Abstract
Voltage-gated calcium channels are required for many key functions in the body. In this review, the different subtypes of voltage-gated calcium channels are described and their physiologic roles and pharmacology are outlined. We describe the current uses of drugs interacting with the different calcium channel subtypes and subunits, as well as specific areas in which there is strong potential for future drug development. Current therapeutic agents include drugs targeting L-type CaV1.2 calcium channels, particularly 1,4-dihydropyridines, which are widely used in the treatment of hypertension. T-type (CaV3) channels are a target of ethosuximide, widely used in absence epilepsy. The auxiliary subunit α2δ-1 is the therapeutic target of the gabapentinoid drugs, which are of value in certain epilepsies and chronic neuropathic pain. The limited use of intrathecal ziconotide, a peptide blocker of N-type (CaV2.2) calcium channels, as a treatment of intractable pain, gives an indication that these channels represent excellent drug targets for various pain conditions. We describe how selectivity for different subtypes of calcium channels (e.g., CaV1.2 and CaV1.3 L-type channels) may be achieved in the future by exploiting differences between channel isoforms in terms of sequence and biophysical properties, variation in splicing in different target tissues, and differences in the properties of the target tissues themselves in terms of membrane potential or firing frequency. Thus, use-dependent blockers of the different isoforms could selectively block calcium channels in particular pathologies, such as nociceptive neurons in pain states or in epileptic brain circuits. Of important future potential are selective CaV1.3 blockers for neuropsychiatric diseases, neuroprotection in Parkinson’s disease, and resistant hypertension. In addition, selective or nonselective T-type channel blockers are considered potential therapeutic targets in epilepsy, pain, obesity, sleep, and anxiety. Use-dependent N-type calcium channel blockers are likely to be of therapeutic use in chronic pain conditions. Thus, more selective calcium channel blockers hold promise for therapeutic intervention.
I. Introduction
Voltage-gated calcium channels are required for key functions in excitable cells, including transmitter release and hormone secretion (Catterall et al., 2013), excitation-transcription coupling (Wheeler et al., 2012), and excitation-contraction coupling (Bannister and Beam, 2013). To determine which calcium channels are involved in specific processes, we can employ a range of selective drugs as blockers of the different channels, as part of the armory of experimental tools. This is particularly important if we are to infer potential therapeutic uses of selective blockers from such experiments.
The first voltage-gated calcium channel complex to be studied was that from skeletal muscle, where it is present in great abundance in the transverse tubules. After purification of the complex, it was found to contain five components: α1 (approximately 170 kDa), α2 (approximately 150 kDa), β (approximately 52 kDa), δ (approximately 17–25 kDa), and γ (approximately 32 kDa) in an approximately stoichiometric ratio (Takahashi et al., 1987; Tanabe et al., 1987) (Fig. 1). The α1 subunit was found to bind the calcium channel blockers 1,4-dihydropyridines (DHPs), and thus was established as the pore-forming subunit. From these seminal studies came the cloning of 10 mammalian α1 subunits, four β subunits, and four or more α2δ subunits. This diversity provides a wealth of sites for selective pharmacological modification (Schroeder et al., 2000; Catterall and Swanson, 2015), which are outlined in Fig. 1. This review concentrates on the actual and potential pharmacology of these voltage-gated calcium channels throughout the body.
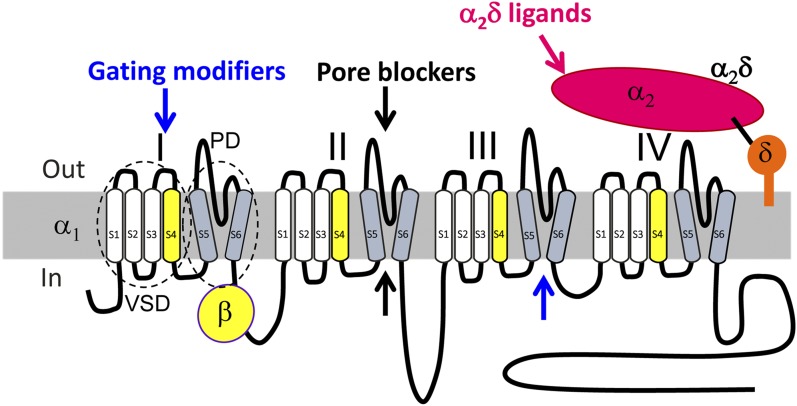
Fig. 1.
Diagram of voltage-gated calcium channel subunit topology. Voltage-gated calcium channel subunit topology showing major drug binding mechanisms. Channel inhibition can be induced by modification of channel gating (blue arrows, gating modifiers) by interaction with extracellular regions within one or more of the four voltage-sensing domains (VSDs) (e.g., peptide toxins, such as ω-agatoxin IVA; section III.D.2), or within the activation gates of the pore domain (PD) channel, formed by all four S5–S6 helices together (e.g., DHP LTCC blocker; section III.D). Direct block of the pore from the extracellular side (by peptide toxins such as ω-conotoxin GVIA; section II.D.2) or small molecules (with access from the cytoplasmic side) can also target regions within the ion conducting pathway and obstruct permeation through the pore (black arrows; pore blockers). Some drugs also act through both mechanisms (e.g., phenylalkylamine LTCC blockers; section II.D.1). For structural features, also see Fig. 4. The α2δ ligands (magenta arrow) can modify channel trafficking.
II. CaV1 Channel Family
A. Genes Encoding CaV1 Pore-Forming α1 Subunits
The CaV1 Ca2+ channel family is also known as the so-called L-type Ca2+ channels (LTCCs). In early studies in cardiac myocytes (Nilius, 1986) and neurons (Carbone and Lux, 1984; Nowycky et al., 1985), they were designated “L” due to their long-lasting inward currents during depolarization, which allowed them to be distinguished from rapidly decaying Ca2+ currents, termed transient or T-type channels (see section IV on CaV3 channels). A feature that distinguishes L-type channels from all other Ca2+ channels is their high sensitivity for organic L-type Ca2+ channel blockers (CCBs), also known as Ca2+ antagonists. These drugs serve not only as essential pharmacological tools to isolate L-type current components in vitro, but they have also been used clinically for decades to treat cardiovascular diseases. Radioactive derivatives of CCBs were subsequently used to reversibly label LTCCs in the brain, heart, and smooth and skeletal muscle. The density of L-type channels was an order of magnitude higher in skeletal muscle than in other tissues, which allowed purification of the channel complex, biochemical characterization of its subunits, and cloning of its pore-forming α1 subunit. The skeletal muscle L-type channel, formed by CaV1.1 α1 subunits, is encoded by the CACNA1S gene (Catterall et al., 2005). This genetic information subsequently allowed homology cloning of CaV1.2 (CACNA1C) and CaV1.3 α1 subunits (CACNA1D). Much later, human genetics finally identified the retinal CaV1.4 channel (CACNA1F) as the fourth member of the LTCC family (Bech-Hansen et al., 1998; Strom et al., 1998).
As we outline below, the four LTCC isoforms possess similar pharmacological properties but differ regarding their tissue distribution and biophysical properties. Moreover, they all undergo extensive alternative splicing that can affect their activity and interaction with other modulatory proteins. This functional heterogeneity allows Ca2+ signals to be adjusted to individual cellular requirements. Human genetic diseases leading to gain or loss of function have been described for all four L-type channel isoforms.
B. Physiology of CaV1 Channels
1. Physiologic Roles of CaV1 Calcium Channels.
Tissue expression of CaV1.1 and CaV1.4 is more restricted than that of CaV1.2 and CaV1.3 (Fig. 2). CaV1.1 is mainly expressed in skeletal muscle and is essential for skeletal muscle contraction. CaV1.4 is primarily restricted to the retina and is required for normal visual function. CaV1.1 and CaV1.4 α1 transcripts are not found at significant levels in the brain, although expression in a limited subset of neurons cannot be excluded (Sinnegger-Brauns et al., 2009). By contrast, in most electrically excitable cells, CaV1.2 and/or CaV1.3 are expressed and both isoforms are often even expressed in the same cell, such as in neurons (Olson et al., 2005; Chan et al., 2007; Dragicevic et al., 2014), adrenal chromaffin cells (Marcantoni et al., 2010), and sinoatrial node (SAN) and atrial cardiomyocytes (Mangoni et al., 2003). Both channels are required for normal brain function and serve different roles in the cardiovascular system and in endocrine functions. Transcripts for all L-type channel isoforms have also been detected in lymphocytes, although their functional role in these cells remains unknown.
Fig. 2.
The most important physiologic functions of the different LTCC isoforms. Except for skeletal muscle Ca2+ channels (a complex of CaV1.1 α1 associated with β1a, α2δ-1, and γ1 subunits) and the working myocardium (CaV1.2 α1 associated with primarily β2 and α2δ-1 subunits), their subunit composition is not known for other tissues. These sites represent actual and potential sites for action of selective LTCC blockers. DA, dopamine; IHC, inner hair cells; OHCs, outer hair cells.
a. CaV1.1.
CaV1.1 is expressed in skeletal muscle within the junctional membranes of the T-tubule system. CaV1.1 channels interact physically with ryanodine-sensitive Ca2+ release channels [ryanodine receptors (RyRs) such as RyR1] in the sarcoplasmic reticulum (SR), where they trigger rapid Ca2+ release and contraction (Tanabe et al., 1987). The direct CaV1.1-RyR1 conformational coupling has been shown to involve the CaV1.1 α1-subunit II–III intracellular loop (Block et al., 1988; Nakai et al., 1998; Grabner et al., 1999). The CaV1.1 channel expressed in adult muscle conducts a very small amplitude, slow-activating Ca2+ current with a very right-shifted voltage sensitivity, making this channel a truly atypical Ca2+ channel (for review, see Bannister and Beam, 2013). Typical intramembrane charge movements (gating currents), voltage-gated SR Ca2+ release, and tetrad formation can all be restored upon reexpression of CaV1.1 α1 subunits in CaV1.1 α1-deficient skeletal muscle myotubes (Tanabe et al., 1988; Takekura et al., 1994), demonstrating the essential role of CaV1.1 in skeletal muscle. RyR1 influences essential properties of skeletal LTCCs and enhances channel function (Nakai et al., 1996; Avila and Dirksen, 2000). The direct mechanical coupling mechanism and small amplitude Ca2+ influx can explain the absence of pharmacological effects of CCBs at therapeutic doses in muscle. Although these drugs bind to CaV1.1 with nanomolar affinity (Glossmann and Striessnig, 1990) and can inhibit Ca2+ inward current in skeletal muscle myocytes in vitro (Benedetti et al., 2015), they do not efficiently inhibit the fast voltage-dependent conformational changes in CaV1.1 α1 subunits that trigger SR Ca2+ release.
b. CaV1.2 and CaV1.3.
As outlined above, both isoforms are expressed in the heart, brain, and endocrine cells. Since they differ only slightly in their sensitivity toward CCBs, their contribution to individual cellular processes and physiologic functions could not be dissected using pharmacological means but required the generation of CaV1.2- and CaV1.3-deficient mice (for reviews, see Striessnig and Koschak, 2008; Hofmann et al., 2014).
i. L-Type Ca2+ Channels in the Heart.
CaV1.2 and CaV1.3 are expressed in the heart but their contribution to L-type current varies in different regions. In cardiomyocytes, CaV1.2 predominates and triggers contraction. By contrast, in the SAN and atrioventricular node (AVN), CaV1.3 is the predominant LTCC isoform. In CaV1.3−/− mice, resting heart rate is reduced and arrhythmic, spontaneous SAN pacemaker frequency is slowed and irregular, and diastolic depolarization is prolonged (Zhang et al., 2002; Mangoni et al., 2003). In humans, normal pacemaking function also requires CaV1.3 channels because loss-of-function mutations in the CaV1.3 α1-subunit gene (CACNA1D) also lead to bradyarrhythmia in humans (Baig et al., 2011). They work in a complex pacemaker network of sarcolemmal electrogenic molecules—including CaV3.1, the hyperpolarization-activated cyclic nucleotide-gated channels (HCNs) HCN-4 and HCN-2, delayed rectifier K+ channels, and the Na/Ca exchanger—and in conjunction with intracellular rhythmic sarcoplasmic Ca2+ oscillations (supported by SR Ca2+ release through RyRs and SR Ca2+ uptake through sarcoplasmic/endoplasmic reticulum calcium transport-ATPase -2) (for a recent review, see Striessnig et al., 2014). Therefore, knockout or pharmacological inhibition of CaV1.3 alone reduces heart rate and induces irregular SAN action but does not completely prevent pacemaking (Striessnig et al., 2014).
SAN cells are an excellent example to demonstrate why both CaV1.2 and CaV1.3 channel isoforms are required for proper function. Differences in their biophysical properties as well as subcellular localization enable them to support SAN activity during different time points of the action potential cycle. CaV1.3 channels activate at more negative membrane potentials than CaV1.2 in SAN (and other) cells (Lipscombe, 2002; Mangoni et al., 2003, 2006; Marcantoni et al., 2010; Bock et al., 2011; Christel et al., 2012). They can therefore sustain Ca2+ entry at threshold potentials and during the diastolic depolarization phase. CaV1.3 also closely colocalizes with sarcomeric RyRs (Christel et al., 2012), which may allow it to contribute to RyR-mediated Ca2+ release during diastolic depolarization (Lakatta and DiFrancesco, 2009). CaV1.2 activates at more positive potentials and colocalizes less with sarcomeric RyRs (Christel et al., 2012). It therefore seems to contribute little to this intracellular Ca2+ release. However, its biophysical properties allow CaV1.2 to support the SAN action potential. CaV1.3 is also the prominent L-type channel in AVN cells and contributes to AVN conduction and pacemaking (Platzer et al., 2000; Marger et al., 2011b).
In the working myocardium, the CaV1.2 channels predominate. They tightly associate with signaling molecules involved in cAMP and protein kinase A (PKA) signaling (Balijepalli et al., 2006) and mediate cardiac inotropy (Sinnegger-Brauns et al., 2004). No CaV1.3 expression is found in ventricular muscle and only low expression is found in the atria. CaV1.2 activation supplies Ca2+ to trigger Ca2+-induced Ca2+ release from the SR RyRs for contraction. CaV1.2 α1-subunit knockout mice die in utero (Seisenberger et al., 2000); therefore, homozygous loss-of-function mutations are likely lethal in humans as well. As shown in knockout mice, even a less than 50% reduction of ICa can lead to heart failure and enhanced lethality (Goonasekera et al., 2012). Cardiac disease can result not only from permanent loss of CaV1.2 activity but also from enhanced CaV1.2 activity. In transgenic mice overexpressing accessory β subunits, the sustained increase in Ca2+ current amplitude (without major kinetic changes) induces cardiac hypertrophy (Chen et al., 2011). As discussed below, de novo mutations in the CaV1.2 α1 gene (CACNA1C) or its auxiliary subunits also cause human cardiac disease.
ii. L-Type Ca2+ Channels in the Brain.
Fast presynaptic neurotransmitter release in neurons depends on the close coupling of presynaptic CaV2 channels to the release machinery. By contrast, CaV1.3 and CaV1.2 are located postsynaptically predominantly on the cell soma and in the spines and shafts of dendrites in neurons (Di Biase et al., 2008; Jenkins et al., 2010). There they shape neuronal firing and activate Ca2+-dependent pathways involved in control of gene expression, termed excitation-transcription coupling (Ma et al., 2013). By supporting neuronal plasticity, they participate in different forms of learning and memory, drug addiction, and neuronal development (for review, see Striessnig et al., 2014). Channel-bound calmodulin (CaM) and calmodulin kinase II (CaMKII) are essential biochemical elements decoding voltage-induced alterations in channel activity (Wheeler et al., 2008; Christel and Lee, 2012; Ma et al., 2013). Approximately 90% of the LTCCs in the brain are CaV1.2 and only 10% are CaV1.3 (Hell et al., 1993; Sinnegger-Brauns et al., 2009), and they often reside within the same neuron (Olson et al., 2005; Chan et al., 2007; Dragicevic et al., 2014).
Studies of the role of CaV1.2 and CaV1.3 in different brain functions in vivo are complicated by the fact that LTCC blockers preferentially act on vascular rather than neuronal LTCCs in vivo, and supratherapeutic doses may be required to effectively inhibit brain channels (see below) (Helton et al., 2005). The quantification of L-type current components is difficult due to the substantial contribution of CaV2 channels to total Ca2+ current in most neurons. Even more complexity is introduced by the fact that CaV1.2 and CaV1.3 α1 subunits can associate with all four β subunits (Pichler et al., 1997) and undergo alternative splicing (Bock et al., 2011; Huang et al., 2013b); CaV1.3 can also undergo RNA editing (Huang et al., 2012). At antihypertensive doses, organic CCBs (e.g., nimodipine, isradipine, or diltiazem) do not affect brain function in humans during chronic treatment. However, subtle central nervous system (CNS) effects of LTCC blockers can be detected in experimental clinical studies in healthy volunteers as changes in corticospinal metaplasticity (Wankerl et al., 2010). Unfortunately, experimental in vivo doses used in animal experiments are usually very high and cause pronounced CaV1.2-mediated cardiovascular effects, which seriously compromises the interpretation of behavioral outcomes of such studies (Waltereit et al., 2008; Busquet et al., 2010).
Genetically modified mice have been instrumental in revealing the physiologic role of the two brain LTCC isoforms (Striessnig and Koschak, 2008; Hofmann et al., 2014; Striessnig et al., 2014). Hippocampal function depends mainly on CaV1.2. This isoform is required for hippocampal spatial memory formation (Moosmang et al., 2005a; White et al., 2008) for protein synthesis-dependent, NMDA receptor–independent late-phase long-term potentiation (LTP) in CA3-CA1 synapses, and for activation of the microtubule-associate protein kinase/cAMP/calcium response element binding protein (CREB) signaling cascade (Moosmang et al., 2005a). In contrast with CaV1.2, CaV1.3 does not contribute to CA3-CA1 hippocampal LTP and the spatial memory encoding in the Morris water maze appeared normal in CaV1.3-deficient mice (McKinney and Murphy, 2006).
These two LTCCs also contribute in different ways to other types of memory, such as fear memory and memory associated with drug-taking behaviors. CaV1.3 is not required for acquisition and extinction of conditioned contextual fear memory (Moosmang et al., 2005a; Busquet et al., 2008) but is required for its consolidation (McKinney et al., 2009). Impaired consolidation in CaV1.3−/− mice was associated with significantly reduced LTP in the basolateral amygdala synapse receiving input from the entorhinal cortex and enhanced excitability of basolateral amygdala neurons (McKinney et al., 2009). CaV1.2 seems to carry most of the measurable L-type current in lateral amygdala neurons and their acute pharmacological inhibition reduces thalamolateral amygdala LTP and auditory cued fear memory acquisition (Langwieser et al., 2010).
CaV1.2 and CaV1.3 deficiency also affects anxiety- and depression-like behaviors. Reduced CaV1.2 activity in mouse forebrain enhances anxiety-like behaviors (Lee et al., 2012a). In one study, enhanced anxiety was only observed in females (Dao et al., 2010) and was associated with an antidepressant phenotype in both sexes. CaV1.3 deficiency induces antidepressant-like behaviors not explained by deafness (Busquet et al., 2010). Conversely, selective stimulation of CaV1.3 channels in vivo by the LTCC activator BayK8644 (1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-3-pyridinecarboxylic acid methyl ester) induces depression-like behavior (Sinnegger-Brauns et al., 2004). Genetic defects resulting in enhanced activity of Cav1.2 (CACNA1C gene mutations) cause Timothy syndrome (TS), which is characterized not only by severe cardiac arrhythmias but also by neurologic and neuropsychiatric abnormalities (see section II.C below).
Neuronal plasticity associated with drug dependence involves signaling cascade controlled by CaV1.2 and CaV1.3 LTCC activity in a different manner. When using locomotor sensitization as a model for psychostimulant-induced long-term plasticity, CaV1.3 mediates the development of sensitization, whereas CaV1.2 is responsible for expression of the sensitized response (Giordano et al., 2010). Signaling pathways involved in acute psychostimulant treatment and activated during development of sensitization have been identified (Schierberl et al., 2011; Striessnig et al., 2014).
LTCCs also appear to contribute to the high vulnerability of substantia nigra pars compacta (SNc) dopamine neurons to cell death in Parkinson’s disease (PD) (Surmeier et al., 2011). In these permanently active neurons, they mediate activity-dependent dendritic Ca2+ transients that contribute to oxidative stress. Transcripts for both CaV1.2 and CaV1.3 have been detected in these cells (Chan et al., 2007; Dragicevic et al., 2014). LTCCs appear to have only a minor stabilizing role for pacemaking itself (Guzman et al., 2009; Dragicevic et al., 2014), but CaV1.3 Ca2+ channels regulate SNc firing rates through dopamine D2 autoreceptors activated by dendritic dopamine release in a negative feedback loop through activation of G protein–coupled K+ channels (GIRKs) such as GIRK2 (KCNJ6) (Dragicevic et al., 2014).
CaV1.3 LTCCs also play a role in maintenance of normal synaptic connectivity. On D2 receptor–expressing striatopallidal medium spiny neurons, they are required for synaptic pruning induced by dopamine depletion (Olson et al., 2005; Fieblinger et al., 2014). A role for CaV1.3 in synaptic refinement has been described in the auditory pathway during development (Hirtz et al., 2012). Together these data point to an important role of CaV1.3 for the generation and maintenance of neuronal connectivity.
iii. L-Type Ca2+ Channels in Endocrine Cells.
LTCCs are present in many endocrine cells but are best characterized in pancreatic islet cells, adrenal chromaffin cells, and aldosterone-producing cells in the adrenal cortex. In mouse pancreatic β cells, CaV1.2 LTCCs control the fast phase of insulin secretion (Barg et al., 2001; Schulla et al., 2003; Sinnegger-Brauns et al., 2004). β-cell–specific ablation of CaV1.2 impairs insulin secretion and glucose tolerance (Schulla et al., 2003). In mice, the CaV1.3 channels do not couple to insulin secretion (Barg et al., 2001; Sinnegger-Brauns et al., 2004) but are required for β-cell proliferation and maintenance of normal β-cell number (Namkung et al., 2001). In contrast with mice, CaV1.3 transcripts seem to predominate in human β cells (Rorsman and Braun, 2013). Therefore, species differences with respect to isoform expression cannot be excluded. In mice, the late phase of insulin secretion is more dependent on CaV2.3 (Jing et al., 2005). Glucagon-secreting α cells express both CaV1.2 and CaV1.3, in addition to CaV2 channels (Vignali et al., 2006). High doses of CCBs (achieved during intoxication) reduce insulin secretion and cause hyperglycemia, supporting the important role of LTCCs for insulin secretion in humans (Levine et al., 2007). However, the therapeutic (vasodilating) plasma concentrations of DHPs that lower blood pressure do not cause a clinically relevant inhibition of pancreatic LTCCs or hormone secretion in the endocrine pancreas.
In mouse chromaffin cells, CaV1.2 and CaV1.3 together carry about 50% of the total Ca2+ current, each isoform contributing equally to the L-type current component. Although CaV2 and CaV3 channels are also present (Marcantoni et al., 2010), LTCCs are those coupled most tightly to catecholamine secretion during long depolarizing stimuli (Marcantoni et al., 2010). Although non-LTCCs contribute about one-half of the total inward Ca2+ current during square pulse depolarizations, they only contribute about 20% of the total Ca2+ charge during a train of action potentials (Vandael et al., 2012). The lower activation voltage range of CaV1.3, compared with CaV1.2, allows them to be active at threshold voltages and sustain a pacemaker current responsible for the spontaneous activity of chromaffin cells (Marcantoni et al., 2010). CaV1.3 channels also engage in a complex coupling to Ca2+-activated large and small conductance Ca2+-activated potassium channels. Accordingly, these K+ currents are reduced in CaV1.3-deficient cells, resulting in changes in the cell’s firing properties. CaV1.3 not only drives action potential pacemaking but also serves as a brake for mouse chromaffin cell firing by activating small conductance Ca2+-activated potassium channels and inducing spike frequency adaptation. This could be of physiologic significance upon high-frequency stimulation of chromaffin cells during stress responses (Vandael et al., 2012).
A recent surprising discovery was that CaV1.3 Ca2+ channels can play a central role for aldosterone secretion in humans. Primary aldosteronism is a common cause of secondary hypertension. In most cases it is attributable to either unilateral aldosterone-producing adenoma (APA) or to bilateral adrenal hyperplasia. Several steps of aldosterone synthesis are controlled by intracellular Ca2+ (Azizan et al., 2013). Therefore, mutations in different ion channels and ATPases, which directly or indirectly increase intracellular Ca2+ signaling, enhance aldosterone production in APAs. These are somatic mutations in the plasma membrane Ca2+ pump PMCA3 (ATP2B3) (Beuschlein et al., 2013), or mutations that depolarize the cell and activate LTCCs. This also includes mutations in GIRK4 K+ channels (KCNJ5) (Choi et al., 2011) and in the Na+/K+-ATPase (ATP1A1) (Azizan et al., 2013; Beuschlein et al., 2013). Many recurrent mutations were also found in the pore-forming α1 subunit of CaV1.3 (CACNA1D) (Azizan et al., 2013; Scholl et al., 2013; Fernandes-Rosa et al., 2014). All of the functionally tested CaV1.3 α1 mutations exhibit a clear gain-of-function phenotype (Azizan et al., 2013; Scholl et al., 2013). This provided direct evidence that CaV1.3 plays a major role for APA-induced aldosteronism. In the human adrenal cortex, CACNA1D transcripts are the most abundant Ca2+ channel subunits (Scholl et al., 2013), suggesting that CaV1.3 channels are also important for regulation of physiologic aldosterone secretion. Despite this important role of LTCCs, CCBs lower blood pressure but do not effectively lower plasma aldosterone levels in most patients with primary hyperaldosteronism (Stimpel et al., 1988; Carpenè et al., 1989). This may be explained by the contribution of other Ca2+ channels to aldosterone secretion, as recently reported for T-type channels in humans (Scholl et al., 2015) and rodents (Hu et al., 2012).
iv. L-Type Ca2+ Channels in Auditory and Vestibular Hair Cells.
CaV1.3 channels play an essential role for hearing and CaV1.3 deficiency leads to deafness in both mice (Platzer et al., 2000) and in humans (Baig et al., 2011). Whereas CaV2 channels form part of the presynaptic active zones of neurons, CaV1 channels are associated with the specialized presynaptic structures providing highly localized Ca2+ signals for neurotransmitter release at ribbon synapses in sensory cells, such as cochlear inner hair cells (CaV1.3) and photoreceptors (mainly CaV1.4). Patch clamp recordings in CaV1.3−/− hair cells revealed that these channels carry 80% to >90% (depending on the hair cell position along the longitudinal axis of the cochlea) of the total Ca2+ current in both inner and outer hair cells (for review, see Koschak et al., 2013).
c. CaV1.4.
In the retina, immunoreactivity for CaV1.4 α1 has been localized in the synapses of the outer and inner plexiform layer, as well as on photoreceptor cell bodies (Morgans, 2001; Regus-Leidig et al., 2009; Busquet et al., 2010; Mercer and Thoreson, 2011). These channels are predominantly expressed at release sites located in close vicinity to the typical horseshoe-shaped ribbon synapses. Retinal photoreceptors are highly specialized, light-sensing cells. Sustained release of glutamate from their ribbon synapses is Ca2+ dependent and LTCCs serve as the predominant source for Ca2+ entry. Heterologously expressed CaV1.4 currents show rapid activation, open at more negative membrane potentials compared CaV1.2, and inactivate slowly. These properties allow the channel to conduct sustained Ca2+ currents at voltages negative to −40 mV (for review, see Koschak et al., 2013). Whereas only a minor fraction of channels might be available at this potential (approximately 10%–15% at −35 mV), the resulting Ca2+ influx is expected to be sufficient to trigger neurotransmitter release. Like CaV1.3, CaV1.4 is slightly less sensitive to block by DHP CCBs than CaV1.2 at negative holding potentials (see also section II.D below). This intermediate DHP sensitivity of CaV1.4 and CaV1.3 is in good accordance with data obtained in retinal cells, in which relatively high concentrations of DHPs are required to efficiently block L-type Ca2+ currents (Wilkinson and Barnes, 1996). In some individuals, nifedipine altered the so-called “light rise” of the electro-oculogram presumably by inhibiting LTCCs (most likely CaV1.3) on the basolateral surface of the retinal pigment epithelium, thereby preventing the slow rise in intracellular Ca2+ required to generate the light rise (Constable, 2011). Thus far, there have been no reports of obvious visual dysfunction in patients receiving CCB medication. Some LTCC blockers have been reported to delay the progression of visual deficits in degenerative retinitis pigmentosa (Barabas et al., 2010; Nakazawa, 2011). However, these findings were not reproduced in all studies, and it remains unclear whether this potential photoreceptor-protective effect is due to block of retinal LTCCs.
2. CaV1 Family Splice Variants.
Alternative splicing is a key mechanism for regulating both the functional properties of Cav1 channels as well as their subcellular targeting to specialized cellular structures. Best understood is the C-terminal splicing of CaV1.3 α1 subunits, which gives rise to fundamentally different channels. These “long” and “short” CaV1.3 channels differ with respect to not only their Ca2+- and voltage-dependent gating properties (Bock et al., 2011; Tan et al., 2011) but also their association with modulatory signaling scaffolds (Olson et al., 2005). Some of the splicing-induced effects influence CaV1.3 channel modulation by CaM (Liu et al., 2010; Bock et al., 2011). CaM preassociates with all CaV1 and CaV2 α1 subunits, even at low intracellular Ca2+ concentrations (Ben Johny et al., 2013). Calcium-induced conformational changes allow CaM to promote inactivation [i.e., Ca2+-dependent inactivation (CDI)], which involves interaction with C- and N-terminal effector sites (for review, see Christel and Lee, 2012; Simms et al., 2014). CDI and voltage-dependent inactivation during depolarization involve conformational rearrangements of the intracellular channel mouth (Tadross et al., 2010). By restraining Ca2+ influx through the channel, CDI prevents excessive Ca2+ influx. Several mechanisms regulate the strength of CaM binding and therefore the effectiveness of CDI. Among those are competing CaM-like Ca2+ binding proteins, which do not support CDI (Yang et al., 2006; Cui et al., 2007) and RNA editing (Huang et al., 2012). CaV1 channels also contain a modulatory domain within the C terminus itself. In CaV1.3 and CaV1.4 channels, a C-terminal modulatory structure (CTM) is formed by noncovalent interaction of a proximal and a distal C-terminal regulatory domain (PCRD and DCRD, respectively) and putative α helices (Singh et al., 2006, 2008). This structure can compete with CaM binding (Liu et al., 2010). It thereby weakens CDI, reduces open probability, and also shifts the voltage dependence of channel activation to more positive voltages (Singh et al., 2006, 2008). As discussed below, this C-terminal intramolecular interaction is also conserved in CaV1.2 channels and is a target for channel modulation by PKA. Proteolytic processing has not yet been reported in CaV1.3, but alternative splicing creates multiple short splice variants that lack the DCRD and therefore allow robust modulation by CaM (Bock et al., 2011; Tan et al., 2011). Accordingly, “short” channel variants exhibit much more pronounced CDI, a more negative activation range, and higher open probability (Bock et al., 2011). Almost complete C-terminal inhibition of CDI also occurs in CaV1.4 (Singh et al., 2006) and thereby enables permanent Ca2+ influx underlying photoreceptor signaling (Singh et al., 2006).
Alternative splicing can also affect the pharmacological properties of LTCCs. Extensive alternative splicing outside the C-terminal tail has been described for CaV1.2 α1 subunits. As outlined below, tissue-specific splicing occurs. Arterial smooth muscle variants can activate and inactivate at more negative membrane potentials than splice variants predominantly found in cardiomyocytes (Liao et al., 2009). Alternative splicing may also change in disease states. For example, this has been reported in hypertrophied rat and human failing hearts, in rat myocardial infarction models, and in human atherosclerotic blood vessels (for an extensive review, see Liao and Soong, 2010).
In CaV1.1 α1 subunits, only 4 of 13 splice variants are likely to encode functional channels (Perez-Reyes et al., 1990; Tuluc et al., 2009). However, one variant is abundantly expressed in mouse and human myotubes but is not in differentiated muscle and may therefore play a special role in developing and regenerating muscle (Tuluc et al., 2009). This variant differs from the adult variant only in the length of the domain IV S3–S4 linker due to skipping of exon 29 (CaV1.1Δ29) (Tuluc et al., 2009). Upon expression in dysgenic myotubes, the CaV1.1Δ29 splice variant is normally targeted into triads and supports skeletal muscle type excitation-contraction coupling, but there is a drastically increased voltage sensitivity and open probability of the channel (Tuluc et al., 2009). Interestingly, the pathogenesis of myotonic dystrophy (DM) types 1 and 2 (DM1 and DM2, respectively)—an autosomal dominant disorder characterized by skeletal myopathy, cardiac arrhythmia, cataracts, hypogonadism, hypersomnolence, insulin resistance, and other symptoms—has been related to the aberrant splicing of several genes, including CaV1.1 (Tang et al., 2012). A marked repression of exon 29 in DM1 and DM2 patients was found. In DM1, the extent of exon 29 skipping was also correlated with muscle strength of the patients. Small interfering RNA studies in mice suggested that two splicing factors previously implicated in DM1, MBNL1, and CUGBP1 (Philips et al., 1998; Lin et al., 2006), regulate exon 29 splicing. Together these findings indicated that DM-associated splicing defects alter CaV1.1 function, with a potential for exacerbation of myopathy. Differences in intracellular Ca2+ entry observed for myotubes from DM1 and DM2 patients might at least in part be related to changes in the expression of the embryonic mRNA isoform lacking exon 29 (Santoro et al., 2014).
In CaV1.4 α1 subunits, a transcript scanning approach identified 19 alternative splice variants (Tan et al., 2012). It is currently unclear how, and to what extent, these naturally occurring alternative splice variants add to the properties of native CaV1.4 currents (Von Gersdorff and Matthews, 1996; Rabl and Thoreson, 2002) or whether their expression is differentially regulated under pathophysiological conditions in the retina. However, one of the splice variants found in 14% of the full-length transcripts screened can be predicted to affect CaV1.4 channel gating. It is generated by inclusion of an alternative exon 43* and inserts a stop codon that truncates the C terminus. This mutation would remove its CTM, which prevents CaV1.4 from undergoing CDI, (see above, Singh et al., 2006). This could lead to expression of channel species undergoing more pronounced inactivation. Overall, alternative splicing in the C terminus was shown to produce at least four splice variants resulting in different lengths of the C-terminal tail (Tan et al., 2012).
C. CaV1 Channel Pathophysiology
1. CaV1.1.
a. Hypokalemic Periodic Paralysis.
Hypokalemic periodic paralysis (HypoPP) is an heterogeneous autosomal dominant disorder, with missense mutations of a Ca2+ channel (CaV1.1, HypoPP-1) or a sodium channel (NaV1.4, HypoPP-2) accounting for 60% and 20% of cases, respectively (Jurkat-Rott et al., 2002). HypoPP symptoms generally manifest around the second decade of life, and they are characteristically exhibited with hypotonia as well as attacks of local or generalized skeletal muscle weakness or paralysis. Muscle fibers of HypoPP patients show a paradoxical, long-lasting depolarization in response to low extracellular K+, which leads to Na+ channel inactivation, loss of membrane excitability, and paralysis, independent of whether Na+ or Ca2+ channels are affected (Jurkat-Rott et al., 2000; Ruff, 2000). S4 arginine mutations of NaV1.4 associated with HypoPP induced a hyperpolarization-activated cationic leak through the voltage sensor of the skeletal muscle NaV1.4 (Sokolov et al., 2007; Struyk et al., 2008), referred to as gating pore current or omega current (Jurkat-Rott et al., 2012). Recently, fibers from a mouse model for HypoPP carrying the mutation CaV1.1 R528H also elicited a small anomalous inward current at the resting potential (Wu et al., 2012), similar to observations in a NaV1.4 HypoPP mouse model (Wu et al., 2011). Therefore, the gating pore current may be a common mechanism for paradoxical depolarization and susceptibility to HypoPP arising from missense mutations in the S4 voltage sensor of either Ca2+ or Na+ channels.
b. Malignant Hyperthermia.
Malignant hyperthermia (MH) is a potentially fatal pharmacogenetic disorder in which susceptible individuals experience a life-threatening hypermetabolic reaction of skeletal muscle after exposure to certain anesthetics or skeletal muscle relaxants (e.g., succinylcholine). This uncontrolled increase in the concentration of free myoplasmic Ca2+ released from the SR Ca2+ stores underlies this phenotype (Jurkat-Rott et al., 2002). Up to 70% of all MH cases are caused by mutations in RyR1 (MHS1), whereas only approximately 1% of cases result from CaV1.1 α1 mutations (MHS5). For deeper insights into both CaV1.1 structure/function and the pathophysiological mechanisms of MH from the functional analysis of CaV1.1 mutants, see Yarotskyy and Dirksen (2013).
2. CaV1.2.
a. Timothy Syndrome.
TS is an autosomal dominant condition caused by de novo gain-of-function mutations in the pore-forming α1 subunit of CaV1.2 [CACNA1C; Online Mendelian Inheritance in Man (OMIM) number 601005]. It is a multiorgan disease characterized by both cardiac and extracardiac symptoms. The underlying mutations reduce voltage-dependent inactivation (Splawski et al., 2004; Barrett and Tsien, 2008). This enhances Ca2+ influx and delays cardiomyocyte repolarization with increased risk of severe ventricular arrhythmias. Lethal tachycardias are the primary cause of death and of reduced average life expectancy (2.5 years). Typical extracardiac features include dysmorphic facial features, syndactyly, and mental retardation (Marks et al., 1995; Splawski et al., 2005; Gillis et al., 2012). Older patients are likely to develop autism (Splawski et al., 2005). TS mutations are located in the S6 segment of the first homologous repeat (IS6; Fig. 1), which forms part of the activation gate. This segment is alternatively spliced (exon 8, 8a). Classic TS type 1 results from a recurrent de novo CACNA1C mutation, G406R in exon 8a. An atypical form (TS type 2) is caused by mutations in G406R or G402S in exon 8. In two patients reported thus far, the G402S mutation shows a stronger cardiac phenotype but without syndactyly (Splawski et al., 2005; Hiippala et al., 2015). Since the original publications of the typical TS mutations in IS6, a number of other CACNA1C mutations have been identified in constitutively expressed exons showing a gain-of-function phenotype with enhanced current amplitudes or slowing of voltage-dependent inactivation and/or enhanced inward currents at negative voltages (Fukuyama et al., 2014; Hennessey et al., 2014; Boczek et al., 2015; Wemhöner et al., 2015). Intriguingly, most of them were identified in patients presenting with long QT and arrhythmias without obvious extracardiac symptoms (Fukuyama et al., 2014; Hennessey et al., 2014; Hiippala et al., 2015; Wemhöner et al., 2015). On the other hand, patients with mutations outside IS6 (I1166T and A1473G in the repeat III and IV activation gates, G1911R in the long C-terminal tail) (Gillis et al., 2012; Hennessey et al., 2014; Boczek et al., 2015) showed additional extracardiac symptoms (e.g., seizures, craniofacial features, developmental delay, microcephaly, dentition abnormalities), including syndactyly in A1473G. CACNA1C mutations can also underlie sudden unexpected infant death (Hennessey et al., 2014). Despite the finding of a CaV1.2 gain of function, CCBs are not established as therapy for TS. The TS type 1 mutation is less sensitive to block by DHPs than wild-type channels (Splawski et al., 2004).
