Abstract
In September 2013, we discovered sooty mould growing on kenaf with the extrafloral nectaries in Iksan, Korea and identified the causative fungus as Leptoxyphium kurandae based on morphological characteristics and phylogenetic analyses. This is the first report of sooty mould caused by L. kurandae on kenaf in Korea and globally.
Keywords: Extrafloral nectaries, Hibiscus cannabinus, Pathogenicity, Phylogenetic analyses
Kenaf (Hibiscus cannabinus L.) is an annual herbaceous plant in the family Malvaceae. The species is ecologically and economically important as a crop plant, because it contains various usable components such as fibers, fiber strands, proteins, oils, and allelopathic chemicals [1]. Kenaf is widely cultivated in many countries as a source of composites, seed oils, and pulp for paper production, as well as for use in other industrial applications [2, 3]. This plant is particularly widespread in the tropics and subtropics, and it is grown as a vegetable and fiber crop in Africa [4]. In addition, it is considered as an alternative raw material to wood in the pulp industry [5]. In Korea, several field trials have been conducted to introduce kenaf as an alternative agricultural crop for animal feeding and as a natural source of high-quality cellulose fibers. The cultivation area of this plant in experimental fields is > 30 ha.
In September 2013, we discovered sooty mould growing on approximately 40% of 5-month-old kenaf plants in an experimental field at Jeollabuk-do Agricultural Research and Extension Services, Iksan, Korea (Fig. 1A). The infection began in the leaf petiole and spread to the leaf veins on the adaxial and abaxial leaf surfaces. A sooty, black, velvety, crust-like coating developed along the veins on the leaf surface. The fungus exhibited a different growth pattern to that of typical sooty mould, namely that growth was limited to specific areas such as the leaf veins and leaf petiole (Fig. 1B).
Fig. 1. Sooty mould caused by Leptoxyphium kurandae on kenaf. A, Sooty mould on kenaf; B, Close-up view of a leaf covered with black mats; C, Conidiomata with long cylindrical part; D, Conidiomata with short cylindrical part; E, Conidiophores; F, Conidia; G, Two-week-old colony on potato dextrose agar.
To examine the morphological characteristics, we mounted fresh samples of fungal structures on a glass slide in a drop of water. We examined these samples by using bright field and differential interference contrast light microscopy, with an Olympus BX51 microscope (Olympus, Tokyo, Japan) for measurements and a Zeiss AX10 microscope equipped with an AxioCam MRc5 (Carl Zeiss, Göttingen, Germany) for imaging.
A representative specimen was housed in the Korea University Herbarium (KUS) under accession number KUSF27692. To obtain a pure isolate, the fungal structures were collected from infected leaf tissues by using forceps under a dissecting microscope and were placed in a drop of sterilized water on a glass slide. A disposable bacterial loop was dipped into the conidial suspension and streaked onto 2% water agar plates supplemented with 100 mg/L of streptomycin sulfate. After 1 day of incubation at 25℃, single conidial colonies were transferred onto potato dextrose agar (PDA). The isolate obtained from KUS-F27692 was deposited in the Korean Agricultural Culture Collection under accession number KACC47659.
Fungal colonies on PDA were flat, dark olivaceous to black with dark synnemata, and produced ivory conidial masses (Fig. 1G). Conidiomata were synnematous, straight to slightly curved, and composed of three parts, namely, a dark brown bulbous base; a brown to pale brown cylindrical part with parallel synnematous hyphae, 45~290 × 13~20 µm; and a funnel-shaped hyphal apex, 25~68 × 18~42 µm (Fig. 1C and 1D). Conidiophores were subcylindrical to subulate, septate, aggregated in the apical part of the synnema, and 15~28 × 2~3 µm (Fig. 1E). Conidiogenous cells were terminal, monophialidic, tapering at the apex. Conidia were oval to broadly ellipsoid with rounded ends, unicellular, aseptate, eguttulate to guttulate, hyaline, 6~9 × 2.5~4.5 µm, and gathered at the apex of the synnemata (Fig. 1F). Based on morphological and cultural characteristics, we identified the causative fungus as Leptoxyphium kurandae Crous & R.G. Shivas [6]. However, the wide size range of the cylindrical parts of the conidiomata and guttulate conidia more closely resembled Park et al.'s description of L. kurandae [7].
