Abstract
An aortoesophageal fistula (AEF) is an extremely rare, potentially fatal condition, and aortic surgery is usually performed together with extracorporeal circulation. However, this surgical method has a high rate of surgical complications and mortality. This report describes an AEF caused by tuberculous esophagitis that was treated successfully using a two-stage operation. A 52-yr-old man was admitted to the hospital with severe hematemesis and syncope. Based on the computed tomography and diagnostic endoscopic findings, he was diagnosed with an AEF and initially underwent thoracic endovascular aortic repair. Esophageal reconstruction was performed after controlling the mediastinal inflammation. The patient suffered postoperative anastomotic leakage, which was treated by an endoscopic procedure, and the patient was discharged without any further problems. The patient received 9 months of anti-tuberculosis treatment after he was diagnosed with histologically confirmed tuberculous esophagitis; subsequently, he was followed as an outpatient and has had no recurrence of the tuberculosis or any further issues.
Keywords: Esophageal Fistula, Aortic Disease, Thoracic Endovascular Aortic Repair (TEVAR)
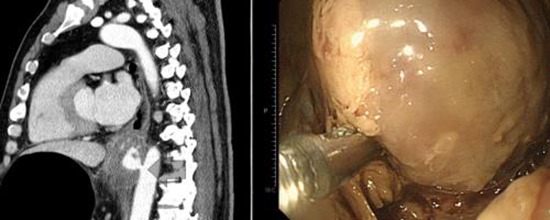
Graphical Abstract
INTRODUCTION
An aortoesophageal fistula (AEF) is a rare condition that is frequently fatal and requires emergency treatment. The potential causes of AEF include surgery, esophageal reflux, tuberculosis, trauma, corrosive esophagitis, aortic aneurysm, foreign body, or malignancy. Irrespective of the cause, the resulting massive upper gastrointestinal hemorrhage requires urgent treatment, such as surgery or embolization. In cases of AEF, conventional aortic surgery requires cardiopulmonary bypass and is associated with high mortality and morbidity. We report a case of AEF with mediastinitis treated using a planned two-stage approach: an initial thoracic endovascular aortic repair (TEVAR) as a bridge to stabilization, followed by esophageal reconstruction surgery. We believe that a two-stage approach is the optimal therapeutic option in certain AEF cases.
CASE DESCRIPTION
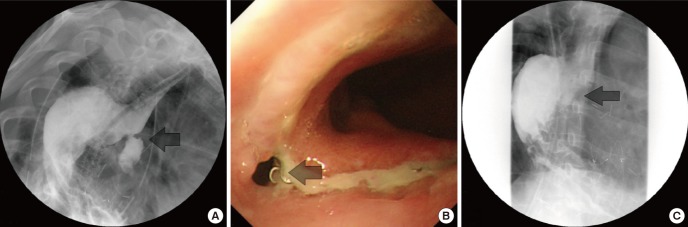
A 52-yr-old man who experienced syncope after severe hematemesis was admitted to the emergency department on September 9, 2012. Chest computed tomography (CT) did not detect any aortic lesions, such as a thoracic aortic aneurysm, while an abscess-like lesion of the esophagus with blood leakage was detected (Fig. 1). Therefore, we performed endoscopy to obtain hemostasis of the upper gastrointestinal bleeding. At endoscopy, the blood loss was found to be too severe to manage, and the patient was prepared for emergency surgery. As the patient's vital signs were unstable, the primary treatment involved stopping the bleeding. We considered reconstruction of the esophagus to be the next step. Aortography was performed before TEVAR. Aortography confirmed the presence of a circular mass beside the esophagus at the T9 level. A SEAL® thoracic stent graft (30 × 100 mm, S&G