Loss-of-function (missense) mutations in CaV1.2 α1 (CACNA1C), CaV1.2 β2 (CACNB2), and CaV1.2 α2δ-1 (CACNA2D1) genes have also been associated with different types of cardiac arrhythmias, including Brugada syndrome (Napolitano and Antzelevitch, 2011; Fukuyama et al., 2014). Together these data indicate that cardiac CaV1.2 must operate within a narrow activity range to ensure normal cardiac excitability.
The role of CaV1.2 dysfunction for extracardiac developmental and neurologic symptoms of TS has also been studied. Craniofacial abnormalities and syndactyly in TS patients can be explained by a role of CaV1.2 during development. For example, CaV1.2 is expressed in pharyngeal arches within the subset of cells that give rise to jaw primordia. Ca2+ influx through CaV1.2 regulates jaw development and affects cellular hypertrophy and hyperplasia in the mandible (Ramachandran et al., 2013).
b. Neuropsychiatric Disease.
Given the expression of CaV1.2 in most brain regions, the gain-of-function phenotype can also alter neuronal function and neuronal development. Autism often develops in older TS patients who survive from arrhythmias. Autistic behavioral traits are replicated in mice expressing a human TS mutation (Bader et al., 2011). Activity-dependent dendrite retraction was observed in induced pluripotent stem cell–derived neurons produced from TS patients (Krey et al., 2013), indicating that normal CaV1.2 activity is essential for synaptic development. On the basis of our current knowledge about the role of CaV1.2 channels for brain physiology (see section II.B), this suggests that CaV1.2 dysfunction may also contribute to human neuropsychiatric disease risk. Indeed, large-scale genome-wide association studies (GWASs) revealed a strong association between susceptibility for various psychiatric disorders, including bipolar disease, schizophrenia, and major depression, and single nucleotide polymorphisms (SNPs) in the CACNA1C gene. These are located within intronic regions (Bhat et al., 2012). SNP rs1006737, a common intronic risk haplotype, is one of the most consistent associations in psychiatric genetics (Bhat et al., 2012; Yoshimizu et al., 2015). It also has an impact on task-based human behaviors and human brain morphology, such as gray matter volume of specific regions (for references, see Yoshimizu et al., 2015). Interestingly, this SNP leads to increased CaV1.2 α1 subunit mRNA expression and L-type current density in fibroblast-derived induced neurons (Yoshimizu et al., 2015). This fits well with the observation that autism associated with TS is also caused by gain-of-function CACNA1C mutations.
3. CaV1.3.
a. Parkinson’s Disease.
As described above, LTCCs serve as an important Ca2+ source in spontaneously active SNc neurons, which preferentially degenerate in PD. In some reports, DHPs were found to protect SNc neurons in neurotoxin-based models of PD in rodents and nonhuman primates (Kupsch et al., 1995, 1996; Chan et al., 2007; Ilijic et al., 2011). This was achieved at low doses of DHPs, considered therapeutic in humans. Further support for a potential therapeutic role for DHPs comes from case-control and cohort studies. These studies reported a significantly reduced risk for a first-time diagnosis of PD in users of brain-permeable CCBs (odds or rate ratios of 0.71–0.78) (Becker et al., 2008; Ritz et al., 2010; Pasternak et al., 2012; Lang et al., 2015). Neuroprotection by CCBs, in particular by DHPs, can be rationalized by inhibition of dendritic Ca2+ entry during action potentials of rhythmic activity or during burst firing (Putzier et al., 2009), which occurs in response to reward-predicting stimuli (Liss and Roeper, 2008). In addition, these drugs may reduce α-synuclein–dependent l-DOPA–induced degeneration of SNc-dopamine neurons (Mosharov et al., 2009).
b. Hearing and Cardiac Dysfunction.
Like in knockout mice, the major symptoms of CaV1.3 deficiency in humans are SAN dysfunction (bradycardia and arrhythmia) and deafness. This has been described in two Pakistani families with autosomal recessive sinoatrial node dysfunction and deafness syndrome (Baig et al., 2011). Thus far, it is unclear to what extent other CACNA1D mutations or polymorphisms contribute risk for hearing disorders or for SAN dysfunction. Despite a normal life span, CaV1.3−/− mice also appear more vulnerable to ventricular extrasystoles (Matthes et al., 2004) and atrial fibrillation due to reduced L-type currents and impaired intracellular Ca2+ handling in atrial myocytes (Zhang et al., 2002; Mancarella et al., 2008).
c. Neuropsychiatric Disease.
As for CaV1.2, human genetics also strongly point to an important role of CaV1.3 LTCCs in the pathophysiology of neuropsychiatric disease, including autism spectrum disorders (ASDs). As described above, somatic CaV1.3 α1-subunit (CACNA1D) gain-of-function mutations cause aldosteronism through excess aldosterone production in APAs (Azizan et al., 2013). Interestingly, two of these mutations were also found as germline de novo mutations in two patients with a severe congenital syndrome presenting not only with primary aldosteronism but also with neurodevelopmental deficits and seizures at early age (PASNA, OMIM number 615474) (Scholl et al., 2013). In addition, de novo CACNA1D mutations have also been reported as high-risk mutations in two patients with sporadic autism and intellectual disability (Iossifov et al., 2012; O’Roak et al., 2012). For both mutations, functional studies also revealed a strong channel gain of function (Pinggera et al., 2015) very similar to the biophysical changes observed for mutations in APAs (Azizan et al., 2013; Scholl et al., 2013). Given the important role of CaV1.3 for many brain functions (see above) and the causal role of CaV1.2 gain of function in autism associated with TS, these data do not prove, but strongly suggest, a direct causal role of the two de novo mutations in the ASD patients (Pinggera et al., 2015). Moreover, these observations prompt several clinically relevant questions: Would patients with ASD and patients with primary aldosteronism with seizures and neurologic abnormalities with CACNA1D mutations benefit from therapy with LTCC blockers? Is aldosterone secretion also enhanced in the two ASD patients or do they show any other symptoms that could result from enhanced Cav1.3 function?
These findings also raise the important question of to what extent more subtle functional changes in CaV1.3 function in known CACNA1D polymorphisms can also contribute to overall neuropsychiatric disease risk.
4. CaV1.4.
a. Congenital Stationary Night Blindness Type 2.
CaV1.4 channels are the most predominant LTCCs in retinal neurons. Their importance is well supported by the fact that CACNA1F gene mutations cause several forms of human retinal diseases (OMIM numbers 300071, 300476, and 300600). The majority of CaV1.4 mutations were identified in patients with congenital stationary night blindness type 2 (CSNB2). Typical symptoms of CSNB2 are low visual acuity, myopia, nystagmus, strabismus, photophobia, and night blindness (Bech-Hansen et al., 1998). The severity of night blindness is a variable symptom, and in some cases, it was not even reported. Because of the X-linked nature of CaV1.4 channel dysfunction, CSNB2 mainly involves male individuals but heterozygote female individuals can also be affected (Hope et al., 2005; Michalakis et al., 2014).
Structural aberrations identified in CSNB2 patients comprise CaV1.4 α1-subunit missense or truncation mutations in addition to insertions or deletions, which can be categorized by their functional effects as loss or gain of function or impairment of the CTM (see above) (Stockner and Koschak, 2013). The complete absence of channel function or altered gating properties is expected to eliminate or decrease CaV1.4-mediated Ca2+ entry required for normal photoreceptor signaling. Both loss of channel function and a strong gain of function can also lead to alterations in photoreceptor synapse formation. This has been demonstrated in mice lacking CaV1.4 (CaV1.4−/−) and mutant mice (CaV1.4 α1 containing the I745T mutation, which induces a gain-of-function phenotype with activation at more hyperpolarized voltages and slowed inactivation) (Tom Dieck, 2013). These data demonstrated the importance of proper CaV1.4 function for efficient photoreceptor synapse maturation, and that dysregulation of CaV1.4 channels in CSNB2 may have synaptopathic consequences. Thus far, no comparable human data are available regarding retinal morphology. However, inner and outer retinal layers were shown to be thinned in CSNB2 patients when evaluated with spectral domain optical coherence tomography (Chen et al., 2012). These animal data suggest that altered Ca2+ signaling in CSNB2 may result in changes in retinal morphology early in development and may contribute to the overall dysfunction of retinal transmission. Potential pharmacotherapeutic interventions might therefore have to be applied early in disease. Such interventions also depend on mechanistic insights into the aberrations caused by the individual mutations. Gene therapeutic approaches focus on recombinant viral vectors as promising vehicles for therapeutic gene delivery to the retina (for reviews, see Boye et al., 2013; Lipinski et al., 2013). Gene replacement strategies may be applicable in patients carrying null mutations (full channel) or with impaired CTM function (C-terminal truncations) (Burtscher et al., 2014). The recent finding that some mutations (e.g., L860P; Burtscher et al., 2014) reduce the expression of functional channels by decreasing protein stability also suggests alternative approaches, such as pharmacochaperoning with ligands that stabilize folding intermediates and reduce endoplasmic reticulum–associated degradation. Valproic acid has been suggested to act as a pharmacological chaperone for unfolded proteins and is being explored in an ongoing clinical trial in patients with autosomal dominant retinitis pigmentosa (ClinicalTrials.gov identifier NCT01233609). Direct pharmacological activation of CaV1.4 channels with known LTCC activators (e.g., BayK8644) is not feasible for clinical application in human retinal disorders due to toxic side effects resulting from activation of CaV1.2 and CaV1.3 in other tissues as outlined below.
D. Pharmacology of CaV1 Channels
1. Molecular Pharmacology.
Clinically used CCBs belong to different chemical classes. The most widely used are DHPs, such as amlodipine, felodipine, or nifedipine (Fig. 3). Like verapamil (a phenylalkylamine) and diltiazem (a benzothiazepine), they interact with overlapping high-affinity drug binding domains close to the pore and to the proposed activation gate of LTCC α1 subunits (Fig. 4) (Hockerman et al., 1997; Striessnig et al., 1998; Tikhonov and Zhorov, 2009; Catterall and Swanson, 2015). Binding is reversible, stereoselective and, in isolated membranes at zero membrane potential, occurs with dissociation constants in the nanomolar range (0.1–50 nM) (Glossmann and Striessnig, 1990). Bound drugs interfere with the normal voltage-dependent cycling of the channel through its resting, open, and inactivated states (modulated receptor model) (Bean et al., 1986; Berjukow and Hering, 2001). The uncharged DHPs primarily stabilize and induce inactivated channel states. They possess much higher affinity for the inactivated channel conformation and thus their IC50 decreases with increased availability of inactivated channel states at more depolarized membrane potentials (voltage-dependent block) (Bean et al., 1986; Hamilton et al., 1987; Berjukow and Hering, 2001; Koschak et al., 2001). Access of phenylalkylamines and benzothiazepines is favored by the open channel state. Direct pore block together with stabilization of inactivated channel states with slowed recovery from inactivation results in pronounced frequency- or use-dependent inhibition (Shabbir et al., 2011). Ca2+ channel activators, such as the DHPs (−)-BayK8644 and (+)-SDZ202-791 [propan-2-yl (4R)-4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-5-nitro-1,4-dihydropyridine-3-carboxylate], also exist (see below).
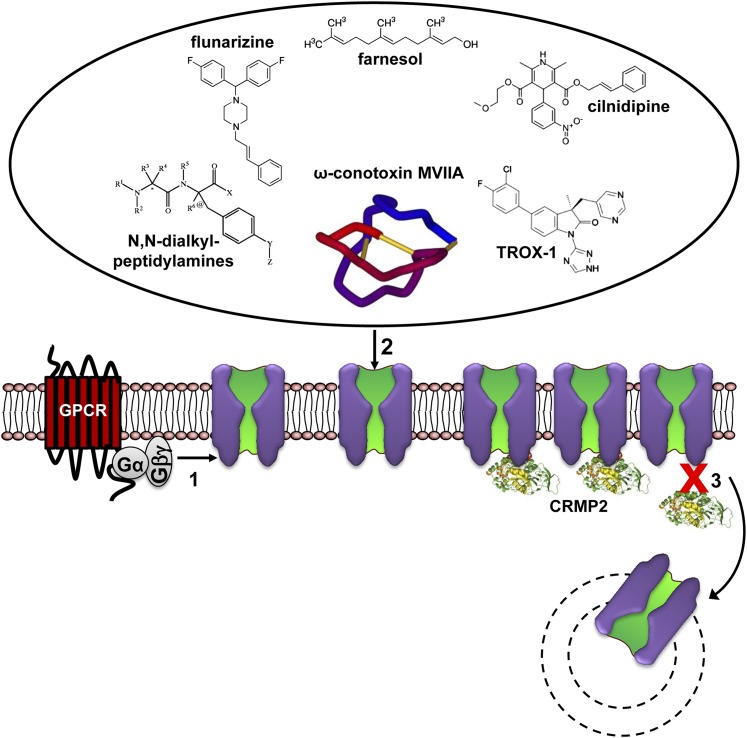
Fig. 3.
Modulation of L-type channels by drugs, toxins, and signaling pathways. (Pathway 1) The major pharmacologically relevant classes of LTCC active drugs are shown. DHPs (prototype nifedipine) are the most selective LTCC blockers. Verapamil and diltiazem also block non- LTCCs at higher concentrations (Diochot et al., 1995; Ishibashi et al., 1995). Tetrandrine is a bis-benzylisoquinoline alkaloid. It was isolated from the Chinese medicinal herb Stephania tetrandra used in China to treat hypertension and angina (King et al., 1988). In addition to LTCCs, it also blocks non–L-type current components (Weinsberg et al., 1994). Note that the apparent potency of classic L-type channel blockers strongly depends on membrane potential and/or stimulation frequency and action potential length. (Pathway 2) L-type channels are modulated by a variety of different signaling pathways either through membrane-delimited actions of activated G proteins (pathway 2a) or enzymes activated by GPCR (pathway 2b) or receptor tyrosine kinase (RTK) (pathway 2c) signaling (for details, see text). The FS2 structure (very similar to calciseptine) was drawn according to PDB ID 1TFS (chain trace, disulfide bonds not shown).
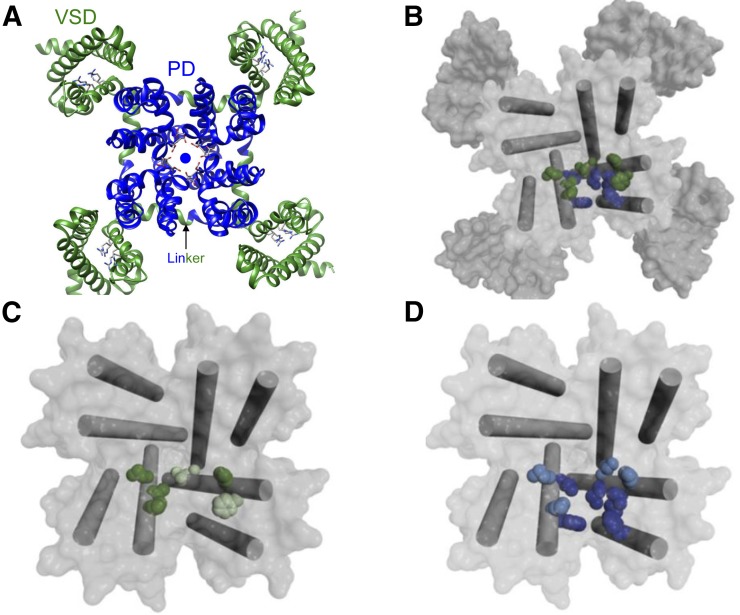
Fig. 4.
Calcium channel structure and ligand binding sites. (A) Extracellular view of the overall structure of CaVAb (preopen state; PDB ID 4MVQ), a homotetrameric voltage-dependent and calcium-selective channel generated by introducing three negatively charged aspartate residues (side chains in the pore are illustrated) (Tang et al., 2014). The four homologous domains of the α1 subunits of voltage-gated calcium channels likely possess a very similar architecture. Each domain contributes a voltage-sensing domain (VSD) (green, segments S1–S4) and a pore-forming domain, which together form the pore domain (PD) with a central ion conducting pathway for calcium ions (sphere). Each voltage sensor contains four positively charged arginines (side chains illustrated) that sense transmembrane voltage changes. Voltage-sensor movements are transmitted to the PD through a linker (arrow indicates one of them) between helices S4 and S5. (B) Top view of the pore module of CaVAb (pore-forming S5 and S6 helices are shown as cylinders) in the preopen state, with amino acid side chains analogous to those implicated in phenylalkylamine binding (green) and amino acid side chains specific for DHP binding illustrated in blue. The CaVAb structure has been used to illustrate how the analogs of amino acid residues important for drug binding in mammalian channels may form drug binding domains (Catterall and Swanson, 2015).The overlapping binding pocket can explain noncompetitive interactions observed in binding experiments in different tissues (Striessnig et al., 1998). (C) Top view of the CaVAb pore module in the preopen state, with the S5 and S6 segments illustrated as cylinders and amino acid side chains analogous to those implicated in phenylalkylamine binding illustrated in dark green for CaV1.2-specific residues and in light green for CaV-conserved residues. (D) Representation of DHP binding residues as in (C) for phenylalkylamines. Images in (B) to (D) were reproduced from Catterall and Swanson (2015), with permission.
The sensitivity of LTCCs for DHP CCBs varies in different tissues for several reasons. One explanation is the variable contribution of these LTCCs to total L-type current. CaV1.3 and CaV1.4 exhibit about 5- to 10-fold lower sensitivity to DHPs than CaV1.2, as demonstrated in heterologous expression systems at negative membrane potentials (Koschak et al., 2001, 2003; Xu and Lipscombe, 2001). This can explain the relatively weak inhibition of L-type pacemaker currents in the SAN, which are dominated by CaV1.3 (Mangoni and Nargeot, 2001). Another factor affecting DHP sensitivity of L-type currents is alternative splicing of α1 subunits. For CaV1.2, it has been demonstrated that DHPs inhibit currents in arterial smooth muscle at lower concentrations than in the working myocardium. A detailed analysis of CaV1.2 α1 splice variants in the heart and smooth muscle revealed the presence of more DHP-sensitive splice variants predominantly expressed in arterial smooth muscle. Some of these splice variants activate at slightly more negative voltages (Liao et al., 2004; Cheng et al., 2009) and are therefore expected to preferentially contribute to a steady-state Ca2+ inward current (window current) close to the smooth muscle resting potential that controls myogenic tone. The more depolarized resting membrane potential in smooth muscle (≥−60 mV) compared with cardiomyocytes (or most neurons) favors inactivated channel states preferentially blocked by DHPs. Some of these splice variants are also prone to more pronounced steady-state inactivation, which also enhances DHP sensitivity (Liao et al., 2007). There is also evidence that alternative splicing of CaV1.2 α1 affects the molecular architecture of the drug binding domain and thus the access of DHPs for inactivated channel states (Welling et al., 1993). Alternative splicing (in the C terminus) also slightly affects the DHP sensitivity of CaV1.3 (Huang et al., 2013b).
2. Clinical Pharmacology.
LTCC blockers have been licensed for decades for the treatment of hypertension and myocardial ischemia, and they belong to the most widely prescribed drugs worldwide. DHPs are arterial vasodilators reducing arterial muscle tone, peripheral vascular resistance, and vasospasms in coronary or peripheral arteries. By lowering arterial blood pressure and afterload, DHPs also reduce cardiac oxygen demand. Together with their spasmolytic effect, this explains most of the antianginal actions of DHPs. At therapeutic doses, DHPs lack negative inotropic actions and do not directly affect SAN and AVN function. In addition to their antihypertensive, vasodilating, and spasmolytic properties, verapamil and diltiazem are also negative chronotropic, dromotropic, and inotropic and thus inhibit exercise-induced increases in heart rate and myocardial oxygen consumption (similar to β-adrenoceptor antagonists). These direct cardiodepressant effects make them suitable for the treatment of angina pectoris in hypertensive patients (Bangalore et al., 2008).
Unwanted effects at therapeutic doses, such as flushing, headache, dizziness, and hypotension, are mostly related to the vasodilating effects of CCBs. Peripheral edema and ankle swelling is often the therapy-limiting side effect upon long-term use of DHPs (Parkinson Study Group, 2013). Constipation is a frequent side effect of verapamil and can be explained by LTCC inhibition in intestinal smooth muscle (Moosmang et al., 2005b). Verapamil (and to a lesser degree, diltiazem) can cause bradycardia, atrioventricular block, or a decrease in left ventricular function, especially in patients who are taking β-adrenoceptor blockers or who have preexisting heart disease. DHPs can also worsen angina, most likely due to a redistribution of coronary blood flow to the nonischemic myocardium in the absence of direct cardiodepressant effects.
At therapeutic doses, CCBs cause no relevant side effects in other tissues where LTCCs serve important functions. There is no evidence for muscle weakness from block of CaV1.1 channels in skeletal muscle, increased hearing thresholds from inhibition of CaV1.3 in cochlear inner hair cells, visual impairment from block of CaV1.4 in retinal photoreceptors, or CNS disturbances from block of CaV1.2 and or CaV1.3 in the brain. Suppression of insulin secretion and hyperglycemia occur only at toxic plasma levels after CCB overdose (Levine et al., 2007). However, this side effect plays no role at therapeutic doses in clinical practice.
3. L-Type Calcium Channels as Potential Targets for Other Indications.
Our increasing understanding regarding the physiologic and pathophysiological role of LTCCs also outside the cardiovascular system raises the important question about the pharmacotherapeutic potential of LTCC block in other tissues. A particularly challenging question relates to the efficient inhibition of LTCCs in the brain. As outlined above, a number of therapeutically highly relevant pharmacological effects can be postulated from findings in mutant mice and from human mutations. This includes neuroprotection in PD as well as treatment of neuropsychiatric disorders, ASDs, and febrile seizures. Since CCBs are well established for clinical use in cardiovascular disease, they could be “repurposed” for other indications.
a. Parkinson’s Disease Neuroprotection.
Based on the strong preclinical findings regarding a key role of LTCC-mediated Ca2+ load in SNc neurons, a phase 3 clinical trial (ClinicalTrials.gov identifier NCT02168842) has already been initiated to study the neuroprotective potential of the DHP isradipine in early PD. Isradipine is currently licensed for the treatment of high blood pressure. At present, the preclinical in vivo findings from neurotoxin-induced PD models do not allow us to predict whether CaV1.2, CaV1.3, or both isoforms contribute to the proposed Ca2+ toxicity. In clinical trials, CaV1.2-mediated side effects, such as hypotension and/or peripheral edema, limit long-term treatment of PD with higher doses of DHPs (Parkinson Study Group, 2013), providing a strong argument for efforts to discover CaV1.3-selective inhibitors that are not yet available (see below). However, it is currently unknown whether CaV1.3-selective inhibitors would miss a neuroprotective component mediated by CaV1.2 channels.
b. Neuropsychiatric Disease.
As described above, GWASs have revealed a strong association of intronic SNPs in CACNA1C and the susceptibility for psychiatric disorders, including bipolar disease, schizophrenia, major depression, and ADs. It is one of the most consistent associations reported in psychiatric genetics (Dao et al., 2010; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Ripke et al., 2013). The recent findings that one of these SNPs (rs1006737) leads to increased CaV1.2 function (Yoshimizu et al., 2015), and that gain-of-function CACNA1C mutations cause autism in TS, strongly motivate the reevaluation of CCBs for the treatment of bipolar disease, schizophrenia, and major depression. In contrast with earlier clinical studies (Hollister and Trevino, 1999; Post et al., 2000), future trials could stratify patients according to risk alleles to define cohorts who may benefit most from the addition of CCBs to standard therapy (Ostacher et al., 2014; Lencz and Malhotra, 2015). Nonselective brain-permeable CCBs, such as isradipine, are expected to block both CaV1.2 and CaV1.3. On the basis of the preclinical findings discussed above, the inhibition of CaV1.3 may contribute antidepressant effects.
c. Febrile Seizures.
CaV1.2 channels appear to contribute critically to the generation of febrile seizures. This has been shown using patch clamp recordings from hippocampal pyramidal cells in acute rat pup brain slices (Radzicki et al., 2013). Nimodipine could block hyperthermia-induced abnormal spontaneous firing of these neurons in vitro as well as in an in vivo model. Nimodipine was applied intraperitoneally, acutely at a dose of 2.5 mg/kg, which was carefully selected to prevent side effects, but it is expected to reach much higher plasma concentrations than during therapeutic dosing in humans. Nimodipine, unlike other CCBs, is also a potent inhibitor of adenosine uptake (Striessnig et al., 1985); therefore, a contribution of this mechanism to the observed in vivo protection of febrile seizure cannot be excluded. Irrespective of these considerations, this study provided compelling evidence for a role of CaV1.2 in febrile seizures and for clinical trials to stop or prevent seizures triggered by high fever and to reduce the risk for long-term neurologic consequences. Parenteral nimodipine is already licensed for the treatment of subarachnoid hemorrhage and is thus already available for interventional studies.
d. Cardiovascular Disease.
The recent discovery that CaV1.3 plays a key role in aldosterone secretion may be one of the reasons why therapeutic doses of the DHP CCBs, which preferentially block CaV1.2 in arterial resistance vessels, show no robust inhibitory effects on aldosterone secretion in humans. This may be achieved in the future with potent CaV1.3-selective inhibitors. They are unlikely to affect cardiac inotropy due to their absence in ventricular myocardium but are expected to cause a bradycardic effect (Platzer et al., 2000; Baig et al., 2011). This combined mechanism of action could be therapeutically meaningful in patients with heart failure, in which heart rate (due to enhanced sympathetic drive) and aldosterone (due to secondary aldosteronism) are both elevated. High heart rate is a risk factor in heart failure, and selective lowering of heart rate, with the HCN (If) channel blocking bradycardic agent ivabradine, improves cardiovascular outcomes (Böhm et al., 2010). However, in this patient cohort, a troublesome side effects of these drugs may be atrial fibrillation risk, which has been shown to be increased in CaV1.3-deficient mice (see above).
4. Pharmacological Targeting of L-Type Calcium Channels in the Brain.
Effective block of LTCCs in the brain is complicated by the fact that negative resting membrane potentials in most neurons and short action potential durations do not favor high sensitivity for DHPs due to their state-dependent action (Helton et al., 2005). At the same time, alternative splicing and more depolarized potentials render CaV1.2 channels highly DHP sensitive in arterial resistance vessels (see above). To minimize cardiovascular side effects and maximize therapeutic actions in the brain, two strategies can be pursued. One consists of the development of CaV1.3-selective drugs. However, if inhibition of neuronal CaV1.2 channels is also desired, then higher CNS activity may be achieved by enhancing brain delivery of CCBs to the brain.
a. CaV1.3-Selective L-Type Calcium Channel Blockers.
In radioligand binding studies, isradipine binds to CaV1.2 and CaV1.3 channels with indistinguishable affinities (Koschak et al., 2001). However, in functional studies, isradipine inhibits recombinant CaV1.2 channel currents with about 5- to 10-fold lower IC50 values (Koschak et al., 2001), indicating differences in the effect of voltage on drug sensitivity. Evidence for more potent inhibition of CaV1.2 by isradipine also comes from experiments in isolated SAN cells, in which 70% of the L-type current is CaV1.3 mediated. In a previous study, 50 nM isradipine inhibited only 26% of the wild-type current (mostly CaV1.3) but 72% of the CaV1.2 component remaining in CaV1.3−/− SAN cells. This implies an IC50 for CaV1.3 well above 50 nM (Mangoni et al., 2003).
Thus far, only one study has described CaV1.3-selective blockers. A detailed structure-activity relationship has been reported for novel pyrimidine-2,4,6-triones (Kang et al., 2012, 2013). The most selective candidate, BPN-4689 [1-(3-chlorophenethyl)-3-cyclopentylpyrimidine-2,4,6-trione; also referred to as compound 8 (Cp8)] (Kang et al., 2012), showed a more than 600-fold selectivity for CaV1.3 compared with CaV1.2 in a fluorescent imaging plate reader assay. Whole-cell patch clamp recordings in human embryonic kidney 293 cells stably expressing LTCC complexes revealed an IC50 of 24.3 µM for CaV1.3 inhibition, whereas CaV1.2 Ba2+ currents were nearly unaffected. A follow-up study (Huang et al., 2014) confirmed the inhibitory activity of Cp8 on transiently expressed LTCC Ca2+ currents in whole-cell patch clamp recordings. However, neither high potency nor relevant CaV1.3 selectivity was confirmed. These experiments also revealed a dependence of the Cp8-mediated effect on the coexpressed auxiliary β subunit. With palmitoylated β2a, CaV1.2 Ca2+ currents were even more sensitive to Cp8 than CaV1.3 currents. A third study found an even more complex modulation of LTCC Ba2+ and Ca2+ currents by Cp8 (Ortner et al., 2014). In whole-cell patch clamp recordings on transiently expressed LTCCs in tsA201 cells, Cp8 induced a pronounced time-dependent change in gating kinetics characterized by a slowing of the activation, inactivation, and deactivation time course and thus closely resembled the activity of known LTCC activators. This effect was also confirmed for native CaV1.2- and CaV1.3-current components in mouse chromaffin cells (Ortner et al., 2014). Taken together, these studies suggest that the CaV1.3 selectivity of Cp8 and related pyrimidine-2,4,6-triones is highly dependent on experimental conditions and that these drugs may even cause channel-activating effects. Therefore, CaV1.3-selective blockers, for use as CaV1.3-selective pharmacological tools and suitable for further clinical development, still remain to be discovered.
b. L-Type Channel Activators as Therapeutics.
In addition to selective blockers, activators of LTCCs have also been successfully used to study the role of LTCCs for cellular signaling and LTCC physiology in vivo. The most widely used experimental compounds are the DHPs (−)-BayK8644 and (+)-SDZ202-791 (Glossmann and Striessnig, 1990) as well as the benzoyl pyrrole FPL 64167 (methyl 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylate) (Zheng et al., 1991). The stereoselective activation of a voltage-gated Ca2+ current component by these drugs is currently the most specific proof for the presence of an L-type current. These compounds exert their activating properties by increasing current amplitudes, shifting activation voltage to more negative voltages, slowing of inactivation, and increasing and slowing tail currents (Tsien et al., 1986; McDonough et al., 2005). Despite their invaluable role in studying the molecular pharmacology of LTCCs in vitro, they are not suitable for clinical use. They activate all four LTCC isoforms and in vivo effects are largely determined by toxic effects through activation of CaV1.2 in the brain and cardiovascular system. BayK8644 increases cardiac contractility (Pelc et al., 1986), induces cardiac arrhythmias (Zhou et al., 2013), and elevates arterial blood pressure (Bourson et al., 1989). Activation of brain LTCCs by BayK8644 induces a severe neurobehavioral dystonic syndrome, including self-biting, mostly due to CaV1.2 activation (Sinnegger-Brauns et al., 2004; Hetzenauer et al., 2006). It is associated with enhanced release of dopamine, glutamate, and other neurotransmitters as well as massive neuronal activation in most brain regions (Sinnegger-Brauns et al., 2004; Hetzenauer et al., 2006). These pharmacological effects preclude the chronic administration of LTCC activators. However, it is currently unclear whether short-term administration of low doses in a controlled clinical setting could lead to long-term changes in brain function, such as those induced by electroconvulsive therapy.
c. Peptide Toxins Inhibiting L-Type Calcium Channels.
As for non-LTCCs, peptides selectively inhibiting L-type channels have been discovered. Calciseptine and FS2 (Fig. 3) are structurally highly related 60–amino acid polypeptides, isolated from venom of the black mamba (Dendroaspis polylepis polylepis). Similar to DHPs, they selectively block LTCCs, and this explains their smooth muscle relaxant and cardiodepressant properties (De Weille et al., 1991; Watanabe et al., 1995). Glacontryphan-M (11 amino acid residues) isolated from the venom of the marine snail Conus marmoreus (Hansson et al., 2004) is also present in the wings of a butterfly, apparently serving as predator defense (Bae et al., 2012). In pancreatic β cells, it inhibits only L-type currents with low nanomolar IC50 values and does not inhibit other (CaV2) Ca2+ channels (Hansson et al., 2004). Selective but less potent inhibition of neuronal LTCCs has also been reported for a peptide, CSTX-1, isolated from the venom of a spider, Cupiennius salei (Kubista et al., 2007). Calcicludine is a 60–amino acid polypeptide from the venom of Dendroaspis angusticeps structurally related to dendrotoxins (Schweitz et al., 1994). In addition to neuronal L-type currents, it also blocks native N-type and other high voltage–activated Ca2+ channels at low nanomolar concentrations (Schweitz et al., 1994). In contrast with N-type CCBs (ziconotide, see section III.D.2 on CaV2 channels), peptide toxins blocking LTCCs have not been developed for clinical use thus far.
5. Indirect Modulation of CaV1 Calcium Channels.
The activity of LTCCs is modulated by neurotransmitters, enzymes, and alternative splicing and protein interactions in a number of ways.
a. cAMP-Dependent Protein Kinase (Protein Kinase A).
Activation of cardiac (CaV1.2) LTCCs by adrenergic stimulation in the “fight-or-flight” response and upon therapy with β-adrenergic receptor agonists is the classic example of ion channel regulation by a signaling pathway (Fig. 3). During the fight-or-flight response, PKA phosphorylates CaV1.2 LTCC currents in cardiomyocytes, and this contributes to increased heart rate and contractility. Modulation requires the proteolytic cleavage of the C-terminal tail by post-translational proteolytic processing. The resulting C-terminal fragment remains noncovalently attached with the remainder of the long C-terminal tail through interaction of two putative α-helices (PCRD and DCRD, see above) (Fuller et al., 2010; Fu et al., 2013). Binding of the C-terminal fragment to the cleaved α1 subunit inhibits channel activity. PKA is anchored to the C-terminal fragment by A kinase–anchoring proteins. PKA phosphorylates two CaV1.2 α1 residues, serine 1700 and threonine 1704, within the PCRD helix (Fuller et al., 2010; Fu et al., 2013). This interferes with PCRD–DCRD interaction, relieves inhibition by the C-terminal fragment, and increases CaV1.2 current. In mutant mice carrying these mutations, the important role of the phosphorylation of these residues for β-adrenergic modulation of CaV1.2 channels in the heart was recently confirmed in vivo (Fu et al., 2013). C-terminally attached phosphatases (including protein phosphatase 2A and 2B/calcineurin) ensure rapid dynamics for regulation by phosphorylation/dephosphorylation in the heart and brain (Murphy et al., 2014).
A kinase–anchoring proteins are also found in a complex with native CaV1.3 channels (Marshall et al., 2011) and are stimulated by PKA. This has been shown in adrenal chromaffin cells (Mahapatra et al., 2012) and in the SAN (Mangoni et al., 2003). For CaV1.3, the molecular details for PKA regulation are less well studied but seem to also involve phosphorylation sites within the C-terminal tail (Liang and Tavalin, 2007; Ramadan et al., 2009).
b. Membrane Phospholipids.
Various G protein–coupled receptors (GPCRs) (e.g., muscarinic acetylcholine receptors) can inhibit voltage-gated calcium channels, including LTCCs (Suh and Hille, 2005; Hille et al., 2015), through activation of phospholipase C. Phosphatidylinositol 4,5-bisphosphate (PIP2) seems to stabilize active channel conformations by tethering cytoplasmic domains, bound to its inositol phosphates, to the plasma membrane to which PIP2 is anchored through its fatty acid side chains (Suh et al., 2012). This can explain the reduction of Ca2+ channel currents by receptor-mediated PIP2 depletion. In superior cervical ganglion neurons, extracellularly applied arachidonic acid can also inhibit Ca2+ channel activity (Heneghan et al., 2009). Current models predict that arachidonic acid released after phospholipase C activation and activation of Ca2+-sensitive phospholipase A2 can occupy the fatty acid binding site of PIP2 and interfere with PIP2 stabilization of the channel. Channel-lipid interactions at the inner leaflet of the membrane bilayer which reduce rather than stabilize channel activity have also been identified (Kaur et al., 2015).
As for CaV2 channels, inhibition of LTCCs by GPCR activation through direct G protein–mediated, membrane-delimited pathways (Fig. 3, pathway 2a) has also been reported (Gilon et al., 1997; Pérez-Garci et al., 2013) but is less well understood on the molecular level.
c. Receptor Tyrosine Kinases.
Activation of receptor tyrosine kinases (e.g., by insulin-like growth factor-1) can activate CaV1.2 and CaV1.3 LTCC function, involving phosphorylation of their pore-forming α1 subunits (Bence-Hanulec et al., 2000; Gao et al., 2006) (Fig. 3, pathway 2c).
d. Protein Interactions with L-Type Calcium Channels.
For a discussion of confirmed protein interaction partners, see separate reviews by Calin-Jageman and Lee (2008) and Striessnig et al. (2014). Protein–protein interactions, as described for LTCCs in the brain and heart, can serve as scaffold proteins, stabilize channel gating, recruit enzymes (e.g., PKA, CaMKII; see above) to the channel, or guide the channel to defined subcellular compartments. In principle, modulation of LTCC may also be achieved by interference with modulatory proteins, including accessory subunits. For example, genetically encoded CCBs can be obtained by anchoring known α1-subunit protein interaction partners (e.g., CaM or CaMKII) to the plasma membrane (Yang et al., 2013).
e. Novel Modulatory Mechanisms.
