula edodes) have been purified to control cucumber anthracnose [26]. In addition, it was also demonstrated that water extract from SMS of hatakeshimeji (Lyophyllum decastes) could control cucumber anthracnose disease and enhance the state of SAR inducing defense genes such as chitinase and β-1,3-glucanase [15].
In the present study, water extract from SMS of H. erinaceus activated defense genes related to SAR. SAR is widely known to suppress a broad spectrum of plant pathogens, including fungi, bacteria, and viruses [21]. Carbohydrate and protein elicitors that induce defense mechanism in plants are released from the mycelia of fungal pathogens. Therefore, SMS of H. erinaceus that diffuses into the mushroom mycelia could be an abundant source of elicitors for SAR. However, future investigations are needed to identify the elicitors associated with SAR induction in tomato plants treated with WESMS of H. erinaceus.
Protective effect of H. erinaceus WESMS against bacterial wilt disease of tomato
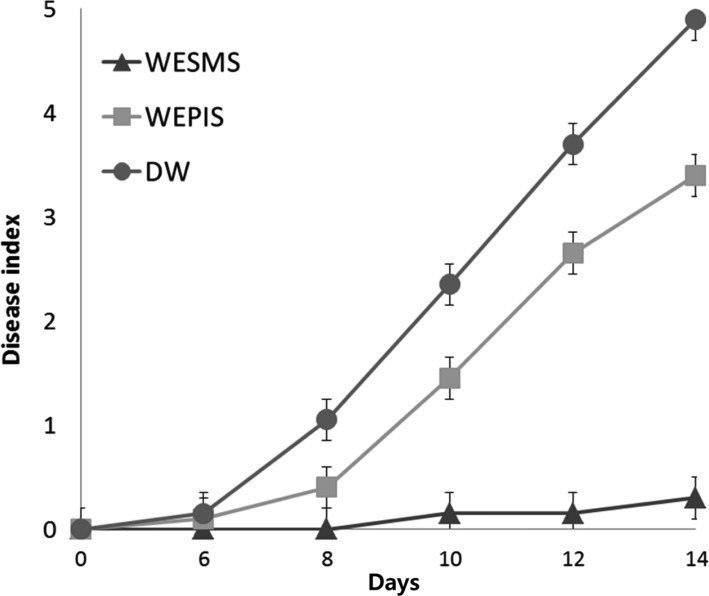
To investigate if the protective effect of WESMS is derived from SMS of H. erinaceus, tomato plants were treated with WESMS before bacterial inoculation of PIS. Plants incubated for 14 days after WESMS, water extract of pre-inoculated substrate substrate (WEPIS), and water treatment showed 15%, 68%, and 98% disease incident, showing highly protective effect of 85% against tomato wilt disease (Fig. 4). As shown in Fig. 5, tomato plants treated with WESMS were healthy; however, all the water-treated tomato plants exhibited wilted symptoms. It is speculated that the antibacterial compounds and elicitors in WESMS of H. erinaceus may be play critical role in suppressing the tomato wilt disease by inhibiting cell density of the pathogen and inducing defense genes in tomatoz.
Fig. 4. Suppression of bacterial wilt disease on tomato plants treated with water extract from spent mushroom substrate of Hericium erinaceus. Ralstonia solanacearum Biovar 3 cells (5 × 106) were inoculated on tomato seedlings in 3 days on the third day after treatment with water extract from spent mushroom substrate (WESMS), water extract of pre-inoculated substrate substrate (WEPIS), and distilled water (DW). Disease symptoms was checked at every two-day interval from 6th day after inoculation. The disease symptoms was evaluated based on the percentage of wilted plants on an index scale of 0 = no visible disease symptoms; 1 = 1~25%; 2 = 26~50%; 3 = 51~70%; 4 = 71~90% of wilted plants; and 5 = completely wilted plant. Each point represents the mean disease index (± SE) for three independent experiments, each containing 20 plants per treatment.
Fig. 5. Effect of water extract from spent mushroom substrate (WESMS) of Hericium erinaceus on disease severity of bacterial wilt of tomato caused by Ralstonia solanacearum B3. Disease symptom was observed after 14 days of inoculation.

The focus of research has mainly been on the role of WESMS in suppression of diseases caused by fungal plant pathogens. However, in a previous study that investigated the suppression effect of SMS of the edible mushrooms Lyophyllum decastes and Pleurotus eryngii against fungal and bacterial diseases in cucumber plants [13], Lyophyllum decastes was found to significantly reduce powdery mildew by Podosphaera xanthii and, angular leaf spot by Pseudomonas syringae pv. lachrymans. It was demonstrated that the bacterial suppression event may be due to induction of SAR-related defense genes such as β-1,3-glucanase [17].