Biotech, Seoul, Korea) was inserted at the T7-10 level, and subsequent aortography confirmed that no bleeding was present (Fig. 2). The patient was monitored for hematemesis in the intensive care unit for 12 hr after surgery. As there was no hematemesis, the patient was transferred to a general ward and prepared for esophageal reconstruction. Since concurrent inflammation of the mediastinum was very likely, the patient was administered preoperative antibiotic therapy and continued fasting. After the white blood cell count and other inflammatory signs normalized, esophageal reconstruction was performed. After preparing for a gastric pull-up through a midline laparotomy, the patient was placed in the posterolateral position for a thoracotomy. Necrosis of the diverticulum was observed directly above the esophagogastric junction, which was found to contain thrombotic masses. The esophagus was dissected until the point beneath the azygos vein; after resection, an end-to-end anastomosis stapler (28 mm) was used for the esophagogastrostomy. After confirming the location of the L-tube, a 28-Fr chest tube was inserted, and the incision was closed. On pathological examination, the subserosal lymphoid tissue in the esophagus showed chronic granulomatous inflammation with caseous necrosis, and polymerase chain reaction analysis of tuberculosis was positive. The diverticulum and thrombotic masses showed acute gangrenous inflammation. Consequently, this AEF was considered to be a complication of tuberculous esophagitis and esophageal diverticulitis. Esophagography performed 7 days after the esophageal reconstruction showed a small esophageal anastomotic leak, and esophagogastroduodenoscopy (EGD) was performed using Dermabond® to seal the anastomotic leak (1). The EGD and esophagography were repeated 7 days later and confirmed the absence of anastomotic leakage. Subsequently, a soft diet was resumed and anti-tuberculosis treatment initiated (Fig. 3). The patient was discharged 20 days after the esophageal reconstruction and underwent anti-tuberculosis treatment for 9 months after the surgery. Based on the results of radiological examinations and a sputum acid-fast bacilli (AFB) smear and culture, the patient was deemed to be cured of tuberculosis. Aortic CT and EGD performed 1 and 2 yr postoperatively confirmed that the patient had recovered without any complications.
Fig. 1. A swelling was seen on the outer wall of the esophagus, through which blood leaked directly from the aorta.
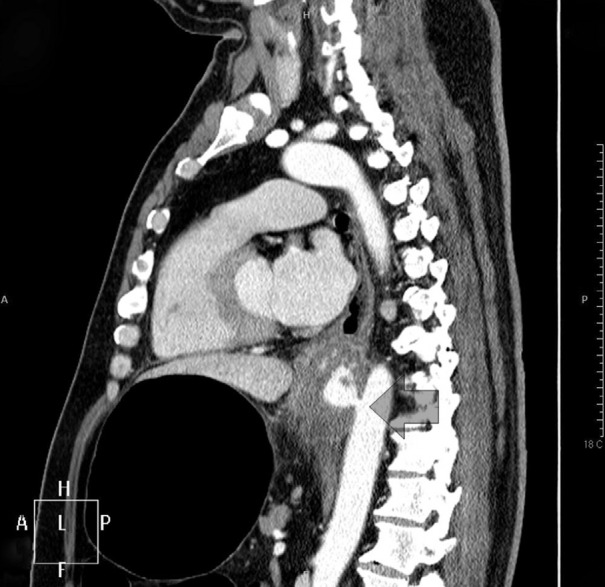
Fig. 2. After thoracic endovascular aortic repair (TEVAR). (A) Esophagogastroduodenoscopy (EGD) showing esophageal swelling. (B) Before TEVAR aortography. (C) After TEVAR aortography, no blood leakage was visible.
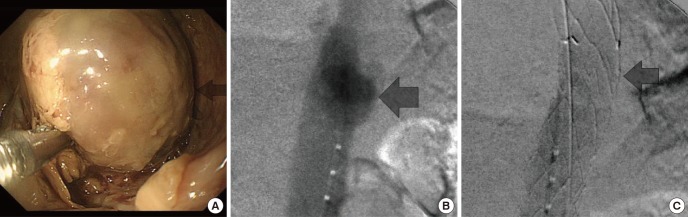
Fig. 3. Esophageal anastomotic leakage. (A) Esophageal anastomotic leakage was observed on esophagography. (B) Esophagogastroduodenoscopy (EGD) was performed to confirm the site of the leakage. (C) After the anastomotic leak site was closed, no further leakage was visible on esophagography.