Since maintenance of LTCC channel activity within a narrow activity range seems to be a prerequisite especially for normal brain and heart function, close control of its activity and expression is required. Recent studies have identified novel modulatory mechanism beyond the usual signaling pathways. Among those are microRNAs (miRs), which have been identified as potential regulators of CaV1.2. For example, miR-1 targets the CaV1.2 α1-subunit gene (CACNA1C) and reduces its expression (Rau et al., 2011). In DM (DM1, DM2) miR-1 is lost, which may account for the observed upregulation of heart CaV1.2 α1 protein and the resulting cardiac pathology in affected individuals (Rau et al., 2011).
E. Conclusion
The recent discovery of important physiologic functions controlled by different LTCC isoforms (particularly CaV1.2 and CaV1.3) identifies these LTCCs as new drug targets. This is especially attractive because nonselective channel blockers have been in clinical use for decades and could therefore be repurposed for novel indications. In addition, a high therapeutic potential for several indications, including neuropsychiatric diseases, can also be predicted for novel, CaV1.3-selective CCBs.
III. CaV2 Channel Family
A. Genes, Gene Products, and Splice Variants
Like the LTCCs described in the preceding section, members of the CaV2 family are heteromultimeric assemblies of a pore-forming CaVα1 subunit plus ancillary CaVβ and CaVα2δ subunits, with the former defining the channel subtype. The CaV2 family is encoded by three genes (CACNA1A, CACNA1B, and CACNA1E) that encode CaVα1 subunits CaV2.1, CaV2.2, and CaV2.3, respectively (Mori et al., 1991; Dubel et al., 1992; Williams et al., 1992). CaV2.1 channels give rise to both P-type and Q-type currents that were described in neurons, with this distinction likely being caused by a combination of associated the CaVβ subunit (Richards et al., 2007) and alternative splice events in the CaV2.1 subunit per se (see below). CaV2.2 and CaV2.3 underlie neuronal N-type and R-type currents, respectively.
Each of the CaV2 channel family members can undergo alternative splicing, thus creating a wide spectrum of CaV2 currents with specific biophysical and pharmacological properties. For example, alternative splicing of CaV2.1 channels in the domain I–II linker region can drastically alter voltage-dependent inactivation, whereas an insertion of asparagine-proline motif in the domain IV S3–S4 loop region drastically alters sensitivity of the channels to the spider toxin ω-agatoxin-IVA (Bourinet et al., 1999). Alternative splicing of exon 37, in the CaV2.1 C-terminal region, results in altered calcium-dependent inactivation and facilitation of the channel (Soong et al., 2002; Chaudhuri et al., 2004; Chang et al., 2007). Alternative splicing of the C-terminal region also affects channel biophysics and functional regulation by the CaVβ subunit (Sandoz et al., 2001). It is interesting to note that splice variation in CaV2.1 channels has been shown to alter the functional effects of mutations linked to familial hemiplegic migraine (FHM) (Adams et al., 2009), such that biophysical consequences of several of these pathologic mutations are weakened in CaV2.1 channels containing exon 47.
Alternative splicing of CaV2.2 channels has also been described. Splicing of sequences in domain III S3–S4 and domain IV S3–S4 gives rise to variants with different biophysical properties and tissue distribution (Lin et al., 1997, 1999). Along these lines, alternative splicing of exon 18 in the intracellular domain II–III linker region alters voltage-dependent inactivation of the channels (Pan and Lipscombe, 2000; Thaler et al., 2004), and splice variants of the human CaV2.2 channel that lack large portions of the II–III linker display very large shifts in the half-inactivation potential of the channel and distinct subcellular distributions in neurons, in addition to preventing the association of the channel with synaptic proteins such as syntaxin 1A (Kaneko et al., 2002; Szabo et al., 2006). Interestingly, similar deletion variants in the CaV2.1 II–III linker have also been described (Rajapaksha et al., 2008). Alternative splicing of exon 31 in the CaV2.2 voltage sensor produces channels with different activation kinetics (Lin et al., 2004). Perhaps the splice event that has gathered the most attention involves exon 37 of the channel. Alternative splicing of this exon (which encodes sequences in the CaV2.2 C terminus) alters current densities, second messenger regulation, and tissue distribution, with the exon 37a–expressing variants being more expressed in small nociceptive neurons (Bell et al., 2004; Castiglioni et al., 2006; Raingo et al., 2007; Andrade et al., 2010). Remarkably, the variant containing exon 37a contributed most prominently to nociceptive signaling (Altier et al., 2007). The effects of splicing of exon 37 on current densities could be correlated with alterations in the ubiquitination state of the channel (Marangoudakis et al., 2012). Truncated forms of CaV2.1 and CaV2.2 channels that lack entire transmembrane domains have also been reported (Scott et al., 1998; Raghib et al., 2001). Coexpression of these truncated forms mediates dominant negative effects on full-length channels due to the activation of unfolded protein response pathways (Page et al., 2004).
Interestingly, splicing of exons 24 and 31 in CaV2.1 and CaV2.2 channels appears to be controlled by the splicing factor Nova-2 (Allen et al., 2010), suggesting a common cellular mechanism for fine-tuning the expression/properties of these two channel subtypes, and perhaps explaining why analogous splice variants are observed in these two calcium channels subtypes (as noted above, large deletions in the domain II–III linker region have been described for both CaV2.1 and CaV2.2 channels; Kaneko et al., 2002; Rajapaksha et al., 2008).
In contrast with CaV2.1 and CaV2.2, investigations into alternate splicing of CaV2.3 channels have been more limited. Six major splice isoforms of CaV2.3 have been identified and shown to differ in their tissue distribution (Marubio et al., 1996; Vajna et al., 1998; Schramm et al., 1999) and in their pharmacological properties (Tottene et al., 1996, 2000).
Overall, the three members of the CaV2 family can give rise to a large number of different types of ionic conductances through alternate splicing of various exons. This diversity is further enhanced by coassembly with the various ancillary CaVβ and CaVα2δ subunits, and their splice variants, thus providing tremendous control of calcium entry in specific tissues at specific times during development. The existence of multiple variants of a single calcium channel type is potentially important for drug design. For example, in the context of developing new analgesics, the ability to selectively target CaV2.2 channels containing exon 37a, although downregulated in experimental neuropathic pain conditions (Altier et al., 2007), might allow for selective inhibition of CaV2.2 channels expressed in nociceptive neurons while sparing channels expressed in other regions of the nervous system.
B. Physiologic Roles of CaV2 Calcium Channels
CaV2 channels are primarily thought of as the drivers of evoked synaptic transmission (Wheeler et al., 1994). Although these channels are expressed at various subcellular loci, they are targeted to presynaptic nerve terminals where they open in response to incoming action potential (Westenbroek et al., 1992, 1995). The ensuing entry of calcium ions then triggers the fusion of synaptic vesicles, culminating in the release of neurotransmitters into the synaptic cleft. The three major CaV2 channel isoforms support not only rapid neurotransmitter release but also hormone release from secretory cells such as chromaffin cells (Santana et al., 1999; Albillos et al., 2000; Wykes et al., 2007; Álvarez et al., 2013).
To facilitate effective coupling between the neurotransmitter release machinery and calcium entry, these CaV2.1 and CaV2.2 channels contain a synaptic protein interaction (synprint) site that interacts with syntaxin 1A and SNAP25 (Sheng et al., 1994, 1996; Rettig et al., 1996). This is one mechanism by which channels can be localized in proximity to synaptic vesicles. It also allows for regulation of calcium channel activity by these synaptic proteins. In particular, syntaxin 1A is a potent regulator of CaV2.1 and CaV2.2 channel availability (Bezprozvanny et al., 1995); furthermore, syntaxin 1A facilitates G protein inhibition of CaV2.2 channels (Jarvis et al., 2000; Jarvis and Zamponi, 2001; for review, see Zamponi, 2003). Several considerations suggest that this syntaxin 1A–mediated regulation of channel activity, rather than coupling to the release apparatus, may be the physiologically more important function of the synaptic protein interaction site. First, invertebrate CaV2 channels do not possess a synprint motif, yet they perfectly support synaptic transmission (Spafford et al., 2003). Second, although they are able to bind to syntaxin 1A in vitro, CaV2.3 calcium channels do not have a synprint-like motif. Finally, work from several groups has identified postsynaptic density protein PSD95, Drosophila disc large tumor suppressor Dlg1, and Zona occludens-1 protein–containing proteins such as Rab3-interacting molecule and MINT-1 as critical anchors between CaV2 channels and synaptic vesicles (Maximov and Bezprozvanny, 2002; Han et al., 2011, 2015; Kaeser et al., 2011; Wong et al., 2013, 2014), with the interactions being critically dependent on the C-terminal region of the channel.
In addition to supporting vesicle release, members of the CaV2 channel family also fulfill other signaling functions. For example, CaV2.1 and CaV2.2 channels interact physically with large conductance calcium-activated potassium channels and provide the calcium influx needed to efficiently activate these channels (Berkefeld et al., 2006; Berkefeld and Fakler, 2008). This in turn allows CaV2 channels to regulate neuronal excitability by altering potassium conductances (Loane et al., 2007). In addition, CaV2 channel activity has been linked to CREB-dependent gene transcription via activation of Ca2+-CaMKII (Wheeler et al., 2012) as well as to the activation of nuclear factor of activated T cells (Hernández-Ochoa et al., 2007). Along these lines, the expression of syntaxin 1A appears to be initiated by activation of CaV2.1 calcium channels (Sutton et al., 1999), again via a CREB-dependent pathway.
These fundamental roles of CaV2 channels for neuronal function and communication manifest themselves in many critical physiologic functions in the whole animal, ranging from motor control to the transmission of sensory information. These roles are exemplified by many pathologic conditions that occur as a result of calcium channel dysfunction, as we discuss in the ensuing section.
C. CaV2 Channel Pathophysiology
Notwithstanding the possibility of compensation, CaV2 channel knockout mouse lines can provide compelling insights into the function of a particular CaV2 channel isoform. This is readily apparent when considering the phenotype of CaV2.1 null mice. These mice exhibit ataxia and absence seizures and die around 4 weeks after birth (Jun et al., 1999). Although CaV2.1 channels control neuromuscular synaptic transmission under normal circumstances, these mice are not paralyzed, likely because of compensation from CaV2.2 and CaV2.3 channels (Jun et al., 1999; Urbano et al., 2003). It is interesting to note that postnatal deletion of the CaV2.1 encoding gene results in a much slower onset of the neurologic deficits (Mark et al., 2011).
In contrast with CaV2.1 null mice, CaV2.2 deficiency leads to only mild consequences, which include reduced pain hypersensitivity in models of inflammatory and neuropathic pain (Hatakeyama et al., 2001; Kim et al., 2001a; Saegusa et al., 2001), hyperactivity (Beuckmann et al., 2003), reduced anxiety (Saegusa et al., 2001), a reduction of voluntary alcohol intake (Newton et al., 2004), and problems with blood pressure control (Mori et al., 2002). The effects of CaV2.2 channel deletion on pain are consistent with the notion that CaV2.2 channels are critical for neurotransmitter release from afferent terminals in the spinal dorsal horn (for review, see Bourinet et al., 2014), and these findings validate CaV2.2 channels as potential targets for analgesics. The link between CaV2.2 channels and behaviors related to addiction and anxiety is less clearly understood. Similar to CaV2.2 channel knockout mice, mice lacking CaV2.3 are viable and show reduced pain sensitivity (Saegusa et al., 2002). These mice are also resistant to certain types of chemically induced seizures, suggesting a role of these channels in thalamocortical network excitability or communication (Weiergräber et al., 2007); these mice also show deficits in hippocampal theta oscillation architecture (Müller et al., 2012). Finally, it has been reported that these CaV2.3 null mice show deficits in second-phase insulin release (Jing et al., 2005).
Another source for insights into the physiologic and pathophysiological roles of channels is derived from channelopathies in both animals and humans. To our knowledge, no mouse mutations in CaV2.2 and CaV2.3 channels have been linked to a disease phenotype, perhaps consistent with the absence of a severe phenotype on the corresponding null mice. However, there is a recent report of an apparent gain-of-function human point mutation in the CaV2.2 gene, leading to a myoclonus-dystonia phenotype (Groen et al., 2015).
A different picture emerges with regard to CaV2.1 channels. There are several mouse lines with mutations in CaV2.1 channels that give rise to ataxic and epileptic phenotypes. This includes “leaner,” “tottering,” and “rocker,” which were previously reviewed in detail (Pietrobon, 2002; Khosravani and Zamponi, 2006). Mutations in CaV2.1 channels have been described in patients with various forms of ataxia, as well as in patients with FHM. Spinal cerebellar ataxia type 6 is a disorder in which there is a polyglutamine expansion within the channel’s C-terminal region (Jodice et al., 1997). The cellular mechanisms by which these expansions trigger the disease phenotype remain a topic of investigation. When introduced into CaV2.1 channels and studied in heterologous systems, the polyglutamine expansions have been shown to cause hyperpolarizing shifts in the half-inactivation potential of the channels (Matsuyama et al., 1999). When introduced into a mouse model, however, channel function in cerebellar Purkinje cells does not appear to be compromised in Purkinje cells (Saegusa et al., 2007). A similar lack of effects on channel biophysics was observed in another mouse model; nontheless, an age-dependent neuronal dysfunction was observed, and there was an accumulation of CaV2.1 aggregates (Watase et al., 2008). Altogether, these data suggest that it is the formation of these aggregates, rather than alterations in channel function, that underlies the clinical phenotype in spinal cerebellar ataxia type 6.
Episodic ataxia type 2 is another form of movement disorder that has been linked to CaV2.1 channel mutations (for review, see Pietrobon, 2010). These mutations include missense mutations, splice site mutations, and frame shifts the lead to the truncation of the CaV2.1 protein junctions and typically lead loss of channel function (Wappl et al., 2002; Kipfer et al., 2013). In addition, dominant negative effects of mutated channels on normal CaV2.1 channels have been reported (Jouvenceau et al., 2001; Jeng et al., 2008; Mezghrani et al., 2008; Page et al., 2010). Consistent with what has been observed with CaV2.1 null mice, loss of function of CaV2.1 due to a premature truncation of the protein has been shown to give rise to absence seizures in one patient with episodic ataxia (Jouvenceau et al., 2001). The observation that loss of CaV2.1 function is linked to adverse events such as movement disorders and seizures suggests that therapeutics with off-target actions on CaV2.1 channels may result in pathophysiological side effects.
On the other hand, gain-of-function mutations in the gene encoding CaV2.1 channels have been associated with FHM (Tottene et al., 2009). Numerous FHM-1 mutations in CACNA1A have been discovered, and a number of these have been examined in both heterologous expression systems and in knock-in mouse models. It has become clear that expression of such mutants in heterologous systems is not ideal, because these mutations appear to manifest themselves differently when the channels are present in a native neuronal environment [compare Hans et al. (1999) with Van den Maagdenberg et al. (2004)]. The various mutations can lead to drastic differences in disease severity consistent with the notion that FHM-1 has a wide spectrum of clinical phenotypes (Pietrobon and Moskowitz, 2013). A mouse model of one of the mutations (S218L) recapitulates the clinical phenotype observed in humans (Van den Maagdenberg et al., 2010), including ataxia, seizures, and brain edema after head trauma. Remarkably, a small organic molecule (tert-butyl dihydroquinone) that normalizes the gain-of-function phenotype of these mutant channels has been shown to counteract the effects of the equivalent of the S218L mutation on Drosophila synaptic physiology (Inagaki et al., 2014). It remains to be determined whether this compound may mediate similar protection in S218L knock-in mice.
Overall, among the CaV2 channel family members, CaV2.1 appears to be the main subtype compromised by multiple genetic mutations. That said, one could speculate that CaV2.2 channel and CaV2.3 channel dysfunction may be more subtle in many cases, except for the gain-of-function mutation recently reported in CaV2.2 (Groen et al., 2015), and could contribute to disorders such as pain hypersensitivity, addiction, or seizures, perhaps via dysregulation by cellular signaling processes rather than genetic abnormalities in the channels themselves.
D. Molecular Pharmacology of CaV2 Channels
1. CaV2.1 and CaV2.3 Channels and Their Potential Roles as Targets for Therapeutics.
Various types of voltage-gated calcium channels can be potently inhibited by peptide toxins isolated from the venoms of a variety of predatory organisms, such as fish-hunting molluscs, scorpions, and spiders. For example, CaV2.1 channels are potently inhibited by ω-agatoxin IVA, a large polypeptide that is isolated from the venom of the North American funnel web spider Agenelopsis aperta (Adams et al., 1993) (for review, see Olivera et al., 1994). However, as noted above, unless they are carefully modulated with compounds that normalize aberrant gain of function (see Inagaki et al., 2014), CaV2.1 channels are not broadly considered as good pharmacological targets. CaV2.3 channel inhibitors could potentially have a beneficial effect in seizure disorders (Dibué et al., 2013) and as pain therapeutics (Matthews et al., 2007); however, these channels do not have a particularly rich pharmacology and selective small organic inhibitors of CaV2.3 channels are lacking (Schneider et al., 2013). They are inhibited by the spider toxin SNX-482 (Newcomb et al., 1998); however, this toxin also targets CaV1.2 LTCCs (Bourinet et al., 2001) and A-type K+ currents (Kimm and Bean, 2014) and is thus not selective.
2. CaV2.2 Channels and Their Roles as Targets for Therapeutics.
In contrast with the CaV2.1 and CaV2.3 channels, there is an extensive body of literature pertaining to N-type calcium channel inhibitors. There are four principal means by which CaV2.2 channel–mediated cellular events can be regulated for therapeutic purposes: 1) direct block of CaV2.2 channel peptides and small organic molecules, 2) activation of a range of GPCRs, 3) interference with CaV2.2 channel trafficking, and 4) direct interference with the coupling of the channels to downstream effectors (Fig. 5). Here, we provide a brief overview of these four mechanisms.
Fig. 5.
Modulation of N-type channels by drugs, toxins, and signaling pathways. The major pharmacologically relevant classes of N-type calcium channel active drugs and toxins are shown in pathway 2. This includes pore-blocking peptide toxins such as ω-conotoxin MVIIA, as well as a series of different types of small organic molecules that include piperazines and piperidines, DHPs, and long-chain carbon molecules. N-type channels are modulated by a variety of different signaling pathways either through membrane-delimited actions of activated G proteins activated by GPCRs (pathway 1), or by interfering with scaffolding proteins such as CRMP-2 (pathway 3) (for details, see the text). The image of ω-conotoxin MVIIA is reproduced from Wikipedia (https://en.wikipedia.org/wiki/Ziconotide).
a. Direct CaV2.2 Channel Blockers.
CaV2.2 channels are potently inhibited by peptide toxins isolated from the venoms of a variety of predatory organisms. In particular, they are selectively and potently inhibited by ω-conotoxin GVIA, a peptide toxin isolated from the fish-hunting cone snail Conus geographus (Olivera et al., 1984) (Fig. 5, pathway 2). Indeed, ω-conotoxin GVIA and ω-agatoxin IVA have been used extensively as experimental tools to help distinguish native N-type and P/Q-type currents in various types of neurons, and this has been made possible largely by the high degree of target selectivity by these peptides. Furthermore, these two toxins also exemplify two major modes of action: pore block and gating modification. ω-Conotoxin GVIA is a small 27–amino acid peptide with a backbone that is constrained by three disulfide bonds and it can be physically lodged into the permeation pathway of the channel, thus acting as a pore blocker (Reynolds et al., 1986; Ellinor et al., 1994; Feng et al., 2001). The unblocking rate constant is quite low, leading to virtually irreversible block (at least over the time course of tens of minutes) unless the plasma membrane is strongly hyperpolarized (Stocker et al., 1997; Feng et al., 2003). To our knowledge, all of the known ω-conotoxins that act on CaV2 channels fall into this category. By contrast, ω-agatoxin IVA is a much larger (83 amino acid) peptide that acts by blocking voltage sensor movement, rather than occluding the pore of the channel (Mintz et al., 1992). It too produces poorly reversible inhibition, unless the membrane is repetitively depolarized—an action that allows the voltage sensors of the channel to dislodge the bound toxin (Mintz et al., 1992). Several other spider toxins fall into the category of gating inhibitors, including α-grammotoxin SIA, a peptide that is isolated from the venom of the tarantula Grammostola spatulata and inhibits both CaV2.2 and CaV2.1 channels (Lampe et al., 1993; McDonough et al., 1997), and the CaV2.3 channel blocker SNX-482 from the tarantula Hysterocrates gigas (Bourinet et al., 2001).
Fish-hunting molluscs have proven to yield a particularly rich palette of CaV2 calcium channel blockers, in many cases with selectivity for CaV2.2 channels. A 25–amino acid CaV2.2 channel pore-blocking toxin, ω-conotoxin MVIIA, has been isolated from the Conus magus snail and mediates potent analgesia after intrathecal delivery to rodents (Chaplan et al., 1994; Bowersox and Luther, 1998; Wang et al., 2000; Scott et al., 2002) and human patients with persistent cancer pain (Atanassoff et al., 2000; Miljanich, 2004; Staats et al., 2004; Thompson et al., 2006; Wallace et al., 2006; Ver Donck et al., 2008). This fits with the important role of CaV2.2 channels in neurotransmitter release from afferent terminals. C. magus also yields a number of other CaV2 channel inhibitors such as ω-conotoxins MVIIB, MVIIC, and MVIID (Olivera et al., 1994). Along these lines, peptides isolated from other types of snails such as Conus striatus, Conus fulman, and Conus catus produce various CaV2 channel blocking peptides. These peptides generally share a similar disulfide bridge arrangement and act as pore blockers. Although most of them act selectively on CaV2.2 channels (e.g., ω-conotoxins SIA, FVIA, or CVID) (Smith et al., 2002; Adams et al., 2003; Lee et al., 2010), others also block CaV2.1 channels (e.g., ω-conotoxins SIB and MVIIC) (Hillyard et al., 1992; Adams et al., 1993; Woppmann et al., 1994). Some of these ω-conotoxins are effective systemically in a mouse model of inflammatory pain (Sadeghi et al., 2013). Of note, ω-conotoxin CVID has been tested as an analgesic in clinical trials (Schroeder et al., 2006).
The blocking site for ω-conotoxin GVIA and MIIVA in the CaV2.2 subunit has been investigated via the construction of chimeric channels (Ellinor et al., 1994) and by site-directed mutagenesis (Feng et al., 2001, 2003). These studies have revealed that the large extracellular domain III S5–S6 region is a key determinant of ω-conotoxin GVIA block, and that mutagenesis of a single glycine residue in this region at position 1326 to proline dramatically enhances the reversibility of ω-conotoxin GVIA and MVIIA block. A subsequent study showed that coexpression of the CaVα2δ subunit alters both the kinetics and extent of inhibition of the channels by ω-conotoxin CVID and MVIIA (Mould et al., 2004), but it is not clear whether this is due to a steric hindrance of toxin access or an allosteric effect.
Although peptide toxins can be highly selective high-affinity blockers of various CaV2 channel subtypes, their clinical use is limited because they do not cross the blood–brain barrier. Furthermore, many of the pore-blocking conotoxins do not act effectively as state-dependent blockers (Feng et al., 2003), which can be a desirable feature in clinically active compounds, as seen with anticonvulsants and local anesthetics (Hille, 1977; Willow et al., 1985; Ragsdale et al., 1991; Zamponi et al., 1993). Both of these issues are overcome with the development of small organic blockers, but often at the expense of selectivity and affinity. Although there are, to our knowledge, no selective small organic inhibitors of CaV2.1 and CaV2.3 channels, several small organic molecules that preferentially block CaV2.2 channels have been identified, likely because of the importance of the latter channel subtype for pain transmission. The peptidylamines are one such class, and they are designed to mimic the pore-blocking actions of the larger conotoxin molecules and are formed by linking N,N-di-substituted leucine acid to a tyrosine amine (Hu et al., 1999b,c; Ryder et al., 2000). High-affinity (approximately 40 nM) block of CaV2.2 channels has been reported in the literature (Ryder et al., 1999). The same authors also identified phenylalanine and benzoxy-aniline derivatives as high-affinity (<1 μM) CaV2.2 channel blockers with efficacy in pain (Hu et al., 1999a,d).
Another distinct class of CaV2.2 channel blockers is derived from compounds that are related to D2 dopamine receptor–blocking antipsychotics (and a subclass of ion channel blockers such as fomocaine and flunarizine; Benjamin et al., 2006; Ye et al., 2011) (Fig. 5, pathway 2). These types of compounds contain a core piperidine, morpholine, or piperazine structure, often linked to one or two diphenyl moieties via alkyl chains, and they have long been known to block N-type channels (Tytgat et al., 1991; Zamponi et al., 1996). Extensive structure-activity work in this compound class has been reported (Zamponi et al., 2009; Pajouhesh et al., 2010, 2012). Furthermore, several lead compounds in this class have been validated in animal models of pain. For the derivatives with a mixed action on CaV2.2 and CaV3 channels, the addictive and intoxicating narcotic properties of ethanol were also abrogated (Newton et al., 2008). Other derivatives in this class include pyrazolpiperidines (Subasinghe et al., 2012) and aminopiperidine sulfonamide (Shao et al., 2012), both of which have analgesic properties by virtue of N-type channel blocking action.
Although most typically thought of as blockers of LTCCs, there is evidence that some DHPs can also block N-type calcium channels with high affinity. One such example is cilnidipine (Uneyama et al., 1997; Kato et al., 2002), which has analgesic properties in rats (Koganei et al., 2009) in addition to being kidney protective and antihypertensive in human patients (Hatta et al., 2012; Kario et al., 2013). These beneficial effects may be attributable to the actions of this compound on N-type channels in the sympathetic nervous system (Takahara, 2009).
Finally, other examples of CaV2.2 channel blockers that have been described in the literature include an oxindole compound termed TROX-1 [(3R)-5-(3-chloro-4-fluorophenyl)-3-methyl-3-(pyrimidin-5-ylmethyl)-1-(1H-1,2,4-triazol-3-yl)-1,3-dihydro-2H-indol-2-one] (Abbadie et al., 2010; Swensen et al., 2012), which is a state-dependent inhibitor that has analgesic properties. In addition, long carbon chain molecules such as aliphatic monoamines (Beedle and Zamponi, 2000) and farnesol (Roullet et al., 1999) block CaV2.2 channels with high affinity (albeit not selectively) and exhibit preferential block of inactivated channels. There are likely many other classes of CaV2.2 channel–inhibiting pharmacophores, altogether indicating that these channels show a rich pharmacology. It is worth reiterating that many of the compounds described above mediate state-dependent block of CaV2.2 channels, which is seen as a leftward shift in the steady-state inactivation curve of the channel and frequency-dependent inhibition of current activity. This contrasts with the tonic blocking action that is typically observed with pore-blocking toxins. Also in contrast with the action of peptide toxins, the blocking sites for the vast majority of small organic CaV2.2 channel blockers are not known. Although the nature of the coexpressed CaVβ subunit and point mutations in the domain I–II region of CaV2.1 both affect piperidine block (Benjamin et al., 2006), it is not clear whether this region is the physical interaction site for these compounds.
b. G Protein Inhibition of CaV2.2 Channels.
Many types of GPCRs are functionally linked to CaV2.2 calcium channels (for reviews, see Dolphin, 2003; Tedford and Zamponi, 2006) (Fig. 5, pathway 1). Activation of these receptors initiates nucleotide exchange in the associated Gα subunit, producing active signaling molecules (Gα-GTP and Gβγ). The Gβγ subunits physically associate with the Cav2.2 channel to mediate a potent voltage-dependent inhibition of channel activity (Herlitze et al., 1996; Ikeda, 1996), which arises from a stabilization of the channel’s closed conformation (Jones et al., 1997). CaV2.1 channels are regulated in an analogous manner, but they undergo a much smaller degree of inhibition (Arnot et al., 2000). Although the majority of clinically used drugs act via various GPCRs, these receptors are coupled to many downstream effector systems; hence, the extent to which the clinical action of receptor agonists and antagonists involves CaV2 calcium channels is unclear. However, opioid receptors are one example in which the clinical action of a receptor agonist is linked closely to CaV2.2. The µ-opioid receptor agonist morphine is a potent clinically used analgesic that interacts with µ-opioid receptors (Mizoguchi et al., 2012), which then inhibits CaV2.2 channels in dorsal horn synapses (Heinke et al., 2011), along with concomitant activation of G protein–coupled inward rectifier potassium channels (Marker et al., 2005). The receptor-induced inhibition of CaV2.2 channels is thought to reduce presynaptic calcium levels, which in turn reduces synaptic transmission between these afferent nerve terminals (Kondo et al., 2005; Beaudry et al., 2011). Morphine also acts at μ-opioid receptors that are expressed in the CNS (Diaz et al., 1995; Goodchild et al., 2004), where a clear correlation between physiologic effects and modulation of CaV2.2 channels is more difficult to establish. Although morphine is considered selective for μ-opioid receptors, selective agonists of the other three members of the extended opioid receptor family (i.e., δ- and κ-opioid receptors, and nociceptin receptors) also functionally inhibit CaV2.2 channels (Gross and Macdonald, 1987; Moises et al., 1994; Motin et al., 1995; Morikawa et al., 1998; Toselli et al., 1999; Larsson et al., 2000; Yeon et al., 2004; Ruiz-Velasco et al., 2005; Evans et al., 2010). As in the case of μ-opioid receptors, their activation induces analgesia in various animal models of pain (King et al., 1997; Darland et al., 1998; Field et al., 1999; Mika et al., 2001; Courteix et al., 2004; Nozaki et al., 2012). Although there are no clinically approved δ-opioid– and nociceptin receptor–targeting analgesics, there is at least one κ-opioid receptor agonist (pentazocine) that is used in humans as an analgesic.
GABAB receptors are another class of receptors that inhibit CaV2.2 calcium channels in dorsal horn synapses (Terrence et al., 1985); however, the associated CNS side effects typically preclude clinical use of systemic GABAB agonists such as baclofen (Schuele et al., 2005; Bortolato et al., 2010). Nevertheless, intrathecal baclofen is used in patients to treat spasticity and associated central pain after brain or spinal cord injury (Slonimski et al., 2004). Interestingly, the α-conotoxin Vc1.1 and the structurally related peptide Rg1A have been shown to activate peripheral GABAB, leading to CaV2.2 channel inhibition and analgesia when delivered intrathecally or intramuscularly (Callaghan et al., 2008; Callaghan and Adams, 2010; Klimis et al., 2011; Cuny et al., 2012; Berecki et al., 2014). To enhance oral bioavailability, a cyclized version of the Vc1.1 has been designed (Carstens et al., 2011); however, it is unclear whether these cyclized peptides could lead to similar central nervous side effects.
Altogether, CaV2.2 channels are important effectors of 7-transmembrane-helix receptors, with the physiologic significance of this regulation being most clearly exemplified in the primary afferent pain pathway. Although it is likely that agonists of other GPCRs mediate their downstream effects via CaV2 calcium channels in many other physiologic processes (see Kisilevsky et al., 2008), systematic studies of the effects of GPCR agonists in CaV2.2 null mice would be required to ascertain the importance of CaV2 channels as physiologic effectors.
c. Inhibition of CaV2.2 Channel Trafficking.
CaV2.2-type calcium channels have been shown to associate with collapsin response mediator protein 2 (CRMP-2; Chi et al., 2009) (Fig. 5, pathway 3). This interaction stabilizes the channels in the plasma membrane, presumably by slowing the rate of channel internalization, and this in turn facilitates CaV2.2 channel–mediated release of neurotransmitters such as calcitonin gene–related peptide (Chi et al., 2009). Conversely, disruption of Cav2.2 channel interactions with CRMP-2 can be achieved by using interfering peptides, attached to cell penetrating sequences such as TAT. TAT peptides reduce CaV2.2 channel density in the plasma membrane, thereby mediating analgesic effects in various pain models (Brittain et al., 2011; Ripsch et al., 2012; Wilson et al., 2012). This is an example of how CaV2.2-mediated calcium entry can be regulated by targeting the mechanism that controls channel density in the plasma membrane without blocking channel function per se. A search for small molecular mimetics of these TAT peptides is ongoing. Another mechanism that is critical for CaV2.2 channel trafficking is the association of these channels with the ancillary CaVα2δ subunit. This mechanism can also be exploited for therapeutic intervention, as discussed in detail in later sections of this review.
d. Interference with CaV2.2 Coupling to the Synaptic Vesicle Release Machinery.
As noted earlier, CaV2.2 channels physically associate with proteins that are involved in fast synaptic transmission (Sheng et al., 1994). It has been shown that competitive disruption of CaV2.2 interactions with syntaxin 1A by using synthetic synprint peptides blocks CaV2.2 channel–mediated synaptic transmission (Mochida et al., 1996). This constitutes an example of how CaV2.2 channel–mediated physiologic processes can be pharmacologically manipulated without alteration of CaV2.2 channel function or density. As with regulators of CaV2.2 channel trafficking, it may be possible to identify small organic mimetics of these synprint peptides that can be used to target CaV2.2 channel–mediated synaptic transmission as a potential approach toward treating conditions such as pain.
E. Conclusion
Altogether, among the CaV2 channel family members, CaV2.2 and to a lesser extent CaV2.3 channels have potential as therapeutic targets. Although CaV2.3 channels may potentially be explored as targets for epileptic seizures and analgesics, CaV2.3 (thus far) has a relatively limited pharmacology that can be exploited for therapeutic purposes. By contrast, substantial efforts have been made in identifying novel classes of CaV2.2 channel blockers with high affinity and selectivity. This effort may have been boosted by the U.S. Food and Drug Administration approval of Prialt (the commercial name of ω-conotoxin MVIIA or ziconotide; Jazz Pharmaceuticals, Dublin, Ireland) and the phenotype of the CaV2.2 null mouse. Beyond their application as analgesics, CaV2.2 channel blockers may well be effective in conditions such as drug dependence and anxiety.
IV. CaV3 Channel Family
A. Genes, Gene Products, and Splice Variants
T-type calcium channels are represented by three genes (CACNA1G, CACNA1H, and CACNA1I) that encode three different types of CaV3α1 subunits: CaV3.1 (Perez-Reyes et al., 1998), CaV3.2 (Cribbs et al., 1998), and CaV3.3 (Lee et al., 1999a). Expression of these subunits gives rise to T-type currents with distinct electrophysiological and pharmacological properties (Mcrory et al., 2001; Santi et al., 2002). Unlike members of the high voltage–activated channel CaV1 and CaV2 families, CaV3 calcium channels do not require coassembly with auxiliary calcium channel subunits. Nonetheless, these channels can be functionally regulated by these ancillary subunits. Coexpression of CaVα2δ subunits has been shown to increase current density of T-type calcium channels; however, no biochemical complexes have been identified (Dolphin et al., 1999; Dubel et al., 2004). Furthermore, CaVγ6 subunits can depress CaV3.1 channel current density (Hansen et al., 2004), and this is due to a physical interaction with the CaV3.1 subunit (Lin et al., 2008). Its effect can be mimicked by small peptide sequences derived from CaVγ6. Both CaVα2δ-2 and CaVγ5 subunits alter gating currents of CaV3.1 channels, which is again indicative of direct functional modulation (Lacinová and Klugbauer, 2004). Nonetheless, these functional interactions do not have the hallmarks of the universal auxiliary subunit regulation of CaV1 and CaV2 calcium channels.
The three CaV3 subunits have all been shown to undergo alternative splicing, which serves to increase functional diversity (Swayne and Bourinet, 2008). Alternative splicing events in the domain I–II linker of CaV3.1, leading to exclusion of exon 8, result in enhanced cell surface expression and thus elevated current densities (Shcheglovitov et al., 2008), indicating that this region may be involved in either endoplasmic reticulum retention or cell surface trafficking. A CaV3.1 splice isoform isolated from the mouse inner ear, including exons 14, 25A, 34, and 35, displays unique permeation characteristics (Nie et al., 2008). Multiple splice isoforms of CaV3.1 in the domain III–IV linker region that arise from different combinations of exons 25A, 25B, and 26 have been identified and shown to exhibit altered activation and inactivation kinetics, as has splicing of exon 14 in the II–III linker region (Chemin et al., 2001a). Notably, the expression of the III–IV linker splice isoforms is altered in samples from human glioma and in retinoblastoma cells, suggesting a possible role of particular CaV3.1 isoforms in tumor growth (Latour et al., 2004; Bertolesi et al., 2006). Alternative splicing has also been described for CaV3.2 channels. Splicing of exons 25 and 26 in the domain III–IV linker of this channel results in changes in activation and inactivation kinetics (Ohkubo et al., 2005). Furthermore, exon 25 influences the functional effects of CaV3.2 channel mutations that have been linked to the development of seizures in a rat model of absence epilepsy (Powell et al., 2009) and may be linked to the development of cardiac hypertrophy (David et al., 2010). Altogether, in CaV3.2 channels, as many as 14 different sites for splice variation have been identified, some of which are capable of producing nonfunctional channels (Zhong et al., 2006). Finally, splicing events in CaV3.3 channels have also been shown to give rise to variants with distinct biophysical properties. Splicing of exon 9 in the domain I–II linker and exons 33 and 34 in the C-terminal region of the channel are important determinants of channel properties (Murbartián et al., 2002, 2004).
In summary, different splice isoforms of all three CaV3 channel subtypes can give rise to a large array of different types of T-type channel conductances that may be expressed in a region-specific manner and can also be developmentally regulated. Understanding which specific splice isoforms contribute to particular physiologic functions is an important consideration for drug discovery.
B. Physiologic Roles of CaV3 Calcium Channels
T-type calcium channels are ideally suited to regulate neuronal excitability, due to their hyperpolarized range of activation and inactivation. At a typical neuronal resting membrane potential, T-type calcium channels are partially inactivated. A brief membrane hyperpolarization (e.g., inhibitory postsynaptic event) can be sufficient to recover channels from inactivation, thus increasing the fraction of the channel that is available for opening (Coulter et al., 1989; Huguenard and Prince, 1992). This in turn facilitates membrane depolarization and thus neuronal firing to give rise to a phenomenon termed “rebound bursting” (McCormick and Huguenard, 1992). This is of particular importance in thalamocortical circuitry (Ulrich and Huguenard, 1997) but also in many other brain circuits such as in the cerebellum (Molineux et al., 2006; Tadayonnejad et al., 2010). In addition, as a result of the overlap between activation and inactivation curves, T-type channels support a window current that is active near neuronal resting membrane potentials, which also contributes to the regulation of neuronal excitability (Chevalier et al., 2006; Dreyfus et al., 2010). In the SAN, CaV3 channels also contribute to pacemaker activity (Mangoni et al., 2006). Finally, T-type calcium channels have been shown to be associated physically and functionally with members of voltage- and calcium-activated potassium channels (Anderson et al., 2010, 2013; Engbers et al., 2012, 2013; Rehak et al., 2013). These associations confer T-type channel–mediated calcium-dependent control of potassium channel activity, which in turn regulates neuronal firing patterns (Turner and Zamponi, 2014). CaV3.2 channels have also been associated functionally with inhibition of KV7 channels to control axonal firing (Martinello et al., 2015) and with HCN channels to regulate presynaptic function at specific cortical synapses (Huang et al., 2011).
