Abstract
A population-based case-control was performed to explore familial aggregation of esophageal squamous cell carcinoma (ESCC). Family history of cancer was assessed by a structured questionnaire, and from which 2 cohorts of relatives of cases and controls were reconstructed. Unconditional logistic regression and Cox proportional hazards regression were applied for case-control design and reconstructed cohort design, respectively. We observed a close to doubled risk of ESCC associated with a positive family history of esophageal cancer among first degree relatives (odds ratio [OR] = 1.85, 95% confidence interval [CI]: 1.42–2.41), after adjusting age, sex, family size and other confounders. The excess risks of ESCC increased with the increasing of first-degree relatives affected by esophageal cancer (p < 0.001). In particular, those individuals whose both parents with esophageal cancer had an 8-fold excess risk of ESCC (95% CI: 1.74–36.32). The reconstructed cohort analysis showed that the cumulative risk of esophageal cancer to age 75 was 12.2% in the first-degree relatives of cases and 7.0% in those of controls (hazard ratio = 1.91, 95% CI: 1.54–2.37). Our results suggest family history of esophageal cancer significantly increases the risk for ESCC. Future studies are needed to understand how the shared genetic susceptibility and/or environmental exposures contribute to the observed excess risk.
The data of GLOBOCAN 2012 have shown a great international variation of esophageal cancer incidence, and China is one of areas with the highest esophageal cancer incidence rates in the world1. Esophageal cancer has two major histopathological subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. The highest incidence rates of esophageal adenocarcinoma are found in the Western countries, whereas ESCC is the predominant subtype in China, in which more than 90% are of this subtype2,3. The striking geographic variation implies that environmental factors might be more important in the development of esophageal cancer. However, genetic factors may also contribute to the susceptibility to esophageal cancer, as suggested by familial aggregation4,5,6,7,8,9 and segregation studies10,11. However, inconsistent results from previous studies have been reported. In addition, according to different study designs, there exist different methods for measuring family history of cancer. In a case-control design, the outcomes are case and control subjects, and the exposure is the disease status in their relatives. In a reconstructed cohort design, the cumulative risks of disease are evaluated for the reconstructed cohorts of relatives of case and control subjects12. To date, exploration for the familial risk of esophageal cancer predominantly uses the case-control design, but rarely the reconstructed cohort design.
We thus conducted a population-based case-control study in Taixing of China, a high-incidence area of ESCC, aiming to explore the role of family history of cancer in ESCC occurrence, using two different approaches, i.e. case-control design and reconstructed cohort design.
Materials and Methods
Subject recruitment and data collection
We conducted a population-based case-control study on the etiology of esophageal cancer in Taixing of Jiangsu Province from October 2010 to March of 2012. We recruited cases mainly from endoscopy units at the four largest hospitals of Taixing (the People’s Hospital of Taixing, the Second People’s Hospital of Taixing, the Third People’s Hospital of Taixing and the Hospital of Traditional Chinese Medicine of Taixing). More than 90% of the patients in this area are referred to these hospitals and were invited to participate. We also supplemented case recruitment by additional linkage to the local Cancer Registry. During the same time period, we enrolled population controls, which were frequency matched to the cases of ESCC on sex and age (in 5-year groups) and were randomly selected from the Taixing Population Registry. All subjects enrolled in the study were local inhabitants ages 40–85 who have lived in Taixing for at least 5 years prior to diagnosis date for cases or interview date for controls.
From October of 2010 to March of 2012, we found 777 suspected cases from the hospitals, among which 752 were recruited. In addition, by linkage with the local Cancer Registry, 485 cases were further identified among whom 226 died before contact, and 101 refused or were too ill to participate, leaving 158 being recruited into the study. For each case, we also tried to collect sections from formalin-fixed and paraffin-embedded tissue blocks. All sections were stained by H.E. method and reviewed by one study pathologist. Finally we enrolled a total of 775 esophageal cancer cases into the study, and we estimated that about 81% incident cases in the study base were included according to estimated number from local Cancer Registry. Among enrolled cases, 718 (93%) were confirmed histopathologically by the study pathologist, including 648 cases of ESCC, 63 cases of esophageal adenocarcinoma, and 7 cases of other types of esophageal cancer (Supplemental Fig. 1). In total 1311 population-based controls were randomly selected, among whom 221 were excluded due to death before contact, outmigration or unfound, leaving 1090 eligible subjects. Finally, 779 controls were recruited to current study (participation rate: 71.0%).
