Abstract
The use of microorganisms and their secreted molecules to prevent plant diseases is considered an attractive alternative and way to supplement synthetic fungicides for the management of plant diseases. Strain BS062 was selected based on its ability to inhibit the mycelial growth of Botrytis cinerea, a major causal fungus of postharvest root rot of ginseng and strawberry gray mold disease. Strain BS062 was found to be closely related to Streptomyces hygroscopicus (99% similarity) on the basis of 16S ribosomal DNA sequence analysis. Postharvest root rot of ginseng and strawberry gray mold disease caused by B. cinerea were controlled up to 73.9% and 58%, respectively, upon treatment with culture broth of Streptomyces sp. BS062. These results suggest that strain BS062 may be a potential agent for controlling ginseng postharvest root rot and strawberry gray mold disease.
Keywords: Biocontrol agent, Botrytis cinerea, Ginseng root rot, Gray mold disease, Streptomyces sp. BS062
Botrytis diseases such as Botrytis blight and gray-mold disease are caused by Botrytis cinerea Pers.: Fr., and are the most problematic diseases among greenhouse-grown crops and storage crops. B. cinerea is normally controlled using fungicides; however, the growing demand of consumers worldwide for a reduction in the use of fungicides as well as the emergence of chemical resistant pathogens has emphasized the need to find alternative methods for controlling gray mold disease [1,2]. Biological control uses microorganisms and/or their secreted molecules to prevent disease and is considered an attractive alternative to fungicides for the management of plant disease. Importantly, biological control is not associated with any of the negative effects of chemical control, although relatively few antagonistic microbes have been commercialized as biocontrol agents due to problems such as inconsistent performance in the field, lack of broad-spectrum disease suppression activity, and/or slower or less complete suppression compared to chemical alternatives [2,3,4,5].
Actinomycetes are recognized as potential sources of antibiotics and lytic enzymes with both medical and industrial value [5,6,7]. While only a few taxa (mainly Streptomyces spp.) have been studied as potential biocontrol agents against fungal phytopathogens, several fungicides based on antibiotics present in Streptomyces are commercially used in pest control. Jinggangmycin, an antibiotic produced by S. hygroscopicus var. jinggangensis, is widely used to control rice sheath blight caused by Rhizoctonia solani in China [5]. Likewise, Mycostop, a product containing mycelium and spore of S. griseoviridis, is used to control Fusarium wilt and Botrytis gray mold of vegetables or ornamentals in Finland [6].
In our screening program to identify microorganisms that have the potential to be useful as microbial fungicide for the control of Botrytis diseases, an effective bacterial strain BS062 was isolated from a soil sample. In this study, we tested the ability of strain BS062 to antagonize the growth of a wide variety of plant pathogenic fungi in vitro. In addition, the effect of BS062 culture broth against Botrytis diseases on strawberry and ginseng were evaluated in vivo and compared with a commercial fungicide.
Identification and fermentation of strain BS062
Strain BS062 showing antagonistic effects against B. cinerea was isolated from a soil sample collected at Mt. Gyeryongsan, Korea. Strain BS062 was identified as belonging to genus Streptomyces on the basis of its structural and morphological characteristics (data not shown), and thus strain BS062 was designated as Streptomyces sp. BS062. 16s rDNA gene sequence analysis showed that strain BS062 was closely related to Streptomyces hygroscopicus (99% similarity, Genbank database accession No. AJ391820).
Strain BS062 was cultured in 100mL of yeast-malt extract broth in a 500-mL Erlenmeyer flask on a rotary shaker at 150 rpm for 3 days at 28℃. A 5-mL aliquot of the culture broth was then transferred into 200 mL of yeast-starch broth (soluble starch 70 g, soybean meal 5 g, yeast extract 17 g, (NH4)2SO4 1 g, CaCO3 1 g in 1 L water) in a 1-L Erlenmeyer flask. A total of 20 flasks containing yeaststarch broth were inoculated with culture broth as described above and incubated for 6 days at 28℃ on a rotary shaker with agitation at 150 rpm. After incubation, the resulting culture broth was centrifuged at 5,000 rpm for 20 min, and the supernatant was used for estimation of in vitro and in vivo biological activity.