In addition to regulating neuronal excitability, T-type calcium channel activity has been linked to evoked hormone secretion, such as the release of catecholamines from chromaffin cells (Carabelli et al., 2007). In addition, T-type calcium channels have been linked to neurotransmitter release from presynaptic afferent nerve terminals in the spinal cord dorsal horn (Jacus et al., 2012; García-Caballero et al., 2014). This function may rely in part on the association of these CaV3 channels with the synaptic vesicle release proteins syntaxin 1A and SNAP25, which in turn have been shown to modulate Cav3 channel activity (Weiss et al., 2012). Furthermore, T-type channels, particularly CaV3.1, are important in sleep-wake cycles and feeding behavior (Uebele et al., 2009).
T-type calcium channel activity is also important for the function of the cardiovascular system and the renin-angiotensin system (Hansen, 2015). Angiotensin results in the upregulation of T-type calcium channels, which then triggers an increase in aldosterone secretion (Chen et al., 1999). This process appears to involve the activation of CaMKII and its phosphorylation of the domain II–III linker region in CaV3.2 channels (Yao et al., 2006). As a result, T-type calcium channels are considered excellent potential targets for the development of novel antihypertensive drugs (Oshima et al., 2005; Perez-Reyes et al., 2009). Aldosterone, in turn, has been shown to upregulate T-type channel expression in cultured cardiac myocytes, thereby altering beating frequency (Lalevée et al., 2005). This highlights the function of T-type calcium channels as regulators of pacemaking in the heart (Mesirca et al., 2014, 2015). A recent study revealed that CaV3.2 T-type calcium channels are also critically important for relaxation of cerebral arteries by contributing to a negative feedback loop that involves calcium-induced calcium release from RyRs and subsequent activation of calcium-dependent potassium conductances (Harraz et al., 2014, 2015). This then would suggest that T-type calcium channel blockers could act in some cases as vasoconstrictors. By contrast, CaV3.3 in human cerebral arteries contributes to smooth muscle cell contraction in cooperation with CaV1.2. (Harraz et al., 2015)
T-type calcium channel activity has also been linked to gene transcription. Activation of T-type channels has been shown to activate nuclear factor of activated T cells in cartilage tissue (Lin et al., 2014) and during the development of cardiac hypertrophy (Hsu et al., 2013; Huang et al., 2013a). T-type channels have also been linked to activation of CREB in cardiomyocytes in response to aldosterone (Ferron et al., 2011). Unlike in the case of CaV1 and CaV2 calcium channels (Wheeler et al., 2012), the mechanism by which T-type calcium channels modulates gene expression remains poorly understood.
C. CaV3 Channel Pathophysiology
Knockout mouse lacking the three CaV3 calcium channel isoforms have been created and examined in detail. Mice lacking the CaV3.1 subunit are viable and have a relatively mild behavioral phenotype. They are resistant to baclofen-induced seizures (Kim et al., 2001b), whereas mice overexpressing CaV3.1 present with absence epilepsy (Ernst et al., 2009). They also show resistance to chemically induced tremor (Park et al., 2010), but the latter is accompanied by increased cerebellar atrophy and a loss of motor coordination (Chang et al., 2011). CaV3.1 knockout mice also appear to show increased visceral pain sensation due to alterations in thalamic neuron firing (Kim et al., 2003), and these mice present with bradycardia (Mangoni et al., 2006), which is consistent with the role of these channels in cardiac pacemaker activity. Furthermore, CaV3.1 knockout mice show resistance to high-fat diet–induced weight gain (Uebele et al., 2009).
CaV3.2 null mice show abnormal development of the trachea and reduced relaxation of vascular tissue in response to acetylcholine (Chen et al., 2003a), the latter fitting with the observations described in the preceding section. These mice also show reduced sensitivity to certain types of peripheral painful stimuli (Choi et al., 2007), as well as heightened anxiety and impaired memory (Gangarossa et al., 2014). CaV3.3 null mice exhibit an increased susceptibility to drug-induced spike and wave discharges (Lee et al., 2014) but appear otherwise normal.
A number of channelopathies linked to CaV3 channels have been described in humans. Although there is no consistent linkage of mutations in human CaV3.1 and CaV3.3 channels to pathophysiology, mutations in CaV3.2 channels have been associated with seizure disorders, autism, and hyperaldosteronism. Many single nucleotide mutations in CaV3.2 have been reported in patients with childhood absence epilepsy and other types of idiopathic generalized epilepsies (Chen et al., 2003b; Heron et al., 2004, 2007). Functional studies in which these mutations were introduced into transiently expressed CaV3.2 channels revealed that a subset of the mutations caused gains of function in channel gating and increases in cell surface expression, whereas others appeared to have no effects on the biophysical properties of the channels (Khosravani et al., 2004, 2005; Vitko et al., 2005, 2007; Peloquin et al., 2006; Heron et al., 2007). The absence of biophysical effects of some of the mutations is curious; however, a recent study examining the consequences of a CaV3.2 mutation in a rat model of absence epilepsy revealed that the biophysical effect of the mutation depended critically on the use of a specific splice variant backbone of the channel (Powell et al., 2009). Gain-of-function mutations in CaV3.2 have also been linked to a genetic form of autism (Splawski et al., 2006), although how these changes in channel function lead to an autistic phenotype is not understood. Gain-of-function mutations in CACNA1H have also recently been associated with early onset hypertension and hyperaldosteronism (Scholl et al., 2015), in agreement with the known role of CaV3.2 in aldosterone secretion from zona glomerulosa cells in the adrenal cortex (Guagliardo et al., 2012).
Dysregulation of T-type calcium channels has been associated with chronic pain in animal models. In particular, the dorsal root ganglion (DRG) subtypes expressing CaV3.2 have recently been characterized in molecular detail (Reynders et al., 2015; Usoskin et al., 2015). In primary afferent fibers, CaV3.2 channels regulate neuronal excitability and synaptic transmission in the dorsal horn (Jacus et al., 2012; Waxman and Zamponi, 2014). Therefore, enhancement of CaV3.2 channel expression/activity contributes to pain hypersensitivity. Although no mutations in CaV3.2 that result in increased pain in humans have been reported in the literature, peripheral nerve injury or inflammation (Jagodic et al., 2008; García-Caballero et al., 2014), diabetes (Jagodic et al., 2007; Messinger et al., 2009), and colonic inflammation (Marger et al., 2011a) all give rise to increased DRG neuron T-type calcium currents in rodents. At least two mechanisms appear to contribute to this phenomenon: an enhancement of CaV3.2 channel trafficking, due to glycosylation in the case of diabetic pain (Orestes et al., 2013; Weiss et al., 2013), and stabilization of these channels as a result of enhanced deubiquitination (García-Caballero et al., 2014). Inhibiting CaV3.2 channels pharmacologically thus mediates analgesia (François et al., 2014). The recent development of a floxed CaV3.2–green fluorescent protein mouse line revealed that this channel is expressed in sensory neurons specialized in detecting mechanical stimuli, termed low-threshold mechanoreceptors. Conditional knockout of the channel in this subtype of DRGs further shows that CaV3.2 is implicated in allodynia linked to neuropathic pain (François et al., 2015).
As noted above, CaV3.2 channels also play a role in pressure overload-induced cardiac hypertrophy (Chiang et al., 2009). They appear to be a contributor to abnormal growth of ventricular cells (Martínez et al., 1999) and may dispose hypertrophic tissue to arrhythmias (Nuss and Houser, 1993). Finally, there is accumulating evidence that T-type calcium channels may also participate in the growth of certain cancers (Ohkubo and Yamazaki, 2012; Rim et al., 2012; Zhang et al., 2012; Das et al., 2013; Gackière et al., 2013; Dziegielewska et al., 2014).
Altogether, it appears as if aberrant expression and function of T-type calcium channels is a factor in multiple disorders. Conversely, targeting these channels pharmacologically may provide a spectrum of therapeutic benefits. Below, we highlight aspects of T-type calcium channel pharmacology.
D. Molecular Pharmacology of CaV3 Channels
1. Inorganic Ions.
One of the key distinguishing features of T-type calcium channels is their sensitivity to extracellularly applied nickel ions (Fox et al., 1987). CaV3.2 calcium channels display a greater affinity for nickel ions compared with CaV3.1 and CaV3.3 channels, by approximately one order of magnitude (Lee et al., 1999b). This is due to the fact that CaV3.2 channels express a unique histidine residue at position 191 within the domain I S3–S4 loop (Kang et al., 2006; Nosal et al., 2013). It was subsequently shown that the same residue also acts as a major redox modulation site in the channel, which leads to inhibition of channel activity by ascorbate (Nelson et al., 2007) and upregulation of channel function in the presence of l-cysteine (Nelson et al., 2005). Differential regulation of CaV3 isoforms also occurs in the presence of another metal ion, zinc. CaV3.2 channels are inhibited more strongly by zinc ions compared with the other two CaV3 isoforms (Traboulsie et al., 2007). Interestingly, under certain circumstances, zinc ions can act as agonists of CaV3.3 channels by slowing the rate of deactivation, giving rise to ultraslow tail currents (Traboulsie et al., 2007; Reynders et al., 2015). By contrast, the deactivation kinetics of CaV3.2 channels is enhanced by zinc ions (Noh et al., 2010). Finally, magnesium ions also appear to modulate T-type channel activity. Importantly, it was shown that differential magnesium blocking affinity in external barium- and calcium-containing solutions underlies the apparent differences in the magnitude of CaV3.1 currents carried by calcium and barium (Serrano et al., 2000).
In addition to divalent metal ions, T-type channels are also potently blocked by trivalent metal ions (Mlinar and Enyeart, 1993). Specifically, for cloned human CaV3.1 channels, yttrium was the most potent of the lanthanides, with an affinity of around 30 nM (Beedle et al., 2002). However, block was greatly attenuated upon increasing the concentration of permeant ions, suggesting that trivalent ions act by physically occluding the pore of the channel.
Altogether, metal ions can be potent inhibitors of T-type calcium channel activity, also showing some selectivity. However, these ions are predominantly useful as research tools, rather than as a therapeutic approach.
2. Peptide Toxins.
Like in the case of inorganic ions, peptide toxins are not particularly useful therapeutic agents because they cannot be administered orally and do not cross the blood–brain barrier. Kurtoxin, a peptide isolated from the venom of the scorpion species Parabuthus transvaalicus, was first reported to inhibit CaV3.1 calcium channels with high affinity (Chuang et al., 1998). This compound acts as a gating modifier in a manner akin to that described for the P-type channel blocker ω-agatoxin IVA (Sidach and Mintz, 2002). However, kurtoxin also targets other calcium channel isoforms, including both N and L types (Sidach and Mintz, 2002), and also has effects on sodium channels (Zhu et al., 2009). The solution structure of kurtoxin has been solved and shown to resemble those of α-scorpion toxins, but nonetheless with unique surface properties that could explain its action on T-type channels (Lee et al., 2012b). KLI and KLII are additional P. transvaalicus scorpion toxins with blocking effects on T-type calcium channels. Both toxins block T-type channels and sodium channels, with only a weak effect on transiently expressed CaV3.3 channels (Olamendi-Portugal et al., 2002).
Protoxins I and II are peptides isolated from the Thrixopelma pruriens tarantula, and they were originally described as sodium channel inhibitors (Schmalhofer et al., 2008). Both peptides were subsequently shown to block CaV3 channels in a subtype-dependent manner (Edgerton et al., 2010). Protoxin I preferentially blocks CaV3.1 channels over CaV3.3 and even more so over CaV3.2 (Ohkubo et al., 2010; Bladen et al., 2014b). Protoxin II appears to act as a gating modifier, with the highest affinity for CaV3.2 channels (Edgerton et al., 2010; Bladen et al., 2014b). Another spider toxin that blocks T-type calcium channels is PsPTx3, a peptide isolated from Theraphosidae tarantula that has apparent selectivity for CaV3.2 calcium channels (French patent application FR2940973).
Overall, compared with N-type calcium channels for which there is a rich peptide toxin pharmacology (in particular in marine snails), peptide toxin inhibitors of CaV3 channels remain relatively scant and derive mostly from arachnids. It should be noted, however, that peptide blockers of T-type calcium channels need not be confined to those derived from venomous species. For example, monocyte chemoattractant protein-1, which is an endogenous agonist of the chemokine receptor CCR2, directly and potently inhibits CaV3.2 T-type calcium channels (You et al., 2010).
3. Small Organic Molecules.
Compared with peptide toxins, there is no dearth of small organic T-type calcium channel blockers. A number of different classes of T-type calcium channel blockers have been identified (Fig. 6). One of the first recognized blockers of T-type calcium channels is the diuretic amiloride (Tang et al., 1988). It blocks CaV3.2 channels with about one order of magnitude higher affinity compared with CaV3.1 and CaV3.3 channels (Lopez-Charcas et al., 2012); however, this compound is by no means a selective T-type calcium channel inhibitor (Kleyman and Cragoe, 1988; Manev et al., 1990). The succinimides are also a group of relatively simple compounds that include the antiepileptic agent ethosuximide (Huguenard, 2002). This compound is a low-affinity blocker of all three CaV3 channel isoforms and displays state-dependent inhibition (Gomora et al., 2001).
Fig. 6.
T-type calcium channel regulators. Examples of classes of blockers known to inhibit T-type calcium channels, including small organic molecules and the peptide toxin kurtoxin. The inhibitors either physically block the pore, or bind to the gating machinery (pathway 1). T-type calcium channels can also be regulated by activation of GPCRs, either directly by G protein βγ subunits (pathway 2a), or indirectly via protein kinases such as Rho kinase, protein kinase C, or CaMKII (pathway 2b). T-type calcium channel expression in the plasma membrane is regulated by ubiquitination and deubiquitinating. The deubiquitinase USP5 removes ubiquitin groups, thus increasing channel stability in the plasma membrane. Interfering with USP5 binding to the channel (pathway 3) leads to channel internalization and degradation. The kurtoxin image is reproduced from the Orientations of Proteins in Membranes database (Lomize et al., 2006; http://opm.phar.umich.edu/protein.php?pdbid=1t1t).
Mibefradil is a compound that initially generated significant excitement in the field, due to its purported selective inhibition of T-type calcium channels (Mishra and Hermsmeyer, 1994; Ertel and Clozel, 1997). This compound was approved by the U.S. Food and Drug Administration for the treatment of hypertension, but it had to be withdrawn from the market because of metabolism by cytochrome P450 and drug–drug interactions (Mullins et al., 1998). Furthermore, this compound was by no means a selective inhibitor of T-type channels. A more recent derivative of mibefradil (NNC55-0396 [(1S,2S)-2-[2-[[3-(1H-benzimidazol-2-yl)propyl]methylamino]ethyl]-6-fluoro-1,2,3,4-tetrahydro-1-(1-methylethyl)-2-naphthalenyl cyclopropanecarboxylate dihydrochloride]) has much lower interactions with CYP3A4 (Bui et al., 2008).
T-type calcium channels also interact with endocannabinoids and synthetic cannabinoid receptor ligands. Anandamide and its derivative Na-Gly (arachidonyl glycine) mediate potent inhibition of CaV3 calcium channels (Chemin et al., 2001b; Barbara et al., 2009). NMP-7 [(9-pentylcarbazol-3-yl)-piperidin-1-ylmethanone] is a synthetic carbazole derivative that acts as an agonist of cannabinoid receptors. This compound and several of its derivatives also potently block T-type calcium channels (You et al., 2011; Gadotti et al., 2013).
Diphenyl-butyl piperidines are a class of neuroleptic drugs that are well known for their actions as D2 dopamine receptor antagonists (Seeman, 1980). Several members of this class of compounds, including pimozide and penfluridol, potently inhibit CaV3 channels in a subtype-dependent manner (Enyeart et al., 1990; Santi et al., 2002). Rational drug discovery efforts centered around the piperidine core pharmacophore have resulted in the discovery of a number of selective and highly potent T-type channel inhibitors, including a compound termed Z944 (N-[[1-[2-(tert-butylamino)-2-oxoethyl]piperidin-4-yl]methyl]-3-chloro-5-fluorobenzamide) (Tringham et al., 2012). This compound has completed phase1b clinical trials for pain and is being advanced into phase 2 trials. A series of compounds that combine the carbazole core of NMP-7 and features of Z944 also mediate potent CaV3 channel inhibition without off-target effects on cannabinoid receptors and with efficacy in several in vivo models of pain (Bladen et al., 2015). Another series of compounds that incorporates features of Z944 includes TTA-A2 [(R)-2-(4-cyclopropylphenyl)-N-(1-(5-(2,2,2-trifluoroethoxy)pyridin-2-yl)ethyl) acetamide] and TTA-P2 [3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide] (Choe et al., 2011; Francois et al., 2013), both of which mediate state-dependent inhibition of T-type currents with a preference for CaV3.2. TTA-A2 increased sleep and prevented high-fat diet–induced weight gain in mice (Uebele et al., 2009).
T-type calcium channels also have the propensity to interact with certain types of DHPs. LTCC-blocking DHPs such as nimodipine and niguldipine also potently block T-type channels (Stengel et al., 1998). Several types of DHP with preferential blocking action on T-type channels over L-type channels have since been identified (Kumar et al., 2002; Bladen et al., 2014a). In contrast with these inhibitors, another compound, ST101 (spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one), exhibits a channel-activating response, and this may be useful to further unravel the role of T-type channels. Its use in vivo showed cognitive-enhancing effects (Moriguchi et al., 2012).
It is interesting to note that many of the classes of compounds described above have been tested in various rodent models of inflammatory and neuropathic pain and have shown to mediate analgesia. Furthermore, we are aware of at least two T-type channel blockers that are being tested in humans for safety and efficacy in pain: Z944 (Lee, 2014) and ABT-639 [5-[(8aR)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazine-2-carbonyl]-4-chloro-2-fluoro-N-(2-fluorophenyl)benzenesulfonamide] (Ziegler et al., 2015). This underscores the importance of T-type calcium channels (particularly CaV3.2) in the primary afferent pain pathway. On the other hand, as noted earlier, T-type calcium channels are important targets for treating absence seizures, with ethosuximide being one of the archetypal T-type channel blocking antiepileptic drugs. Other clinically used antiepileptic drugs with at least partial action on T-type calcium channels include zonisamide (Matar et al., 2009) and valproic acid (Todorovic and Lingle, 1998).
4. Interference with CaV3 Channel Regulation.
T-type calcium channels can be regulated by extracellular signaling molecules, and this can potentially be exploited for therapeutic purposes. For example, it has been shown that T-type channels (most notably CaV3.2 channels) are regulated by redox modulation. Ascorbate inhibits CaV3.2 channel activity via metal catalyzed oxidization (Nelson et al., 2007), whereas l-cysteine increases CaV3.2 current amplitudes (Nelson et al., 2005; Joksovic et al., 2006) through redox activity. This redox modulation occurs at a specific residue (His-191) (Nelson et al., 2007), which is also involved in Ni2+ block of these channels (Kang et al., 2006) and results in hyperalgesia (Pathirathna et al., 2006). Along these lines, hydrogen sulfide induces hyperalgesia via actions on CaV3.2 calcium channels (Maeda et al., 2009). In this context, it is interesting to note that administration of polaprezinc can mediate analgesia in a model for interstitial cystitis, presumably through its antioxidant activity (Murakami-Nakayama et al., 2015).
Another means of altering CaV3 channel activity is via intracellular messenger regulation. This includes effects of protein kinases (Welsby et al., 2003; Yao et al., 2006; Iftinca et al., 2007), direct binding of G proteins (Wolfe et al., 2003), and phosphatases (Huang et al., 2013a). A detailed description of second messenger regulation of T-type channels has been the focus of several previous review articles (Huc et al., 2009; Iftinca and Zamponi, 2009). Here, we focus on one example in which T-type channel regulation can potentially be exploited as a therapeutic strategy for pain. As noted earlier, CaV3.2 channels are under control by ubiquitinating and deubiquitinating enzymes. The deubiquitinase USP5 is upregulated after injury or inflammation, leading to increased T-type channel activity and thus chronic pain. Uncoupling USP5 from the channel via interfering TAT peptides reverses the pain phenotype (García-Caballero et al., 2014), as do small organic mimetics (Gadotti et al., 2015) (Fig. 6). This is reminiscent of the approach described above for the interaction between CaV2.2 channels and CRMP-2 (Ripsch et al., 2012) and supports the idea of therapeutic interventions that are not targeted at blocking channel activity, but instead interfere with channel trafficking.
E. Conclusion
T-type channels are important regulators of neuronal firing and neuronal communication, and they play important roles in the cardiovascular system. Their dysregulation can give rise to conditions such as epilepsy, pain, cardiac hypertrophy, and cancer; consequently, they are potential drug targets for these conditions. However, despite the fact that there are many classes of potential T-type channel blocking small organic molecules, their clinical use to date has been restricted largely to the treatment of absence seizures.
V. Auxiliary α2δ and β Subunits
Purification of the channel complexes showed the CaV1.1 and CaV1.2 channels, as well as the CaV2.1 and CaV2.2 channels, to be associated with auxiliary α2δ and β subunits (Takahashi et al., 1987; Tanabe et al., 1987; Witcher et al., 1993; Liu et al., 1996). Subsequent expression studies have shown that the other CaV1 and CaV2 channels also require these subunits for cell surface and functional expression (see sections II and III). However, from purification studies, the association of the α2δ subunit with the complex was found to be looser than that of the β subunit and was dependent on the solubilization conditions used to extract the channel complex from the lipid bilayer (Müller et al., 2010). Whether the T-type channels are associated with auxiliary α2δ subunits is still under investigation (see section IV). This section concentrates on α2δ subunits, because of their important role as an established therapeutic target; however, the function of β subunits is also discussed briefly below (section V.F), because they are functionally very important in CaV1 and CaV2 calcium channel complexes and also since disruption of the interaction between α1 and β subunits has been the subject of drug discovery projects.
A. α2δ Subunit Genes and Gene Products
Four mammalian genes encoding α2δ subunits have been cloned (CACNA2D1–CACNA2D4). A further gene was also identified by homology (Whittaker and Hynes, 2002). The first to be cloned was α2δ-1, after purification of the protein as part of the skeletal muscle calcium channel complex. CACNA2D1 encodes α2δ-1, whose distribution is fairly ubiquitous in excitable cells and some other cell types. In addition to being present in skeletal muscle, it is also found in cardiac and smooth muscle as well as in both the central and peripheral nervous systems and in secretory tissue. In skeletal, cardiac, and smooth muscle, α2δ-1 is associated with the LTCCs CaV1.1 in skeletal muscle and CaV1.2 in cardiac and smooth muscle (Ellis et al., 1988; Jay et al., 1991; Klugbauer et al., 1999; Wolf et al., 2003; Walsh et al., 2009). CACNA2D2 and CACNA2D3, encoding α2δ-2 and α2δ-3, were identified by homology with CACNA2D1. They are expressed in neurons and some other tissues (Klugbauer et al., 1999; Barclay et al., 2001). CACNA2D4, encoding α2δ-4, is present in retinal neurons and elsewhere (Qin et al., 2002; Wycisk et al., 2006b). The exon structure is similar in all α2δ subunit genes; for example, CACNA2D1 has 39 exons.
Several other related genes have been found bioinformatically to have a comparable domain structure. A gene incorrectly termed CACNA2D5 in an article by Whittaker and Hynes (2002) has the sequence of α2δ-4 (CACNA2D4), and the more remotely related gene, erroneously termed CACNA2D4 in that review corresponds to the more distantly related CACHD1, and has not yet been characterized. Homologous genes are also found in Drosophila melanogaster and Caenorhabditis elegans (Dickman et al., 2008; Saheki and Bargmann, 2009) and have been characterized to affect both calcium channel and presynaptic functions. Furthermore, proteins of the CLCA gene family, which have a related domain structure, have been identified to be auxiliary subunits of calcium-activated chloride channels (Yurtsever et al., 2012).
1. α2δ Subunit Splice Variants.
It was noted that the cDNA sequence of α2δ-1 subunit isoforms expressed in rat brain and skeletal muscle showed some divergence (Kim et al., 1992). Three regions, termed A, B, and C, were later identified to be alternatively spliced, with ΔA + B + C being the major splice variant in brain and in peripheral DRG neurons (Angelotti and Hofmann, 1996; Lana et al., 2014). A change in α2δ-1 splicing was recently found in rat DRG neurons, after spinal nerve ligation. Increased expression was observed of a minor splice variant (ΔA + BΔC), particularly in small DRG neurons, and this showed a lower affinity for gabapentin (Lana et al., 2014). There are also splice variants of the other α2δ subunits, but their expression has not been studied in detail (Klugbauer et al., 1999; Barclay and Rees, 2000; Qin et al., 2002).
B. Physiologic Roles of α2δ Proteins
1. Roles in Calcium Channel Function.
The α2δ subunits were originally described as transmembrane proteins, but evidence suggests they are glycosylphosphatidylinositol anchored (Davies et al., 2010) (Fig. 1). In coexpression studies, the α2δ-1 to α2δ-4 subunits results in increased currents formed by high voltage–activated calcium channels (CaV1 and CaV2 families). The current density is markedly increased, and there are also a number of effects on biophysical parameters of the current, including hyperpolarization of inactivation and increase in inactivation kinetics as well as an increase in the coupling of channel opening to changes in voltage (Gurnett et al., 1996, 1997; Qin et al., 1998; Wakamori et al., 1999; Barclay et al., 2001; Brodbeck et al., 2002; Klugbauer et al., 2003; Yasuda et al., 2004; Cantí et al., 2005; Tuluc et al., 2007). Several of these studies show that α2δ and β subunits produce synergistic effects on current density for several channel subtypes (see Yasuda et al., 2004). For the CaV1.1 channel complex in skeletal muscle, α2δ-1 was also found to have a functional role in excitation-coupled entry of Ca2+ into skeletal myotubes, although not in the formation of the CaV1.1 tetrad structure or in excitation-contraction coupling (Obermair et al., 2005; Gach et al., 2008). In mice in which α2δ subunits were depleted, there was a reduction of calcium currents in relevant cell types (Barclay et al., 2001; Fuller-Bicer et al., 2009; Patel et al., 2013; Pirone et al., 2014).
It was recently shown that α2δ-1 subunits increased the expression of CaV2 channels in the plasma membrane (Cassidy et al., 2014); therefore, at least part of the effect of α2δ subunits on current density relates to trafficking of channel complexes. The effect of α2δ-1 was largely dependent on the additional presence of a β subunit that produced a marked increase of the density of channels in the plasma membrane (Cassidy et al., 2014). However, no effect of α2δ-1 was found on the rate of endocytosis of CaV2.2 (Cassidy et al., 2014), making it likely that α2δ subunits increase plasma membrane expression by enhancing forward trafficking of the channel complexes. In agreement with an effect of α2δ subunits on trafficking, α2δ-1 knockdown, using short hairpin RNA, reduced plasma membrane expression of CaV1.2 in smooth muscle cells (Bannister et al., 2009) and reduced CaV2.1 levels in synaptic boutons (Hoppa et al., 2012).
Several studies also found a small enhancement of T-type (CaV3) channel expression by α2δ subunits (Dolphin et al., 1999; Dubel et al., 2004), although these channels express very well without α2δ subunits. This leaves open the possibility that CaV3 channel trafficking and function may be enhanced by auxiliary subunit expression.
2. Structural Roles of α2δ-1 Subunits.
The α2δ subunits have a similar domain structure to many proteins involved in extracellular matrix and extracellular protein–protein interactions (Whittaker and Hynes, 2002). Indeed, α2δ-1 has been reported to interact with thrombospondins and to have a structural role in synapse formation (Eroglu et al., 2009), and the α2δ proteins in Drosophila have been shown to have an effect on presynaptic morphology (Dickman et al., 2008; Kurshan et al., 2009), as is also the case for α2δ-3 in an auditory system synapse (Pirone et al., 2014). Since α2δ subunits are involved in calcium channel trafficking as well as function, it may be difficult to tease apart their roles independent of calcium channels.
C. Pathophysiological Roles of the α2δ Subunits in Disease
1. Cardiac Dysfunction.
Human mutations in CACNA2D1 have been associated with cardiac dysfunction in a small number of patients, including Brugada (Burashnikov et al., 2010) and short QT (Templin et al., 2011; Bourdin et al., 2015) syndromes. Furthermore, knockout of cacna2d1 in mice resulted in a cardiac phenotype; the mice had compromised cardiac function and smaller cardiac calcium channel current density (Fuller-Bicer et al., 2009).
2. Epilepsies and Cerebellar Ataxia.
Mutations in cacna2d2 give rise to a phenotype of cerebellar ataxia and absence epilepsy in the spontaneously arising mouse mutants ducky and ducky2J (Barclay et al., 2001; Brodbeck et al., 2002; Donato et al., 2006). Entla is another mouse strain with a mutation in cacna2d2, and these mice display ataxia and tonic-clonic epilepsy (Brill et al., 2004). This is also seen in the targeted knockout of cacna2d2 (Ivanov et al., 2004). These mutations are all recessive, with the heterozygotes showing no significant behavioral effects (Barclay et al., 2001). Interestingly, ataxia is one of the adverse events reported when gabapentin and pregabalin are used therapeutically (Beal et al., 2012; Zaccara et al., 2012).
The ataxic phenotype is thought to result from the loss of α2δ-2 from cerebellar Purkinje cells, in which it is normally strongly expressed (Barclay et al., 2001). Mutations in CACNA2D2 in humans have been described to cause rare cases of recessive epileptic encephalopathy and mental retardation (Edvardson et al., 2013; Pippucci et al., 2013; Vergult et al., 2015). Family members with one mutated copy of this gene were unaffected. Regarding the role of α2δ-1 in epilepsy, in two rat models of epileptic seizures, no upregulation of brain α2δ-1 was observed, although there were regions of dysregulated α2δ-1 distribution associated with neuronal cell loss (Nieto-Rostro et al., 2014).
3. Neuropathic Pain.
Peripheral nerve injury has, as one consequence, an increase of α2δ-1 mRNA in damaged DRG neurons, as evidenced by in situ hybridization (Newton et al., 2001), microarray analysis (Wang et al., 2002), and quantitative polymerase chain reaction (Bauer et al., 2009; Lana et al., 2014). This results in an increase of α2δ-1 protein in DRGs and their terminals within the spinal cord (Luo et al., 2001; Bauer et al., 2009). Furthermore, α2δ-1–overexpressing mice show a neuropathic phenotype (tactile allodynia and hyperalgesia) in the absence of nerve injury (Li et al., 2006), indicating that α2δ-1 regulates the excitability of DRG neurons. In agreement with this, cacna2d1 knockout mice showed a phenotype of reduced sensitivity to mechanical stimulation and delayed onset of neuropathic mechanical hypersensitivity after peripheral nerve injury (Patel et al., 2013). It has been found that expression of α2δ-1 reduced the on rate of ω-conotoxins including MVIIA and CVID as well as their apparent affinity for CaV2.2 in the oocyte expression system (Mould et al., 2004). Given that α2δ-1 is upregulated in damaged sensory neurons in neuropathic pain models, this may limit the efficacy of these toxins.
A role for α2δ-3 in central pain processing in mice and humans has also been elucidated, on the basis of a Drosophila screen that identified the importance of straightjacket (stj), the Drosophila homolog of α2δ-3, in thermal nociception (Neely et al., 2010).
4. Psychiatric Disorders.
SNPs in the α2δ subunit and other calcium channel genes have been associated with a spectrum of psychiatric diseases in a five-disorder meta-analysis of GWASs (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013). The data were gathered from patients with bipolar disorder, schizophrenia, major depressive disorder, ASD, and attention-deficit disorder. In this study, SNPs in the α2δ genes CACNA2D2 and CACNA2D4 (as well as other calcium channel genes CACNA1C, CACNA1S, CACNA1D, CACNA1E, and CACNB2) were found to show significant association with illness, across these disorders. SNPs in CACNA1I were also recently associated with schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). In a more recent study, an excess of several rare disruptive mutations in CACNA2D1, CACNA2D2, and CACNA2D3, as well as other calcium channel genes and a variety of other genes involved in synaptic function, were found in schizophrenic patients (Purcell et al., 2014). Furthermore, a splice site mutation in CACNA2D3 was identified previously as a “likely gene-disrupting mutation” in ASDs (Iossifov et al., 2012).
5. Night Blindness.
It was initially found that CACNA2D4, encoding α2δ-4, was distributed in a limited number of cell types, including the pituitary gland, adrenal gland, and colon, suggesting that it might have a role in secretory tissue (Qin et al., 2002). However, widespread distribution of α2δ-4 transcripts was observed in mouse tissue (Wycisk et al., 2006a). Mutation in the CACNA2D4 can lead to dysfunction of photoreceptors, causing certain forms of night blindness. These include spontaneous mutation in the mouse, as well as human mutations (Wycisk et al., 2006a,b). These mutations are both associated with autosomal recessive cone dystrophy and night blindness. It is possible that the phenotype resulting from α2δ-4 loss or dysfunction is confined to the retina because of a unique role in photoreceptors and lack of compensation by other α2δ subunits.
6. Hearing.
A role for α2δ-3 in hearing has been identified using a knockout mouse for α2δ-3 (Pirone et al., 2014). This study identified reduced presynaptic Ca2+ channels and smaller auditory nerve fiber terminals synapsing on cochlear nucleus bushy cells to be associated with a specific hearing impairment resulting from the loss of α2δ-3.
D. Pharmacology of α2δ Ligands
1. Ligand Binding Sites on α2δ Subunits.
The α2δ-1 and α2δ-2 subunits (but not α2δ-3 or α2δ-4) possess a binding site for the antiepileptic drugs gabapentin (2-[1-(aminomethyl)-cyclohexyl] acetic acid) and pregabalin [S(+)-3-isobutyl GABA].
Other related compounds include 4-methylpregabalin and similar molecules (Ohashi et al., 2008; Corrigan et al., 2009). These compounds were not designed as drugs with α2δ binding in mind; rather, they were intended to increase GABAA receptor activation. They were synthesized to be rigid lipophilic analogs of GABA (Belliotti et al., 2005; Silverman, 2008). However, it was identified that gabapentin did not affect GABA receptors or GABA levels, despite being effective in various forms of experimental epilepsy models and in some human epilepsies (for review, see Taylor et al., 2007). To unravel the function of gabapentin, a key step was to purify and identify its binding sites in the brain by using radiolabeled gabapentin and proteomic approaches (Gee et al., 1996). By these means, the primary binding site was found to be α2δ-1. 3H-gabapentin was then observed to also bind to α2δ-2 but not α2δ-3 (Klugbauer et al., 1999; Gong et al., 2001). It is worth emphasizing that α2δ subunits, as accessory proteins of voltage-gated calcium channels, would not have been considered as drug targets. Indeed, until α2δ-1 was identified as the binding site of gabapentin, the α2δ proteins were not considered to have any ligand binding sites. A single affinity binding site was observed, and the Hill coefficient was reported as near to 1, indicating a lack of binding cooperativity (Dissanayake et al., 1997).
In the initial studies in which α2δ-1 was identified as the binding site for gabapentin, the apparent affinity for gabapentin binding showed a sequential increase during the steps of purification of α2δ from pig brain, from 92 nM in membranes to 9.4 nM for purified α2δ-1 protein (Brown et al., 1998). In our studies, a marked increase in gabapentin binding affinity was observed for both α2δ-1 and α2δ-2 purified in detergent-resistant membrane (lipid raft) fractions compared with total membrane fractions (Davies et al., 2006; Lana et al., 2014). For example, the binding of 3H-gabapentin to α2δ from membranes from mouse cerebellum (predominantly α2δ-2, which is strongly expressed in Purkinje neurons) gave a KD of approximately 385 nM, whereas the KD was approximately 80 nM in detergent-resistant membranes from the same tissue (Davies et al., 2006). For α2δ-2 expressed in Cos-7 cells, the KD was approximately 470 nM in membranes and approximately 50 nM in the lipid raft fraction (Davies et al., 2006). The affinity of 3H-gabapentin binding to α2δ-1 was similarly found to increase 3-fold on dialysis of brain membranes, and this was attributed to the removal of diffusible factor of molecular mass < 12 kDa (Dissanayake et al., 1997). These results could indicate that there may be an endogenous ligand that occupies this site, although its function still remains obscure and its nature remains unknown; however, many endogenous amino acids, including l-leucine, are able to bind to α2δ subunits (Brown et al., 1998). Structure-function studies showed that C-terminal truncation of α2δ-1 abrogated binding to 3H-gabapentin (Brown and Gee, 1998). Subsequently, residues were identified in α2δ-1, which were essential for gabapentin binding, particularly the third arginine (R) in an RRR motif (Wang et al., 1999). Mutation of RRR to RRA in both α2δ-1 and α2δ-2 consistently reduced the functionality of the α2δ-1 and α2δ-2 subunits, both in terms of calcium current enhancement (Field et al., 2006; Hendrich et al., 2008) and calcium channel trafficking (Tran-Van-Minh and Dolphin, 2010; Cassidy et al., 2014). Whether this suggests that binding of the endogenous “agonist” ligand is necessary for full functionality of the α2δ subunits remains unclear, because the mutation itself could reduce α2δ function. However, in this scenario, gabapentinoid drugs acting as “antagonists” would displace the “endogenous agonist.” Although a number of endogenous small molecules have been shown to bind to α2δ-1 and α2δ-2, including l-leucine (Gee et al., 1996; Dissanayake et al., 1997), it has not been shown that they fulfill an endogenous agonist role.