DISCUSSION
Although AEF is a rare disease, it requires urgent treatment and can be fatal. In most cases, it involves an aortic aneurysm, and the classical treatment is aortic aneurysm surgery under cardiopulmonary bypass, followed by esophageal reconstruction or closing of the esophagus. However, mediastinitis is also seen in many cases, and the mortality rates associated with aortic aneurysm surgery performed under cardiopulmonary bypass are very high due to contamination of the surgical field (2). In recent years, aortic stents have been used to treat such cases (3,4). However, one report stated that the treatment of a thoracic aortic aneurysm using a stent graft can result in AEF (5). Moreover, in cases where mediastinitis is also seen, the infection can progress to recurrent AEF. In our case, however, the AEF was believed to have been caused by esophageal diverticulum inflammation rather than by an aortic aneurysm. In addition, due to the severe hematemesis, the risk of cardiopulmonary bypass was considered to be high. Therefore, an emergency TEVAR was performed. After excluding the AEF and before reconstructing the esophagus, the patient was administered preoperative antibiotic therapy to minimize the complications caused by mediastinal infection. As there have been no reports on the duration of antibiotic treatment, we performed the reconstructive surgery once the white blood cell count, erythrocyte sedimentation rate, and C-reactive protein values were normalized, although this might not be sufficient. The esophageal diverticulum was found to occupy half of the lower esophagus, as assessed by EGD, and was accompanied by esophageal inflammation over a wide area; consequently, primary repair was considered impossible. Therefore, esophageal reconstruction using the stomach was performed. The upper one-third of the esophagus appeared normal, while the middle and lower thirds showed severe adhesions, edematous changes, and esophageal swelling, although there were no gross signs of infection, such as pus. To ensure that the anastomosis was performed on the normal portion of the esophagus, the anastomosis was performed above the azygos vein. The postoperative anastomotic leakage was believed to be due to the insertion of the aortic stent graft, which reduced the esophageal blood supply. As the anastomotic leakage was the size of a pin point, an endoscopic intervention was considered to be better than a revision thoracotomy.
The treatment of AEF using an aortic stent graft is controversial. A large portion of AEFs are located at the connection between a thoracic aortic aneurysm and the esophagus; therefore, in such cases, using an aortic stent graft can reduce the risk of bleeding. However, the fistula between the false lumen and esophagus remains intact and can potentially result in complications, such as graft infection. One recent report described a patient with no prior AEF who developed an AEF after TEVAR (5). In general, TEVAR involves the use of a stent graft that is slightly larger in diameter than that of the aorta; the resulting structural friction and reduced blood supply were believed to have caused the AEF (6). After TEVAR, depending on the location of the AEF, aortic replacement can be performed under cardiopulmonary bypass or under a temporary bypass connecting the left subclavian artery to the distal thoracic aorta. The AEF that occurs after TEVAR is very likely to be accompanied by an aortic stent graft infection; therefore, the stent graft is removed, and extra-anatomical bypass or aortic replacement surgery is conducted (6,7).
Tuberculous esophageal disease does not arise primarily in the esophagus, but instead occurs by local extension from a nearby anatomical position (8). Compared with other locations in the gastrointestinal tract, tuberculosis in the esophagus is rare. The most common symptom is dysphagia, while hematemesis is very rare. If the esophagus and trachea are connected, liquid intake can lead to respiratory symptoms, such as coughing. The systemic symptoms are accompanied by weight loss in many cases. As the symptoms alone cannot be used to distinguish such cases from those of esophageal cancer, esophagoscopy is essential for determining the diagnosis. Esophagoscopy can show signs of external compression, and ulcerative lesions are often detected. Such complications due to tuberculous esophagitis can lead to the development of fistulas into the mediastinum (9).