Trained staff interviewed study subjects face-to-face using a structured questionnaire, which covers information on demographic characteristics, lifestyles and family history of cancer. The staff inquired thoroughly about how many brothers, sisters and children they had, and whether their parents, siblings or children had ever been afflicted with any cancer. For those relatives without a history of cancer, we collected their vital status at the time of interview, including current age or age at death. For those relatives with a history of cancer, we further gathered the information about site of the tumor and age at cancer diagnosis.
Statistical analysis
We used unconditional logistic regression models to estimate the odds ratios (ORs) with 95% confidence intervals (CIs) of ESCC associated with a family history of cancers of the esophagus, stomach, liver, colorectum, pancreas, and all sites combined. We defined a positive family history of cancer as having at least one first-degree relative affected with cancer. We separately assessed the associations of cancers in parents and siblings with ESCC risk. We also estimated whether cases and controls differed with the number of first-degree relatives with cancer. P value for trend was derived from Wald test by entering regression models the number of first-degree relatives with cancer as a continuous variable. In analysis of any cancers, unexposed subjects were those without a positive family history of any cancer. In analysis of digestive cancers, unexposed subjects were those without a positive family history of these cancers. In multivariate analysis, we adjusted for age (continuous), sex, family size (continuous), education (illiteracy/primary school/primary high school/secondary high school and above), tobacco smoking (never/ever smoker of any tobacco), alcohol drinking (never/ever), missing & filled teeth (MFT, none/1 ~ 4/ ≥ 4), times of tooth brushing per day (<2 times/ ≥ 2 times), daily consumption of pickled vegetables (<10 g/ ≥ 10 g) and daily consumption of fresh fruits (<27.5 g/ ≥ 27.5 g).
Based on the kin-cohort data, two cohorts were reconstructed, containing the first-degree relatives of cases and those of controls (171 first-degree relatives were excluded due to missing information of age). Study subjects were followed from birth until the occurrence of esophageal cancer, death, age of 85, or the date of the study interview, whichever occurred first. The exposed cohort included first-degree relatives of cases, and the unexposed cohort those relatives of controls. Cumulative risks of esophageal cancer over time among 2 kin-cohorts were calculated and plotted using Kaplan-Meier method. The relative risk (in term of hazard ratio) of esophageal cancer for the relatives of cases compared to those of controls was derived from Cox proportional hazards regression, which included exposure status (case/control) and sex as covariates, and relative-type (parent/sibling/offspring) as stratification variable. The proportional hazards assumption was checked for each covariate in the Cox model by the method of cumulative sums of Martingale-based residuals13, and found not violated. To avoid the influence of familial aggregation, a method proposed by Lee was used to account for the intracluster dependence14.
All statistical analyses were carried out with SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina).
Ethics
This study was approved by the Institutional Review Board of School of Life Sciences, Fudan University and the Institutional Review Board of Qilu Hospital, Shandong University. This study was carried out in accordance with the approved guidelines, and all participants provided written informed consent.
Results
Demographics
Current study was based on 648 ESCC cases which were independently reviewed and confirmed by the study pathologist and 779 frequency-matched controls. After deleting 36 records (29 cases and 7 controls) with incomplete questionnaire information on family history of cancer, 619 cases and 772 controls were finally include in the analysis.
Table 1 presents selected characteristics and distribution of potential risk factors among cases and controls. Controls were a bit older than cases, while there was no difference concerning the distribution of sex and education. Compared with controls, cases consumed alcohol more often but brushed teeth less often, while there were no differences concerning smoking, number of missing and filled teeth, and consumption of pickled vegetables and fresh fruits. These factors were considered as potential confounders as well as important risk factors reported in previous literature, and were thus included in the regression model for adjustment in subsequent analyses.
