Abstract
Background
With the widespread use of general health examinations, the detection rate of pulmonary nodules has increased; however, locating the pulmonary nodules is still a challenge.
Methods
We reviewed cases that underwent computed tomography (CT)-guided coil localization followed by real-time digital subtraction angiography (DSA)-guided accurate resection of solitary pulmonary nodules (SPNs) using video-assisted thoracoscopic surgery (VATS) at our hospital, and we evaluated the clinical value. From September 2011 to October 2014, 116 cases with SPNs were treated in our unit. The lesion was preoperatively localized using coil placement under CT guidance, and the patients were subsequently transferred to the hybrid operating room. VATS wedge resection with real-time DSA guidance was performed, and further processing was conducted in accordance with the intraoperative pathological diagnosis for these lesions.
Results
Coil localization, which averaged 15.30±3.20 min, was successful in all patients (100%), while VATS wedge resection took 24.20±12.10 min and lobectomy or segmentectomy took 88.8±36 min. The pathological results revealed malignant lesions in 61 cases and benign lesions in 55 cases.
Conclusions
Preoperative CT-guided coil localization for SPNs had a high accuracy with no serious complications. Following real-time DSA-guided VATS resection, the lesions could be accurately removed with a cutting edge distance of >2 cm to the lesion, which may help diagnose and treat the SPN simultaneously.
Keywords: Lung, lung cancer, thoracoscopy/video-assisted thoracoscopic surgery (VATS), surgery, complications
Introduction
The accepted definition for solitary pulmonary nodule (SPN) is a single periphery defined well and visible on a computed tomography (CT) scan, a lesion of ≤30 mm in diameter that is completely surrounded by pulmonary parenchyma, and a lesion without pulmonary atelectasis, pulmonary hilar enlargement, or pleural effusions (1,2). With the popularization of CT examination and low-dose CT screening, SPNs can be discovered easily. When the malignant probability is >60%, video-assisted thoracoscopic surgery (VATS) is a recommended processing strategy for diagnosis and radical treatment (3) to obtain an integrated diagnosis and treatment. However, the intraoperative quick finding and accurate positioning of SPNs that are >2 cm deep with a nodule <8 mm in diameter is difficult. Most intrapulmonary focal ground-glass opacities (fGGOs) increase the difficulty because they are soft, and some surgeons have even converted to thoracotomy, which is not minimally invasive (4).
From September 2011 to October 2014, we assessed 116 patients according to the diagnosis and treatment of the clinical pathway (5). We localized the SPNs by using CT-guided coil placement followed by resection of the SPNs by VATS in combination with digital subtraction angiography (DSA) in a hybrid operating room. We report on this method of quick localization and accurate resection, which achieved satisfactory results.
Technology
Equipment
The following equipment was used to conduct our study: a double spiral CT (Siemens Medical Solution, Forchheim, Germany), FD20 DSA (Philips Healthcare, Best, the Netherlands),Tornado Embolization Coil (MWCE-35-3-4, diameter: 4 mm; Cook Inc., Bloomington, IN, USA), percutaneous transhepatic cholangiography needle (18 G × 150 mm); and high-definition endoscopic camera system (Echelon Flex 60; Johnson & Johnson, New Brunswick, NJ, USA).