The RRR motif is situated just upstream of the VWA domain, and it is therefore predicted to sit structurally at the base of the VWA domain, between it and the first chemosensory-like domain (Dolphin, 2012). Mutation of RRR to RRA reduced the affinity of gabapentin binding, for both α2δ-1 (Wang et al., 1999) and α2δ-2 (Davies et al., 2006). Knock-in mice were created with the same point mutation in either α2δ-1 or α2δ-2, such that gabapentin binding affinity was markedly reduced (Field et al., 2006; Lotarski et al., 2011). Using the α2δ-1 knock-in mouse model, it was found that binding of gabapentin and pregabalin to the α2δ-1 subunit is essential for their therapeutic effect, both in neuropathic pain and in several epilepsy models (Field et al., 2006; Lotarski et al., 2014). Furthermore, anxiolytic-like effects of pregabalin in mice were also mediated by drug binding to α2δ-1 rather than α2δ-2 (Lotarski et al., 2011). Nevertheless, gabapentin and pregabalin show similar affinities for α2δ-1 and α2δ-2 (Gong et al., 2001; Li et al., 2011); therefore, α2δ-1–selective ligands might show an improved side effect profile.
Using ligand binding assays, it has been possible to identify many compounds that displace 3H-gabapentin or 3H-pregabalin, some with greater affinity for α2δ-1 or with selectivity toward α2δ-1 relative to α2δ-2, as well as with improved pharmacokinetics (Cundy et al., 2004; Mortell et al., 2006; Field et al., 2007; Rawson et al., 2011); however, none are yet in clinical use, except extended-release prodrugs of gabapentin. Ataxia is reported to be one of the side effects of gabapentin that might be mediated via binding to α2δ-2, arguing that selective α2δ-1 ligands could be therapeutically useful (Field et al., 2007). Many other gabapentin-like compounds have also been found to bind to α2δ-1 (Blakemore et al., 2010)
It is interesting to speculate whether ligands (endogenous or otherwise) might also bind to and modulate the function of α2δ-3 (and α2δ-4), neither of which possess an RRR motif, and whether drugs binding to the fairly ubiquitous α2δ-3 subunits might have therapeutic potential, if they could be identified. Development of an assay for such drugs would be challenging, but structural and modeling studies will have a part to play.
2. Binding of Gabapentin to α2δ-1 Splice Variants.
The minor splice variant of α2δ-1 (ΔA + BΔC), whose relative expression was increased after nerve injury in DRG neurons, showed a reduced affinity for gabapentin (Lana et al., 2014). This finding points toward the possibility that variation in the relative expression level of this splice variant in patients with neuropathic pain may influence the therapeutic efficacy of these drugs.
3. Mechanism of Action of α2δ Ligands.
In terms of molecular mechanism of action (downstream of binding to α2δ subunits), most studies indicate that gabapentin and pregabalin produce very little or no acute inhibition of calcium currents in transfected cells or in neuronal cell bodies in most studies (Rock et al., 1993; Davies et al., 2006; Hendrich et al., 2008), although some studies reported small acute inhibitory effects (Stefani et al., 1998; Martin et al., 2002; Sutton et al., 2002). It was also found that although gabapentin did not inhibit calcium currents in wild-type mouse DRG neurons, the currents in DRGs from α2δ-1–overexpressing mice were inhibited by gabapentin (Li et al., 2006). It was then found that chronic but not acute application of gabapentin markedly reduced calcium currents formed by several different α1/β/α2δ subunit combinations (Hendrich et al., 2008). This effect occurred when using either α2δ-1 or α2δ-2; however, it did not occur in the absence of α2δ subunits or when α2δ-3 or mutant α2δ subunits that bind gabapentin very poorly were used in the experiments (Hendrich et al., 2008), strongly pointing to the view that effects of gabapentin were indeed occurring via binding to α2δ subunits. More recently, it has been found that chronic gabapentin reduces the cell surface expression of α2δ-1, α2δ-2, and the associated α1 subunits CaV2.1 and CaV2.2 (Hendrich et al., 2008; Tran-Van-Minh and Dolphin, 2010; Cassidy et al., 2014), by disrupting the recycling process (Tran-Van-Minh and Dolphin, 2010). In agreement with the hypothesis that gabapentinoid drugs reduce trafficking of these α2δ subunits, it was also found that chronic in vivo pregabalin application reduced the amount of α2δ-1 in presynaptic terminals in the dorsal horn of the spinal cord, interpreted as an effect on trafficking from DRG cell bodies (Bauer et al., 2009).
Other potential mechanisms of action of gabapentin have been put forward. Thrombospondins are secreted extracellular matrix proteins that promote synaptogenesis via binding to a postsynaptic receptor, which was identified to be α2δ-1 (Eroglu et al., 2009). Gabapentin was found to disrupt this interaction between α2δ-1 and thrombospondins (Eroglu et al., 2009) and has been found to interfere with synaptogenesis, while at the same time not affecting the stability of preformed synapses (Eroglu et al., 2009). Thrombospondins are elevated during synaptogenesis associated with development and are also elevated after nerve injury. However, thrombospondins promote formation of nonfunctional silent synapses (Christopherson et al., 2005), and thrombospondins destabilize postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors (Hennekinne et al., 2013). The involvement of gabapentin in these processes remains unclear.
As mentioned above, it is possible that selective ligands of α2δ-1 might be of therapeutic use, with fewer side effects, because the therapeutic antiepileptic, anxiolytic, and antihyperalgesic effects in animal models of gabapentin and pregabalin were via binding to α2δ-1 (Field et al., 2006; Lotarski et al., 2011, 2014). Nevertheless, α2δ-2 binds these drugs with similar affinity and is expressed in brain regions associated with movement and other behaviors (Barclay et al., 2001).
4. Role of Amino Acid Transporters in the Action of Gabapentin.
Pregabalin and gabapentin do not inhibit the transport of GABA in vitro (Su et al., 2005) However, both gabapentin and pregabalin are zwitterions at neutral pH and use the large neutral amino acid transporter “system L” for uptake across cell membranes (Belliotti et al., 2005; Su et al., 2005; Dickens et al., 2013).
5. Effects of α2δ Ligand Drugs on Synaptic Transmission and Transmitter Release.
α2δ-1 subunits are strongly expressed in presynaptic terminals (Taylor and Garrido, 2008; Bauer et al., 2009). Indeed, chronic effects of gabapentinoid drugs have been observed in pain pathways (Biggs et al., 2014). Acute effects of gabapentinoid drugs to inhibit transmitter release and synaptic transmission have been observed in some, but not all, in vitro systems (reviewed in Davies et al., 2007; Taylor et al., 2007). It is possible that, despite their lack of acute effect on somatic calcium currents in several systems (for review, see Dolphin, 2013), the gabapentinoid drugs might have differential effects on calcium currents in presynaptic terminals compared with cell bodies, resulting in more rapid inhibition. It is also possible that calcium channel turnover is higher in presynaptic terminals than in somata, so that effects on calcium channel trafficking are observed more acutely. Thus, gabapentinoid drugs might act rapidly or more slowly, depending on the interplay between these different processes, and possibly also depending on presynaptic sensitization, such as by activation of protein kinase C (Maneuf and McKnight, 2001). However, gabapentin had no effect on transmitter release at hippocampal synapses in culture (Hoppa et al., 2012).
6. Effects of Gabapentinoids in Preclinical Animal Models.
a. Seizure Models.
Gabapentin and pregabalin are effective in several models of anticonvulsant action (Welty et al., 1993; Vartanian et al., 2006). By contrast, pregabalin did not reduce the incidence of spontaneous absence seizures in a genetic rat model of absence seizures (Vartanian et al., 2006). The effect of these drugs in several epilepsy models has been shown to result from binding to α2δ-1, rather than α2δ-2 (Lotarski et al., 2014), although there was no widespread upregulation of brain α2δ-1 in two epilepsy models studied (Nieto-Rostro et al., 2014). It is possible that gabapentin has its effect by preventing new synapse formation as previously described (Eroglu et al., 2009), giving rise to the possibility that gabapentin might be protective against epileptogenesis by these means (Radzik et al., 2015).
b. Pain Models.
The effect of the gabapentinoid drugs is said to be “state dependent,” meaning they have no effect on normal acute pain perception in naive animals, whereas they are efficacious in chronic pain models (Dickenson et al., 2005). Some behavioral effects of gabapentinoid drugs on pain phenotypes may be observed acutely (Field et al., 2006), but chronic treatment is generally more effective (Hao et al., 2000; Fox et al., 2003; Xiao et al., 2007). In agreement with this, it was observed that the effect of pregabalin increased with time of chronic treatment (Bauer et al., 2009).
E. Therapeutic Uses of α2δ Ligands
1. Epilepsy.
Gabapentin was first developed as a GABA analog; although it is now known that this does not represent its mechanism of action, it was shown to be effective as an antiepileptic drug in clinical trials. Gabapentin was approved for use as an adjunct drug to improve control of partial seizures (Crawford et al., 1987; Marson et al., 2000). Pregabalin is also effective as adjunct therapy for partial seizures (French et al., 2003). It is possible that gabapentin also has a protective mechanism against seizure development (Rossi et al., 2013).
2. Neuropathic Pain.
Gabapentin and pregabalin are widely used in neuropathic pain treatment, including diabetic neuropathy, chronic neuropathic pain induced by chemotherapeutic and other drugs, as well as postherpetic and trigeminal neuralgia (Rosenstock et al., 2004; Richter et al., 2005; Stacey et al., 2008; O’Connor and Dworkin, 2009; Moore et al., 2009, 2014). These drugs have relatively slow onsets of action and no effect on acute pain (Moore et al., 2009). Their efficacy is low in terms of numbers of patients that must be treated to observe a therapeutic response in one patient (e.g., 4.4 in postherpetic neuralgia), but the efficacy of gabapentin is consistent with that of other drug therapies in postherpetic neuralgia and painful diabetic neuropathy (Moore et al., 2014; Johnson and Rice, 2014).
Fibromyalgia is a poorly understood pain syndrome, including persistent, widespread pain and tenderness, sleep problems, and fatigue. Although gabapentin and pregabalin are used in this condition (Traynor et al., 2011; Wiffen et al., 2013), a systematic review of clinical trials for gabapentin concluded that there was currently insufficient evidence to confirm its efficacy (Moore et al., 2014).
3. Other Indications.
Restless legs syndrome, also known as Willis–Ekbom disease, is a common neurologic disorder with an approximate adult prevalence of 1.9–15.0%. Disruption of sleep due to symptoms of restless legs syndrome may result in fatigue and depression. Gabapentin and the longer-acting prodrug gabapentin enacarbil have been found to have some efficacy in the treatment of this disorder (Happe et al., 2003; Garcia-Borreguero et al., 2013). Pregabalin has also been examined for use in generalized anxiety disorder (Wensel et al., 2012).
F. Physiologic and Potential Pharmacological Roles of CaVβ Subunits
1. CaVβ Subunit Biochemistry.
CaVβ subunits were first identified in the purified skeletal muscle voltage-gated calcium channel complex and the gene for CaVβ1 was then cloned (Ruth et al., 1989). Three more CaVβ subunit genes were then identified by homology: CaVβ2, CaVβ3, and CaVβ4 (for reviews, see Dolphin, 2003; Buraei and Yang, 2010). The CaVβ subunits are cytoplasmic proteins that bind with high affinity to the part of the intracellular loop between domains I and II of the CaV1 and CaV2 α1 subunits. This binding motif is an 18–amino acid region in the proximal part of the I–II linker, termed the α-interaction domain (AID) (Pragnell et al., 1994).
CaVβ subunits were found by homology modeling to contain a conserved src homology-3 domain and a guanylate kinase-like domain, linked by a flexible loop (Hanlon et al., 1999). However, the guanylate kinase-like domain has no kinase activity, because there are mutations in the active site. Three studies solved the crystal structure of the conserved domains in several β subunits (Chen et al., 2004; Opatowsky et al., 2004; Van Petegem et al., 2004). They showed that the interaction site of the AID peptide is in a groove in the guanylate kinase-like domain (Van Petegem et al., 2004). In the intact I–II loop, the α-helical structure of the AID, which is imposed by binding to the CaVβ-subunit, is predicted to continue to the end of S6 in transmembrane domain I (Opatowsky et al., 2004). Thus, β subunits may be considered as chaperones to induce correct folding of the α1 subunit.
2. Physiology of CaVβ Subunits.
CaVβ subunits increase the functional expression and influence the biophysical properties of the CaV1 and CaV2 channels, and at least two processes have been proposed to account for this. All of the CaVβ subunits increase the maximum single channel open probability, which will increase current through individual channels, and will thus result in increased macroscopic current density (Matsuyama et al., 1999; Meir et al., 2000; Neely et al., 2004). However, CaVβ subunits also increase the amount of channel protein in the cell membrane, as measured by imaging, gating charge determination, or various biochemical means (Josephson and Varadi, 1996; Kamp et al., 1996; Brice et al., 1997; Bichet et al., 2000; Altier et al., 2002; Cohen et al., 2005; Leroy et al., 2005; Cassidy et al., 2014). CaVβ subunits were postulated to mask an endoplasmic reticulum retention signal in the I–II linker of CaVα1 subunits (Bichet et al., 2000; Cornet et al., 2002), although no specific motif was identified (Cornet et al., 2002). It was then found that a mutation (W391A) in the I–II loop of CaV2.2 disrupts functional interaction with CaVβ subunits (Leroy et al., 2005), and this was used this to probe the mechanism of action of CaVβ subunits. CaV2.2 (W391A) was found to have a more rapid rate of degradation than wild-type CaV2.2, and this was blocked by proteasomal inhibitors (Waithe et al., 2011). A similar conclusion was reached for the effects of CaVβ subunits on LTCCs (Altier et al., 2011); these findings may represent a general function of CaVβ subunits in protecting the α1 subunit from endoplasmic reticulum–associated proteasomal degradation and thus promoting forward trafficking of the channels to the plasma membrane.
Moreover, calcium channel–independent functions of β subunits have also been reported. Several studies demonstrated targeting of β4 subunits into the nucleus, suggesting a direct function in activity-dependent gene regulation (Colecraft et al., 2002; Hibino et al., 2003; Subramanyam et al., 2009; Tadmouri et al., 2012). Isoform-specific functions of β4 splice variants were recently observed in neurons (Etemad et al., 2014). Although many aspects of the regulation and function of this new signaling pathway are still controversial, a lack of this nonconventional β4 function could contribute to the ataxic phenotype in patients and mice with mutations in the β4 gene.
3. Pathophysiology and Potential Pharmacology Involving CaVβ Subunits.
CaVβ subunit pathology has been implicated in epilepsy, cardiac dysfunction, and other diseases (for review, see Buraei and Yang, 2010). It has been hypothesized that development of a drug targeting the groove within CaVβ into which the AID peptide is inserted could inhibit the interaction between the CaVα1 and β subunits and thus reduce calcium channel function, which could be beneficial in certain conditions such as hypertension and chronic pain. Indeed, such a lead molecule was identified (Young et al., 1998). However, since the interaction between CaVβ and the AID region is of very high affinity and involves a number of residues, it is difficult to identify first how a small molecule would compete for binding and second how selectivity between the different CaVα1 and β subunits would be obtained. Nevertheless, the use of cell-permeant peptides described above to interfere with the interaction between CaV2.2 and CRMP-2 (Ripsch et al., 2012) might also be employed to disrupt the α1–β interaction.
VI. Conclusions
The identification of specific roles for different calcium channel isoforms, as well as their different splice variants and auxiliary subunits, has been aided by knockout mouse models and the existence of human mutations in many of these channels and their auxiliary subunits. For example, the recent discovery of important physiologic functions differentially controlled by CaV1.2 and CaV1.3 identifies both channels as potentially novel drug targets, if selectivity can be achieved. Nonselective blockers of these channels (DHPs and other CCBs) have been of use in the treatment of hypertension for many years and their side effect profile has been well studied. Some selectivity of DHPs is nevertheless achieved in vivo for targeting vascular CaV1.2 because of the depolarized potentials found in this tissue, since DHPs bind with higher affinity to inactivated channels. If selective drugs can be developed, there is a strong therapeutic potential for selective CaV1.3 blockers for several indications, including neuropsychiatric diseases, PD neuroprotection, and resistant hypertension associated with hyperaldosteronism.
For the CaV2 channel family members, CaV2.2 has particular potential as a therapeutic target. Major effort has been put into identifying novel classes of CaV2.2 channel blockers with high affinity, selectivity, and use dependence. This effort has been spurred on by the success, albeit limited, of ziconotide (the peptide ω-conotoxin MVIIA) as an intrathecally administered drug for use in intractable pain, as well as the phenotype of the CaV2.2 knockout mouse. Beyond their application as analgesics, CaV2.2 channel blockers may well be effective in conditions such as drug dependence and anxiety.
T-type (CaV3) channels are important regulators of neuronal firing and pacemaker activity, and they play important roles in the cardiovascular system. Their dysregulation contributes to a number of chronic conditions, including epilepsy and pain; consequently, they are both potential and actual (ethosuximide) drug targets for these conditions. However, despite the fact that many classes of potential T-type channel blocking small organic molecules have been developed and studied, their clinical use to date has been restricted largely to the prophylaxis of absence seizures by ethosuximide.
Of the CaV auxiliary subunits, α2δ-1 is a well established drug target for the drugs gabapentin and pregabalin, used in chronic neuropathic pain and in combination with other drugs in several forms of epilepsy. These drugs also bind to α2δ-2, but not α2δ-3. Whether a selective or more potent α2δ-1 ligand would have fewer side effects and whether a similar ligand targeting α2δ-3 might be of therapeutic use remain to be determined. Although targeting the interaction between α1 and β might be of therapeutic relevance in principle, this has not yet proven possible.
In conclusion, it is clear that selective calcium channel blockers are likely to hold great promise for therapeutic intervention in the future.
Acknowledgments
The authors thank Dr. Emmanuel Bourinet for very useful suggestions on section IV.
Abbreviations
- ABT-639
5-[(8aR)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazine-2-carbonyl]-4-chloro-2-fluoro-N-(2-fluorophenyl)benzenesulfonamide
- AID
α-interaction domain
- APA
aldosterone-producing adenoma
- ASD
autism spectrum disorder
- AVN
atrioventricular node
- BayK8644
1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-3-pyridinecarboxylic acid methyl ester
- BPN-4689
Cp8, 1-(3-chlorophenethyl)-3-cyclopentylpyrimidine-2,4,6-trione
- CaM
calmodulin
- CaMKII
calmodulin kinase II
- CCB
Ca2+ channel blocker
- CDI
Ca2+-dependent inactivation
- CNS
central nervous system
- CREB
cAMP/calcium response element binding protein
- CTM
C-terminal modulatory structure
- CSNB2
congenital stationary night blindness type 2
- DCRD
distal C-terminal regulatory domain
- DHP
dihydropyridine
- DRG
dorsal root ganglion
- DM
myotonic dystrophy
- FHM
familial hemiplegic migraine
- FPL 64167
2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester
- GIRK
G protein–coupled K+ channel
- GPCR
G protein–coupled receptor
- GWAS
genome-wide association study
- HCN
hyperpolarization-activated cyclic nucleotide-gated channel
- HypoPP
hypokalemic periodic paralysis
- LTCC
L-type calcium channel
- LTP
long-term potentiation
- MH
malignant hyperthermia
- miR
microRNA
- NMP-7
(9-pentylcarbazol-3-yl)-piperidin-1-ylmethanone
- NNC55-0396
(1S,2S)-2-[2-[[3-(1H-benzimidazol-2-yl)propyl]methylamino]ethyl]-6-fluoro-1,2,3,4-tetrahydro-1-(1-methylethyl)-2-naphthalenyl cyclopropanecarboxylate dihydrochloride
- OMIM
Online Mendelian Inheritance in Man
- PCRD
proximal C-terminal regulatory domain
- PD
Parkinson’s disease
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A
- RyR
ryanodine receptor
- SAN
sinoatrial node
- SDZ202-791
propan-2-yl (4R)-4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-5-nitro-1,4-dihydropyridine-3-carboxylate
- SNc
substantia nigra pars compacta
- SNP
single nucleotide polymorphism
- SR
sarcoplasmic reticulum
- ST101
spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one
- TROX-1
(3R)-5-(3-chloro-4-fluorophenyl)-3-methyl-3-(pyrimidin-5-ylmethyl)-1-(1H-1,2,4-triazol-3-yl)-1,3-dihydro-2H-indol-2-one
- TS
Timothy syndrome
- TTA-A2
(R)-2-(4-cyclopropylphenyl)-N-(1-(5-(2,2,2-trifluoroethoxy)pyridin-2-yl)ethyl) acetamide
- TTA-P2
3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide
- Z944
N-[[1-[2-(tert-butylamino)-2-oxoethyl]piperidin-4-yl]methyl]-3-chloro-5-fluorobenzamide
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Zamponi, Striessnig, Koschak, Dolphin.
Footnotes
This research was supported in part by the Wellcome Trust [Senior Investigator Award 098360/Z/12/Z], the Research Councils UK Medical Research Council [Grants G0801756 and G0901758 (to A.C.D.)], and the Austrian Research Fund [Grants FWF F44020 (to J.S.) and P26881 (to A.K.)].
References
- Abbadie C, McManus OB, Sun SY, Bugianesi RM, Dai G, Haedo RJ, Herrington JB, Kaczorowski GJ, Smith MM, Swensen AM, et al. (2010) Analgesic effects of a substituted N-triazole oxindole (TROX-1), a state-dependent, voltage-gated calcium channel 2 blocker. J Pharmacol Exp Ther 334:545–555. [DOI] [PubMed] [Google Scholar]
- Adams DJ, Smith AB, Schroeder CI, Yasuda T, Lewis RJ. (2003) Omega-conotoxin CVID inhibits a pharmacologically distinct voltage-sensitive calcium channel associated with transmitter release from preganglionic nerve terminals. J Biol Chem 278:4057–4062. [DOI] [PubMed] [Google Scholar]
- Adams ME, Myers RA, Imperial JS, Olivera BM. (1993) Toxityping rat brain calcium channels with omega-toxins from spider and cone snail venoms. Biochemistry 32:12566–12570. [DOI] [PubMed] [Google Scholar]
- Adams PJ, Garcia E, David LS, Mulatz KJ, Spacey SD, Snutch TP. (2009) Ca(V)2.1 P/Q-type calcium channel alternative splicing affects the functional impact of familial hemiplegic migraine mutations: implications for calcium channelopathies. Channels (Austin) 3:110–121. [DOI] [PubMed] [Google Scholar]
- Albillos A, Neher E, Moser T. (2000) R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci 20:8323–8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SE, Darnell RB, Lipscombe D. (2010) The neuronal splicing factor Nova controls alternative splicing in N-type and P-type CaV2 calcium channels. Channels (Austin) 4:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, et al. (2007) Differential role of N-type calcium channel splice isoforms in pain. J Neurosci 27:6363–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dubel SJ, Barrère C, Jarvis SE, Stotz SC, Spaetgens RL, Scott JD, Cornet V, De Waard M, Zamponi GW, et al. (2002) Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J Biol Chem 277:33598–33603. [DOI] [PubMed] [Google Scholar]
- Altier C, Garcia-Caballero A, Simms B, You H, Chen L, Walcher J, Tedford HW, Hermosilla T, Zamponi GW. (2011) The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci 14:173–180. [DOI] [PubMed] [Google Scholar]
- Álvarez YD, Belingheri AV, Perez Bay AE, Javis SE, Tedford HW, Zamponi G, Marengo FD. (2013) The immediately releasable pool of mouse chromaffin cell vesicles is coupled to P/Q-type calcium channels via the synaptic protein interaction site. PLoS One 8:e54846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Engbers JD, Heath NC, Bartoletti TM, Mehaffey WH, Zamponi GW, Turner RW. (2013) The Cav3-Kv4 complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations. J Neurosci 33:7811–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, Zamponi GW, Turner RW. (2010) Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci 13:333–337. [DOI] [PubMed] [Google Scholar]
- Andrade A, Denome S, Jiang YQ, Marangoudakis S, Lipscombe D. (2010) Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat Neurosci 13:1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti T, Hofmann F. (1996) Tissue-specific expression of splice variants of the mouse voltage-gated calcium channel alpha2/δ subunit. FEBS Lett 397:331–337. [DOI] [PubMed] [Google Scholar]
- Arnot MI, Stotz SC, Jarvis SE, Zamponi GW. (2000) Differential modulation of N-type 1B and P/Q-type 1A calcium channels by different G protein subunit isoforms. J Physiol 527:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassoff PG, Hartmannsgruber MW, Thrasher J, Wermeling D, Longton W, Gaeta R, Singh T, Mayo M, McGuire D, Luther RR. (2000) Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain. Reg Anesth Pain Med 25:274–278. [DOI] [PubMed] [Google Scholar]
- Avila G, Dirksen RT. (2000) Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca(2+) channel. J Gen Physiol 115:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, et al. (2013) Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 45:1055–1060. [DOI] [PubMed] [Google Scholar]
- Bader PL, Faizi M, Kim LH, Owen SF, Tadross MR, Alfa RW, Bett GC, Tsien RW, Rasmusson RL, Shamloo M. (2011) Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc Natl Acad Sci USA 108:15432–15437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae N, Li L, Lödl M, Lubec G. (2012) Peptide toxin glacontryphan-M is present in the wings of the butterfly Hebomoia glaucippe (Linnaeus, 1758) (Lepidoptera: Pieridae). Proc Natl Acad Sci USA 109:17920–17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, et al. (2011) Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci 14:77–84. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. (2006) Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA 103:7500–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore S, Messerli FH, Cohen JD, Bacher PH, Sleight P, Mancia G, Kowey P, Zhou Q, Champion A, Pepine CJ, INVEST Investigators (2008) Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J 156:241–247. [DOI] [PubMed] [Google Scholar]
- Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. (2009) Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res 105:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister RA, Beam KG. (2013) Ca(V)1.1: The atypical prototypical voltage-gated Ca²⁺ channel. Biochim Biophys Acta 1828:1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas P, Cutler Peck C, Krizaj D. (2010) Do calcium channel blockers rescue dying photoreceptors in the Pde6b ( rd1 ) mouse? Adv Exp Med Biol 664:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G, Alloui A, Nargeot J, Lory P, Eschalier A, Bourinet E, Chemin J. (2009) T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. J Neurosci 29:13106–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, et al. (2001) Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci 21:6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J, Rees M. (2000) Genomic organization of the mouse and human alpha2δ2 voltage-dependent calcium channel subunit genes. Mamm Genome 11:1142–1144. [DOI] [PubMed] [Google Scholar]
- Barg S, Ma X, Eliasson L, Galvanovskis J, Göpel SO, Obermüller S, Platzer J, Renström E, Trus M, Atlas D, et al. (2001) Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys J 81:3308–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CF, Tsien RW. (2008) The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc Natl Acad Sci USA 105:2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, et al. (2009) The increased trafficking of the calcium channel subunit alpha2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2δ ligand pregabalin. J Neurosci 29:4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal B, Moeller-Bertram T, Schilling JM, Wallace MS. (2012) Gabapentin for once-daily treatment of post-herpetic neuralgia: a review. Clin Interv Aging 7:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Sturek M, Puga A, Hermsmeyer K. (1986) Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res 59:229–235. [DOI] [PubMed] [Google Scholar]
- Beaudry H, Dubois D, Gendron L. (2011) Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci 31:13068–13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. (1998) Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19:264–267. [DOI] [PubMed] [Google Scholar]
- Becker C, Jick SS, Meier CR. (2008) Use of antihypertensives and the risk of Parkinson disease. Neurology 70:1438–1444. [DOI] [PubMed] [Google Scholar]
- Beedle AM, Hamid J, Zamponi GW. (2002) Inhibition of transiently expressed low- and high-voltage-activated calcium channels by trivalent metal cations. J Membr Biol 187:225–238. [DOI] [PubMed] [Google Scholar]
- Beedle AM, Zamponi GW. (2000) Block of voltage-dependent calcium channels by aliphatic monoamines. Biophys J 79:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. (2004) Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron 41:127–138. [DOI] [PubMed] [Google Scholar]
- Belliotti TR, Capiris T, Ekhato IV, Kinsora JJ, Field MJ, Heffner TG, Meltzer LT, Schwarz JB, Taylor CP, Thorpe AJ, et al. (2005) Structure-activity relationships of pregabalin and analogues that target the alpha(2)-delta protein. J Med Chem 48:2294–2307. [DOI] [PubMed] [Google Scholar]
- Ben Johny M, Yang PS, Bazzazi H, Yue DT. (2013) Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nat Commun 4:1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence-Hanulec KK, Marshall J, Blair LAC. (2000) Potentiation of neuronal L calcium channels by IGF-1 requires phosphorylation of the alpha1 subunit on a specific tyrosine residue. Neuron 27:121–131. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Tuluc P, Mastrolia V, Dlaska C, Flucher BE. (2015) Physiological and pharmacological modulation of the embryonic skeletal muscle calcium channel splice variant CaV1.1e. Biophys J 108:1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin ER, Pruthi F, Olanrewaju S, Shan S, Hanway D, Liu X, Cerne R, Lavery D, Valenzano KJ, Woodward RM, et al. (2006) Pharmacological characterization of recombinant N-type calcium channel (Cav2.2) mediated calcium mobilization using FLIPR. Biochem Pharmacol 72:770–782. [DOI] [PubMed] [Google Scholar]
- Berecki G, McArthur JR, Cuny H, Clark RJ, Adams DJ. (2014) Differential Cav2.1 and Cav2.3 channel inhibition by baclofen and α-conotoxin Vc1.1 via GABAB receptor activation. J Gen Physiol 143:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjukow S, Hering S. (2001) Voltage-dependent acceleration of Ca(v)1.2 channel current decay by (+)- and (-)-isradipine. Br J Pharmacol 133:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Fakler B. (2008) Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J Neurosci 28:8238–8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, et al. (2006) BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314:615–620. [DOI] [PubMed] [Google Scholar]
- Bertolesi GE, Walia Da Silva R, Jollimore CA, Shi C, Barnes S, Kelly ME. (2006) Ca(v)3.1 splice variant expression during neuronal differentiation of Y-79 retinoblastoma cells. Neuroscience 141:259–268. [DOI] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Miyamoto N, Ino M, Yanagisawa M. (2003) N-type calcium channel alpha1B subunit (Cav2.2) knock-out mice display hyperactivity and vigilance state differences. J Neurosci 23:6793–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, et al. (2013) Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 45:440–444, e1–e2. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Scheller RH, Tsien RW. (1995) Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature 378:623–626. [DOI] [PubMed] [Google Scholar]
- Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, Gould TD. (2012) CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol 99:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. (2000) The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron 25:177–190. [DOI] [PubMed] [Google Scholar]
- Biggs JE, Boakye PA, Ganesan N, Stemkowski PL, Lantero A, Ballanyi K, Smith PA. (2014) Analysis of the long-term actions of gabapentin and pregabalin in dorsal root ganglia and substantia gelatinosa. J Neurophysiol 112:2398–2412. [DOI] [PubMed] [Google Scholar]
- Bladen C, Gündüz MG, Şimşek R, Şafak C, Zamponi GW. (2014a) Synthesis and evaluation of 1,4-dihydropyridine derivatives with calcium channel blocking activity. Pflugers Arch 466:1355–1363. [DOI] [PubMed] [Google Scholar]
- Bladen C, Hamid J, Souza IA, Zamponi GW. (2014b) Block of T-type calcium channels by protoxins I and II. Mol Brain 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen C, McDaniel SW, Gadotti VM, Petrov RR, Berger ND, Diaz P, Zamponi GW. (2015) Characterization of novel cannabinoid based T-type calcium channel blockers with analgesic effects. ACS Chem Neurosci 6:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore DC, Bryans JS, Carnell P, Field MJ, Kinsella N, Kinsora JK, Meltzer LT, Osborne SA, Thompson LR, Williams SC. (2010) Synthesis and in vivo evaluation of 3,4-disubstituted gababutins. Bioorg Med Chem Lett 20:248–251. [DOI] [PubMed] [Google Scholar]
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. (1988) Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107:2587–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, et al. (2011) Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. J Biol Chem 286:42736–42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek NJ, Miller EM, Ye D, Nesterenko VV, Tester DJ, Antzelevitch C, Czosek RJ, Ackerman MJ, Ware SM. (2015) Novel Timothy syndrome mutation leading to increase in CACNA1C window current. Heart Rhythm 12:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators (2010) Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 376:886–894. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Fà M, Dessì C, Puligheddu M, Barberini L, Pillolla G, Polizzi L, Santoni F, et al. (2010) GABAB receptor activation exacerbates spontaneous spike-and-wave discharges in DBA/2J mice. Seizure 19:226–231. [DOI] [PubMed] [Google Scholar]
- Bourdin B, Shakeri B, Tétreault MP, Sauvé R, Lesage S, Parent L. (2015) Functional characterization of CaVα2δ mutations associated with sudden cardiac death. J Biol Chem 290:2854–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. (2014) Calcium-permeable ion channels in pain signaling. Physiol Rev 94:81–140. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. (1999) Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci 2:407–415. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Stotz SC, Spaetgens RL, Dayanithi G, Lemos J, Nargeot J, Zamponi GW. (2001) Interaction of SNX482 with domains III and IV inhibits activation gating of alpha(1E) (Ca(V)2.3) calcium channels. Biophys J 81:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourson A, Moser PC, Gower AJ, Mir AK. (1989) Central and peripheral effects of the dihydropyridine calcium channel activator BAY K 8644 in the rat. Eur J Pharmacol 160:339–347. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Luther R. (1998) Pharmacotherapeutic potential of omega-conotoxin MVIIA (SNX-111), an N-type neuronal calcium channel blocker found in the venom of Conus magus. Toxicon 36:1651–1658. [DOI] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Lewin AS, Hauswirth WW. (2013) A comprehensive review of retinal gene therapy. Mol Ther 21:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. (1997) Importance of the different β subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci 9:749–759. [DOI] [PubMed] [Google Scholar]
- Brill J, Klocke R, Paul D, Boison D, Gouder N, Klugbauer N, Hofmann F, Becker CM, Becker K. (2004) entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem 279:7322–7330. [DOI] [PubMed] [Google Scholar]
- Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, et al. (2011) Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca²⁺ channel complex. Nat Med 17:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck J, Davies A, Courtney J-M, Meir A, Balaguero N, Canti C, Moss FJ, Page KM, Pratt WS, Hunt SP, et al. (2002) The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated alpha 2 delta-2 protein with abnormal function. J Biol Chem 277:7684–7693. [DOI] [PubMed] [Google Scholar]
- Brown JP, Dissanayake VU, Briggs AR, Milic MR, Gee NS. (1998) Isolation of the [3H]gabapentin-binding protein/alpha 2 delta Ca2+ channel subunit from porcine brain: development of a radioligand binding assay for alpha 2 delta subunits using [3H]leucine. Anal Biochem 255:236–243. [DOI] [PubMed] [Google Scholar]
- Brown JP, Gee NS. (1998) Cloning and deletion mutagenesis of the alpha2 delta calcium channel subunit from porcine cerebral cortex. Expression of a soluble form of the protein that retains [3H]gabapentin binding activity. J Biol Chem 273:25458–25465. [DOI] [PubMed] [Google Scholar]
- Bui PH, Quesada A, Handforth A, Hankinson O. (2008) The mibefradil derivative NNC55-0396, a specific T-type calcium channel antagonist, exhibits less CYP3A4 inhibition than mibefradil. Drug Metab Dispos 36:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraei Z, Yang J. (2010) The ß subunit of voltage-gated Ca2+ channels. Physiol Rev 90:1461–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpón E, Hu D, Desai M, Borggrefe M, Häissaguerre M, Kanter R, Pollevick GD, et al. (2010) Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 7:1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher V, Schicker K, Novikova E, Pöhn B, Stockner T, Kugler C, Singh A, Zeitz C, Lancelot ME, Audo I, et al. (2014) Spectrum of Cav1.4 dysfunction in congenital stationary night blindness type 2. Biochim Biophys Acta 1838:2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquet P, Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. (2008) Role of L-type Ca2+ channel isoforms in the extinction of conditioned fear. Learn Mem 15:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N. (2010) CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol 13:499–513. [DOI] [PubMed] [Google Scholar]
- Calin-Jageman I, Lee A. (2008) Ca(v)1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem 105:573–583. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Adams DJ. (2010) Analgesic α-conotoxins Vc1.1 and RgIA inhibit N-type calcium channels in sensory neurons of α9 nicotinic receptor knockout mice. Channels (Austin) 4:51–54. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Haythornthwaite A, Berecki G, Clark RJ, Craik DJ, Adams DJ. (2008) Analgesic alpha-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J Neurosci 28:10943–10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantí C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, et al. (2005) The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA 102:11230–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, Marcantoni A, Comunanza V, de Luca A, Díaz J, Borges R, Carbone E. (2007) Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol 584:149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. (1984) A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature 310:501–502. [DOI] [PubMed] [Google Scholar]
- Carpenè G, Rocco S, Opocher G, Mantero F. (1989) Acute and chronic effect of nifedipine in primary aldosteronism. Clin Exp Hypertens A 11:1263–1272. [DOI] [PubMed] [Google Scholar]
- Carstens BB, Clark RJ, Daly NL, Harvey PJ, Kaas Q, Craik DJ. (2011) Engineering of conotoxins for the treatment of pain. Curr Pharm Des 17:4242–4253. [DOI] [PubMed] [Google Scholar]
- Cassidy JS, Ferron L, Kadurin I, Pratt WS, Dolphin AC. (2014) Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary α2δ-1 subunits. Proc Natl Acad Sci USA 111:8979–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni AJ, Raingo J, Lipscombe D. (2006) Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels. J Physiol 576:119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Leal K, Nanou E. (2013) Calcium channels and short-term synaptic plasticity. J Biol Chem 288:10742–10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Swanson TM. (2015) Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels. Mol Pharmacol 88:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447:1081–1086. [DOI] [PubMed] [Google Scholar]
- Chang KY, Park YG, Park HY, Homanics GE, Kim J, Kim D. (2011) Lack of CaV3.1 channels causes severe motor coordination defects and an age-dependent cerebellar atrophy in a genetic model of essential tremor. Biochem Biophys Res Commun 410:19–23. [DOI] [PubMed] [Google Scholar]
- Chang SY, Yong TF, Yu CY, Liang MC, Pletnikova O, Troncoso J, Burgunder JM, Soong TW. (2007) Age and gender-dependent alternative splicing of P/Q-type calcium channel EF-hand. Neuroscience 145:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. (1994) Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther 269:1117–1123. [PubMed] [Google Scholar]
- Chaudhuri D, Chang SY, DeMaria CD, Alvania RS, Soong TW, Yue DT. (2004) Alternative splicing as a molecular switch for Ca2+/calmodulin-dependent facilitation of P/Q-type Ca2+ channels. J Neurosci 24:6334–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. (2001a) Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J 80:1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P. (2001b) Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J 20:7033–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, et al. (2003a) Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 302:1416–1418. [DOI] [PubMed] [Google Scholar]