Table 1. Demographic information of the study subjects enrolled in a case-control study on esophageal squamous cell carcinoma, Taixing, China.
| Variables | Cases (n = 619) | Controls (n = 772) | Pvalue* |
|---|---|---|---|
| Age (Mean ± SD, years) | 65.9 ± 8.7 | 67.9 ± 7.9 | <0.001 |
| Sex (%) | |||
| Men | 431 (70) | 548 (71) | 0.582 |
| Women | 188(30) | 224 (29) | |
| Education (%) | |||
| Illiteracy | 203 (33) | 238 (31) | 0.714 |
| Primary school | 242 (39) | 296 (38) | |
| Primary high school | 128 (21) | 173 (22) | |
| Secondary high school and above | 46 (7) | 65 (8) | |
| Alcohol drinking (%) | <0.001 | ||
| Never | 263 (43) | 424 (55) | |
| Ever | 355 (57) | 347 (45) | |
| Missing | 1 | 1 | |
| Smoking (%) | 0.538 | ||
| Never | 252 (41) | 332(43) | |
| Ever | 366(59) | 439 (57) | |
| Missing | 1 | 1 | |
| MFT† | 0.609 | ||
| None | 113 (18) | 154 (20) | |
| 1 ~ 4 | 153 (25) | 177 (23) | |
| ≥4 | 344 (56) | 434 (56) | |
| Missing | 9 (1) | 7 (1) | |
| Times of tooth brushing per day | <0.001 | ||
| <2 | 489 (79) | 512 (66) | |
| ≥2 | 127 (21) | 254 (33) | |
| Missing | 3 | 6 (1) | |
| Average daily consumption of pickled vegetables | 0.483 | ||
| <10 | 243 (39) | 292 (38) | |
| ≥10 g | 350 (57) | 455 (59) | |
| Missing | 26 (4) | 25 (3) | |
| Average daily consumption of fresh fruits | 0.698 | ||
| <27.5 g | 290 (47) | 371 (48) | |
| ≥27.5 g | 280 (45) | 374 (48) | |
| Missing | 49 (8) | 27 (4) | |
*P values were based on Wilcoxon rank-sum test for continuous variables, and chi-squared test for categorical variables (two-sided).
†MFT referred to sum of missing and filled teeth.
Family size distribution
Table 2 shows family size, age and sex distributions among siblings and offspring for the case and control groups, respectively. Overall, cases and controls had similar numbers of first-degree relatives (median 8 vs. 8). The median number of siblings and sex distribution were similar between case and control groups, but siblings of control group were on average older than those of case group. For offspring, control group tended to have more offspring than case group, whereas sex distribution and mean age distribution among offspring were similar between the two groups.
Table 2. Distribution of family size, number of siblings and number of offspring in case and control subjects.
| Cases(n = 619) | Controls(n = 772) | Pvalue* | |
|---|---|---|---|
| Family size, median (range)† | 8 (2–16) | 8 (2–15) | 0.864 |
| Siblings | |||
| No. of siblings, Median (range) | 3 (0–12) | 3 (0–10) | 0.093 |
| No. of brothers (%) | 1085(52%) | 1298(52%) | 0.838 § |
| Mean age of brothers‡ | 60.3 | 61.8 | 0.008 |
| No. of sisters (%) | 1017 (48%) | 1202(48%) | |
| Mean age of sisters ‡ | 61.5 | 63.5 | 0.001 |
| Offspring | |||
| No. of offspring, Median (range) | 2 (0–7) | 3 (0-8) | 0.018 |
| No. of sons (%) | 819(53%) | 1115(54%) | 0.398 § |
| Mean age of sons‡ | 41.2 | 42.0 | 0.063 |
| No. of daughters (%) | 734(47%) | 944(46%) | |
| Mean age of daughters‡ | 41.4 | 41.9 | 0.189 |
*P values were based on Wilcoxon rank-sum test for continuous variables, and chi-squared test for categorical variables (two-sided).
†Family size count excluded the index persons.
‡Age at the date of interview of their index persons. For those who died before the interview, age at death was used.
§P values were based on Chi-squared test comparing the difference between sexes (brothers vs sisters, or sons vs daughters).