Surgical procedure
Preoperative localization
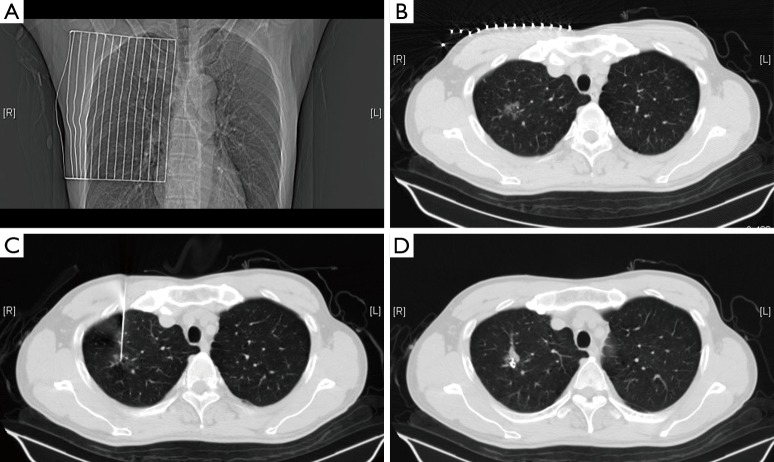
In the following description of the surgical procedure, we have selected one patient as an example. A 64-year-old man was admitted for a shadow in the right upper lobe. The CT scan showed a ground-glass opacity located in the right upper lobe (diameter, 1.3 cm; the distance to the pleura, 2.1 cm). Early in the operating day, the patient was sent to the CT room, and the supine position was selected as the shortest distance to perform a needle biopsy. The superficial needling point was localized using a ruler in combination with three-dimensional reconstruction, and careful attention was paid to avoid structures such as the heart, trachea, blood vessels in the mediastinum and lungs, ribs, scapula, and liver. The best pathway for measuring the depth and angle of needle insertion was chosen. After local disinfection and anesthesia, we inserted the needle according to the depth and angle previously measured, and placement of the needlepoint was adjusted again using a CT scan. The stylet was removed, and a Cook vascular embolization coil was inserted to localize the nodule (Figure 1). The needle was removed, and another CT scan was performed to ensure that the coil was in the right place and that no complications (e.g., hemothorax or pneumothorax) had occurred. The CT image was sent to the hybrid operation room for VATS operation.
Figure 1.
(A) The ruler is placed on the surface of the chest wall; (B) the depth and angle of the needle insertion is measured according to the location of focal ground-glass opacities (fGGOs) guided by computed tomography (CT); (C) the needle is inserted along the optimal path closest to the lesion; (D) the stylet is removed, the coil is placed, and the patient is assessed for any serious complications.
Anesthesia administration and the surgical incision
The patient was ventilated with a double-lumen endotracheal tube while under general anesthesia, and he was placed in the lateral decubitus position (on the side that had a collapsed lung) (Figure 2). The surgeon was on the patient’s ventral side.
Figure 2.
The hybrid operating room and the patient’s position.
A 1-mm incision was made in the seventh intercostal space at the site of the midaxillary line, which was used for thoracoscope insertion. The second incision was made depending on where the lesion was located. For example, if in the upper lobes, a 1.5 cm port was created in the line of the anterior axillary; if in middle and lower lobes, then the port was created at the site of the fourth intercostal space. A 1.5 cm utility port was placed in the ninth intercostal space at the posterior axillary line as auxiliary ports. This port was extended when we removed the specimen during the lobectomy.
Real-time DSA-guided VATS excision
To ensure coil localization with DSA, we adjusted the C-shaped arm and grasped the surrounding lung tissues accurately using sponge forceps with discontinuous and multi-angle real-time fluoroscopic guidance in order to confirm the scope of the operation (Figure 3). Wedge resection was performed using a stapler (Echelon Flex 60) about 2-3 cm around the lesion (Figure 4). The specimen was withdrawn in an endoscopic retrieval bag. The coil was visualized again using fluoroscopy, which was followed by an immediate frozen-section histopathologic examination. If benign, the bleeding was stopped, and a small chest tube was placed without any further resection. If malignant, a lobectomy followed by lymph node dissection was performed, and the specimen was sent for routine histologic examination.
Figure 3.