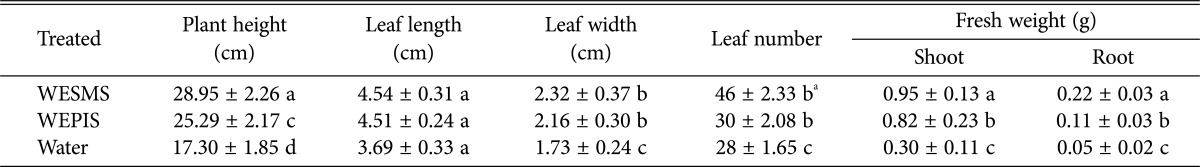
In addition, we investigated if WESMS of H. erinaceus promotes tomato growth, by measuring plant height, leaf number, and fresh weights of root and shoot. Interestingly, WESMS-treatment dramatically promoted the growth of tomato plants compared to water- and WEPIS-treatment (Table 4). Particularly, the highest mean number of 48 leaves in WESMS-treated plants; this is significant since leaves are vital sites of photosynthesis in green plants. Moreover, WESMS treatment also increased the fresh weight of root and shoot from 2 to 20 folds. These results demonstrate that WESMS of H. erinaceus are beneficial to tomato plants with respect to growth promotion as well as disease suppression. Recently, it was reported that SMS of oyster mushroom and button mushroom promoted growth of pepper plant [27]. SMS is considered to be a good source of organic matter and rich in macro- and microelements, thus promoting plant growth, disease suppression and help to increase the soil biological activity [7,28].
Table 4. Effect on tomato growth promotion of WESMS of Hericium erinaceus.
WESMS, water extract spent mushroom substrate; WEPIS, water extract of pre-inoculated substrate substrate.
aThe different letters are significantly different (p < 0.05) according to Duncan's multiple range test.
To conclude, this study reveals that WESMS from H. erinaceus has multiple effects including antibacterial activity, defense gene induction, plant growth promotion and in suppressing the bacterial wilt disease of tomato. Hence, SMS of the edible mushroom H. erinaceus can be used as a eco-friendly control of this disease. Further, this is a new way of recycling the waste from mushroom cultivation, which helps alleviate the waste disposal problem in the mushroom industry. However, identification of the key antibacterial compound and main elicitor of SAR response in WESMS of H. erinaceus requires further investigation.
ACKNOWLEDGEMENTS
This research is supported by research grant (Agenda project No. PJ009969) from Rural Development Administration, Wanju, Korea.
References
- 1.Lim SH, Lee YH, Kang HW. Efficient recovery of lignocellulolytic enzymes of spent mushroom compost from oyster mushrooms, Pleurotus spp., and potential use in dye decolorization. Mycobiology. 2013;41:214–220. doi: 10.5941/MYCO.2013.41.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedict RG, Brady LR. Antimicrobial activity of mushroom metabolites. J Pharm Sci. 1972;61:1820–1822. doi: 10.1002/jps.2600611130. [DOI] [PubMed] [Google Scholar]
- 3.Suay I, Arenal F, Asensio FJ, Basilio A, Cabello MA, Díez MT, García JB, del Val AG, Gorrochategui J, Hernández P, et al. Screening of basidiomycetes for antimicrobial activities. Antonie Van Leeuwenhoek. 2000;78:129–139. doi: 10.1023/a:1026552024021. [DOI] [PubMed] [Google Scholar]
- 4.Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012;78:1707–1718. doi: 10.1055/s-0032-1315370. [DOI] [PubMed] [Google Scholar]
- 5.Subramaniyam R, Vimala R. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat. 2012;3:480–486. [Google Scholar]
- 6.Vikineswary S, Kumaran KS, Ling SK, Dinesh N, Shim YL. Solid substrate fermentation of agroresidues for value added products: the Malaysian experience. In: Wise DL, editor. Global environmental biotechnology. Dordrecht: Springer; 1997. pp. 301–305. [Google Scholar]
- 7.Suess A, Curtis J. Report: value-added strategies for spent mushroom substrate in BC. British Columbia Mushroom Industry; 2006. [Google Scholar]
- 8.Lim SH, Lee YH, Kang HW. Optimal extraction and characteristics of lignocellulytic enzymes from various spent mushroom composts. Korean J Mycol. 2013;41:160–166. [Google Scholar]
- 9.Sochtig H, Grabbe K. The production and utilization of organic-mineral fertilizer from spent mushroom compost. Mushroom Sci. 1995;14:907–915. [Google Scholar]