Family history of cancer and risk of ESCC
The first-degree relatives of the cases were more often reported to have been affected by esophageal cancer than those relatives of the controls (34.7% vs. 21.9%), which renders an OR of 1.85(95% CI: 1.42–2.41) (Table 3). The results were consistent when the analyses were limited to parents (OR = 1.63) or siblings (OR = 2.04), separately. The excess risks were evident when the analyses were stratified by type of relatives. The excess risks of ESCC increased monotonically with the increasing number of first-degree relatives reportedly afflicted with esophageal cancer (p for trend< 0.001). In particular, the individuals whose both parents were diagnosed with esophageal cancer had an 8-fold excess risk of ESCC, compared with those without any parents affected by esophageal cancer (adjusted OR = 7.96, 95% CI: 1.74-36.32). However, increasing number of affected siblings did not seem to further increase the relative risks (Table 3). Table 4 shows the associations for family history of cancer, either overall or at selected specific sites, in relation to ESCC risk. Overall 55.6% of cases and 46.6% of controls had a positive family history of any cancer. Excess ESCC risks were associated with a positive family history of any cancer (adjusted OR = 1.43, 95% CI: 1.13–1.81) or digestive tract cancer (adjusted OR = 1.55, 95% CI: 1.23–1.96). Among specific sites, however, although a family history of stomach cancer, pancreas cancer and colorectal cancer tended to increase the risk of ESCC, none of the estimates was statistically significant. The results were similar when family history was examined among parents or siblings separately. However, no association was found when the analysis was limited to offspring, although the number of affected offspring was very small.
Table 3. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for esophageal squamous cell carcinoma according to family history of esophageal cancer in first-degree relatives*.
| Family history of esophageal cancer in relatives | Cases (n = 619) | Controls (n = 772) | OR (95% CI)† | OR (95% CI)‡ |
|---|---|---|---|---|
| First degree relatives | ||||
| No | 367 | 545 | Reference | Reference |
| Yes | 195 | 153 | 1.91 (1.49–2.46) | 1.85 (1.42–2.41) |
| 1 affected | 150 | 132 | 1.70 (1.29–2.22) | 1.68 (1.27–2.23) |
| ≥2 affected | 45 | 21 | 3.28 (1.92–5.62) | 2.93 (1.67–5.12) |
| P for trend | <0.001 | <0.001 | ||
| Parents | ||||
| No | 439 | 614 | Reference | Reference |
| Yes | 129 | 102 | 1.68 (1.26–2.24) | 1.63 (1.20–2.20) |
| 1 affected of parents | 116 | 100 | 1.53 (1.14–2.06) | 1.49 (1.09–2.03) |
| 2 affected of parents | 13 | 2 | 9.11 (2.04–40.72) | 7.96 (1.74–36.32) |
| P for trend | <0.001 | <0.001 | ||
| Father | 60 | 50 | 1.58 (1.06–2.36) | 1.46 (0.97–2.21) |
| Mother | 82 | 54 | 2.04 (1.41–2.94) | 2.02 (1.39–2.95) |
| Siblings | ||||
| No | 483 | 635 | Reference | Reference |
| Yes | 92 | 64 | 2.09 (1.48–2.95) | 2.04 (1.41–2.94) |
| 1 affected of siblings | 82 | 57 | 2.07 (1.44–2.97) | 2.04 (1.39–2.99) |
| ≥2 affected of siblings | 10 | 7 | 2.30 (0.86–6.17) | 2.04 (0.73–5.69) |
| P for trend | 0.03 | 0.02 | ||
*Occurrence of esophageal cancer was very rare in offspring, so the result was not shown.
†Adjusted for age (continuous) and sex.
‡Adjusted for age (continuous), family size (continuous), sex, education (Illiteracy/primary school/primary high school/secondary high school and above), tobacco smoking (never/ever smoker of any tobacco), alcohol drinking (never/ever), missing & filled teeth (MFT, none/1 ~ 4/ ≥ 4), times of tooth brushing per day (<2 times/ ≥ 2 times), daily consumption of pickled vegetables (<10 g/≥ 10 g) and daily consumption of fresh fruits (<27.5 g/ ≥ 27.5 g).