- Chen RW, Greenberg JP, Lazow MA, Ramachandran R, Lima LH, Hwang JC, Schubert C, Braunstein A, Allikmets R, Tsang SH. (2012) Autofluorescence imaging and spectral-domain optical coherence tomography in incomplete congenital stationary night blindness and comparison with retinitis pigmentosa. Am J Ophthalmol 153:143–54.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, et al. (2011) Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol 50:460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Bayliss DA, Fern RJ, Barrett PQ. (1999) A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+. Am J Physiol 276:F674–F683. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, et al. (2003b) Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol 54:239–243. [DOI] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. (2004) Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature 429:675–680. [DOI] [PubMed] [Google Scholar]
- Cheng X, Pachuau J, Blaskova E, Asuncion-Chin M, Liu J, Dopico AM, Jaggar JH. (2009) Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties. Am J Physiol Heart Circ Physiol 297:H680–H688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier M, Lory P, Mironneau C, Macrez N, Quignard JF. (2006) T-type CaV3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. Eur J Neurosci 23:2321–2329. [DOI] [PubMed] [Google Scholar]
- Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, Khanna R. (2009) Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci 122:4351–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, et al. (2009) The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res 104:522–530. [DOI] [PubMed] [Google Scholar]
- Choe W, Messinger RB, Leach E, Eckle VS, Obradovic A, Salajegheh R, Jevtovic-Todorovic V, Todorovic SM. (2011) TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Mol Pharmacol 80:900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, et al. (2011) K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. (2007) Attenuated pain responses in mice lacking Ca(V)3.2 T-type channels. Genes Brain Behav 6:425–431. [DOI] [PubMed] [Google Scholar]
- Christel C, Lee A. (2012) Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim Biophys Acta 1820:1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christel CJ, Cardona N, Mesirca P, Herrmann S, Hofmann F, Striessnig J, Ludwig A, Mangoni ME, Lee A. (2012) Distinct localization and modulation of Cav1.2 and Cav1.3 L-type Ca2+ channels in mouse sinoatrial node. J Physiol 590:6327–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120:421–433. [DOI] [PubMed] [Google Scholar]
- Chuang RSI, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. (1998) Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat Neurosci 1:668–674. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Foell JD, Balijepalli RC, Shah V, Hell JW, Kamp TJ. (2005) Unique modulation of L-type Ca2+ channels by short auxiliary beta1d subunit present in cardiac muscle. Am J Physiol Heart Circ Physiol 288:H2363–H2374. [DOI] [PubMed] [Google Scholar]
- Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marbán E, Yue DT. (2002) Novel functional properties of Ca(2+) channel β subunits revealed by their expression in adult rat heart cells. J Physiol 541:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable PA. (2011) Nifedipine alters the light-rise of the electro-oculogram in man. Graefes Arch Clin Exp Ophthalmol 249:677–684. [DOI] [PubMed] [Google Scholar]
- Cornet V, Bichet D, Sandoz G, Marty I, Brocard J, Bourinet E, Mori Y, Villaz M, De Waard M. (2002) Multiple determinants in voltage-dependent P/Q calcium channels control their retention in the endoplasmic reticulum. Eur J Neurosci 16:883–895. [DOI] [PubMed] [Google Scholar]
- Corrigan B, Feltner DE, Ouellet D, Werth JL, Moton AE, Gibson G. (2009) Effect of renal impairment on the pharmacokinetics of PD 0200390, a novel ligand for the voltage-gated calcium channel alpha-2-delta subunit. Br J Clin Pharmacol 68:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. (1989) Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol 25:582–593. [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudoré-Civiale MA, Privat AM, Pélissier T, Eschalier A, Fialip J. (2004) Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 110:236–245. [DOI] [PubMed] [Google Scholar]
- Crawford P, Ghadiali E, Lane R, Blumhardt L, Chadwick D. (1987) Gabapentin As an Antiepileptic Drug in Man. J Neurol Neurosurg Psychiatry 50:682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, et al. (1998) Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res 83:103–109. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T, Lee A. (2007) Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells. J Physiol 585:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundy KC, Annamalai T, Bu L, De Vera J, Estrela J, Luo W, Shirsat P, Torneros A, Yao F, Zou J, et al. (2004) XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther 311:324–333. [DOI] [PubMed] [Google Scholar]
- Cuny H, de Faoite A, Huynh TG, Yasuda T, Berecki G, Adams DJ. (2012) γ-Aminobutyric acid type B (GABAB) receptor expression is needed for inhibition of N-type (Cav2.2) calcium channels by analgesic α-conotoxins. J Biol Chem 287:23948–23957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O’Donnell P, Knowles JA, et al. Bipolar Genome Study (BiGS) Consortium (2010) Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 68:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. (1998) Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci 21:215–221. [DOI] [PubMed] [Google Scholar]
- Das A, Pushparaj C, Herreros J, Nager M, Vilella R, Portero M, Pamplona R, Matias-Guiu X, Martí RM, Cantí C. (2013) T-type calcium channel blockers inhibit autophagy and promote apoptosis of malignant melanoma cells. Pigment Cell Melanoma Res 26:874–885. [DOI] [PubMed] [Google Scholar]
- David LS, Garcia E, Cain SM, Thau E, Tyson JR, Snutch TP. (2010) Splice-variant changes of the Ca(V)3.2 T-type calcium channel mediate voltage-dependent facilitation and associate with cardiac hypertrophy and development. Channels (Austin) 4:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Douglas L, Hendrich J, Wratten J, Tran Van Minh A, Foucault I, Koch D, Pratt WS, Saibil HR, Dolphin AC. (2006) The calcium channel alpha2δ-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J Neurosci 26:8748–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. (2007) Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci 28:220–228. [DOI] [PubMed] [Google Scholar]
- Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC. (2010) The alpha2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci USA 107:1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weille JR, Schweitz H, Maes P, Tartar A, Lazdunski M. (1991) Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc Natl Acad Sci USA 88:2437–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase V, Obermair GJ, Szabo Z, Altier C, Sanguesa J, Bourinet E, Flucher BE. (2008) Stable membrane expression of postsynaptic CaV1.2 calcium channel clusters is independent of interactions with AKAP79/150 and PDZ proteins. J Neurosci 28:13845–13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Ruiz F, Flórez J, Hurlé MA, Pazos A. (1995) Mu-opioid receptor regulation during opioid tolerance and supersensitivity in rat central nervous system. J Pharmacol Exp Ther 274:1545–1551. [PubMed] [Google Scholar]
- Dibué M, Kamp MA, Alpdogan S, Tevoufouet EE, Neiss WF, Hescheler J, Schneider T. (2013) Cav 2.3 (R-type) calcium channels are critical for mediating anticonvulsive and neuroprotective properties of lamotrigine in vivo. Epilepsia 54:1542–1550. [DOI] [PubMed] [Google Scholar]
- Dickens D, Webb SD, Antonyuk S, Giannoudis A, Owen A, Rädisch S, Hasnain SS, Pirmohamed M. (2013) Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol 85:1672–1683. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Bee LA, Suzuki R. (2005) Pains, gains, and midbrains. Proc Natl Acad Sci USA 102:17885–17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Kurshan PT, Schwarz TL. (2008) Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci 28:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Richard S, Baldy-Moulinier M, Nargeot J, Valmier J. (1995) Dihydropyridines, phenylalkylamines and benzothiazepines block N-, P/Q- and R-type calcium currents. Pflugers Arch 431:10–19. [DOI] [PubMed] [Google Scholar]
- Dissanayake VUK, Gee NS, Brown JP, Woodruff GN. (1997) Spermine modulation of specific [3H]-gabapentin binding to the detergent-solubilized porcine cerebral cortex alpha 2 δ calcium channel subunit. Br J Pharmacol 120:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. (2003) G protein modulation of voltage-gated calcium channels. Pharmacol Rev 55:607–627. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. (2012) Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat Rev Neurosci 13:542–555. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. (2013) The α2δ subunits of voltage-gated calcium channels. Biochim Biophys Acta 1828:1541–1549. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Wyatt CN, Richards J, Beattie RE, Craig P, Lee JH, Cribbs LL, Volsen SG, Perez-Reyes E. (1999) The effect of alpha2-δ and other accessory subunits on expression and properties of the calcium channel alpha1G. J Physiol 519:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Page KM, Koch D, Nieto-Rostro M, Foucault I, Davies A, Wilkinson T, Rees M, Edwards FA, Dolphin AC. (2006) The ducky(2J) mutation in Cacna2d2 results in reduced spontaneous Purkinje cell activity and altered gene expression. J Neurosci 26:12576–12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, Fauler M, Hetzel A, Watanabe M, Lujan R, et al. (2014) Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain 137:2287–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. (2010) Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci 30:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubel SJ, Altier C, Chaumont S, Lory P, Bourinet E, Nargeot J. (2004) Plasma membrane expression of T-type calcium channel alpha(1) subunits is modulated by high voltage-activated auxiliary subunits. J Biol Chem 279:29263–29269. [DOI] [PubMed] [Google Scholar]
- Dubel SJ, Starr TVB, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. (1992) Molecular cloning of the α-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci USA 89:5058–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska B, Brautigan DL, Larner JM, Dziegielewski J. (2014) T-type Ca2+ channel inhibition induces p53-dependent cell growth arrest and apoptosis through activation of p38-MAPK in colon cancer cells. Mol Cancer Res 12:348–358. [DOI] [PubMed] [Google Scholar]
- Edgerton GB, Blumenthal KM, Hanck DA. (2010) Inhibition of the activation pathway of the T-type calcium channel Ca(V)3.1 by ProTxII. Toxicon 56:624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson S, Oz S, Abulhijaa FA, Taher FB, Shaag A, Zenvirt S, Dascal N, Elpeleg O. (2013) Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. J Med Genet 50:118–123. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Zhang JF, Horne WA, Tsien RW. (1994) Structural determinants of the blockade of N-type calcium channels by a peptide neurotoxin. Nature 372:272–275. [DOI] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, et al. (1988) Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science 241:1661–1664. [DOI] [PubMed] [Google Scholar]
- Engbers JD, Anderson D, Asmara H, Rehak R, Mehaffey WH, Hameed S, McKay BE, Kruskic M, Zamponi GW, Turner RW. (2012) Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci USA 109:2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbers JD, Zamponi GW, Turner RW. (2013) Modeling interactions between voltage-gated Ca (2+) channels and KCa1.1 channels. Channels (Austin) 7:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart JJ, Biagi BA, Day RN, Sheu SS, Maurer RA. (1990) Blockade of low and high threshold Ca2+ channels by diphenylbutylpiperidine antipsychotics linked to inhibition of prolactin gene expression. J Biol Chem 265:16373–16379. [PubMed] [Google Scholar]
- Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. (2009) Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci 29:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. (2009) Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139:380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel SI, Clozel JP. (1997) Mibefradil (Ro 40-5967): the first selective T-type Ca2+ channel blocker. Expert Opin Investig Drugs 6:569–582. [DOI] [PubMed] [Google Scholar]
- Etemad S, Obermair GJ, Bindreither D, Benedetti A, Stanika R, Di Biase V, Burtscher V, Koschak A, Kofler R, Geley S, et al. (2014) Differential neuronal targeting of a new and two known calcium channel β4 subunit splice variants correlates with their regulation of gene expression. J Neurosci 34:1446–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, Zamponi GW. (2010) Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem 285:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZP, Doering CJ, Winkfein RJ, Beedle AM, Spafford JD, Zamponi GW. (2003) Determinants of inhibition of transiently expressed voltage-gated calcium channels by omega-conotoxins GVIA and MVIIA. J Biol Chem 278:20171–20178. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Hamid J, Doering C, Bosey GM, Snutch TP, Zamponi GW. (2001) Residue Gly1326 of the N-type calcium channel alpha 1B subunit controls reversibility of omega-conotoxin GVIA and MVIIA block. J Biol Chem 276:15728–15735. [DOI] [PubMed] [Google Scholar]
- Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, et al. (2014) Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 64:354–361. [DOI] [PubMed] [Google Scholar]
- Ferron L, Ruchon Y, Renaud JF, Capuano V. (2011) T-type Ca²+ signalling regulates aldosterone-induced CREB activation and cell death through PP2A activation in neonatal cardiomyocytes. Cardiovasc Res 90:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M, Greengard P, Cenci MA, et al. (2014) Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun 5:5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Carnell AJ, Gonzalez MI, McCleary S, Oles RJ, Smith R, Hughes J, Singh L. (1999) Enadoline, a selective kappa-opioid receptor agonist shows potent antihyperalgesic and antiallodynic actions in a rat model of surgical pain. Pain 80:383–389. [DOI] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, et al. (2006) Identification of the alpha2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA 103:17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Li Z, Schwarz JB. (2007) Ca2+ channel alpha2-delta ligands for the treatment of neuropathic pain. J Med Chem 50:2569–2575. [DOI] [PubMed] [Google Scholar]
- Fox A, Gentry C, Patel S, Kesingland A, Bevan S. (2003) Comparative activity of the anti-convulsants oxcarbazepine, carbamazepine, lamotrigine and gabapentin in a model of neuropathic pain in the rat and guinea-pig. Pain 105:355–362. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. (1987) Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol 394:149–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois A, Kerckhove N, Meleine M, Alloui A, Barrere C, Gelot A, Uebele VN, Renger JJ, Eschalier A, Ardid D, et al. (2013) State-dependent properties of a new T-type calcium channel blocker enhance Ca(V)3.2 selectivity and support analgesic effects. Pain 154:283–293. [DOI] [PubMed] [Google Scholar]
- François A, Laffray S, Pizzoccaro A, Eschalier A, Bourinet E. (2014) T-type calcium channels in chronic pain: mouse models and specific blockers. Pflugers Arch 466:707–717. [DOI] [PubMed] [Google Scholar]
- François A, Schuetter N, Laffray S, Sanguesa J, Pizzoccaro A, Dubel S, Mantilleri A, Nargeot J, Noel J, Wood JN, et al. (2015) The low-threshold calcium channel Cav3.2 determines low-threshold mechanoreceptor function. Cell Rep DOI: 10.1016/j.celrep.2014.12.042 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. (2003) Dose-Response Trial of Pregabalin Adjunctive Therapy in Patients With Partial Seizures. Neurology 60:1631–1637. [DOI] [PubMed] [Google Scholar]
- Fu Y, Westenbroek RE, Scheuer T, Catterall WA. (2013) Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc Natl Acad Sci USA 110:19621–19626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M, Wang Q, Kato K, Ohno S, Ding WG, Toyoda F, Itoh H, Kimura H, Makiyama T, Ito M, et al. (2014) Long QT syndrome type 8: novel CACNA1C mutations causing QT prolongation and variant phenotypes. Europace 16:1828–1837. [DOI] [PubMed] [Google Scholar]
- Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. (2010) Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal 3:ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, et al. (2009) Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am J Physiol Heart Circ Physiol 297:H117–H124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gach MP, Cherednichenko G, Haarmann C, Lopez JR, Beam KG, Pessah IN, Franzini-Armstrong C, Allen PD. (2008) Alpha2delta1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes. Biophys J 94:3023–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackière F, Warnier M, Katsogiannou M, Derouiche S, Delcourt P, Dewailly E, Slomianny C, Humez S, Prevarskaya N, Roudbaraki M, et al. (2013) Functional coupling between large-conductance potassium channels and Cav3.2 voltage-dependent calcium channels participates in prostate cancer cell growth. Biol Open 2:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadotti VM, Caballero AG, Berger ND, Gladding CM, Chen L, Pfeifer TA, Zamponi GW. (2015) Small organic molecule disruptors of Cav3.2 - USP5 interactions reverse inflammatory and neuropathic pain. Mol Pain 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadotti VM, You H, Petrov RR, Berger ND, Diaz P, Zamponi GW. (2013) Analgesic effect of a mixed T-type channel inhibitor/CB2 receptor agonist. Mol Pain 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Laffray S, Bourinet E, Valjent E. (2014) T-type calcium channel Cav3.2 deficient mice show elevated anxiety, impaired memory and reduced sensitivity to psychostimulants. Front Behav Neurosci 8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Blair LA, Salinas GD, Needleman LA, Marshall J. (2006) Insulin-like growth factor-1 modulation of CaV1.3 calcium channels depends on Ca2+ release from IP3-sensitive stores and calcium/calmodulin kinase II phosphorylation of the alpha1 subunit EF hand. J Neurosci 26:6259–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Kohnen R, Silber MH, Winkelman JW, Earley CJ, Högl B, Manconi M, Montplaisir J, Inoue Y, Allen RP. (2013) The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med 14:675–684. [DOI] [PubMed] [Google Scholar]
- García-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, Bladen C, Chen L, Hamid J, Pizzoccaro A, et al. (2014) The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron 83:1144–1158. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VUK, Offord J, Thurlow R, Woodruff GN. (1996) The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2δ subunit of a calcium channel. J Biol Chem 271:5768–5776. [DOI] [PubMed] [Google Scholar]
- Gillis J, Burashnikov E, Antzelevitch C, Blaser S, Gross G, Turner L, Babul-Hirji R, Chitayat D. (2012) Long QT, syndactyly, joint contractures, stroke and novel CACNA1C mutation: expanding the spectrum of Timothy syndrome. Am J Med Genet A 158A:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P, Yakel J, Gromada J, Zhu Y, Henquin JC, Rorsman P. (1997) G protein-dependent inhibition of L-type Ca2+ currents by acetylcholine in mouse pancreatic B-cells. J Physiol 499:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Tropea TF, Satpute SS, Sinnegger-Brauns MJ, Striessnig J, Kosofsky BE, Rajadhyaksha AM. (2010) Molecular switch from L-type Ca v 1.3 to Ca v 1.2 Ca2+ channel signaling underlies long-term psychostimulant-induced behavioral and molecular plasticity. J Neurosci 30:17051–17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H, Striessnig J. (1990) Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol 114:1–105. [DOI] [PubMed] [Google Scholar]
- Gomora JC, Daud AN, Weiergräber M, Perez-Reyes E. (2001) Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Mol Pharmacol 60:1121–1132. [PubMed] [Google Scholar]
- Gong HC, Hang J, Kohler W, Li L, Su TZ. (2001) Tissue-specific expression and gabapentin-binding properties of calcium channel alpha2delta subunit subtypes. J Membr Biol 184:35–43. [DOI] [PubMed] [Google Scholar]
- Goodchild CS, Nadeson R, Cohen E. (2004) Supraspinal and spinal cord opioid receptors are responsible for antinociception following intrathecal morphine injections. Eur J Anaesthesiol 21:179–185. [DOI] [PubMed] [Google Scholar]
- Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, et al. (2012) Decreased cardiac L-type Ca²⁺ channel activity induces hypertrophy and heart failure in mice. J Clin Invest 122:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Suda N, Beam KG. (1999) The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J Biol Chem 274:21913–21919. [DOI] [PubMed] [Google Scholar]
- Groen JL, Andrade A, Ritz K, Jalalzadeh H, Haagmans M, Bradley TE, Jongejan A, Verbeek DS, Nürnberg P, Denome S, et al. (2015) CACNA1B mutation is linked to unique myoclonus-dystonia syndrome. Hum Mol Genet 24:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RA, Macdonald RL. (1987) Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci USA 84:5469–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardo NA, Yao J, Hu C, Barrett PQ. (2012) Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology 153:3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. (1996) Dual function of the voltage-dependent Ca2+ channel alpha 2 δ subunit in current stimulation and subunit interaction. Neuron 16:431–440. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Felix R, Campbell KP. (1997) Extracellular interaction of the voltage-dependent Ca2+ channel alpha2δ and alpha1 subunits. J Biol Chem 272:18508–18512. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sánchez-Padilla J, Chan CS, Surmeier DJ. (2009) Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci 29:11011–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SL, Yatani A, Brush K, Schwartz A, Brown AM. (1987) A comparison between the binding and electrophysiological effects of dihydropyridines on cardiac membranes. Mol Pharmacol 31:221–231. [PubMed] [Google Scholar]
- Han Y, Babai N, Kaeser P, Südhof TC, Schneggenburger R. (2015) RIM1 and RIM2 redundantly determine Ca2+ channel density and readily releasable pool size at a large hindbrain synapse. J Neurophysiol 113:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. (2011) RIM determines Ca²+ channel density and vesicle docking at the presynaptic active zone. Neuron 69:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon MR, Berrow NS, Dolphin AC, Wallace BA. (1999) Modelling of a voltage-dependent Ca2+ channel β subunit as a basis for understanding its functional properties. FEBS Lett 445:366–370. [DOI] [PubMed] [Google Scholar]
- Hans M, Luvisetto S, Williams ME, Spagnolo M, Urrutia A, Tottene A, Brust PF, Johnson EC, Harpold MM, Stauderman KA, et al. (1999) Functional consequences of mutations in the human alpha1A calcium channel subunit linked to familial hemiplegic migraine. J Neurosci 19:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JP, Chen RS, Larsen JK, Chu PJ, Janes DM, Weis KE, Best PM. (2004) Calcium channel gamma6 subunits are unique modulators of low voltage-activated (Cav3.1) calcium current. J Mol Cell Cardiol 37:1147–1158. [DOI] [PubMed] [Google Scholar]
- Hansen PB. (2015) Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: news from the world of knockout mice. Am J Physiol Regul Integr Comp Physiol 308:R227–R237. [DOI] [PubMed] [Google Scholar]
- Hansson K, Ma X, Eliasson L, Czerwiec E, Furie B, Furie BC, Rorsman P, Stenflo J. (2004) The first gamma-carboxyglutamic acid-containing contryphan. A selective L-type calcium ion channel blocker isolated from the venom of Conus marmoreus. J Biol Chem 279:32453–32463. [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu XJ, Urban L, Wiesenfeld-Hallin Z. (2000) Repeated administration of systemic gabapentin alleviates allodynia-like behaviors in spinally injured rats. Neurosci Lett 280:211–214. [DOI] [PubMed] [Google Scholar]
- Happe S, Sauter C, Klösch G, Saletu B, Zeitlhofer J. (2003) Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology 48:82–86. [DOI] [PubMed] [Google Scholar]
- Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Gonzales AL, Earley S, Vigmond EJ, Nygren A, et al. (2014) Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res 115:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz OF, Visser F, Brett SE, Goldman D, Zechariah A, Hashad AM, Menon BK, Watson T, Starreveld Y, Welsh DG. (2015) CaV1.2/CaV3.x channels mediate divergent vasomotor responses in human cerebral arteries. J Gen Physiol 145:405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S, Wakamori M, Ino M, Miyamoto N, Takahashi E, Yoshinaga T, Sawada K, Imoto K, Tanaka I, Yoshizawa T, et al. (2001) Differential nociceptive responses in mice lacking the alpha(1B) subunit of N-type Ca(2+) channels. Neuroreport 12:2423–2427. [DOI] [PubMed] [Google Scholar]
- Hatta T, Takeda K, Shiotsu Y, Sugishita C, Adachi T, Kimura T, Sonomura K, Kusaba T, Kishimioto N, Narumiya H, et al. (2012) Switching to an L/N-type calcium channel blocker shows renoprotective effects in patients with chronic kidney disease: the Kyoto Cilnidipine Study. J Int Med Res 40:1417–1428. [DOI] [PubMed] [Google Scholar]
- Heinke B, Gingl E, Sandkühler J. (2011) Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ- and C-fibers. J Neurosci 31:1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. (1993) Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol 123:949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton TD, Xu W, Lipscombe D. (2005) Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci 25:10247–10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. (2008) Pharmacological disruption of calcium channel trafficking by the alpha2δ ligand gabapentin. Proc Natl Acad Sci USA 105:3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan JF, Mitra-Ganguli T, Stanish LF, Liu L, Zhao R, Rittenhouse AR. (2009) The Ca2+ channel beta subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J Gen Physiol 134:369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekinne L, Colasse S, Triller A, Renner M. (2013) Differential control of thrombospondin over synaptic glycine and AMPA receptors in spinal cord neurons. J Neurosci 33:11432–11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey JA, Boczek NJ, Jiang YH, Miller JD, Patrick W, Pfeiffer R, Sutphin BS, Tester DJ, Barajas-Martinez H, Ackerman MJ, et al. (2014) A CACNA1C variant associated with reduced voltage-dependent inactivation, increased CaV1.2 channel window current, and arrhythmogenesis. PLoS One 9:e106982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. (1996) Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380:258–262. [DOI] [PubMed] [Google Scholar]
- Hernández-Ochoa EO, Contreras M, Cseresnyés Z, Schneider MF. (2007) Ca2+ signal summation and NFATc1 nuclear translocation in sympathetic ganglion neurons during repetitive action potentials. Cell Calcium 41:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron SE, Khosravani H, Varela D, Bladen C, Williams TC, Newman MR, Scheffer IE, Berkovic SF, Mulley JC, Zamponi GW. (2007) Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann Neurol 62:560–568. [DOI] [PubMed] [Google Scholar]
- Heron SE, Phillips HA, Mulley JC, Mazarib A, Neufeld MY, Berkovic SF, Scheffer IE. (2004) Genetic variation of CACNA1H in idiopathic generalized epilepsy. Ann Neurol 55:595–596. [DOI] [PubMed] [Google Scholar]
- Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. (2006) Brain activation pattern induced by stimulation of L-type Ca2+-channels: contribution of Ca(V)1.3 and Ca(V)1.2 isoforms. Neuroscience 139:1005–1015. [DOI] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, Hudspeth AJ, Lesage F. (2003) Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel beta 4 subunit. Proc Natl Acad Sci USA 100:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiippala A, Tallila J, Myllykangas S, Koskenvuo JW, Alastalo TP. (2015) Expanding the phenotype of Timothy syndrome type 2: an adolescent with ventricular fibrillation but normal development. Am J Med Genet A 167A:629–634. [DOI] [PubMed] [Google Scholar]
- Hille B. (1977) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC. (2015) Phosphoinositides regulate ion channels. Biochim Biophys Acta 1851:844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, McIntosh JM, Cruz LJ, et al. (1992) A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron 9:69–77. [DOI] [PubMed] [Google Scholar]
- Hirtz JJ, Braun N, Griesemer D, Hannes C, Janz K, Löhrke S, Müller B, Friauf E. (2012) Synaptic refinement of an inhibitory topographic map in the auditory brainstem requires functional Cav1.3 calcium channels. J Neurosci 32:14602–14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockerman GH, Johnson BD, Abbott MR, Scheuer T, Catterall WA. (1997) Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels in transmembrane segment IIIS6 and the pore region of the alpha1 subunit. J Biol Chem 272:18759–18765. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Flockerzi V, Kahl S, Wegener JW. (2014) L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev 94:303–326. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Trevino ES. (1999) Calcium channel blockers in psychiatric disorders: a review of the literature. Can J Psychiatry 44:658–664. [DOI] [PubMed] [Google Scholar]
- Hope CI, Sharp DM, Hemara-Wahanui A, Sissingh JI, Lundon P, Mitchell EA, Maw MA, Clover GM. (2005) Clinical manifestations of a unique X-linked retinal disorder in a large New Zealand family with a novel mutation in CACNA1F, the gene responsible for CSNB2. Clin Experiment Ophthalmol 33:129–136. [DOI] [PubMed] [Google Scholar]
- Hoppa M, Lana B, Margas W, Dolphin AC, Ryan TA. (2012) α2δ expression sets presynaptic calcium channel abundance and release probability. Nature 486:122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Chang YT, Chen CC. (2013) Early growth response 1 is an early signal inducing Cav3.2 T-type calcium channels during cardiac hypertrophy. Cardiovasc Res 100:222–230. [DOI] [PubMed] [Google Scholar]
- Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ. (2012) Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest 122:2046–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LY, Ryder TR, Nikam SS, Millerman E, Szoke BG, Rafferty MF. (1999a) Synthesis and biological evaluation of substituted 4-(OBz)phenylalanine derivatives as novel N-type calcium channel blockers. Bioorg Med Chem Lett 9:1121–1126. [DOI] [PubMed] [Google Scholar]
- Hu LY, Ryder TR, Rafferty MF, Cody WL, Lotarski SM, Miljanich GP, Millerman E, Rock DM, Song Y, Stoehr SJ, et al. (1999b) N,N-dialkyl-dipeptidylamines as novel N-type calcium channel blockers. Bioorg Med Chem Lett 9:907–912. [DOI] [PubMed] [Google Scholar]
- Hu LY, Ryder TR, Rafferty MF, Dooley DJ, Geer JJ, Lotarski SM, Miljanich GP, Millerman E, Rock DM, Stoehr SJ, et al. (1999c) Structure-activity relationship of N-methyl-N-aralkyl-peptidylamines as novel N-type calcium channel blockers. Bioorg Med Chem Lett 9:2151–2156. [DOI] [PubMed] [Google Scholar]
- Hu LY, Ryder TR, Rafferty MF, Feng MR, Lotarski SM, Rock DM, Sinz M, Stoehr SJ, Taylor CP, Weber ML, et al. (1999d) Synthesis of a series of 4-benzyloxyaniline analogues as neuronal N-type calcium channel blockers with improved anticonvulsant and analgesic properties. J Med Chem 42:4239–4249. [DOI] [PubMed] [Google Scholar]
- Huang CH, Chen YC, Chen CC. (2013a) Physical interaction between calcineurin and Cav3.2 T-type Ca2+ channel modulates their functions. FEBS Lett 587:1723–1730. [DOI] [PubMed] [Google Scholar]
- Huang H, Ng CY, Yu D, Zhai J, Lam Y, Soong TW. (2014) Modest CaV1.342-selective inhibition by compound 8 is β-subunit dependent. Nat Commun 5:4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tan BZ, Shen Y, Tao J, Jiang F, Sung YY, Ng CK, Raida M, Köhr G, Higuchi M, et al. (2012) RNA editing of the IQ domain in Ca(v)1.3 channels modulates their Ca²⁺-dependent inactivation. Neuron 73:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Yu D, Soong TW. (2013b) C-terminal alternative splicing of CaV1.3 channels distinctively modulates their dihydropyridine sensitivity. Mol Pharmacol 84:643–653. [DOI] [PubMed] [Google Scholar]
- Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, Shah MM. (2011) Presynaptic HCN1 channels regulate Cav3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci 14:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huc S, Monteil A, Bidaud I, Barbara G, Chemin J, Lory P. (2009) Regulation of T-type calcium channels: signalling pathways and functional implications. Biochim Biophys Acta 1793:947–952. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. (2002) Block of T-type Ca(2+) channels is an important action of succinimide antiabsence drugs. Epilepsy Curr 2:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. (1992) A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci 12:3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, Turner RW, Zamponi GW. (2007) Regulation of T-type calcium channels by Rho-associated kinase. Nat Neurosci 10:854–860. [DOI] [PubMed] [Google Scholar]
- Iftinca MC, Zamponi GW. (2009) Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci 30:32–40. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. (1996) Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380:255–258. [DOI] [PubMed] [Google Scholar]
- Ilijic E, Guzman JN, Surmeier DJ. (2011) The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis 43:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki A, Frank CA, Usachev YM, Benveniste M, Lee A. (2014) Pharmacological correction of gating defects in the voltage-gated Ca(v)2.1 Ca²⁺ channel due to a familial hemiplegic migraine mutation. Neuron 81:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, et al. (2012) De novo gene disruptions in children on the autistic spectrum. Neuron 74:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Yatani A, Akaike N. (1995) Block of P-type Ca2+ channels in freshly dissociated rat cerebellar Purkinje neurons by diltiazem and verapamil. Brain Res 695:88–91. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Ward JM, Tessarollo L, McAreavey D, Sachdev V, Fananapazir L, Banks MK, Morris N, Djurickovic D, Devor-Henneman DE, et al. (2004) Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am J Pathol 165:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacus MO, Uebele VN, Renger JJ, Todorovic SM. (2012) Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J Neurosci 32:9374–9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. (2008) Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol 99:3151–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM. (2007) Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci 27:3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SE, Magga JM, Beedle AM, Braun JEA, Zamponi GW. (2000) G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gbetagamma. J Biol Chem 275:6388–6394. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. (2001) Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J Neurosci 21:2939–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. (1991) Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated δ peptides. J Biol Chem 266:3287–3293. [PubMed] [Google Scholar]
- Jeng CJ, Sun MC, Chen YW, Tang CY. (2008) Dominant-negative effects of episodic ataxia type 2 mutations involve disruption of membrane trafficking of human P/Q-type Ca2+ channels. J Cell Physiol 214:422–433. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A. (2010) Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30:5125–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Li DQ, Olofsson CS, Salehi A, Surve VV, Caballero J, Ivarsson R, Lundquist I, Pereverzev A, Schneider T, et al. (2005) CaV2.3 calcium channels control second-phase insulin release. J Clin Invest 115:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodice C, Mantuano E, Veneziano L, Trettel F, Sabbadini G, Calandriello L, Francia A, Spadaro M, Pierelli F, Salvi F, et al. (1997) Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet 6:1973–1978. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Rice AS. (2014) Clinical practice. Postherpetic neuralgia. N Engl J Med 371:1526–1533. [DOI] [PubMed] [Google Scholar]
- Joksovic PM, Nelson MT, Jevtovic-Todorovic V, Patel MK, Perez-Reyes E, Campbell KP, Chen CC, Todorovic SM. (2006) CaV3.2 is the major molecular substrate for redox regulation of T-type Ca2+ channels in the rat and mouse thalamus. J Physiol 574:415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Patil PG, Snutch TP, Yue DT. (1997) G-protein modulation of N-type calcium channel gating current in human embryonic kidney cells (HEK 293). J Physiol 498:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson IR, Varadi G. (1996) The β subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+ channels. Biophys J 70:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenceau A, Eunson LH, Spauschus A, Ramesh V, Zuberi SM, Kullmann DM, Hanna MG. (2001) Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet 358:801–807. [DOI] [PubMed] [Google Scholar]
- Jun K, Piedras-Rentería ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, et al. (1999) Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci USA 96:15245–15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K, Groome J, Lehmann-Horn F. (2012) Pathophysiological role of omega pore current in channelopathies. Front Pharmacol 3:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K, Lerche H, Lehmann-Horn F. (2002) Skeletal muscle channelopathies. J Neurol 249:1493–1502. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K, Mitrovic N, Hang C, Kouzmekine A, Iaizzo P, Herzog J, Lerche H, Nicole S, Vale-Santos J, Chauveau D, et al. (2000) Voltage-sensor sodium channel mutations cause hypokalemic periodic paralysis type 2 by enhanced inactivation and reduced current. Proc Natl Acad Sci USA 97:9549–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. (2011) RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp TJ, Pérez-García MT, Marban E. (1996) Enhancement of ionic current and charge movement by coexpression of calcium channel beta 1A subunit with alpha 1C subunit in a human embryonic kidney cell line. J Physiol 492:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Cooper CB, Nishioka N, Yamasaki H, Suzuki A, Jarvis SE, Akaike A, Satoh M, Zamponi GW. (2002) Identification and characterization of novel human Ca(v)2.2 (alpha 1B) calcium channel variants lacking the synaptic protein interaction site. J Neurosci 22:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HW, Park JY, Jeong SW, Kim JA, Moon HJ, Perez-Reyes E, Lee JH. (2006) A molecular determinant of nickel inhibition in Cav3.2 T-type calcium channels. J Biol Chem 281:4823–4830. [DOI] [PubMed] [Google Scholar]
- Kang S, Cooper G, Dunne SF, Dusel B, Luan CH, Surmeier DJ, Silverman RB. (2012) CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat Commun 3:1146. [DOI] [PubMed] [Google Scholar]
- Kang S, Cooper G, Dunne SF, Luan CH, Surmeier DJ, Silverman RB. (2013) Structure-activity relationship of N,N′-disubstituted pyrimidinetriones as Ca(V)1.3 calcium channel-selective antagonists for Parkinson’s disease. J Med Chem 56:4786–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kario K, Ando S, Kido H, Nariyama J, Takiuchi S, Yagi T, Shimizu T, Eguchi K, Ohno M, Kinoshita O, et al. (2013) The effects of the L/N-type calcium channel blocker (cilnidipine) on sympathetic hyperactive morning hypertension: results from ACHIEVE-ONE. J Clin Hypertens (Greenwich) 15:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wakamori M, Mori Y, Imoto K, Kitamura K. (2002) Inhibitory effects of cilnidipine on peripheral and brain N-type Ca2+ channels expressed in BHK cells. Neuropharmacology 42:1099–1108. [DOI] [PubMed] [Google Scholar]
- Kaur G, Pinggera A, Ortner NJ, Lieb A, Sinnegger-Brauns MJ, Yarov-Yarovoy V, Obermair GJ, Flucher BE, Striessnig J. (2015) A Polybasic Plasma Membrane Binding Motif in the I-II Linker Stabilizes Voltage-Gated Cav1.2 Calcium Channel Function. J Biol Chem [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H, Altier C, Simms B, Hamming KS, Snutch TP, Mezeyova J, McRory JE, Zamponi GW. (2004) Gating effects of mutations in the Cav3.2 T-type calcium channel associated with childhood absence epilepsy. J Biol Chem 279:9681–9684. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Bladen C, Parker DB, Snutch TP, McRory JE, Zamponi GW. (2005) Effects of Cav3.2 channel mutations linked to idiopathic generalized epilepsy. Ann Neurol 57:745–749. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Zamponi GW. (2006) Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev 86:941–966. [DOI] [PubMed] [Google Scholar]
- Kim C, Jun K, Lee T, Kim SS, McEnery MW, Chin H, Kim HL, Park JM, Kim DK, Jung SJ, et al. (2001a) Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol Cell Neurosci 18:235–245. [DOI] [PubMed] [Google Scholar]
- Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin HS. (2003) Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science 302:117–119. [DOI] [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. (2001b) Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron 31:35–45. [DOI] [PubMed] [Google Scholar]
- Kim HL, Kim H, Lee P, King RG, Chin H. (1992) Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel alpha 2 subunit. Proc Natl Acad Sci USA 89:3251–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm T, Bean BP. (2014) Inhibition of A-type potassium current by the peptide toxin SNX-482. J Neurosci 34:9182–9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Rossi GC, Chang AH, Williams L, Pasternak GW. (1997) Spinal analgesic activity of orphanin FQ/nociceptin and its fragments. Neurosci Lett 223:113–116. [DOI] [PubMed] [Google Scholar]