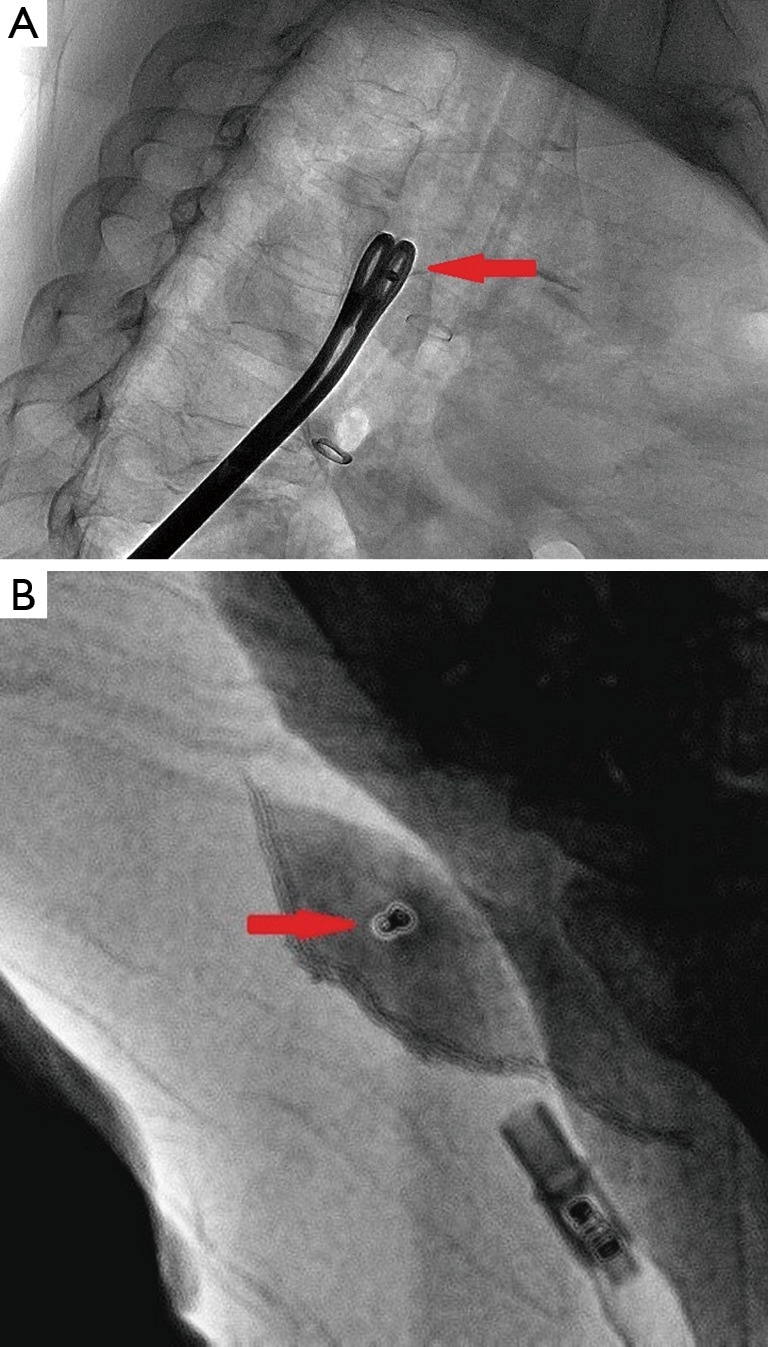
(A) Real-time digital subtraction angiography (DSA) is used to ensure the placement of the coil (red arrow) and to accurately grasp the surrounding lung tissues; (B) the coil is visualized in the specimen, and the range is >2 cm from the incisional margin to the solitary pulmonary nodules (SPNs).
Figure 4.
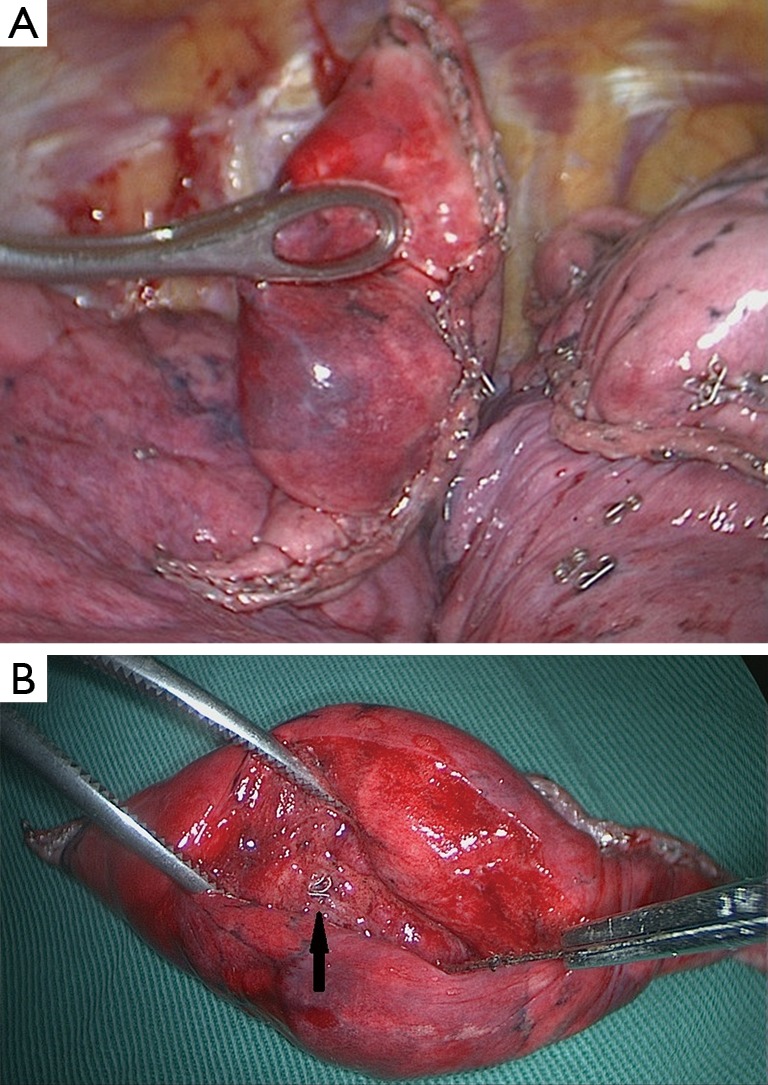
(A) Wedge resection is performed using Echelon Flex 60 staplers; (B) dissection is used to identify the coil and then the lesion is located (black arrow).