Table 4. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for esophageal squamous cell carcinoma according to family history of other types of cancer in first-degree relatives*.
| Family history of cancer | First-degree relatives |
Parents |
Siblings |
Offspring |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (N = 619) | Control (n = 772) | OR (95% CI) | Case (N = 619) | Control (n = 772) | OR (95% CI) | Case (N = 602) | Control (n = 730) | OR (95% CI) | Case (N = 590) | Control (n = 732) | OR (95% CI) | ||
| All cancers combined | |||||||||||||
| No | 238 | 358 | Reference | 340 | 499 | Reference | 401 | 516 | Reference | 575 | 701 | Reference | |
| Yes | 344 | 360 | 1.43(1.13–1.81) | 234 | 223 | 1.46(1.14–1.87) | 181 | 189 | 1.32(1.02–1.72) | 8 | 20 | 0.57(0.24–1.35) | |
| Digestive tract cancer† | |||||||||||||
| No | 273 | 420 | Reference | 364 | 538 | Reference | 421 | 553 | Reference | 576 | 709 | Reference | |
| Yes | 304 | 293 | 1.55(1.23–1.96) | 206 | 181 | 1.57(1.22–2.03) | 158 | 148 | 1.52(1.15–2.00) | 7 | 12 | 0.74(0.28–1.97) | |
| Stomach | |||||||||||||
| No | 470 | 611 | Reference | 506 | 667 | Reference | 532 | 656 | Reference | 583 | 720 | Reference | |
| Yes | 82 | 79 | 1.36(0.96–1.92) | 53 | 45 | 1.57(1.01–2.43) | 38 | 40 | 1.18(0.73–1.92) | 0 | 1 | – | |
| Liver | |||||||||||||
| No | 473 | 601 | Reference | 524 | 677 | Reference | 532 | 647 | Reference | 577 | 713 | Reference | |
| Yes | 72 | 83 | 1.09(0.76–1.55) | 33 | 33 | 1.20(0.71–2.03) | 36 | 50 | 0.89(0.56–1.42) | 6 | 8 | 0.95(0.31–2.88) | |
| Pancreas | |||||||||||||
| No | 524 | 673 | Reference | 550 | 707 | Reference | 561 | 691 | Reference | 583 | 721 | Reference | |
| Yes | 12 | 8 | 2.03(0.79–5.20) | 7 | 4 | 2.82(0.76–10.54) | 5 | 5 | 1.20(0.34–4.24) | 0 | 0 | – | |
| Colorectum | |||||||||||||
| No | 516 | 662 | Reference | 545 | 702 | Reference | 557 | 687 | Reference | 583 | 720 | Reference | |
| Yes | 21 | 19 | 1.33(0.69–2.58) | 12 | 8 | 1.72(0.68–4.37) | 9 | 10 | 1.20(0.46–3.11) | 0 | 1 | – | |
*Adjusted for age (continuous), family size (continuous), sex, education (Illiteracy/primary school/primary high school/secondary high school and above), tobacco smoking (never/ever smoker of any tobacco), alcohol drinking (never/ever), missing & filled teeth (MFT, none/1 ~ 4/ ≥ 4), times of tooth brushing per day (<2 times/ ≥ 2 times), daily consumption of pickled vegetables (<10 g/ ≥ 10 g) and daily consumption of fresh fruits (<27.5 g/ ≥ 27.5 g).
†Digestive tract cancer includes esophageal cancer, stomach cancer, liver cancer, pancreas cancer and colorectum cancer.
Reconstructed cohort analysis
Among 4803 first-degree relatives of cases, 244 (5.1%) were reportedly diagnosed with esophageal cancer. The corresponding figure was 2.9% for those relatives of the controls (171 out of 6010). The cumulative risk of esophageal cancer to age 75 was 12.2% in the first-degree relatives of cases and 7.0% in those of controls (hazard ratio = 1.91, 95% CI: 1.54–2.37) (Fig. 1).
Figure 1. Comparison of cumulative risk of developing esophageal cancer in the first-degree relatives of cases and controls.