Antifungal activity of Streptomyces sp. BS062
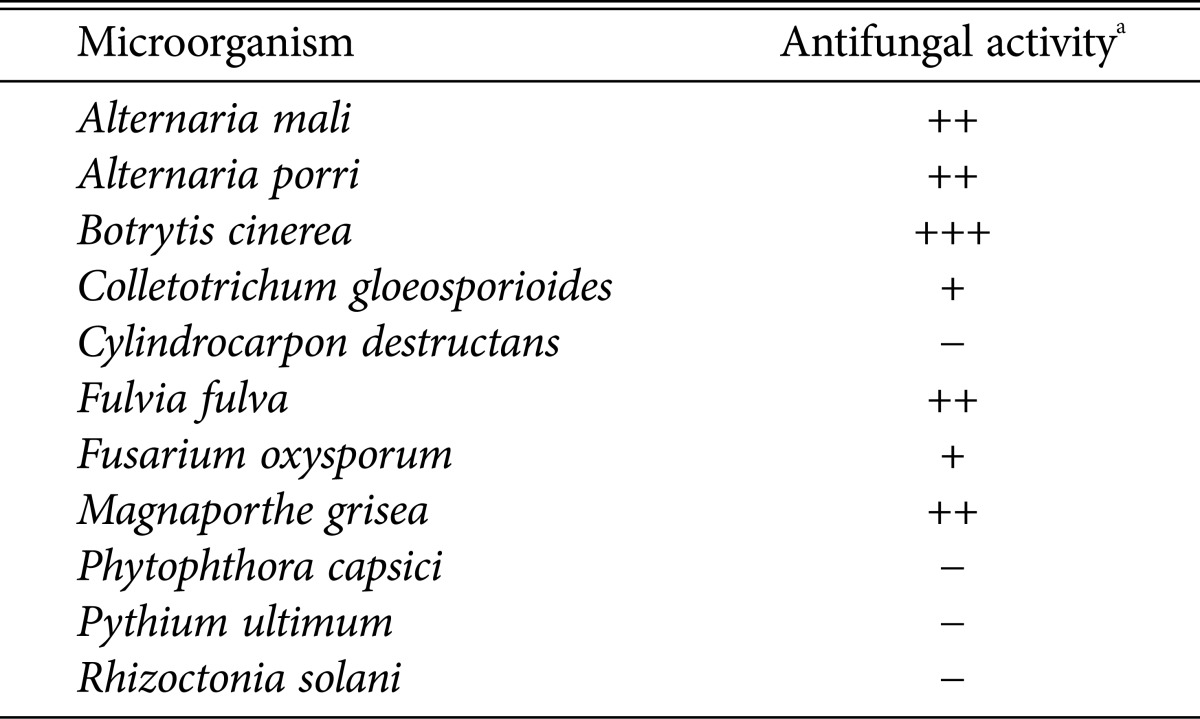
The ability of strain BS062 to inhibit the growth of several plant pathogenic fungi was estimated using the method described by El-Tarabily et al. [8], with minor modifications. Briefly, antifungal activity was evaluated based on its ability to inhibit mycelial growth (Table 1). Streptomyces sp. BS062 exhibited potent inhibitory activity against B. cinerea, Alternaria mali, Alternaria porri, Fulvia fulva, and Magnaporthe grisea, but had no activity against Phytophthora capsici, Pythium ultimum, Cylindrocarpon destructans, and Rhizoctonia solani. Notably, BS062 specifically and potently inhibited the growth of B. cinerea mycelia.
Table 1. Antifungal activity of strain BS062 against plant pathogenic fungi.
aThe antifungal activity of the strain BS062 against plant pathogenic fungi was assessed by dual culture technique. The levels of growth inhibition were determined by the differences between the diameters of the radial fungal growth of a control culture (γ0) and the radial fungal growth of paired-cultures (γ) in the direction of strain BS062 as determined by the equation; Δγ = γ0 - γ; +++, Δγ ≥ 20 mm; ++, Δγ ≥ 10~19 mm; +, Δγ ≥ 5~9 mm; -, Δγ < 5 mm (no antifungal activity).