- King VF, Garcia ML, Himmel D, Reuben JP, Lam YK, Pan JX, Han GQ, Kaczorowski GJ. (1988) Interaction of tetrandrine with slowly inactivating calcium channels. Characterization of calcium channel modulation by an alkaloid of Chinese medicinal herb origin. J Biol Chem 263:2238–2244. [PubMed] [Google Scholar]
- Kipfer S, Jung S, Lemke JR, Kipfer-Kauer A, Howell JP, Kaelin-Lang A, Nyffeler T, Gutbrod K, Abicht A, Müri RM. (2013) Novel CACNA1A mutation(s) associated with slow saccade velocities. J Neurol 260:3010–3014. [DOI] [PubMed] [Google Scholar]
- Kisilevsky AE, Mulligan SJ, Altier C, Iftinca MC, Varela D, Tai C, Chen L, Hameed S, Hamid J, Macvicar BA, et al. (2008) D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron 58:557–570. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr (1988) Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105:1–21. [DOI] [PubMed] [Google Scholar]
- Klimis H, Adams DJ, Callaghan B, Nevin S, Alewood PF, Vaughan CW, Mozar CA, Christie MJ. (2011) A novel mechanism of inhibition of high-voltage activated calcium channels by α-conotoxins contributes to relief of nerve injury-induced neuropathic pain. Pain 152:259–266. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinová L, Marais E, Hobom M, Hofmann F. (1999) Molecular diversity of the calcium channel alpha2δ subunit. J Neurosci 19:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Hofmann F. (2003) Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr 35:639–647. [DOI] [PubMed] [Google Scholar]
- Koganei H, Shoji M, Iwata S. (2009) Suppression of formalin-induced nociception by cilnidipine, a voltage-dependent calcium channel blocker. Biol Pharm Bull 32:1695–1700. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. (2005) Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci 25:3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Pinggera A, Schicker A, Striessnig J. (2013) Role of L-type Ca2+ channels in sensory cells, in Pathologies of Calcium Channels (Weiss N, Kosckak A, eds) pp 47–96, Springer Science & Business Media, Berlin. [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. (2001) alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem 276:22100–22106. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Walter D, Hoda JC, Heinzle T, Grabner M, Striessnig J. (2003) Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci 23:6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Paşca SP, Shcheglovitov A, Yazawa M, Schwemberger R, Rasmusson R, Dolmetsch RE. (2013) Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci 16:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubista H, Mafra RA, Chong Y, Nicholson GM, Beirão PS, Cruz JS, Boehm S, Nentwig W, Kuhn-Nentwig L. (2007) CSTX-1, a toxin from the venom of the hunting spider Cupiennius salei, is a selective blocker of L-type calcium channels in mammalian neurons. Neuropharmacology 52:1650–1662. [DOI] [PubMed] [Google Scholar]
- Kumar PP, Stotz SC, Paramashivappa R, Beedle AM, Zamponi GW, Rao AS. (2002) Synthesis and evaluation of a new class of nifedipine analogs with T-type calcium channel blocking activity. Mol Pharmacol 61:649–658. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Gerlach M, Pupeter SC, Sautter J, Dirr A, Arnold G, Opitz W, Przuntek H, Riederer P, Oertel WH. (1995) Pretreatment with nimodipine prevents MPTP-induced neurotoxicity at the nigral, but not at the striatal level in mice. Neuroreport 6:621–625. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Sautter J, Schwarz J, Riederer P, Gerlach M, Oertel WH. (1996) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res 741:185–196. [DOI] [PubMed] [Google Scholar]
- Kurshan PT, Oztan A, Schwarz TL. (2009) Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci 12:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacinová L, Klugbauer N. (2004) Modulation of gating currents of the Ca(v)3.1 calcium channel by alpha 2 delta 2 and gamma 5 subunits. Arch Biochem Biophys 425:207–213. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, DiFrancesco D. (2009) What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalevée N, Rebsamen MC, Barrère-Lemaire S, Perrier E, Nargeot J, Bénitah JP, Rossier MF. (2005) Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res 67:216–224. [DOI] [PubMed] [Google Scholar]
- Lampe RA, Defeo PA, Davison MD, Young J, Herman JL, Spreen RC, Horn MB, Mangano TJ, Keith RA. (1993) Isolation and pharmacological characterization of omega-grammotoxin SIA, a novel peptide inhibitor of neuronal voltage-sensitive calcium channel responses. Mol Pharmacol 44:451–460. [PubMed] [Google Scholar]
- Lana B, Schlick B, Martin S, Pratt WS, Page KM, Goncalves L, Rahman W, Dickenson AH, Bauer CS, Dolphin AC. (2014) Differential upregulation in DRG neurons of an α2δ-1 splice variant with a lower affinity for gabapentin after peripheral sensory nerve injury. Pain 155:522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Y, Gong D, Fan Y. (2015) Calcium channel blocker use and risk of Parkinson’s disease: a meta-analysis. Pharmacoepidemiol Drug Saf 24:559–566. [DOI] [PubMed] [Google Scholar]
- Langwieser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S. (2010) Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neurosci 30:8367–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson KP, Olsen UB, Hansen AJ. (2000) Nociceptin is a potent inhibitor of N-type Ca(2+) channels in rat sympathetic ganglion neurons. Neurosci Lett 296:121–124. [DOI] [PubMed] [Google Scholar]
- Latour I, Louw DF, Beedle AM, Hamid J, Sutherland GR, Zamponi GW. (2004) Expression of T-type calcium channel splice variants in human glioma. Glia 48:112–119. [DOI] [PubMed] [Google Scholar]
- Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, Lee A, Moosmang S, Hofmann F, Pieper AA, et al. (2012a) Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry 17:1054–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Bae C, Lee J, Ryu JH, Kim HH, Kohno T, Swartz KJ, Kim JI. (2012b) Solution structure of kurtoxin: a gating modifier selective for Cav3 voltage-gated Ca(2+) channels. Biochemistry 51:1862–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klöckner U, Schneider T, Perez-Reyes E. (1999a) Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci 19:1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. (1999b) Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J 77:3034–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. (2014) Z944: a first in class T-type calcium channel modulator for the treatment of pain. J Peripher Nerv Syst 19 (Suppl 2):S11–S12. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim Y, Back SK, Choi HW, Lee JY, Jung HH, Ryu JH, Suh HW, Na HS, Kim HJ, et al. (2010) Analgesic effect of highly reversible ω-conotoxin FVIA on N type Ca2+ channels. Mol Pain 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Lee J, Latchoumane C, Lee B, Oh SJ, Saud ZA, Park C, Sun N, Cheong E, Chen CC, et al. (2014) Rebound burst firing in the reticular thalamus is not essential for pharmacological absence seizures in mice. Proc Natl Acad Sci USA 111:11828–11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Malhotra AK. (2015) Targeting the schizophrenia genome: a fast track strategy from GWAS to clinic. Mol Psychiatry 20:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J, Richards MW, Butcher AJ, Nieto-Rostro M, Pratt WS, Davies A, Dolphin AC. (2005) Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for beta1 but not for palmitoylated beta2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J Neurosci 25:6984–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Boyer EW, Pozner CN, Geib AJ, Thomsen T, Mick N, Thomas SH. (2007) Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit Care Med 35:2071–2075. [DOI] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, et al. (2006) Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain 125:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Taylor CP, Weber M, Piechan J, Prior F, Bian F, Cui M, Hoffman D, Donevan S. (2011) Pregabalin is a potent and selective ligand for α(2)δ-1 and α(2)δ-2 calcium channel subunits. Eur J Pharmacol 667:80–90. [DOI] [PubMed] [Google Scholar]
- Liang Y, Tavalin SJ. (2007) Auxiliary beta subunits differentially determine pka utilization of distinct regulatory sites on Cav1.3 L type Ca2+ channels. Channels (Austin) 1:102–112. [DOI] [PubMed] [Google Scholar]
- Liao P, Soong TW. (2010) CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch 460:353–359. [DOI] [PubMed] [Google Scholar]
- Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. (2007) A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem 282:35133–35142. [DOI] [PubMed] [Google Scholar]
- Liao P, Yu D, Lu S, Tang Z, Liang MC, Zeng S, Lin W, Soong TW. (2004) Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1.2 calcium channels. J Biol Chem 279:50329–50335. [DOI] [PubMed] [Google Scholar]
- Liao P, Zhang HY, Soong TW. (2009) Alternative splicing of voltage-gated calcium channels: from molecular biology to disease. Pflugers Arch 458:481–487. [DOI] [PubMed] [Google Scholar]
- Lin SS, Tzeng BH, Lee KR, Smith RJ, Campbell KP, Chen CC. (2014) Cav3.2 T-type calcium channel is required for the NFAT-dependent Sox9 expression in tracheal cartilage. Proc Natl Acad Sci USA 111:E1990–E1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Miller JW, Mankodi A, Kanadia RN, Yuan Y, Moxley RT, Swanson MS, Thornton CA. (2006) Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet 15:2087–2097. [DOI] [PubMed] [Google Scholar]
- Lin Y, McDonough SI, Lipscombe D. (2004) Alternative splicing in the voltage-sensing region of N-Type CaV2.2 channels modulates channel kinetics. J Neurophysiol 92:2820–2830. [DOI] [PubMed] [Google Scholar]
- Lin Z, Haus S, Edgerton J, Lipscombe D. (1997) Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron 18:153–166. [DOI] [PubMed] [Google Scholar]
- Lin Z, Lin Y, Schorge S, Pan JQ, Beierlein M, Lipscombe D. (1999) Alternative splicing of a short cassette exon in alpha1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci 19:5322–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Witschas K, Garcia T, Chen RS, Hansen JP, Sellers ZM, Kuzmenkina E, Herzig S, Best PM. (2008) A critical GxxxA motif in the gamma6 calcium channel subunit mediates its inhibitory effect on Cav3.1 calcium current. J Physiol 586:5349–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski DM, Thake M, MacLaren RE. (2013) Clinical applications of retinal gene therapy. Prog Retin Eye Res 32:22–47. [DOI] [PubMed] [Google Scholar]
- Lipscombe D. (2002) L-type calcium channels: highs and new lows. Circ Res 90:933–935. [DOI] [PubMed] [Google Scholar]
- Liss B, Roeper J. (2008) Individual dopamine midbrain neurons: functional diversity and flexibility in health and disease. Brain Res Brain Res Rev 58:314–321. [DOI] [PubMed] [Google Scholar]
- Liu H, De Waard M, Scott VES, Gurnett CA, Lennon VA, Campbell KP. (1996) Identification of three subunits of the high affinity omega-conotoxin MVIIC-sensitive Ca2+ channel. J Biol Chem 271:13804–13810. [PubMed] [Google Scholar]
- Liu X, Yang PS, Yang W, Yue DT. (2010) Enzyme-inhibitor-like tuning of Ca(2+) channel connectivity with calmodulin. Nature 463:968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Lima PA, Marrion NV. (2007) Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci 120:985–995. [DOI] [PubMed] [Google Scholar]
- Lomize MA, Lomize AL, Pogozheva ID, Mosberg HI. (2006) OPM: orientations of proteins in membranes database. Bioinformatics 22:623–625. [DOI] [PubMed] [Google Scholar]
- Lopez-Charcas O, Rivera M, Gomora JC. (2012) Block of human CaV3 channels by the diuretic amiloride. Mol Pharmacol 82:658–667. [DOI] [PubMed] [Google Scholar]
- Lotarski S, Hain H, Peterson J, Galvin S, Strenkowski B, Donevan S, Offord J. (2014) Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in α2δ1 (R217A) and α2δ2 (R279A) mouse mutants. Epilepsy Res 108:833–842. [DOI] [PubMed] [Google Scholar]
- Lotarski SM, Donevan S, El-Kattan A, Osgood S, Poe J, Taylor CP, Offord J. (2011) Anxiolytic-like activity of pregabalin in the Vogel conflict test in α2δ-1 (R217A) and α2δ-2 (R279A) mouse mutants. J Pharmacol Exp Ther 338:615–621. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. (2001) Upregulation of dorsal root ganglion (alpha)2(δ) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci 21:1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Cohen S, Li B, Tsien RW. (2013) Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci Rep 33:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Aoki Y, Sekiguchi F, Matsunami M, Takahashi T, Nishikawa H, Kawabata A. (2009) Hyperalgesia induced by spinal and peripheral hydrogen sulfide: evidence for involvement of Cav3.2 T-type calcium channels. Pain 142:127–132. [DOI] [PubMed] [Google Scholar]
- Mahapatra S, Marcantoni A, Zuccotti A, Carabelli V, Carbone E. (2012) Equal sensitivity of Cav1.2 and Cav1.3 channels to the opposing modulations of PKA and PKG in mouse chromaffin cells. J Physiol 590:5053–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella S, Yue Y, Karnabi E, Qu Y, El-Sherif N, Boutjdir M. (2008) Impaired Ca2+ homeostasis is associated with atrial fibrillation in the alpha1D L-type Ca2+ channel KO mouse. Am J Physiol Heart Circ Physiol 295:H2017–H2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, McKnight AT. (2001) Block by gabapentin of the facilitation of glutamate release from rat trigeminal nucleus following activation of protein kinase C or adenylyl cyclase. Br J Pharmacol 134:237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Bertolino M, DeErausquin G. (1990) Amiloride blocks glutamate-operated cationic channels and protects neurons in culture from glutamate-induced death. Neuropharmacology 29:1103–1110. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. (2003) Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA 100:5543–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ME, Nargeot J. (2001) Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res 52:51–64. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, et al. (2006) Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 98:1422–1430. [DOI] [PubMed] [Google Scholar]
- Marangoudakis S, Andrade A, Helton TD, Denome S, Castiglioni AJ, Lipscombe D. (2012) Differential ubiquitination and proteasome regulation of Ca(V)2.2 N-type channel splice isoforms. J Neurosci 32:10365–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantoni A, Vandael DH, Mahapatra S, Carabelli V, Sinnegger-Brauns MJ, Striessnig J, Carbone E. (2010) Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci 30:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrère C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, et al. (2011a) T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci USA 108:11268–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marger L, Mesirca P, Alig J, Torrente A, Dubel S, Engeland B, Kanani S, Fontanaud P, Striessnig J, Shin HS, et al. (2011b) Functional roles of Ca(v)1.3, Ca(v)3.1 and HCN channels in automaticity of mouse atrioventricular cells: insights into the atrioventricular pacemaker mechanism. Channels (Austin) 5:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark MD, Maejima T, Kuckelsberg D, Yoo JW, Hyde RA, Shah V, Gutierrez D, Moreno RL, Kruse W, Noebels JL, et al. (2011) Delayed postnatal loss of P/Q-type calcium channels recapitulates the absence epilepsy, dyskinesia, and ataxia phenotypes of genomic Cacna1a mutations. J Neurosci 31:4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Luján R, Loh HH, Wickman K. (2005) Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci 25:3551–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks ML, Trippel DL, Keating MT. (1995) Long QT syndrome associated with syndactyly identified in females. Am J Cardiol 76:744–745. [DOI] [PubMed] [Google Scholar]
- Marshall MR, Clark JP, 3rd, Westenbroek R, Yu FH, Scheuer T, Catterall WA. (2011) Functional roles of a C-terminal signaling complex of CaV1 channels and A-kinase anchoring protein 15 in brain neurons. J Biol Chem 286:12627–12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson AG, Kadir ZA, Hutton JL, Chadwick DW. (2000) Gabapentin Add-on for Drug-Resistant Partial Epilepsy. Cochrane Database Syst Rev 7:CD001415. [DOI] [PubMed] [Google Scholar]
- Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. (2002) Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology 42:353–366. [DOI] [PubMed] [Google Scholar]
- Martinello K, Huang Z, Lujan R, Tran B, Watanabe M, Cooper EC, Brown DA, Shah MM. (2015) Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron 85:346–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez ML, Heredia MP, Delgado C. (1999) Expression of T-type Ca(2+) channels in ventricular cells from hypertrophied rat hearts. J Mol Cell Cardiol 31:1617–1625. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Röenfeld M, Dasgupta S, Miller RJ, Philipson LH. (1996) Isoform expression of the voltage-dependent calcium channel alpha 1E. Receptors Channels 4:243–251. [PubMed] [Google Scholar]
- Matar N, Jin W, Wrubel H, Hescheler J, Schneider T, Weiergräber M. (2009) Zonisamide block of cloned human T-type voltage-gated calcium channels. Epilepsy Res 83:224–234. [DOI] [PubMed] [Google Scholar]
- Matthes J, Yildirim L, Wietzorrek G, Reimer D, Striessnig J, Herzig S. (2004) Disturbed atrio-ventricular conduction and normal contractile function in isolated hearts from Cav1.3-knockout mice. Naunyn-Schmiedeberg’s Archives of Pharmacology 369:554–562. [DOI] [PubMed] [Google Scholar]
- Matsuyama Z, Wakamori M, Mori Y, Kawakami H, Nakamura S, Imoto K. (1999) Direct alteration of the P/Q-type Ca2+ channel property by polyglutamine expansion in spinocerebellar ataxia 6. J Neurosci 19:RC14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Bee LA, Stephens GJ, Dickenson AH. (2007) The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur J Neurosci 25:3561–3569. [DOI] [PubMed] [Google Scholar]
- Maximov A, Bezprozvanny I. (2002) Synaptic targeting of N-type calcium channels in hippocampal neurons. J Neurosci 22:6939–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. (1992) A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol 68:1384–1400. [DOI] [PubMed] [Google Scholar]
- McDonough SI, Lampe RA, Keith RA, Bean BP. (1997) Voltage-dependent inhibition of N- and P-type calcium channels by the peptide toxin omega-grammotoxin-SIA. Mol Pharmacol 52:1095–1104. [DOI] [PubMed] [Google Scholar]
- McDonough SI, Mori Y, Bean BP. (2005) FPL 64176 modification of Ca(V)1.2 L-type calcium channels: dissociation of effects on ionic current and gating current. Biophys J 88:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. (2006) The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem 13:584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, Lee B, Murphy GG. (2009) Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol Learn Mem 92:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRory JE, Santi CM, Hamming KSC, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. (2001) Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem 276:3999–4011. [DOI] [PubMed] [Google Scholar]
- Meir A, Bell DC, Stephens GJ, Page KM, Dolphin AC. (2000) Calcium channel β subunit promotes voltage-dependent modulation of alpha 1 B by G β γ. Biophys J 79:731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Thoreson WB. (2011) The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis Neurosci 28:453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesirca P, Torrente AG, Mangoni ME. (2014) T-type channels in the sino-atrial and atrioventricular pacemaker mechanism. Pflugers Arch 466:791–799. [DOI] [PubMed] [Google Scholar]
- Mesirca P, Torrente AG, Mangoni ME. (2015) Functional role of voltage gated Ca(2+) channels in heart automaticity. Front Physiol 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger RB, Naik AK, Jagodic MM, Nelson MT, Lee WY, Choe WJ, Orestes P, Latham JR, Todorovic SM, Jevtovic-Todorovic V. (2009) In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain 145:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezghrani A, Monteil A, Watschinger K, Sinnegger-Brauns MJ, Barrère C, Bourinet E, Nargeot J, Striessnig J, Lory P. (2008) A destructive interaction mechanism accounts for dominant-negative effects of misfolded mutants of voltage-gated calcium channels. J Neurosci 28:4501–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Shaltiel L, Sothilingam V, Koch S, Schludi V, Krause S, Zeitz C, Audo I, Lancelot ME, Hamel C, et al. (2014) Mosaic synaptopathy and functional defects in Cav1.4 heterozygous mice and human carriers of CSNB2. Hum Mol Genet 23:1538–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J, Przewłocki R, Przewłocka B. (2001) The role of delta-opioid receptor subtypes in neuropathic pain. Eur J Pharmacol 415:31–37. [DOI] [PubMed] [Google Scholar]
- Miljanich GP. (2004) Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem 11:3029–3040. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME. (1992) P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature 355:827–829. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hermsmeyer K. (1994) Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ Res 75:144–148. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Watanabe C, Sakurada T, Sakurada S. (2012) New vistas in opioid control of pain. Curr Opin Pharmacol 12:87–91. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Enyeart JJ. (1993) Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol 469:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA. (1996) Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron 17:781–788. [DOI] [PubMed] [Google Scholar]
- Moises HC, Rusin KI, Macdonald RL. (1994) mu-Opioid receptor-mediated reduction of neuronal calcium current occurs via a G(o)-type GTP-binding protein. J Neurosci 14:3842–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. (2006) Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci USA 103:5555–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. (2009) Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev (3):CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. (2014) Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 4:CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Müller J, Stiess M, Marais E, Schulla V, Lacinova L, et al. (2005a) Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25:9883–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Lenhardt P, Haider N, Hofmann F, Wegener JW. (2005b) Mouse models to study L-type calcium channel function. Pharmacol Ther 106:347–355. [DOI] [PubMed] [Google Scholar]
- Morgans CW. (2001) Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci 42:2414–2418. [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, et al. (1991) Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature 350:398–402. [DOI] [PubMed] [Google Scholar]
- Mori Y, Nishida M, Shimizu S, Ishii M, Yoshinaga T, Ino M, Sawada K, Niidome T. (2002) Ca(2+) channel alpha(1B) subunit (Ca(V) 2.2) knockout mouse reveals a predominant role of N-type channels in the sympathetic regulation of the circulatory system. Trends Cardiovasc Med 12:270–275. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Shioda N, Yamamoto Y, Tagashira H, Fukunaga K. (2012) The T-type voltage-gated calcium channel as a molecular target of the novel cognitive enhancer ST101: enhancement of long-term potentiation and CaMKII autophosphorylation in rat cortical slices. J Neurochem 121:44–53. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Fukuda K, Mima H, Shoda T, Kato S, Mori K. (1998) Nociceptin receptor-mediated Ca2+ channel inhibition and its desensitization in NG108-15 cells. Eur J Pharmacol 351:247–252. [DOI] [PubMed] [Google Scholar]
- Mortell KH, Anderson DJ, Lynch JJ, 3rd, Nelson SL, Sarris K, McDonald H, Sabet R, Baker S, Honore P, Lee CH, et al. (2006) Structure-activity relationships of alpha-amino acid ligands for the alpha2delta subunit of voltage-gated calcium channels. Bioorg Med Chem Lett 16:1138–1141. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. (2009) Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motin LG, Bennett MR, Christie MJ. (1995) Opioids acting on delta-receptors modulate Ca2+ currents in cultured postganglionic neurones of avian ciliary ganglia. Neurosci Lett 193:21–24. [DOI] [PubMed] [Google Scholar]
- Mould J, Yasuda T, Schroeder CI, Beedle AM, Doering CJ, Zamponi GW, Adams DJ, Lewis RJ. (2004) The alpha2delta auxiliary subunit reduces affinity of omega-conotoxins for recombinant N-type (Cav2.2) calcium channels. J Biol Chem 279:34705–34714. [DOI] [PubMed] [Google Scholar]
- Müller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, et al. (2010) Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci USA 107:14950–14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Struck H, Ho MS, Brockhaus-Dumke A, Klosterkötter J, Broich K, Hescheler J, Schneider T, Weiergräber M. (2012) Atropine-sensitive hippocampal θ oscillations are mediated by Cav2.3 R-type Ca²⁺ channels. Neuroscience 205:125–139. [DOI] [PubMed] [Google Scholar]
- Mullins ME, Horowitz BZ, Linden DH, Smith GW, Norton RL, Stump J. (1998) Life-threatening interaction of mibefradil and beta-blockers with dihydropyridine calcium channel blockers. JAMA 280:157–158. [DOI] [PubMed] [Google Scholar]
- Murakami-Nakayama M, Tsubota M, Hiruma S, Sekiguchi F, Matsuyama K, Kimura T, Moriyama M, Kawabata A. (2015) Polaprezinc attenuates cyclophosphamide-induced cystitis and related bladder pain in mice. J Pharmacol Sci 127:223–228. [DOI] [PubMed] [Google Scholar]
- Murbartián J, Arias JM, Lee JH, Gomora JC, Perez-Reyes E. (2002) Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1). FEBS Lett 528:272–278. [DOI] [PubMed] [Google Scholar]
- Murbartián J, Arias JM, Perez-Reyes E. (2004) Functional impact of alternative splicing of human T-type Cav3.3 calcium channels. J Neurophysiol 92:3399–3407. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Sanderson JL, Gorski JA, Scott JD, Catterall WA, Sather WA, Dell’Acqua ML. (2014) AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Reports 7:1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. (1996) Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380:72–75. [DOI] [PubMed] [Google Scholar]
- Nakai J, Tanabe T, Konno T, Adams B, Beam KG. (1998) Localization in the II-III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J Biol Chem 273:24983–24986. [DOI] [PubMed] [Google Scholar]
- Nakazawa M. (2011) Effects of calcium ion, calpains, and calcium channel blockers on retinitis pigmentosa. J Ophthalmol 2011:292040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS. (2001) Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J Clin Invest 108:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano C, Antzelevitch C. (2011) Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac voltage-dependent L-type calcium channel. Circ Res 108:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A, Garcia-Olivares J, Voswinkel S, Horstkott H, Hidalgo P. (2004) Folding of active calcium channel beta(1b) -subunit by size-exclusion chromatography and its role on channel function. J Biol Chem 279:21689–21694. [DOI] [PubMed] [Google Scholar]
- Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, et al. (2010) A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell 143:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Joksovic PM, Perez-Reyes E, Todorovic SM. (2005) The endogenous redox agent L-cysteine induces T-type Ca2+ channel-dependent sensitization of a novel subpopulation of rat peripheral nociceptors. J Neurosci 25:8766–8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Joksovic PM, Su P, Kang HW, Van Deusen A, Baumgart JP, David LS, Snutch TP, Barrett PQ, Lee JH, et al. (2007) Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J Neurosci 27:12577–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen Xh, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, et al. (1998) Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry 37:15353–15362. [DOI] [PubMed] [Google Scholar]
- Newton PM, Orr CJ, Wallace MJ, Kim C, Shin HS, Messing RO. (2004) Deletion of N-type calcium channels alters ethanol reward and reduces ethanol consumption in mice. J Neurosci 24:9862–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Zeng L, Wang V, Connolly J, Wallace MJ, Kim C, Shin HS, Belardetti F, Snutch TP, Messing RO. (2008) A blocker of N- and T-type voltage-gated calcium channels attenuates ethanol-induced intoxication, place preference, self-administration, and reinstatement. J Neurosci 28:11712–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. (2001) Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res 95:1–8. [DOI] [PubMed] [Google Scholar]
- Nie L, Zhu J, Gratton MA, Liao A, Mu KJ, Nonner W, Richardson GP, Yamoah EN. (2008) Molecular identity and functional properties of a novel T-type Ca2+ channel cloned from the sensory epithelia of the mouse inner ear. J Neurophysiol 100:2287–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Rostro M, Sandhu G, Bauer CS, Jiruska P, Jefferys JG, Dolphin AC. (2014) Altered expression of the voltage-gated calcium channel subunit α₂δ-1: a comparison between two experimental models of epilepsy and a sensory nerve ligation model of neuropathic pain. Neuroscience 283:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. (1986) Possible functional significance of a novel type of cardiac Ca channel. Biomed Biochim Acta 45:K37–K45. [PubMed] [Google Scholar]
- Noh J, Kim MK, Chung JM. (2010) A novel mechanism of zinc block on alpha1G-like low-threshold T-type Ca2+ channels in a rat thalamic relay neuron. Neurosci Res 66:353–358. [DOI] [PubMed] [Google Scholar]
- Nosal OV, Lyubanova OP, Naidenov VG, Shuba YM. (2013) Complex modulation of Ca(v)3.1 T-type calcium channel by nickel. Cell Mol Life Sci 70:1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. (1985) Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316:440–443. [DOI] [PubMed] [Google Scholar]
- Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, Kieffer BL, Gavériaux-Ruff C. (2012) δ-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther 342:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss HB, Houser SR. (1993) T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res 73:777–782. [DOI] [PubMed] [Google Scholar]
- O’Connor AB, Dworkin RH. (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122(Suppl):S22–S32. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, et al. (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. (2005) The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J Biol Chem 280:2229–2237. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Kawai M, Ninomiya N, Taylor C, Kurebayashi Y. (2008) Effect of a new alpha 2 delta ligand PD-217014 on visceral hypersensitivity induced by 2,4,6-trinitrobenzene sulfonic acid in rats. Pharmacology 81:144–150. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Inoue Y, Kawarabayashi T, Kitamura K. (2005) Identification and electrophysiological characteristics of isoforms of T-type calcium channel Ca(v)3.2 expressed in pregnant human uterus. Cell Physiol Biochem 16:245–254. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Yamazaki J. (2012) T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int J Oncol 41:267–275. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Yamazaki J, Kitamura K. (2010) Tarantula toxin ProTx-I differentiates between human T-type voltage-gated Ca2+ Channels Cav3.1 and Cav3.2. J Pharmacol Sci 112:452–458. [DOI] [PubMed] [Google Scholar]
- Olamendi-Portugal T, García BI, López-González I, Van Der Walt J, Dyason K, Ulens C, Tytgat J, Felix R, Darszon A, Possani LD. (2002) Two new scorpion toxins that target voltage-gated Ca2+ and Na+ channels. Biochem Biophys Res Commun 299:562–568. [DOI] [PubMed] [Google Scholar]
- Olivera BM, McIntosh JM, Cruz LJ, Luque FA, Gray WR. (1984) Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry 23:5087–5090. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Miljanich GP, Ramachandran J, Adams ME. (1994) Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem 63:823–867. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. (2005) G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci 25:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. (2004) Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron 42:387–399. [DOI] [PubMed] [Google Scholar]
- Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, et al. (2013) Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels. Diabetes 62:3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner NJ, Bock G, Vandael DH, Mauersberger R, Draheim HJ, Gust R, Carbone E, Tuluc P, Striessnig J. (2014) Pyrimidine-2,4,6-triones are a new class of voltage-gated L-type Ca2+ channel activators. Nat Commun 5:3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Ozono R, Yano Y, Higashi Y, Teragawa H, Miho N, Ishida T, Ishida M, Yoshizumi M, Kambe M. (2005) Beneficial effect of T-type calcium channel blockers on endothelial function in patients with essential hypertension. Hypertens Res 28:889–894. [DOI] [PubMed] [Google Scholar]
- Ostacher MJ, Iosifescu DV, Hay A, Blumenthal SR, Sklar P, Perlis RH. (2014) Pilot investigation of isradipine in the treatment of bipolar depression motivated by genome-wide association. Bipolar Disord 16:199–203. [DOI] [PubMed] [Google Scholar]
- Page KM, Heblich F, Davies A, Butcher AJ, Leroy J, Bertaso F, Pratt WS, Dolphin AC. (2004) Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J Neurosci 24:5400–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KM, Heblich F, Margas W, Pratt WS, Nieto-Rostro M, Chaggar K, Sandhu K, Davies A, Dolphin AC. (2010) N terminus is key to the dominant negative suppression of Ca(V)2 calcium channels: implications for episodic ataxia type 2. J Biol Chem 285:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajouhesh H, Feng ZP, Ding Y, Zhang L, Pajouhesh H, Morrison JL, Belardetti F, Tringham E, Simonson E, Vanderah TW, et al. (2010) Structure-activity relationships of diphenylpiperazine N-type calcium channel inhibitors. Bioorg Med Chem Lett 20:1378–1383. [DOI] [PubMed] [Google Scholar]
- Pajouhesh H, Feng ZP, Zhang L, Pajouhesh H, Jiang X, Hendricson A, Dong H, Tringham E, Ding Y, Vanderah TW, et al. (2012) Structure-activity relationships of trimethoxybenzyl piperazine N-type calcium channel inhibitors. Bioorg Med Chem Lett 22:4153–4158. [DOI] [PubMed] [Google Scholar]
- Pan JQ, Lipscombe D. (2000) Alternative splicing in the cytoplasmic II-III loop of the N-type Ca channel alpha 1B subunit: functional differences are β subunit-specific. J Neurosci 20:4769–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YG, Park HY, Lee CJ, Choi S, Jo S, Choi H, Kim YH, Shin HS, Llinas RR, Kim D. (2010) Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci USA 107:10731–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group (2013) Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord 28:1823–1831. [DOI] [PubMed] [Google Scholar]
- Pasternak B, Svanström H, Nielsen NM, Fugger L, Melbye M, Hviid A. (2012) Use of calcium channel blockers and Parkinson’s disease. Am J Epidemiol 175:627–635. [DOI] [PubMed] [Google Scholar]
- Patel R, Bauer CS, Nieto-Rostro M, Margas W, Ferron L, Chaggar K, Crews K, Ramirez JD, Bennett DL, Schwartz A, et al. (2013) α2δ-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci 33:16412–16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirathna S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. (2006) Differential effects of endogenous cysteine analogs on peripheral thermal nociception in intact rats. Pain 125:53–64. [DOI] [PubMed] [Google Scholar]
- Pelc LR, Gross GJ, Brooks HL, Warltier DC. (1986) Effects of intracoronary Bay k 8644, a calcium channel agonist, in anesthetized dogs. J Cardiovasc Pharmacol 8:1223–1228. [DOI] [PubMed] [Google Scholar]
- Peloquin JB, Khosravani H, Barr W, Bladen C, Evans R, Mezeyova J, Parker D, Snutch TP, McRory JE, Zamponi GW. (2006) Functional analysis of Ca3.2 T-type calcium channel mutations linked to childhood absence epilepsy. Epilepsia 47:655–658. [DOI] [PubMed] [Google Scholar]
- Pérez-Garci E, Larkum ME, Nevian T. (2013) Inhibition of dendritic Ca2+ spikes by GABAB receptors in cortical pyramidal neurons is mediated by a direct Gi/o-β-subunit interaction with Cav1 channels. J Physiol 591:1599–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J-H. (1998) Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 391:896–900. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Van Deusen AL, Vitko I. (2009) Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. J Pharmacol Exp Ther 328:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E, Wei XY, Castellano A, Birnbaumer L. (1990) Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J Biol Chem 265:20430–20436. [PubMed] [Google Scholar]
- Philips AV, Timchenko LT, Cooper TA. (1998) Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280:737–741. [DOI] [PubMed] [Google Scholar]
- Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. (1997) Beta subunit heterogeneity in neuronal L-type Ca2+ channels. J Biol Chem 272:13877–13882. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. (2002) Calcium channels and channelopathies of the central nervous system. Mol Neurobiol 25:31–50. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. (2010) CaV2.1 channelopathies. Pflugers Arch 460:375–393. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. (2013) Pathophysiology of migraine. Annu Rev Physiol 75:365–391. [DOI] [PubMed] [Google Scholar]
- Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J. (2015) CACNA1D De Novo Mutations in Autism Spectrum Disorders Activate Cav1.3 L-Type Calcium Channels. Biol Psychiatry 77:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippucci T, Parmeggiani A, Palombo F, Maresca A, Angius A, Crisponi L, Cucca F, Liguori R, Valentino ML, Seri M, et al. (2013) A novel null homozygous mutation confirms CACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS One 8:e82154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone A, Kurt S, Zuccotti A, Rüttiger L, Pilz P, Brown DH, Franz C, Schweizer M, Rust MB, Rübsamen R, et al. (2014) α2δ3 is essential for normal structure and function of auditory nerve synapses and is a novel candidate for auditory processing disorders. J Neurosci 34:434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. (2000) Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102:89–97. [DOI] [PubMed] [Google Scholar]
- Post RM, Frye MA, Denicoff KD, Leverich GS, Dunn RT, Osuch EA, Speer AM, Obrocea G, Jajodia K. (2000) Emerging trends in the treatment of rapid cycling bipolar disorder: a selected review. Bipolar Disord 2:305–315. [DOI] [PubMed] [Google Scholar]
- Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, et al. (2009) A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci 29:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. (1994) Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature 368:67–70. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kähler A, et al. (2014) A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzier I, Kullmann PH, Horn JP, Levitan ES. (2009) Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci 29:15414–15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Olcese R, Stefani E, Birnbaumer L. (1998) Modulation of human neuronal alpha 1E-type calcium channel by alpha 2 δ-subunit. Am J Physiol 274:C1324–C1331. [DOI] [PubMed] [Google Scholar]
- Qin N, Yagel S, Momplaisir ML, Codd EE, D’Andrea MR. (2002) Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)δ-4 subunit. Mol Pharmacol 62:485–496. [DOI] [PubMed] [Google Scholar]
- Rabl K, Thoreson WB. (2002) Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur J Neurosci 16:2070–2077. [DOI] [PubMed] [Google Scholar]
- Radzicki D, Yau HJ, Pollema-Mays SL, Mlsna L, Cho K, Koh S, Martina M. (2013) Temperature-sensitive Cav1.2 calcium channels support intrinsic firing of pyramidal neurons and provide a target for the treatment of febrile seizures. J Neurosci 33:9920–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzik I, Miziak B, Dudka J, Chrościńska-Krawczyk M, Czuczwar SJ. (2015) Prospects of epileptogenesis prevention. Pharmacol Rep 67:663–668. [DOI] [PubMed] [Google Scholar]
- Raghib A, Bertaso F, Davies A, Page KM, Meir A, Bogdanov Y, Dolphin AC. (2001) Dominant-negative synthesis suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs. J Neurosci 21:8495–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, Scheuer T, Catterall WA. (1991) Frequency and voltage-dependent inhibition of type IIA Na+ channels, expressed in a mammalian cell line, by local anesthetic, antiarrhythmic, and anticonvulsant drugs. Mol Pharmacol 40:756–765. [PubMed] [Google Scholar]
- Raingo J, Castiglioni AJ, Lipscombe D. (2007) Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat Neurosci 10:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksha WR, Wang D, Davies JN, Chen L, Zamponi GW, Fisher TE. (2008) Novel splice variants of rat CaV2.1 that lack much of the synaptic protein interaction site are expressed in neuroendocrine cells. J Biol Chem 283:15997–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran KV, Hennessey JA, Barnett AS, Yin X, Stadt HA, Foster E, Shah RA, Yazawa M, Dolmetsch RE, Kirby ML, et al. (2013) Calcium influx through L-type CaV1.2 Ca2+ channels regulates mandibular development. J Clin Invest 123:1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan O, Qu Y, Wadgaonkar R, Baroudi G, Karnabi E, Chahine M, Boutjdir M. (2009) Phosphorylation of the consensus sites of protein kinase A on alpha1D L-type calcium channel. J Biol Chem 284:5042–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau F, Freyermuth F, Fugier C, Villemin JP, Fischer MC, Jost B, Dembele D, Gourdon G, Nicole A, Duboc D, et al. (2011) Misregulation of miR-1 processing is associated with heart defects in myotonic dystrophy. Nat Struct Mol Biol 18:840–845. [DOI] [PubMed] [Google Scholar]
- Rawson DJ, Brugier D, Harrison A, Hough J, Newman J, Otterburn J, Maw GN, Price J, Thompson LR, Turnpenny P, et al. (2011) Part 3: Design and synthesis of proline-derived α2δ ligands. Bioorg Med Chem Lett 21:3771–3773. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstätter JH. (2009) Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol 512:814–824. [DOI] [PubMed] [Google Scholar]