Clinical experience
After excluding patients with multiple pulmonary nodules or confirmed malignant or metastatic tumors, 116 SPN cases were treated in our unit from September 2011 to October 2014. Among them, 49 were men and 67 were women with a mean age of 55.2±23.5 years, and the mean diameter of the lesions was 12.2±5.12 mm (Table 1). Sixty-one cases had pure ground glass opacity, 21 had a high-density nodule, and 34 had a mixed density ground glass opacity; the distance to the visceral pleura was (15.13±12.54 mm). The distribution of the lesions was as follows: 31 in the left upper lung, 22 in the left lower lung, 40 in the right upper lung, 4 in the right middle lung, and 19 in the right lower lung. All the medical procedures were approved by the Jinling Hospital’s ethics committee.
Table 1. Patients’ clinical data.
| Characteristics | N=116 |
|---|---|
| Age (years) | 55.20±23.50 |
| Sex (male/female) | 52/64 |
| Lesion location | |
| Left upper lobe | 31 |
| Left lower lobe | 22 |
| Right upper lobe | 40 |
| Right middle lobe | 4 |
| Right lower lobe | 19 |
| Diameter (mm) | 12.20±5.12 |
| Distance to the pleura (mm) | 15.13±12.54 |
| GGO/high density nodules | 61/21 |
| Time (from locating the nodule to the beginning of the operation, min) | 67.50±48.10 |
| Operation time 1 (from skin incision to completion of wedge resection, min) | 24.20±12.10 |
| Operation time 2 (VATS lobectomy and lymph node dissection, min) | 88.80±36.00 |
| Volume of chest drainage 1 day postoperatively (mL) | 245.00±165.60 |
| Hospital stay (days) | 5.10±3.80 |
| Complications after locating the nodules | |
| Pneumothorax (no. of cases) | 11 |
| Hemorrhage (no. of cases) | 8 |
VATS, video-assisted thoracoscopic surgery.
SPNs in all 116 cases were successfully localized, and the mean duration for location was 15.3±3.2 min. There were 11 (9.48%) and 8 (6.89%) patients who developed asymptomatic pneumothorax and hemorrhage, respectively. Among them, three had pneumothorax and hemorrhage; however, none required a chest tube placement.
The time interval between puncture and surgery was 67.5±48.10 min. Wedge resection was successful in 100% of all the SPN cases. The operative time (from skin incision until the completion of wedge resection) was 24.20±12.10 min. Four patients had a prolonged operative time due to pleural adhesions. There were no conversions to thoracotomy, and no accidental injuries occurred.
The results of the frozen-section intraoperative and routine pathological postoperative examinations were matched in all the cases. The postoperative pathological results are displayed in Table 2. The SPNs were malignant in 61 patients, and 49 of which underwent complete lobectomies and lymph node dissection with VATS. The pathological diagnoses on the lymph nodes postoperatively were negative. Twelve patients had adenocarcinoma in situ and underwent wedge resection, and segmentectomies with lymph node dissection or sampling were performed.