Suppression of ginseng root rot by culture broth of Streptomyces sp. BS062
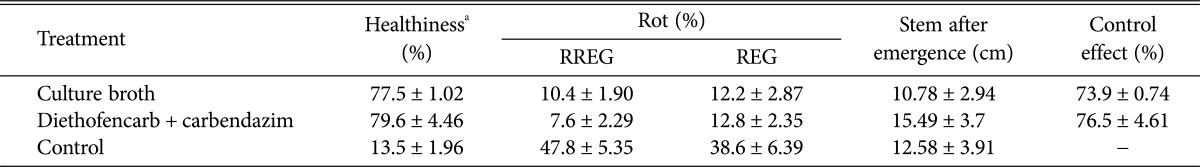
One-yr-old ginseng roots were surface-disinfected in a solution of 1% sodium hypochlorite for 20 min and rinsed with distilled water five times. The resulting ginseng roots were dipped in 10-fold diluted culture broth of strain BS062, diethofencarb + carbendazim (500 ppm) or distilled water. After drying at room temperature for 2 hr, the ginseng roots were inoculated by spraying with a conidial suspension (106 conidia/mL) of B. cinerea. Roots inoculated by B. cinerea were kept for 30 days at 4℃. Disease severity (%) was measured by estimating the percentage of root rot at 15 and 30 days of inoculation. In addition, the numbers of ginseng plants exhibiting symptoms of infection were counted to determine efficacy with respect to controlling root rot. As shown in Table 2, treatment with culture filtrate significantly reduced the severity of ginseng root rot caused by B. cinerea (73.9% compared to controls) during storage at 4℃. In addition, the ability of strain BS062 to control root rot was comparable to that of the fungicide, a complex of diethofencarb and carbendazim. Effectiveness was positively correlated with the concentrations of culture broth (data not shown).
Table 2. Effect of Streptomyces sp. BS062 culture broth on the suppression of postharvest ginseng root rot by Botrytis cinerea.
Experiments were done in triplicate with 100 ginseng roots for each group.
RREG, rotten rhizome and epidermis of ginseng root; REG, rotten epidermis of ginseng root.
aHealthy ginseng root.
Suppression of strawberry gray mold disease by culture broth of Streptomyces sp. BS062
In order to further evaluate the antagonistic activity of strain BS062 against B. cinerea, strawberry fruits were dipped in 10-fold diluted culture filtrate of strain BS062, diethofencarb (1,000-fold dilute, w/v), or sterile water before pathogen inoculation as described below. Briefly, B. cinerea spores were suspended in a solution of 20% tomato juice containing 0.1M KH2PO4 to a concentration of 106 conidia/mL. Aliquots of the B. cinerea spore suspension were sprayed on strawberry fruits, which were then incubated in growth chambers at 20℃ for 7 days. Strawberry fruits were individually rated for disease severity using a scale consisting of 0, 2, 4, 6, and 8; where 0, no symptoms; 2, ≤ 1/4 of the fruit surface afflicted by gray mold disease; 4, 1/4~1/2 of the surface afflicted by gray mold disease; 6, 1/2~3/4 of the surface afflicted by gray mold disease; and 8, entire surface of the fruit afflicted by gray mold disease and covered with profuse aerial mycelium [9]. Efficacy was calculated by adding together the individual strawberry ratings and dividing by the total number of strawberry fruits. This bioassay was repeated three times.
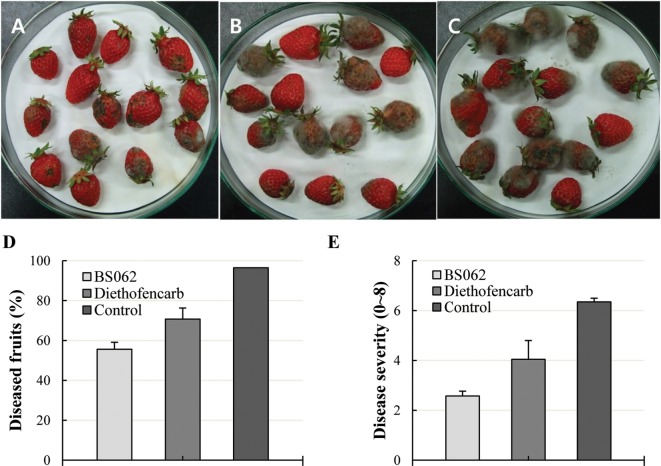
After incubation for 7 days at 20℃, B. cinerea-inoculated strawberry fruits developed gray mold disease with formation of profuse mycelium on the fruit surface (Fig. 1C). In contrast, fruits treated with culture broth of BS062 ranged from healthy to the appearance of light symptoms (Fig. 1A). The efficacy of strain BS062 against strawberry gray mold disease was 58%. The average severity rating of strawberries infected with B. cinerea and treated with sterile water (control) and diethofencarb was 4.04 and 6.34, respectively, while that of strawberries treated with 10-fold diluted culture broth of strain BS062 was 2.5 (Fig. 1). Together, these results indicated that Streptomyces sp. BS062 was effective at controlling strawberry gray mold disease.