- Rehak R, Bartoletti TM, Engbers JD, Berecki G, Turner RW, Zamponi GW. (2013) Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS One 8:e61844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. (1996) Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci USA 93:7363–7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders A, Mantilleri A, Malapert P, Rialle S, Nidelet S, Laffray S, Beurrier C, Bourinet E, Moqrich A. (2015) Transcriptional Profiling of Cutaneous MRGPRD Free Nerve Endings and C-LTMRs. Cell Reports 10:1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ, Wagner JA, Snyder SH, Thayer SA, Olivera BM, Miller RJ. (1986) Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci USA 83:8804–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KS, Swensen AM, Lipscombe D, Bommert K. (2007) Novel CaV2.1 clone replicates many properties of Purkinje cell CaV2.1 current. Eur J Neurosci 26:2950–2961. [DOI] [PubMed] [Google Scholar]
- Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. (2005) Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 6:253–260. [DOI] [PubMed] [Google Scholar]
- Rim HK, Lee HW, Choi IS, Park JY, Choi HW, Choi JH, Cho YW, Lee JY, Lee KT. (2012) T-type Ca2+ channel blocker, KYS05047 induces G1 phase cell cycle arrest by decreasing intracellular Ca2+ levels in human lung adenocarcinoma A549 cells. Bioorg Med Chem Lett 22:7123–7126. [DOI] [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer M, et al. Multicenter Genetic Studies of Schizophrenia Consortium. Psychosis Endophenotypes International Consortium. Wellcome Trust Case Control Consortium 2 (2013) Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripsch MS, Ballard CJ, Khanna M, Hurley JH, White FA, Khanna R. (2012) A peptide uncoupling CRMP-2 from the presynaptic Ca(2+) channel complex demonstrates efficacy in animal models of migraine and aids therapy-induced neuropathy. Transl Neurosci 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. (2010) L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol 67:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock DM, Kelly KM, Macdonald RL. (1993) Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Res 16:89–98. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Braun M. (2013) Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75:155–179. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. (2004) Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 110:628–638. [DOI] [PubMed] [Google Scholar]
- Rossi AR, Angelo MF, Villarreal A, Lukin J, Ramos AJ. (2013) Gabapentin administration reduces reactive gliosis and neurodegeneration after pilocarpine-induced status epilepticus. PLoS One 8:e78516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet JB, Spaetgens RL, Burlingame T, Feng ZP, Zamponi GW. (1999) Modulation of neuronal voltage-gated calcium channels by farnesol. J Biol Chem 274: 25439–25446. [DOI] [PubMed] [Google Scholar]
- Ruff RL. (2000) Skeletal muscle sodium current is reduced in hypokalemic periodic paralysis. Proc Natl Acad Sci USA 97:9832–9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Velasco V, Puhl HL, Fuller BC, Sumner AD. (2005) Modulation of Ca2+ channels by opioid receptor-like 1 receptors natively expressed in rat stellate ganglion neurons innervating cardiac muscle. J Pharmacol Exp Ther 314:987–994. [DOI] [PubMed] [Google Scholar]
- Ruth P, Röhrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. (1989) Primary structure of the β subunit of the DHP-sensitive calcium channel from skeletal muscle. Science 245:1115–1118. [DOI] [PubMed] [Google Scholar]
- Ryder TR, Hu LY, Rafferty MF, Lotarski SM, Rock DM, Stoehr SJ, Taylor CP, Weber ML, Miljanich GP, Millerman E, et al. (2000) Structure-activity relationship at the leucine side chain in a series of N,N-dialkyldipeptidyl-amines as N-type calcium channel blockers. Drug Des Discov 16:317–322. [PubMed] [Google Scholar]
- Ryder TR, Hu LY, Rafferty MF, Millerman E, Szoke BG, Tarczy-Hornoch K. (1999) Multiple parallel synthesis of N,N-dialkyldipeptidylamines as N-type calcium channel blockers. Bioorg Med Chem Lett 9:1813–1818. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Murali SS, Lewis RJ, Alewood PF, Mohammadi S, Christie MJ, Catus Reverse Signs of Mouse Inflammatory Pain After Systemic Administration (2013) Novel ω-conotoxins from C. catus reverse signs of mouse inflammatory pain after systemic administration. Mol Pain 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Kazuno A, Matsuda Y, Nonaka T, Han W, Toriyama H, Tanabe T. (2001) Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J 20:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H, Matsuda Y, Tanabe T. (2002) Effects of ablation of N- and R-type Ca(2+) channels on pain transmission. Neurosci Res 43:1–7. [DOI] [PubMed] [Google Scholar]
- Saegusa H, Wakamori M, Matsuda Y, Wang J, Mori Y, Zong S, Tanabe T. (2007) Properties of human Cav2.1 channel with a spinocerebellar ataxia type 6 mutation expressed in Purkinje cells. Mol Cell Neurosci 34:261–270. [DOI] [PubMed] [Google Scholar]
- Saheki Y, Bargmann CI. (2009) Presynaptic CaV2 calcium channel traffic requires CALF-1 and the alpha(2)delta subunit UNC-36. Nat Neurosci 12:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Bichet D, Cornet V, Mori Y, Felix R, De Waard M. (2001) Distinct properties and differential beta subunit regulation of two C-terminal isoforms of the P/Q-type Ca(2+)-channel alpha(1A) subunit. Eur J Neurosci 14:987–997. [DOI] [PubMed] [Google Scholar]
- Santana F, Michelena P, Jaén R, García AG, Borges R. (1999) Calcium channel subtypes and exocytosis in chromaffin cells: a different view from the intact rat adrenal. Naunyn Schmiedebergs Arch Pharmacol 360:33–37. [DOI] [PubMed] [Google Scholar]
- Santi CM, Cayabyab FS, Sutton KG, McRory JE, Mezeyova J, Hamming KS, Parker D, Stea A, Snutch TP. (2002) Differential inhibition of T-type calcium channels by neuroleptics. J Neurosci 22:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Piacentini R, Masciullo M, Bianchi ML, Modoni A, Podda MV, Ricci E, Silvestri G, Grassi C. (2014) Alternative splicing alterations of Ca2+ handling genes are associated with Ca2+ signal dysregulation in myotonic dystrophy type 1 (DM1) and type 2 (DM2) myotubes. Neuropathol Appl Neurobiol 40:464–476. [DOI] [PubMed] [Google Scholar]
- Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, et al. (2011) Cav1.2 L-type Ca²⁺ channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1.3 channels. J Neurosci 31:13562–13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, Kaczorowski GJ, Garcia ML, Koltzenburg M, Priest BT. (2008) ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol 74:1476–1484. [DOI] [PubMed] [Google Scholar]
- Schneider T, Dibué M, Hescheler J. (2013) How “pharmacoresistant” is Cav2.3, the major component of voltage-gated R-type Ca2+ channels? Pharmaceuticals (Basel) 6:759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, et al. (2013) Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 45:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, et al. (2015) Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife 4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M, Vajna R, Pereverzev A, Tottene A, Klöckner U, Pietrobon D, Hescheler J, Schneider T. (1999) Isoforms of alpha1E voltage-gated calcium channels in rat cerebellar granule cells--detection of major calcium channel alpha1-transcripts by reverse transcription-polymerase chain reaction. Neuroscience 92:565–575. [DOI] [PubMed] [Google Scholar]
- Schroeder CI, Doering CJ, Zamponi GW, Lewis RJ. (2006) N-type calcium channel blockers: novel therapeutics for the treatment of pain. Med Chem 2:535–543. [DOI] [PubMed] [Google Scholar]
- Schroeder CI, Lewis RJ, Adams DJ. (2000) Block of voltage-gated calcium channels by peptide toxins, in Madame Curie Bioscience Database, Landes Bioscience, Austin, TX. [Google Scholar]
- Schuele SU, Kellinghaus C, Shook SJ, Boulis N, Bethoux FA, Loddenkemper T. (2005) Incidence of seizures in patients with multiple sclerosis treated with intrathecal baclofen. Neurology 64:1086–1087. [DOI] [PubMed] [Google Scholar]
- Schulla V, Renström E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, et al. (2003) Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J 22:3844–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitz H, Heurteaux C, Bois P, Moinier D, Romey G, Lazdunski M. (1994) Calcicludine, a venom peptide of the Kunitz-type protease inhibitor family, is a potent blocker of high-threshold Ca2+ channels with a high affinity for L-type channels in cerebellar granule neurons. Proc Natl Acad Sci USA 91:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. (2002) Actions of intrathecal omega-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur J Pharmacol 451:279–286. [DOI] [PubMed] [Google Scholar]
- Scott VES, Felix R, Arikkath J, Campbell KP. (1998) Evidence for a 95 kDa short form of the alpha1A subunit associated with the omega-conotoxin MVIIC receptor of the P/Q-type Ca2+ channels. J Neurosci 18:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. (1980) Brain dopamine receptors. Pharmacol Rev 32:229–313. [PubMed] [Google Scholar]
- Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kühbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. (2000) Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 275:39193–39199. [DOI] [PubMed] [Google Scholar]
- Serrano JR, Dashti SR, Perez-Reyes E, Jones SW. (2000) Mg(2+) block unmasks Ca(2+)/Ba(2+) selectivity of alpha1G T-type calcium channels. Biophys J 79:3052–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir W, Beyl S, Timin EN, Schellmann D, Erker T, Hohaus A, Hockerman GH, Hering S. (2011) Interaction of diltiazem with an intracellularly accessible binding site on Ca(V)1.2. Br J Pharmacol 162:1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao PP, Ye F, Chakravarty PK, Varughese DJ, Herrington JB, Dai G, Bugianesi RM, Haedo RJ, Swensen AM, Warren VA, et al. (2012) Aminopiperidine sulfonamide Cav2.2 channel inhibitors for the treatment of chronic pain. J Med Chem 55:9847–9855. [DOI] [PubMed] [Google Scholar]
- Shcheglovitov A, Vitko I, Bidaud I, Baumgart JP, Navarro-Gonzalez MF, Grayson TH, Lory P, Hill CE, Perez-Reyes E. (2008) Alternative splicing within the I-II loop controls surface expression of T-type Ca(v)3.1 calcium channels. FEBS Lett 582:3765–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Rettig J, Cook T, Catterall WA. (1996) Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature 379:451–454. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Rettig J, Takahashi M, Catterall WA. (1994) Identification of a syntaxin-binding site on N-type calcium channels. Neuron 13:1303–1313. [DOI] [PubMed] [Google Scholar]
- Sidach SS, Mintz IM. (2002) Kurtoxin, a gating modifier of neuronal high- and low-threshold ca channels. J Neurosci 22:2023–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RB. (2008) From basic science to blockbuster drug: the discovery of Lyrica. Angew Chem Int Ed Engl 47:3500–3504. [DOI] [PubMed] [Google Scholar]
- Simms BA, Souza IA, Zamponi GW. (2014) A novel calmodulin site in the Cav1.2 N-terminus regulates calcium-dependent inactivation. Pflugers Arch 466:1793–1803. [DOI] [PubMed] [Google Scholar]
- Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A. (2008) Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem 283:20733–20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Hamedinger D, Hoda JC, Gebhart M, Koschak A, Romanin C, Striessnig J. (2006) C-terminal modulator controls Ca2+-dependent gating of Ca(v)1.4 L-type Ca2+ channels. Nat Neurosci 9:1108–1116. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renström E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschütz R, et al. (2004) Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca 2+ channels. J Clin Invest 113:1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. (2009) Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol 75:407–414. [DOI] [PubMed] [Google Scholar]
- Slonimski M, Abram SE, Zuniga RE. (2004) Intrathecal baclofen in pain management. Reg Anesth Pain Med 29:269–276. [DOI] [PubMed] [Google Scholar]
- Smith MT, Cabot PJ, Ross FB, Robertson AD, Lewis RJ. (2002) The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain 96:119–127. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. (2007) Gating pore current in an inherited ion channelopathy. Nature 446:76–78. [DOI] [PubMed] [Google Scholar]
- Soong TW, DeMaria CD, Alvania RS, Zweifel LS, Liang MC, Mittman S, Agnew WS, Yue DT. (2002) Systematic identification of splice variants in human P/Q-type channel alpha1(2.1) subunits: implications for current density and Ca2+-dependent inactivation. J Neurosci 22:10142–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spafford JD, Munno DW, Van Nierop P, Feng ZP, Jarvis SE, Gallin WJ, Smit AB, Zamponi GW, Syed NI. (2003) Calcium channel structural determinants of synaptic transmission between identified invertebrate neurons. J Biol Chem 278:4258–4267. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. (2005) Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA 102:8089–8096, discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, et al. (2004) Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119:19–31. [DOI] [PubMed] [Google Scholar]
- Splawski I, Yoo DS, Stotz SC, Cherry A, Clapham DE, Keating MT. (2006) CACNA1H mutations in autism spectrum disorders. J Biol Chem 281:22085–22091. [DOI] [PubMed] [Google Scholar]
- Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR, et al. (2004) Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA 291:63–70. [DOI] [PubMed] [Google Scholar]
- Stacey BR, Barrett JA, Whalen E, Phillips KF, Rowbotham MC. (2008) Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain 9:1006–1017. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. (1998) Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology 37:83–91. [DOI] [PubMed] [Google Scholar]
- Stengel W, Jainz M, Andreas K. (1998) Different potencies of dihydropyridine derivatives in blocking T-type but not L-type Ca2+ channels in neuroblastoma-glioma hybrid cells. Eur J Pharmacol 342:339–345. [DOI] [PubMed] [Google Scholar]
- Stimpel M, Ivens K, Wambach G, Kaufmann W. (1988) Are calcium antagonists helpful in the management of primary aldosteronism? J Cardiovasc Pharmacol 12 (Suppl 6):S131–S134. [DOI] [PubMed] [Google Scholar]
- Stocker JW, Nadasdi L, Aldrich RW, Tsien RW. (1997) Preferential interaction of omega-conotoxins with inactivated N-type Ca2+ channels. J Neurosci 17:3002–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockner T, Koschak A. (2013) What can naturally occurring mutations tell us about Ca(v)1.x channel function? Biochim Biophys Acta 1828:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H. (1998) Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol Sci 19:108–115. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Koschak A. (2008) Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene knockout models. Channels (Austin) 2:233–251. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Pinggera A, Kaur G, Bock G, Tuluc P. (2014) L-type Ca(2+) channels in heart and brain. Wiley Interdiscip Rev Membr Transp Signal 3:15–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J, Zernig G, Glossmann H. (1985) Human red-blood-cell Ca2+-antagonist binding sites. Evidence for an unusual receptor coupled to the nucleoside transporter. Eur J Biochem 150:67–77. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Rüther K, Drescher B, et al. (1998) An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet 19:260–263. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Markin VS, Francis D, Cannon SC. (2008) Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis. J Gen Physiol 132:447–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TZ, Feng MR, Weber ML. (2005) Mediation of highly concentrative uptake of pregabalin by L-type amino acid transport in Chinese hamster ovary and Caco-2 cells. J Pharmacol Exp Ther 313:1406–1415. [DOI] [PubMed] [Google Scholar]
- Subasinghe NL, Wall MJ, Winters MP, Qin N, Lubin ML, Finley MF, Brandt MR, Neeper MP, Schneider CR, Colburn RW, et al. (2012) A novel series of pyrazolylpiperidine N-type calcium channel blockers. Bioorg Med Chem Lett 22:4080–4083. [DOI] [PubMed] [Google Scholar]
- Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, Geley S, Flucher BE. (2009) Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels (Austin) 3:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. (2005) Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol 15:370–378. [DOI] [PubMed] [Google Scholar]
- Suh BC, Kim DI, Falkenburger BH, Hille B. (2012) Membrane-localized β-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc Natl Acad Sci USA 109:3161–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA. (2011) The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid Redox Signal 14:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. (2002) Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol 135:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton KG, McRory JE, Guthrie H, Murphy TH, Snutch TP. (1999) P/Q-type calcium channels mediate the activity-dependent feedback of syntaxin-1A. Nature 401:800–804. [DOI] [PubMed] [Google Scholar]
- Swayne LA, Bourinet E. (2008) Voltage-gated calcium channels in chronic pain: emerging role of alternative splicing. Pflugers Arch 456:459–466. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Herrington J, Bugianesi RM, Dai G, Haedo RJ, Ratliff KS, Smith MM, Warren VA, Arneric SP, Eduljee C, et al. (2012) Characterization of the substituted N-triazole oxindole TROX-1, a small-molecule, state-dependent inhibitor of Ca(V)2 calcium channels. Mol Pharmacol 81:488–497. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Obermair GJ, Cooper CB, Zamponi GW, Flucher BE. (2006) Role of the synprint site in presynaptic targeting of the calcium channel CaV2.2 in hippocampal neurons. Eur J Neurosci 24:709–718. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R, Anderson D, Molineux ML, Mehaffey WH, Jayasuriya K, Turner RW. (2010) Rebound discharge in deep cerebellar nuclear neurons in vitro. Cerebellum 9:352–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadmouri A, Kiyonaka S, Barbado M, Rousset M, Fablet K, Sawamura S, Bahembera E, Pernet-Gallay K, Arnoult C, Miki T, et al. (2012) Cacnb4 directly couples electrical activity to gene expression, a process defective in juvenile epilepsy. EMBO J 31:3730–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadross MR, Ben Johny M, Yue DT. (2010) Molecular endpoints of Ca2+/calmodulin- and voltage-dependent inactivation of Ca(v)1.3 channels. J Gen Physiol 135:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara A. (2009) Cilnidipine: a new generation Ca channel blocker with inhibitory action on sympathetic neurotransmitter release. Cardiovasc Ther 27:124–139. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Seagar MJ, Jones JF, Reber BFX, Catterall WA. (1987) Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA 84:5478–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekura H, Bennett L, Tanabe T, Beam KG, Franzini-Armstrong C. (1994) Restoration of junctional tetrads in dysgenic myotubes by dihydropyridine receptor cDNA. Biophys J 67:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BZ, Jiang F, Tan MY, Yu D, Huang H, Shen Y, Soong TW. (2011) Functional characterization of alternative splicing in the C terminus of L-type CaV1.3 channels. J Biol Chem 286:42725–42735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GM, Yu D, Wang J, Soong TW. (2012) Alternative splicing at C terminus of Ca(V)1.4 calcium channel modulates calcium-dependent inactivation, activation potential, and current density. J Biol Chem 287:832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Beam KG, Powell JA, Numa S. (1988) Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 336:134–139. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. (1987) Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 328:313–318. [DOI] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. (1988) Amiloride selectively blocks the low threshold (T) calcium channel. Science 240:213–215. [DOI] [PubMed] [Google Scholar]
- Tang L, Gamal El-Din TM, Payandeh J, Martinez GQ, Heard TM, Scheuer T, Zheng N, Catterall WA. (2014) Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 505:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZZ, Yarotskyy V, Wei L, Sobczak K, Nakamori M, Eichinger K, Moxley RT, Dirksen RT, Thornton CA. (2012) Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of Ca(V)1.1 calcium channel. Hum Mol Genet 21:1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP, Angelotti T, Fauman E. (2007) Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 73:137–150. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Garrido R. (2008) Immunostaining of rat brain, spinal cord, sensory neurons and skeletal muscle for calcium channel alpha2-delta (alpha2-delta) type 1 protein. Neuroscience 155:510–521. [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. (2006) Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev 58:837–862. [DOI] [PubMed] [Google Scholar]
- Templin C, Ghadri JR, Rougier JS, Baumer A, Kaplan V, Albesa M, Sticht H, Rauch A, Puleo C, Hu D, et al. (2011) Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur Heart J 32:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrence CF, Fromm GH, Tenicela R. (1985) Baclofen as an analgesic in chronic peripheral nerve disease. Eur Neurol 24:380–385. [DOI] [PubMed] [Google Scholar]
- Thaler C, Gray AC, Lipscombe D. (2004) Cumulative inactivation of N-type CaV2.2 calcium channels modified by alternative splicing. Proc Natl Acad Sci USA 101:5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JC, Dunbar E, Laye RR. (2006) Treatment challenges and complications with ziconotide monotherapy in established pump patients. Pain Physician 9:147–152. [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. (2009) Structural model for dihydropyridine binding to L-type calcium channels. J Biol Chem 284:19006–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Lingle CJ. (1998) Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol 79:240–252. [DOI] [PubMed] [Google Scholar]
- Tom Dieck S. (2013) Keeping the balance. Channels (Austin) 7:418–419. [DOI] [PubMed] [Google Scholar]
- Toselli M, Tosetti P, Taglietti V. (1999) Kinetic study of N-type calcium current modulation by delta-opioid receptor activation in the mammalian cell line NG108-15. Biophys J 76:2560–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, van den Maagdenberg AM, Ferrari MD, Pietrobon D. (2009) Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 61:762–773. [DOI] [PubMed] [Google Scholar]
- Tottene A, Moretti A, Pietrobon D. (1996) Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci 16:6353–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. (2000) alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci 20:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traboulsie A, Chemin J, Chevalier M, Quignard JF, Nargeot J, Lory P. (2007) Subunit-specific modulation of T-type calcium channels by zinc. J Physiol 578:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Van-Minh A, Dolphin AC. (2010) The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci 30:12856–12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor LM, Thiessen CN, Traynor AP. (2011) Pharmacotherapy of fibromyalgia. Am J Health Syst Pharm 68:1307–1319. [DOI] [PubMed] [Google Scholar]
- Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, Eduljee C, Jiang X, Smith P, Morrison JL, et al. (2012) T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med 4:121ra19. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. (1986) Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol 18:691–710. [DOI] [PubMed] [Google Scholar]
- Tuluc P, Kern G, Obermair GJ, Flucher BE. (2007) Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc Natl Acad Sci USA 104:11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. (2009) A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J 96:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RW, Zamponi GW. (2014) T-type channels buddy up. Pflugers Arch 466:661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat J, Pauwels PJ, Vereecke J, Carmeliet E. (1991) Flunarizine inhibits a high-threshold inactivating calcium channel (N-type) in isolated hippocampal neurons. Brain Res 549:112–117. [DOI] [PubMed] [Google Scholar]
- Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, et al. (2009) Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest 119:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Huguenard JR. (1997) GABA(A)-receptor-mediated rebound burst firing and burst shunting in thalamus. J Neurophysiol 78:1748–1751. [DOI] [PubMed] [Google Scholar]
- Uneyama H, Takahara A, Dohmoto H, Yoshimoto R, Inoue K, Akaike N. (1997) Blockade of N-type Ca2+ current by cilnidipine (FRC-8653) in acutely dissociated rat sympathetic neurones. Br J Pharmacol 122:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Piedras-Rentería ES, Jun K, Shin HS, Uchitel OD, Tsien RW. (2003) Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci USA 100:3491–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, et al. (2015) Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18:145–153. [DOI] [PubMed] [Google Scholar]
- Vajna R, Schramm M, Pereverzev A, Arnhold S, Grabsch H, Klöckner U, Perez-Reyes E, Hescheler J, Schneider T. (1998) New isoform of the neuronal Ca2+ channel alpha1E subunit in islets of Langerhans and kidney--distribution of voltage-gated Ca2+ channel alpha1 subunits in cell lines and tissues. Eur J Biochem 257:274–285. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, et al. (2004) A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 41:701–710. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, Barrett CF, Gherardini L, van de Ven RC, Todorov B, et al. (2010) High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol 67:85–98. [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr (2004) Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature 429:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandael DH, Zuccotti A, Striessnig J, Carbone E. (2012) Ca(V)1.3-driven SK channel activation regulates pacemaking and spike frequency adaptation in mouse chromaffin cells. J Neurosci 32:16345–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian MG, Radulovic LL, Kinsora JJ, Serpa KA, Vergnes M, Bertram E, Taylor CP. (2006) Activity profile of pregabalin in rodent models of epilepsy and ataxia. Epilepsy Res 68:189–205. [DOI] [PubMed] [Google Scholar]
- Ver Donck A, Collins R, Rauck RL, Nitescu P. (2008) An open-label, multicenter study of the safety and efficacy of intrathecal ziconotide for severe chronic pain when delivered via an external pump. Neuromodulation 11:103–111. [DOI] [PubMed] [Google Scholar]
- Vergult S, Dheedene A, Meurs A, Faes F, Isidor B, Janssens S, Gautier A, Le Caignec C, Menten B. (2015) Genomic aberrations of the CACNA2D1 gene in three patients with epilepsy and intellectual disability. Eur J Hum Genet 23:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali S, Leiss V, Karl R, Hofmann F, Welling A. (2006) Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A- and B-cells. J Physiol 572:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitko I, Bidaud I, Arias JM, Mezghrani A, Lory P, Perez-Reyes E. (2007) The I-II loop controls plasma membrane expression and gating of Ca(v)3.2 T-type Ca2+ channels: a paradigm for childhood absence epilepsy mutations. J Neurosci 27:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitko I, Chen Y, Arias JM, Shen Y, Wu XR, Perez-Reyes E. (2005) Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci 25:4844–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. (1996) Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J Neurosci 16:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waithe D, Ferron L, Page KM, Chaggar K, Dolphin AC. (2011) Beta-subunits promote the expression of Ca(V)2.2 channels by reducing their proteasomal degradation. J Biol Chem 286:9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M, Mikala G, Mori Y. (1999) Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J Physiol 517:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MS, Charapata SG, Fisher R, Byas-Smith M, Staats PS, Mayo M, McGuire D, Ellis D, Ziconotide Nonmalignant Pain Study 96-002 Group (2006) Intrathecal ziconotide in the treatment of chronic nonmalignant pain: a randomized, double-blind, placebo-controlled clinical trial. Neuromodulation 9:75–86. [DOI] [PubMed] [Google Scholar]
- Walsh CP, Davies A, Butcher AJ, Dolphin AC, Kitmitto A. (2009) Three-dimensional structure of CaV3.1: comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J Biol Chem 284:22310–22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Mannhardt S, Nescholta S, Maser-Gluth C, Bartsch D. (2008) Selective and protracted effect of nifedipine on fear memory extinction correlates with induced stress response. Learn Mem 15:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. (2002) Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience 114:529–546. [DOI] [PubMed] [Google Scholar]
- Wang M, Offord J, Oxender DL, Su TZ. (1999) Structural requirement of the calcium-channel subunit alpha2δ for gabapentin binding. Biochem J 342:313–320. [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Pettus M, Gao D, Phillips C, Scott Bowersox S. (2000) Effects of intrathecal administration of ziconotide, a selective neuronal N-type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain 84:151–158. [DOI] [PubMed] [Google Scholar]
- Wankerl K, Weise D, Gentner R, Rumpf JJ, Classen J. (2010) L-type voltage-gated Ca2+ channels: a single molecular switch for long-term potentiation/long-term depression-like plasticity and activity-dependent metaplasticity in humans. J Neurosci 30:6197–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wappl E, Koschak A, Poteser M, Sinnegger MJ, Walter D, Eberhart A, Groschner K, Glossmann H, Kraus RL, Grabner M, et al. (2002) Functional consequences of P/Q-type Ca2+ channel Cav2.1 missense mutations associated with episodic ataxia type 2 and progressive ataxia. J Biol Chem 277:6960–6966. [DOI] [PubMed] [Google Scholar]
- Watanabe TX, Itahara Y, Kuroda H, Chen YN, Kimura T, Sakakibara S. (1995) Smooth muscle relaxing and hypotensive activities of synthetic calciseptine and the homologous snake venom peptide FS2. Jpn J Pharmacol 68:305–313. [DOI] [PubMed] [Google Scholar]
- Watase K, Barrett CF, Miyazaki T, Ishiguro T, Ishikawa K, Hu Y, Unno T, Sun Y, Kasai S, Watanabe M, et al. (2008) Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci USA 105:11987–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Zamponi GW. (2014) Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 17:153–163. [DOI] [PubMed] [Google Scholar]
- Weiergräber M, Henry M, Radhakrishnan K, Hescheler J, Schneider T. (2007) Hippocampal seizure resistance and reduced neuronal excitotoxicity in mice lacking the Cav2.3 E/R-type voltage-gated calcium channel. J Neurophysiol 97:3660–3669. [DOI] [PubMed] [Google Scholar]
- Weinsberg F, Bickmeyer U, Wiegand H. (1994) Effects of tetrandrine on calcium channel currents of bovine chromaffin cells. Neuropharmacology 33:885–890. [DOI] [PubMed] [Google Scholar]
- Weiss N, Black SA, Bladen C, Chen L, Zamponi GW. (2013) Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch 465:1159–1170. [DOI] [PubMed] [Google Scholar]
- Weiss N, Hameed S, Fernández-Fernández JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, et al. (2012) A Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem 287:2810–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling A, Kwan YW, Bosse E, Flockerzi V, Hofmann F, Kass RS. (1993) Subunit-dependent modulation of recombinant L-type calcium channels. Molecular basis for dihydropyridine tissue selectivity. Circ Res 73:974–980. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ. (2003) A mechanism for the direct regulation of T-type calcium channels by Ca2+/calmodulin-dependent kinase II. J Neurosci 23:10116–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty DF, Schielke GP, Vartanian MG, Taylor CP. (1993) Gabapentin anticonvulsant action in rats: disequilibrium with peak drug concentrations in plasma and brain microdialysate. Epilepsy Res 16:175–181. [DOI] [PubMed] [Google Scholar]
- Wemhöner K, Friedrich C, Stallmeyer B, Coffey AJ, Grace A, Zumhagen S, Seebohm G, Ortiz-Bonnin B, Rinné S, Sachse FB, et al. (2015) Gain-of-function mutations in the calcium channel CACNA1C (Cav1.2) cause non-syndromic long-QT but not Timothy syndrome. J Mol Cell Cardiol 80:186–195. [DOI] [PubMed] [Google Scholar]
- Wensel TM, Powe KW, Cates ME. (2012) Pregabalin for the treatment of generalized anxiety disorder. Ann Pharmacother 46:424–429. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. (1992) Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron 9:1099–1115. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TVB, Snutch TP, Catterall WA. (1995) Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci 15:6403–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. (1994) Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science 264:107–111. [DOI] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. (2008) CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol 183:849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. (2012) Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell 149:1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. (2008) Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem 15:1–5. [DOI] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO. (2002) Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell 13:3369–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice AS, Lunn MP, Hamunen K, Haanpaa M, Kalso EA. (2013) Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev 11:CD010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MF, Barnes S. (1996) The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol 107:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. (1992) Structure and functional expression of alpha 1, alpha 2, and β subunits of a novel human neuronal calcium channel subtype. Neuron 8:71–84. [DOI] [PubMed] [Google Scholar]
- Willow M, Gonoi T, Catterall WA. (1985) Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol 27:549–558. [PubMed] [Google Scholar]
- Wilson SM, Schmutzler BS, Brittain JM, Dustrude ET, Ripsch MS, Pellman JJ, Yeum TS, Hurley JH, Hingtgen CM, White FA, et al. (2012) Inhibition of transmitter release and attenuation of anti-retroviral-associated and tibial nerve injury-related painful peripheral neuropathy by novel synthetic Ca2+ channel peptides. J Biol Chem 287:35065–35077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher DR, De Waard M, Sakamoto J, Franzini-Armstrong C, Pragnell M, Kahl SD, Campbell KP. (1993) Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science 261:486–489. [DOI] [PubMed] [Google Scholar]
- Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N. (2003) Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J Mol Biol 332:171–182. [DOI] [PubMed] [Google Scholar]
- Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. (2003) T-type calcium channel regulation by specific G-protein betagamma subunits. Nature 424:209–213. [DOI] [PubMed] [Google Scholar]
- Wong FK, Li Q, Stanley EF. (2013) Synaptic vesicle capture by CaV2.2 calcium channels. Front Cell Neurosci 7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FK, Nath AR, Chen RH, Gardezi SR, Li Q, Stanley EF. (2014) Synaptic vesicle tethering and the CaV2.2 distal C-terminal. Front Cell Neurosci 8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woppmann A, Ramachandran J, Miljanich GP. (1994) Calcium channel subtypes in rat brain: biochemical characterization of the high-affinity receptors for omega-conopeptides SNX-230 (synthetic MVIIC), SNX-183 (SVIB), and SNX-111 (MVIIA). Mol Cell Neurosci 5:350–357. [DOI] [PubMed] [Google Scholar]
- Wu F, Mi W, Burns DK, Fu Y, Gray HF, Struyk AF, Cannon SC. (2011) A sodium channel knockin mutant (NaV1.4-R669H) mouse model of hypokalemic periodic paralysis. J Clin Invest 121:4082–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Mi W, Hernández-Ochoa EO, Burns DK, Fu Y, Gray HF, Struyk AF, Schneider MF, Cannon SC. (2012) A calcium channel mutant mouse model of hypokalemic periodic paralysis. J Clin Invest 122:4580–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. (2006a) Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci 47:3523–3530. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J, Wissinger B, Zrenner E, Wilke R, Kohl S, et al. (2006b) Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am J Hum Genet 79:973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes RC, Bauer CS, Khan SU, Weiss JL, Seward EP. (2007) Differential regulation of endogenous N- and P/Q-type Ca2+ channel inactivation by Ca2+/calmodulin impacts on their ability to support exocytosis in chromaffin cells. J Neurosci 27:5236–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. (2007) Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience 144:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. (2001) Neuronal Ca(V)1.3α(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21:5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA, Yue DT. (2006) Switching of Ca2+-dependent inactivation of Ca(v)1.3 channels by calcium binding proteins of auditory hair cells. J Neurosci 26:10677–10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, He LL, Chen M, Fang K, Colecraft HM. (2013) Bio-inspired voltage-dependent calcium channel blockers. Nat Commun 4:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Davies LA, Howard JD, Adney SK, Welsby PJ, Howell N, Carey RM, Colbran RJ, Barrett PQ. (2006) Molecular basis for the modulation of native T-type Ca2+ channels in vivo by Ca2+/calmodulin-dependent protein kinase II. J Clin Invest 116:2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarotskyy V, Dirksen RT. (2013) Cav1.1 in malignant hyperthermia, in Pathologies of Calcium Channels (Weiss N, Koschak A, eds) pp 151–165, Springer Science & Business Media, Berlin. [Google Scholar]
- Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. (2004) Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci 20:1–13. [DOI] [PubMed] [Google Scholar]
- Ye Q, Yan LY, Xue LJ, Wang Q, Zhou ZK, Xiao H, Wan Q. (2011) Flunarizine blocks voltage-gated Na(+) and Ca(2+) currents in cultured rat cortical neurons: A possible locus of action in the prevention of migraine. Neurosci Lett 487:394–399. [DOI] [PubMed] [Google Scholar]
- Yeon KY, Sim MY, Choi SY, Lee SJ, Park K, Kim JS, Lee JH, Lee KM, Oh SB. (2004) Molecular mechanisms underlying calcium current modulation by nociceptin. Neuroreport 15:2205–2209. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, Ongur D, McPhie D, Cohen B, Perlis R, et al. (2015) Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry 20:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Altier C, Zamponi GW. (2010) CCR2 receptor ligands inhibit Cav3.2 T-type calcium channels. Mol Pharmacol 77:211–217. [DOI] [PubMed] [Google Scholar]
- You H, Gadotti VM, Petrov RR, Zamponi GW, Diaz P. (2011) Functional characterization and analgesic effects of mixed cannabinoid receptor/T-type channel ligands. Mol Pain 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K, Lin S, Sun L, Lee E, Modi M, Hellings S, Husbands M, Ozenberger B, Franco R. (1998) Identification of a calcium channel modulator using a high throughput yeast two-hybrid screen. Nat Biotechnol 16:946–950. [DOI] [PubMed] [Google Scholar]
- Yurtsever Z, Sala-Rabanal M, Randolph DT, Scheaffer SM, Roswit WT, Alevy YG, Patel AC, Heier RF, Romero AG, Nichols CG, et al. (2012) Self-cleavage of human CLCA1 protein by a novel internal metalloprotease domain controls calcium-activated chloride channel activation. J Biol Chem 287:42138–42149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccara G, Perucca P, Gangemi PF. (2012) The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol 68:903–912. [DOI] [PubMed] [Google Scholar]
- Zamponi GW. (2003) Regulation of presynaptic calcium channels by synaptic proteins. J Pharmacol Sci 92:79–83. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Doyle DD, French RJ. (1993) State-dependent block underlies the tissue specificity of lidocaine action on batrachotoxin-activated cardiac sodium channels. Biophys J 65:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Feng ZP, Zhang L, Pajouhesh H, Ding Y, Belardetti F, Pajouhesh H, Dolphin D, Mitscher LA, Snutch TP. (2009) Scaffold-based design and synthesis of potent N-type calcium channel blockers. Bioorg Med Chem Lett 19:6467–6472. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Soong TW, Bourinet E, Snutch TP. (1996) β subunit coexpression and the alpha1 subunit domain I-II linker affect piperidine block of neuronal calcium channels. J Neurosci 16:2430–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Jiang D, Zhang D, Qian Z, Liu C, Tao J. (2012) Inhibition of T-type Ca²⁺ channels by endostatin attenuates human glioblastoma cell proliferation and migration. Br J Pharmacol 166:1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. (2002) Functional roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res 90:981–987. [DOI] [PubMed] [Google Scholar]
- Zheng W, Rampe D, Triggle DJ. (1991) Pharmacological, radioligand binding, and electrophysiological characteristics of FPL 64176, a novel nondihydropyridine Ca2+ channel activator, in cardiac and vascular preparations. Mol Pharmacol 40:734–741. [PubMed] [Google Scholar]
- Zhong X, Liu JR, Kyle JW, Hanck DA, Agnew WS. (2006) A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Genet 15:1497–1512. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zhang SM, Wang QL, Wu Q, Chen M, Pei JM. (2013) Anti-arrhythmic effect of verapamil is accompanied by preservation of cx43 protein in rat heart. PLoS One 8:e71567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HL, Wassall RD, Cunnane TC, Teramoto N. (2009) Actions of kurtoxin on tetrodotoxin-sensitive voltage-gated Na+ currents, NaV1.6, in murine vas deferens myocytes. Naunyn Schmiedebergs Arch Pharmacol 379:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Duan WR, An G, Thomas JW, Nothaft W. (2015) A randomized double-blind, placebo- and active-controlled study of T-type calcium channel blocker ABT-639 in diabetic patients with peripheral neuropathic pain. Pain DOI: 10.1097/j.pain.0000000000000263 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]