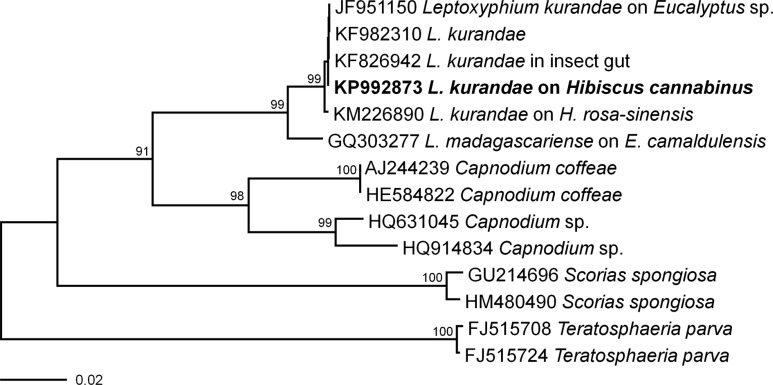
Fungal mycelia were harvested from the surface of PDA and were used in molecular analyses. Genomic DNA was extracted by using the DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA, USA). The internal transcribed spacer (ITS) region of rDNA was amplified by using the primers ITS1 and ITS4 [8]. The PCR products were separated by electrophoresis on a 1% agarose gel to confirm the target size and were purified by using a QIAquick PCR purification Kit (Qiagen Inc.). The purified DNA was sequenced directly by using the Macrogen Sequencing Service (Macrogen, Seoul, Korea). The obtained 503-bp ITS sequence was deposited in GenBank (accession No. KP992873). A GenBank BLAST search revealed that the ITS sequence showed 100% similarity with that of L. kurandae (KF826942). For phylogenetic analyses, all the available ITS rDNA sequences of several species belonging to the Capnodiaceae were retrieved from GenBank. Teratosphaeria parva belonging to the Teratosphaeriaceae was used as an outgroup taxon. A neighbor-joining phylogenetic tree was constructed by using the maximum composite likelihood method with MEGA6 [9] and bootstrap values calculated from 1,000 replicate runs. The phylogenetic relationship inferred from the ITS rDNA sequences revealed a separate clade distinct from other species in the Capnodiaceae; in the neighborjoining tree, this clade was clustered with L. kurandae (Fig. 2). Sequences from L. kurandae isolated from H. cannabinus (KP992873) and L. kurandae isolated from H. rosa-sinensis (KM226890) differed by two base pairs; however, the two isolates belonged to the same clade in the phylogram.
Fig. 2. Neighbor-joining phylogram of Leptoxyphium kurandae and related taxa based on the sequences of the internal transcribed spacer rDNA region. Bootstrap values are shown for branches with > 70% support. The isolate obtained from the present study is shown in boldface.
To fulfill Koch's postulates, three healthy kenaf seedlings were sprayed with a conidial suspension (ca. 5 × 104 conidia/mL) obtained from isolate KACC47659. Three additional kenaf seedlings were treated with sterile distilled water, to serve as controls. Treated and control plants were individually covered with polythene bags to maintain 100% relative humidity for 24 hr and were then transferred to separate rooms in a greenhouse and maintained at 28 ± 2℃ and high (> 80%) relative humidity. Typical symptoms of sooty mould appeared on infected kenaf plants 4 days after inoculation. Black synnematous hyphae were formed on the extrafloral nectaries; these symptoms were identical to those observed in the field. Control plants remained symptomless. The pathogenicity test was performed twice, with similar results.
Sooty mould associated with the extrafloral nectaries was previously reported in H. rosa-sinensis [7]; hence, our present study is the second record of an association between sooty mould and the extrafloral nectaries of Hibiscus spp. However, we are the first to report L. kurandae infection of H. cannabinus [10]. We observed a slightly different fungal growth pattern on H. cannabinus than on H. rosa-sinensis-growth spread to the veins not only of the abaxial surface but also of the adaxial surface. This variation may be related to the excretion of extrafloral nectar by the host plant. There are no previously published literature reports regarding diseases of kenaf in Korea. Therefore, to the best of our knowledge, this is the first record of L. kurandae on kenaf in Korea. Our findings indicate that sooty mould can occur on a plant bearing extrafloral nectaries but not having honeydew-secreting insects. However, sooty mould caused by L. kurandae poses no major threat to kenaf plants, and therefore, economic losses are likely to be negligible.
ACKNOWLEDGEMENTS
This study was partially funded by the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009251052015)" of the Rural Development Administration, Republic of Korea. Part of this work was also supported by the BK21 PLUS program in 2013 funded by the National Research Foundation of Korea (NRF).
References
- 1.Webber CL, III, Bledsoe VK. Kenaf yield components and plant composition. In: Janick J, Whipkey A, editors. Trends in new crops and new uses. Alexandria (VA): ASHS Press; 2002. pp. 348–357. [Google Scholar]
- 2.Mohamed A, Bhardwaj H, Hamama A, Webber CL., III Chemical composition of kenaf (Hibiscus cannabinus L.) seed oil. Ind Crops Prod. 1995;4:157–165. [Google Scholar]
- 3.Akil HM, Omar MF, Mazuki AAM, Safiee S, Ishak ZAM, Bakar AA. Kenaf fiber reinforced composites: a review. Mater Des. 2011;32:4107–4121. [Google Scholar]
- 4.Plant Resource of Tropical Africa. Hibiscus cannabinus. PROTA4U [Internet]. Wageningen: Wageningen University; 2015. [cited 2015 Mar 16]. Available from: http://www.prota4u.info/ [Google Scholar]
- 5.Nishino T, Hirao K, Kotera M, Nakamae K, Inagaki H. Kenaf reinforced biodegradable composite. Compos Sci Technol. 2003;63:1281–1286. [Google Scholar]
- 6.Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, Alfenas AC, Alfenas RF, Burgess TI, Carnegie AJ, Hardy GE, et al. Fungal planet description sheets: 69-91. Persoonia. 2011;26:108–156. doi: 10.3767/003158511X581723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Cho SE, Hong SH, Choi IY, Shin HD. Sooty mould on Hibiscus rosa-sinensis caused by Leptoxyphium kurandae is associated with extrafloral nectaries. J Phytopathol. 2014 Oct 17; doi: 10.1111/jph.12332. [Epub] [DOI] [Google Scholar]
- 8.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; 1990. pp. 315–322. [Google Scholar]
- 9.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farr DF, Rossman AY. Fungal Databases [Internet] Washington, DC: Systematic Mycology & Microbiology Laboratory, ARS, USDA; 2015. [cited 2015 Mar 16]. Available from: http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]