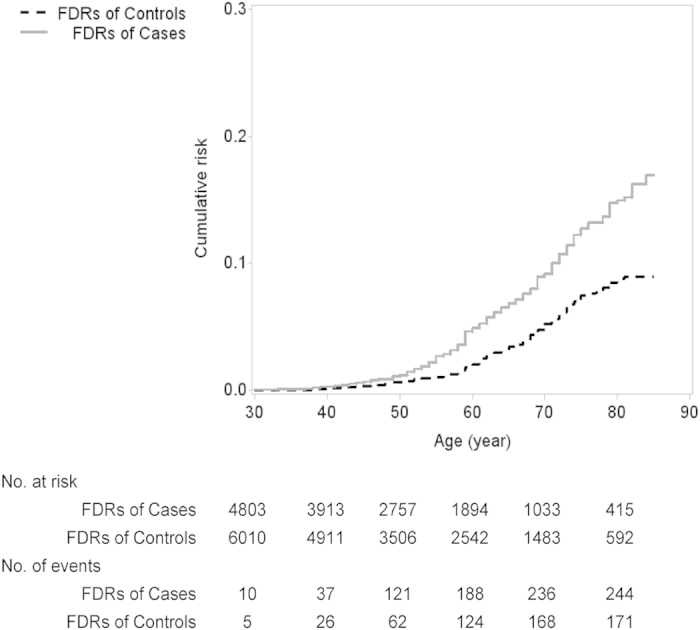
FDR: first-degree relatives.
Sensitivity analysis
We also performed a sensitivity analysis by excluding 70 cases whose information of family history was gathered after pathological diagnosis had been made and/or treatment had started (Supplementary Fig 1). In this sub-analysis, 33.91% of cases reported a positive family history of esophageal cancer. The adjusted OR by unconditional logistic regression was 1.79 (95% CI: 1.35–2.37), and the hazard ratio by the Cox regression was 1.90 (95% CI: 1.53–2.37). In another sensitivity analysis, we treated missing family history of cancer as ‘no reported history of cancer’. The results remained virtually unchanged compared with those in the main analysis (data not shown).
Discussion
In this large population-based case-control study, we confirmed a strong association between a family history of esophageal cancer and the risk of ESCC. The excess risks increased monotonically with increasing number of affected relatives. In particular, if both parents were affected, it rendered an 8-fold excess risk of ESCC for their offspring. By the age of 75, it was estimated that about 12% of the first-degree relatives of ESCC patients might develop the malignancy, while the corresponding figure was 7% for those relatives of the normal control subjects.
Previous epidemiological studies conducted in endemic areas (including mainly Linxian and Shanxi) in China have demonstrated that individuals with a family history of esophageal cancer have a higher risk of such cancer6,8,15. In a case-control study from Turkmen population (another endemic area for ESCC), a more than 2-fold excess risk for esophageal cancer was noted using reconstructed cohort design4. However, a multicenter population-based case-control study performed in the United States did not find any familial link for ESCC16. A Swedish case-control study also revealed no association between a history of esophageal cancer in first-degree relatives and the risk of ESCC17. The inconsistency in results might arise from different frequency of ESCC susceptibility alleles (genetic susceptibility) and variation in attributable environmental or lifestyle risk factors, or a combination of both. It appears that genetic factors play a minor role in the etiology of the “Western type” of ESCC, whereas tobacco and alcohol consumption account for the vast majority of the etiology of this disease18. But neither the relative risk associated with smoking nor that of alcohol drinking is of sufficient magnitude to explain the extremely high incidence in China. Because we don’t have exposure information in the first-degree relatives, we cannot distinguish the effects of genetic susceptibility from environmental factors. But for a disease with notable familial aggregation, environmental factors alone cannot account for such a strong aggregation19. Recent exome and whole-genome studies also reveal the importance contribution of genetic susceptibility to the occurrence of ESCC20,21,22.
In the present study, we found elevated risks of ESCC among individuals with affected parents (OR = 1.63) or siblings (OR = 2.04) and an obvious dose-response relationship with the increase of affected relatives. The OR was slightly higher for siblings, compared with parents, indicating that recessive or X-linked susceptibility genes might be involved in the occurrence of ESCC, or that siblings share more lifestyle risk factors. In addition, we found the excess risk was remarkably higher if both parents were affected than that of individuals with more than one sibling was affected. This suggested that heredity does seem to take a more important role in the etiology of esophageal cancer in endemic areas.