- 10.Hayward AC. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol. 1991;29:65–87. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- 11.Denny TP. Ralstonia solanacearum: a plant pathogen in touch with its host. Trends Microbiol. 2000;8:486–489. doi: 10.1016/s0966-842x(00)01860-6. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki R, Yamaguchi A. Spent substrate of shiitake (Lentinula edodes) inhibits symptoms of anthracnose in cucumber. Mushroom Sci Biotechnol. 2009;17:113–115. [Google Scholar]
- 13.Parada RY, Murakami S, Shimomura N, Otani H. Suppression of fungal and bacterial diseases of cucumber plants by using the spent mushroom substrate of Lyophyllum decastes and Pleurotus eryngii. J Phytopathol. 2012;160:390–396. [Google Scholar]
- 14.Pacumbaba RP, Beyl CA, Pacumbaba RO., Jr Shiitake mycelial leachate suppresses growth of some bacterial species and symptoms of bacterial wilt of tomato and lima bean in vitro. Plant Dis. 1999;83:20–23. doi: 10.1094/PDIS.1999.83.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Parada RY, Murakami S, Shimomura N, Egusa M, Otani H. Autoclaved spent substrate of hatakeshimeji mushroom (Lyophyllum decastes Sing.) and its water extract protect cucumber from anthracnose. Crop Prot. 2011;30:443–450. [Google Scholar]
- 16.Wong KH, Sabaratnam V, Abdullah N, Kuppusamy UR, Naidu M. Effects of cultivation techniques and processing on antimicrobial and antioxidant activities of Hericium erinaceus (Bull.:Fr.) Pers. extracts. Food Technol Biotechnol. 2009;47:47–55. [Google Scholar]
- 17.Milling A, Babujee L, Allen C. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One. 2011;6:e15853. doi: 10.1371/journal.pone.0015853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JT, Huang JW. Antimicrobial activity of edible mushroom culture filtrates on plant pathogens. Plant Pathol Bull. 2010;19:261–270. [Google Scholar]
- 19.Rydberg J, Cox M, Musikas C, Choppin GR. Principles and practice of solvent extraction. 2nd ed. New York: Marcel Dekker, Inc.; 1992. [Google Scholar]
- 20.Walters DR, Fountaine JM. Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J Agric Sci. 2009;147:523–535. [Google Scholar]
- 21.Walters DR, Ratsep J, Havis ND. Controlling crop diseases using induced resistance: challenges for the future. J Exp Bot. 2013;64:1263–1280. doi: 10.1093/jxb/ert026. [DOI] [PubMed] [Google Scholar]
- 22.Herman MA, Restrepo S, Smart CD. Defense gene expression patterns of three SAR-induced tomato cultivars in the field. Physiol Mol Plant Pathol. 2007;71:192–200. [Google Scholar]
- 23.Hassan MA, Abo-Elyousr KA. Activation of tomato plant defence responses against bacterial wilt caused by Ralstonia solanacearum using DL-3-aminobutyric acid (BABA) Eur J Plant Pathol. 2013;136:145–157. [Google Scholar]
- 24.Shibuya N, Minami E. Oligosaccharide signalling for defense responses in plant. Physiol Mol Plant Pathol. 2001;59:223–233. [Google Scholar]
- 25.Walters D, Walsh D, Newton A, Lyon G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology. 2005;95:1368–1373. doi: 10.1094/PHYTO-95-1368. [DOI] [PubMed] [Google Scholar]
- 26.Di Piero RM, Wulff NA, Pascholati SF. Partial purification of elicitors from Lentinula edodes basidiocarps protecting cucumber seedlings against Colletotrichum lagenarium. Braz J Microbiol. 2006;37:175–180. [Google Scholar]
- 27.Roy S, Barman S, Chakraborty U, Chakraborty B. Evaluation of spent mushroom substrate as biofertilizer for growth improvement of Capsicum annuum L. J Appl Biol Biotechnol. 2015;3:022–027. [Google Scholar]
- 28.Ahlawat OP, Manikandan K, Sagar MP, Rai D, Gupta P, Vijay B. Effect of composted button mushroom spent substrate on yield, quality and disease incidence of Pea (Pisum sativum) Mushroom Res. 2011;20:87–94. [Google Scholar]