Table 2. Distribution of the pathological findings in the 116 patients.
| Pathological results | No. of cases |
|---|---|
| Primary lung adenocarcinoma | 49 |
| Atypical adenomatous hyperplasia | 7 |
| Inflammatory lesions | 15 |
| Adenocarcinoma in situ | 12 |
| Pulmonary cyst | 8 |
| Sclerosing hemangioma | 5 |
| Smooth muscle lipoma | 3 |
| Hamartoma | 13 |
| Fibrosis nodules with carbon foam deposition | 4 |
The length of stay was 5.1±3.8 days. There were no severe postoperative complications, and no perioperative deaths occurred. We followed 108 (95.12%) patients, with a mean follow-up duration of 7.2±10.5 months. No recurrence or distant metastases were detected in the 61 patients with malignant tumors.
Comments
Lung cancer is the number one cause of mortality worldwide; overall, the survival rate is only 15% within 5 years. The survival rate of early stage lung cancer (especially Ia stage lung cancer) can be >80% postoperatively (6). It is difficult to determine the difference between benign and malignant tumors that are <2 cm on CT scans. They may be a malignant tumor such as adenocarcinoma in situ, minimally invasive adenocarcinoma (MIA), invasive adenocarcinoma, or invasive mucous adenocarcinoma. Alternatively, they may be precancerous lesions for atypical adenomatous hyperplasia. Benign lesions include hamartoma, focal interstitial fibrosis, organized pneumonia, inflammation, hemorrhage, etc. If patients with AIS or MIA undergo radical surgery, the 5-year disease-free survival rate may be close to 100%. Therefore, making a definite diagnosis and effective treatment as soon as possible is a clinical dilemma. Clinical treatment urgently requires a method for integrating diagnosis and treatment.
With the rapid development of imaging technology and equipment, especially the popularity of multi detector CT, there are increasing detected rates of SPNs. However, qualitative diagnosis still poses to be a problem. Statistically, >40% of SPNs <2 cm in diameter are malignant. Thus, >28% of lung mini nodule (<1 cm in diameter) may also be malignant (7). In this study, 52.58% of the resected lesions were malignant. If SPNs do not receive effective treatment in a timely manner, the malignant lesions could spread or become metastasized. Percutaneous pulmonary aspiration biopsy guided by CT has been widely used in the clinical setting. However, for SPNs <1 cm in diameter, a needle biopsy is still problematic, because the malignant tumor can metastasize along the needle path and the positive rate is low. Therefore, surgery is still required.
Within the past few years, VATS has developed rapidly, and its advantages are reduced pain, minimal trauma, quick recovery, and higher safety. Now an increasing number of surgeons and patients prefer minimally invasive operations for resecting SPNs in the early stage. However, the precise localization of SPNs, especially solid nodules, has always been problematic. Lesions may be approximately localized by reviewing CT scans and three-dimensional reconstruction and by palpating with instruments during surgery. However, if the SPN is <1 cm in diameter and far from the visceral pleura or even GGO, locating it may be difficult. Sometimes surgeons have to extend the resection. Consequently, this extension not only eliminates the advantages of minimally invasive surgery, but it also increases the risk of misjudgment. As a result, it is increasingly important to find an effective way to precisely localize the SPNs preoperatively. Native and foreign surgeons often use CT-guided intralesional injection of methylene blue, HOOK-wire placement, or intraoperative ultrasonic location; yet, localization may not be precise enough and the location marker may shift during operation (8). Methylene blue injection preoperation works well for SPNs close to the pleura, but if the distance between the nodules and visceral pleura is >1.5 cm, locating the SPN with this method is difficult. Methylene blue may diffuse because of the patients’ breathing movement; thus, it is difficult to identify the specific location of the lesion. This situation also occurred when the patients were old or smoked a lot, because the color of their lung surface was too dark to identify any lesions (9). The HOOK-wire location is simple, effective, and quick. HOOK-wire can also lift the lesion to a superficial location. However, sometimes the lesion is so close to the pleura for the wire to hook onto (10), so the methylene blue has to be used in combination (9), which wastes a lot of time. If the nodule is >2 cm in depth or >1 cm in diameter and is located previously using the HOOK-wire method, lobectomy with VATS would still face the problems of a positive surgical margin with too much tissue being removed, making it difficult to guarantee the distance between the nodule and incisional margin. Sometimes the wire is cut off and remains in the body (11,12). Intraoperative ultrasonic location is a useful for the non-invasive detection of nodules that cannot be palpated. However, the operation is complex and highly depends on the operator, and if the nodule is of low density, especially with GGO, this method is useless (13).