In many case-control studies of familial aggregation of esophageal cancer, positive family history among first-degree relatives is often used as a risk factor, and odd ratio is calculated. Instead of treating family history information as an “exposure” in case-control design, an alternative analytic strategy is to transform the case-control design into a cohort design. Based on reconstructed cohort design, Akbari et al. have estimated the cumulative risk of esophageal cancer to age 75 was 34% in the first-degree relatives of cases and 14% in those of controls in northern Iran4. In our study, we observed a similar relative risk, but a much lower absolute risk. Since the incidence rates of ESCC are comparable between the two areas, theoretically we should observe similar cumulative risks among the relatives of control subjects. The more than doubled cumulative risk observed in the previous study might indicate the influence of misclassification of cancer types.
There is controversy about the validity of different designs for assessing familial aggregation. Khoury and Flanders demonstrated that the case-control design would yield a biased risk estimate, with age of relatives and family size being viewed as confounding variables in assessing the disease risk estimate23. On the other hand, the reconstructed cohort design is thought to show no such biases. However, Zimmerman concluded that neither family size nor age fulfills the criteria for confounding factors, thus the case-control and reconstructed cohort designs are both valid in estimating familial aggregation of disease24. Therefore the investigators should be free to choose the design and measures that best suit the available data to assess familial aggregation. Our results support this notion as similar relative risks were observed for both designs. However, different family history measures may have various implications for counseling using empiric genetic risks. The case-control design is useful when a subject wants to know his/her disease risk given his/her family history. When the disease status of one index person is considered as a risk factor, the reconstructed cohort design is useful to quantify the risk of disease for the relatives of an affected individual.
There are several strengths to this study. First, the detailed information about the family history allowed us to reconstruct cohorts of the relatives of cases and controls, from which we obtained the cumulative risk estimates of esophageal cancer among exposed relatives compared with that among the unexposed relatives. Second, all of the ESCC cases were carefully reviewed and verified by the study pathologist. All subjects underwent detailed in-person interviews which provided necessary information of potential confounders. Finally, the sample size is relatively large and response rates were reasonably high among both cases and controls.
Relying on self-reported information about family history of cancer is a major shortcoming in this study, especially when no validation study has been performed in the study area. This might result in potential misclassification of cancer types in first-degree relatives. However, validation studies in other settings have proved that self-reported family history of cancer could reflect the actual information when compared with retrieving information from medical records25,26. Study subjects were generally unware of subtypes of esophageal cancer, thus it was impossible for us to distinguish the histological subtypes of esophageal cancers in the relatives. But based on recent report, more than 95% of these esophageal cancers should be ESCC subtype in China3. Recall bias is another concern, as case subjects might tend to exaggerate their family history of cancer. However, most cases in this study were enrolled before they were aware of the diagnosis. Further, the similar results from the sensitivity analysis, after we excluded case subjects enrolled after pathological diagnosis being made and/or treatment started, allayed such a concern.
In conclusion, our results indicate that familial aggregation of ESCC in endemic area is notable. The shared genetic susceptibility and environmental exposures, or possibility their interaction, might contribute to this phenomenon which urges future studies to explore the underlying mechanisms.
Additional Information
How to cite this article: Chen, T. et al. Family history of esophageal cancer increases the risk of esophageal squamous cell carcinoma. Sci. Rep. 5, 16038; doi: 10.1038/srep16038 (2015).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 81072358 and 81273151), Key Projects in the National Science & Technology Pillar Program (2011BAI09B00) and Key Scientific and Technological Projects of Shandong Province (2012GSF11857). The authors would like to acknowledge the interviewers of Fudan-Taizhou Institute of Health Sciences for the data collection, and the staff of Taixing Center for Disease Control and Prevention for the help in field work. We also sincerely thank Dr. Chenjun Zhou for carefully reviewing pathological slides for the study.
Footnotes
Author Contributions C.T. and C.H. conducted the data analyses, interpreted the data and wrote the initial draft of the manuscript; C.X. provided statistical guidance in the study design and data analyses; Y.Z. participated in the study implementation and field supervision; Y.X. and Z.M. assisted in manuscript revisions; L.M. contributed to the study design and implementation, interpreted the data and revised the initial draft; J.L. and Y.W. contributed to the study design and implementation, and interpreted the data. All authors contributed to the editing of the final version of the manuscript.