Fig. 1. The incidence and severity of Botrytis gray mold disease of strawberries in the absence or presence of Streptomyces sp. BS062. A, 10-fold dilution of the culture broth of Streptomyces sp. BS062; B, Diethofencarb (250 ppm); C, Control (sterilized water); D, Percent of diseased fruits; E, Disease severity. The statistical results for incidence and severity of each disease are expressed as the mean ± standard error.
Inhibition of mycelia growth of B. cinerea by culture broth of Streptomyces sp. BS062
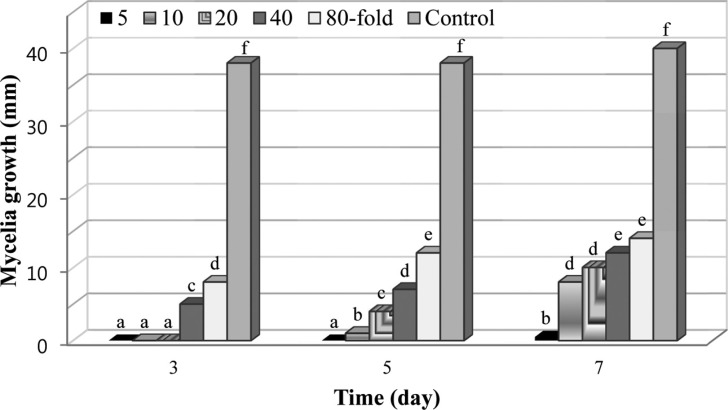
We next investigated the inhibitory effect of BS062 on mycelia growth of B. cinerea on potato dextrose agar medium according to the method described by Droby et al. [10]. Briefly, a 5-mm diameter plug of mycelia agar was obtained from the growing edge of 5-day-old cultures of B. cinerea and placed in the center of a plate containing potato dextrose agar medium and different concentrations (5-, 10-, 20-, 40-, and 80-fold dilutions) of culture broth, which was then filtered using a 0.2-µm filter (Millipore) before addition into autoclaved potato dextrose agar medium. The resulting diameters of B. cinerea growth were observed after incubation at 23℃ for 3, 5, and 7 days, and results were recorded as the diameter of fungal growth (Fig. 2). The culture filtrate of strain BS062 potently inhibited the mycelial growth of B. cinerea. Specifically, after incubation for 3 or 5 days, the growth of B. cinerea was completely inhibited by the 5-fold dilution of the culture filtrate. While inhibition decreased slightly with increasing incubation time, we noted a positive correlation between the concentration of the culture filtrate and inhibition of mycelia growth. This result suggested that strain BS062 inhibited the B. cinerea mycelia growth through the production of extracellular antifungal antibiotics. Consistent with this possibility, production of secondary antifungal substances such as antibiotics has been reported in many species of Streptomyces [11,12,13]. In addition, according to a literature survey, S. hygroscopicus inhibits a broad range of fungal pathogens such as Rhizoctonia solani, Pythium ultimum, Fusarium oxysporum, and Sclerotinia homeocarpa [14,15]; however, the antifungal spectrum of BS062 was different from that reported for S. hygroscopicus. Thus, the isolation of active substance from the culture broth of Streptomyces sp. BS062 is in progress.
Fig. 2. Effect of various dilutions of Streptomyces sp. BS062 culture broth on Botrytis cinerea mycelia growth. Mycelial growth was monitored by measuring the colony diameter at 2-day intervals. Bars with different letters within each sampling interval are statistically different according to Ducan's multiple range test (p < 0.05).