SPNs can be resected precisely because of coil placement guided by preoperative CT in combination with intraoperative real-time DSA. The advantages of the COOK coil are as follows. First, the coil is a spiral steel wire coated with fibers; therefore, dislodgement because of respiratory movement or surgical procedure can be avoided. All the cases were successfully localized in this study. Second, the metal material can be easily discovered before and detected after surgery with DSA, so the resection range can be ensured. Third, the nodule adjacent to the coil can be easily found by pathologists using palpation. All lesions <1 cm in diameter were found; thus, the accuracy of the pathological diagnosis can be ensured.
SPN localization performed preoperatively was followed by VATS guided by real-time DSA in the hybrid operation room. A drainage chest tube also prevented complications such as pneumothorax and hemothorax caused by needle biopsy. In the example patient, the involved lung collapsed during surgery. According to the CT image, the puncture site was observed directly on the visceral pleural surface, so the surgeon could grasp the approximate location. Adjustment was performed guided by multi-angle real-time fluoroscopic DSA. Before the incision was closed, the stapler was placed >2 cm away from the SPN. Removal of the specimen and DSA again detected that the coil was visualized, and there was >2 cm from the incisional margin to the SPN. Neither positive surgical margin nor operation error occurred. We achieved a precise excision. This method also provides a new minimally invasive treatment method for patients with poor lung function. This method maximized the remaining lung tissue with a safety distance between the incisional margin and lesion to decrease the incidence of complications. Echelon Flex 60 staplers were used in this study because of their adjustable angle. In our experience, keeping the stapler closed for about 15 s may reduce bleeding and avoid some complications.
We discussed the development status and advancement of CT-guided coil location combined with real-time DSA guided SPN VATS resection. The limitation was obvious, including the single center study design and insufficiencies of the cases. Currently, real-time DSA guided VATS resection of SPN using CT-guided coil placement showed greater advantages than other location methods before operation. This method makes for a precise, effective, and minimally invasive resection of SPN, achieving an integrated diagnosis and treatment that is worth popularizing. Interdisciplinary cooperation will also be an inevitable development direction in the future medicine.
Acknowledgements
None.
Footnotes
Conflicts of Interest: No funds were used to perform the evaluation. The tested technology was not purchased, borrowed or donated to the study. All authors stated that they had full control of the design of the study, methods used, outcome parameters and results, analysis of data and production of the written report.
References
- 1.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [DOI] [PubMed] [Google Scholar]
- 2.Wu YL, Jiang GL, Liao ML, et al. Chinese Consensus on Treatment of Solitary Pulmonary Nodule. The Journal of Evidence-Based Medicine 2009;9:243-6. [Google Scholar]
- 3.Song Y, Yao YW. Recent state in diagnosis and treatment of small pulmonary nodules. Clin J Lung Dis (Electronic Edition) 2012;5:295-9. [Google Scholar]
- 4.Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705-9. [DOI] [PubMed] [Google Scholar]
- 5.Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology 2009;250:264-72. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Sui XZ, Yang DS, et al. Solitary pulmonary nodules: a risk factor analysisi. Clin J Thorac Cardiovasc Surg 2010;26:161-4. [Google Scholar]
- 7.Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi K, Toyooka S, Gobara H, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36:378-82. [DOI] [PubMed] [Google Scholar]
- 9.Yu TF, Xu H, Liu XS, et al. CT-guided localization with combination of methylene blue and a Hookwire system for small pulmonary nodules before video-assisted thoracoscopic resection: the clinic application. Clin J Thorac Cardiovasc Surg 2012;28:401-3. [Google Scholar]
- 10.Jia CY, Chen HQ, Wang QW, et al. The diagnosis value of CT guidance Hookwire positioning thoracoscopic surgery for solitary pulmonary nodule. China Oncology 2013;23:917-20. [Google Scholar]
- 11.Lv X, Yang Y, Hu J, et al. Clinical application of CT-guided preoperative pulmonary nodule localization technique. Zhongguo Fei Ai Za Zhi 2011;14:418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyoshi K, Toyooka S, Gobara H, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36:378-82. [DOI] [PubMed] [Google Scholar]
- 13.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [DOI] [PubMed]