In our case, TEVAR was performed as the initial treatment. However, when considering the long-term benefits, the use of TEVAR remains controversial, and many reports have been published on this subject (5). In cases such as ours, when a state of shock is suspected due to sudden, severe hematemesis, TEVAR can stop the bleeding quickly and accurately. We believe that this is an added value associated with the use of TEVAR as the primary treatment procedure, which is followed by planned secondary surgery. Case reports on secondary surgery of the aorta performed after TEVAR have also been published (10). When an AEF is caused by an aneurysm, open surgery might be better than TEVAR. However, when an AEF is caused by lesions in the esophagus, TEVAR is a good alternative.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and coordination of the study: Shin HK, Choi CW. Design of ethical issues: Her K. Acquisition of data: Lim JW. Manuscript approval: all authors.
References
- 1.Petersen B, Barkun A, Carpenter S, Chotiprasidhi P, Chuttani R, Silverman W, Hussain N, Liu J, Taitelbaum G, Ginsberg GG, et al. Tissue adhesives and fibrin glues. Gastrointest Endosc. 2004;60:327–333. doi: 10.1016/s0016-5107(04)01564-0. [DOI] [PubMed] [Google Scholar]
- 2.von Segesser LK, Tkebuchava T, Niederhäuser U, Künzli A, Lachat M, Genoni M, Vogt P, Jenni R, Turina MI. Aortobronchial and aortoesophageal fistulae as risk factors in surgery of descending thoracic aortic aneurysms. Eur J Cardiothorac Surg. 1997;12:195–201. doi: 10.1016/s1010-7940(97)00142-5. [DOI] [PubMed] [Google Scholar]
- 3.Czerny M, Zimpfer D, Fleck T, Gottardi R, Cejna M, Schoder M, Lammer J, Wolner E, Grabenwoger M, Mueller MR. Successful treatment of an aortoesophageal fistula after emergency endovascular thoracic aortic stent-graft placement. Ann Thorac Surg. 2005;80:1117–1120. doi: 10.1016/j.athoracsur.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 4.Metz R, Kimmings AN, Verhagen HJ, Rinkes IH, van Hillegersberg R. Aortoesophageal fistula successfully treated by endovascular stent-graft. Ann Thorac Surg. 2006;82:1117–1119. doi: 10.1016/j.athoracsur.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 5.Eggebrecht H, Mehta RH, Dechene A, Tsagakis K, Kühl H, Huptas S, Gerken G, Jakob HG, Erbel R. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv. 2009;2:570–576. doi: 10.1016/j.jcin.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Hance KA, Hsu J, Eskew T, Hermreck AS. Secondary aortoesophageal fistula after endoluminal exclusion because of thoracic aortic transection. J Vasc Surg. 2003;37:886–888. doi: 10.1067/mva.2003.159. [DOI] [PubMed] [Google Scholar]
- 7.De Masi M, Amabile P, Bal L, Piquet P. Management of endograft infection coupled with aortoesophageal fistula: extra-anatomic aortic bypass and endograft explantation. J Thorac Cardiovasc Surg. 2013;146:e11–e13. doi: 10.1016/j.jtcvs.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 8.Mokoena T, Shama DM, Ngakane H, Bryer JV. Oesophageal tuberculosis: a review of eleven cases. Postgrad Med J. 1992;68:110–115. doi: 10.1136/pgmj.68.796.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rämö OJ, Salo JA, Isolauri J, Luostarinen M, Mattila SP. Tuberculous fistula of the esophagus. Ann Thorac Surg. 1996;62:1030–1032. doi: 10.1016/0003-4975(96)00471-7. [DOI] [PubMed] [Google Scholar]
- 10.Vallabhajosyula P, Komlo C, Wallen T, Szeto WY. Two-stage surgical strategy for aortoesophageal fistula: emergent thoracic endovascular aortic repair followed by definitive open aortic and esophageal reconstruction. J Thorac Cardiovasc Surg. 2012;144:1266–1268. doi: 10.1016/j.jtcvs.2012.07.084. [DOI] [PubMed] [Google Scholar]