References
- Ferlay J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, E359–386 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao J., He, Y. T., Zheng R. S., Zhang S. W. & Chen W. Q. Analysis of esophageal cancer time trends in China, 1989–2008. Asian Pac J Cancer Prev 13, 4613–4617 (2012). [DOI] [PubMed] [Google Scholar]
- Arnold M., Soerjomataram I., Ferlay J. & Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64, 381–387 (2015). [DOI] [PubMed] [Google Scholar]
- Akbari M. R. et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. Int J Cancer 119, 1047–1051 (2006). [DOI] [PubMed] [Google Scholar]
- Chang-Claude J. et al. Familial aggregation of oesophageal cancer in a high incidence area in China. Int J Epidemiol 26, 1159–1165 (1997). [DOI] [PubMed] [Google Scholar]
- Gao Y. et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer 9, 269 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavello W. et al. Family history of cancer, its combination with smoking and drinking, and risk of squamous cell carcinoma of the esophagus. Cancer Epidemiol Biomarkers Prev 14, 1390–1393 (2005). [DOI] [PubMed] [Google Scholar]
- Hu N. et al. Familial aggregation of oesophageal cancer in Yangcheng County, Shanxi Province, China. Int J Epidemiol 21, 877–882 (1992). [DOI] [PubMed] [Google Scholar]
- Wang Y. P. et al. Esophageal cancer in Shanxi Province, People’s Republic of China: a case-control study in high and moderate risk areas. Cancer Causes Control 3, 107–113 (1992). [DOI] [PubMed] [Google Scholar]
- Carter C. L. et al. Segregation analysis of esophageal cancer in 221 high-risk Chinese families. J Natl Cancer Inst 84, 771–776 (1992). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Segregation analysis of esophageal cancer in a moderately high-incidence area of northern China. Am J Hum Genet 67, 110–119 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E. & Susser M. Familial aggregation studies. A note on their epidemiologic properties. Am J Epidemiol 129, 23–30 (1989). [DOI] [PubMed] [Google Scholar]
- Lin D. Y., Wei L. J. & Ying Z. Checking the cox model with cumulative sums of martingale-based residuals. Biometrika 80, 557–572 (1993). [Google Scholar]
- Lin D. Y. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med 13, 2233–2247 (1994). [DOI] [PubMed] [Google Scholar]
- Li J. Y. et al. A case-control study of cancer of the esophagus and gastric cardia in Linxian. Int J Cancer 43, 755–761 (1989). [DOI] [PubMed] [Google Scholar]
- Dhillon P. K. et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer 93, 148–152 (2001). [DOI] [PubMed] [Google Scholar]
- Lagergren J., Ye W., Lindgren A. & Nyren O. Heredity and risk of cancer of the esophagus and gastric cardia. Cancer Epidemiol Biomarkers Prev 9, 757–760 (2000). [PubMed] [Google Scholar]
- Umar S. B. & Fleischer D. E. Esophageal cancer: epidemiology, pathogenesis and prevention. Nature Clinical Practice Gastroenterology & Hepatology 5, 517–526 (2008). [DOI] [PubMed] [Google Scholar]
- Khoury M. J., Beaty T. H. & Liang K. Y. Can familial aggregation of disease be explained by familial aggregation of environmental risk factors? Am J Epidemiol 127, 674–683 (1988). [DOI] [PubMed] [Google Scholar]
- Song Y. et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509, 91–95 (2014). [DOI] [PubMed] [Google Scholar]
- Dulak A. M. et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nature genetics 45, 478–486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. Esophageal squamous cell carcinoma (ESCC): advance in genomics and molecular genetics. Dis Esophagus 28, 84–89 (2015). [DOI] [PubMed] [Google Scholar]
- Khoury M. J. & Flanders W. D. Bias in using family history as a risk factor in case-control studies of disease. Epidemiology 6, 511–519 (1995). [DOI] [PubMed] [Google Scholar]
- Zimmerman R., Pal D. K., Tin A., Ahsan H. & Greenberg D. A. Methods for assessing familial aggregation: family history measures and confounding in the standard cohort, reconstructed cohort and case-control designs. Hum Hered 68, 201–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber R. A. & Slattery M. L. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol 146, 244–248 (1997). [DOI] [PubMed] [Google Scholar]
- Ziogas A. & Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med 24, 190–198 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