Conclusion
In the course of screening for microorganisms with the potential to be used as biocontrol agents, Streptomyces sp. BS062 was identified as having potent antifungal activity against Botrytis cinerea, a major causal fungus of postharvest root rot of ginseng and strawberry gray mold disease. According to in vivo tests, Streptomyces sp. BS062 significantly reduced the severity of postharvest root rot of ginseng and gray mold disease of strawberry caused by B. cinerea with an efficacy of 74% and 58%, respectively. In addition, strain BS062 was found to be closely related to S. hygroscopicus, having a 99% similarity on the basis of 16S ribosomal DNA sequence analysis. However, despite this similarity, the antifungal spectrum of BS062 was distinct from what was previously reported for S. hygroscopicus. Although the specific antifungal substances produced by Streptomyces sp. BS062 remain to be identified, this strain has the potential to be a useful agent for the biological control of postharvest root rot in ginseng and strawberry gray mold disease.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Agenda Project (grant No. PJ00995102) of the Rural Development Administration (RDA), Republic of Korea.
References
- 1.Wardlow LR, O'Neill TM. Management strategies for controlling pests and diseases in glasshouse crops. Pestic Sci. 1992;36:341–347. [Google Scholar]
- 2.Kim JH, Lee SH, Kim CS, Lim EK, Choi KH, Kong HG, Kim DW, Lee SW, Moon BJ. Biological control of strawberry gray mold caused by Botrytis cinerea using Bacillus licheniformis N1 formulation. J Microbiol Biotechnol. 2007;17:438–444. [PubMed] [Google Scholar]
- 3.Jacobsen BJ, Zidack NK, Larson BJ. The role of Bacillus-based biological control agents in integrated pest management systems: plant diseases. Phytopathology. 2004;94:1272–1275. doi: 10.1094/PHYTO.2004.94.11.1272. [DOI] [PubMed] [Google Scholar]
- 4.Larkin RP, Roberts DP, Gracia-Garza JA. Biological control of fungal diseases. In: Hutson D, Miyamoto J, editors. Fungicidal activity: chemical and biological approaches to plant protection. New York: Wiley; 1998. pp. 141–191. [Google Scholar]
- 5.Roberts DP, Lohrke SM, Meyer SL, Buyer JS, Bowers JH, Baker CJ, Li W, de Souza JT, Lewis JA, Chung S. Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Prot. 2005;24:141–155. [Google Scholar]
- 6.Goodfellow M, Williams ST. Ecology of actinomycetes. Annu Rev Microbiol. 1983;37:189–216. doi: 10.1146/annurev.mi.37.100183.001201. [DOI] [PubMed] [Google Scholar]
- 7.Srinivansan MC, Laxman RS, Deshpande MV. Physiology and nutritional aspects of actinomycetes: an overview. World J Microbiol Biotechnol. 1991;7:171–184. doi: 10.1007/BF00328987. [DOI] [PubMed] [Google Scholar]
- 8.El-Tarabily KA, Soliman MH, Nassar AH, Al-Hassani HA, Sivasithamparam K, McKenna F, Hardy GE. Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathol. 2000;49:573–583. [Google Scholar]
- 9.Wan M, Li G, Zhang J, Jiang D, Huang HC. Effect of volatile substances of Steptomyces platensis F-1 on control of plant fungal diseases. Biol Control. 2008;46:552–559. [Google Scholar]
- 10.Droby S, Wisniewski M, El Ghaouth A, Wilson C. Influence of food additives on the control of postharvest rots of apple and peach and efficacy of the yeast-based biocontrol product aspire. Postharvest Biol Technol. 2003;27:127–135. [Google Scholar]
- 11.Xiao K, Kinkel LL, Samac DA. Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol Control. 2002;23:285–295. [Google Scholar]
- 12.Fguira LF, Fotso S, Ameur-Mehdi RB, Mellouli L, Laatsch H. Purification and structure elucidation of antifungal and antibacterial activities of newly isolated Streptomyces sp. strain US80. Res Microbiol. 2005;156:341–347. doi: 10.1016/j.resmic.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Taechowisan T, Lu C, Shen Y, Lumyong S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc 130 and their antifungal activity. Microbiology. 2005;151:1691–1695. doi: 10.1099/mic.0.27758-0. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain K, Crawford DL. In vitro and in vivo antagonism of pathogenic turfgrass fungi by Streptomyces hygroscopicus strains YCED9 and WYE53. J Ind Microbiol Biotechnol. 1999;23:641–646. doi: 10.1038/sj.jim.2900671. [DOI] [PubMed] [Google Scholar]
- 15.Rothrock CS, Gottlieb D. Role of antibiosis in antagonism of Streptomyces hygroscopicus var. geldanus to Rhizoctonia solani in soil. Can J Microbiol. 1984;30:1440–1447. [Google Scholar]