Synopsis
This review covers the rationale, mechanisms, and availability of commercially available virtual environment-based interventions for stroke rehabilitation. It describes interventions for motor, speech, cognitive, and sensory dysfunction. Also discussed are the important features and mechanisms that allow virtual environments to facilitate motor relearning. A common challenge facing the field is inability to translate success in small trials to efficacy in larger populations. The heterogeneity of stroke pathophysiology has been blamed and experts advocate for the study of multimodal approaches. Therefore, this article also introduces a framework to help define new therapy combinations that may be necessary to address stroke heterogeneity.
Keywords: virtual reality, motor relearning, hemiparesis, neuroplasticity, stroke
I. Introduction
Despite our best efforts, stroke continues to be a leading cause of acquired disability throughout the world and is responsible for approximately 102 million disability adjusted life years annually1. Even more concerning to care providers – 66% of the 666K new stroke survivors each year may suffer chronic cognitive or physical impairment after 6 mo. of conventional care2,3.
Evidence for neurologic recovery through cortical reorganization4 has lead to new interventions that try to accelerate functional recovery. One promising approach uses virtual environments (VE) in the form of video games or therapeutic tasks to train impairments. A definition of VEs are computer-simulated objects that respond to speech or motor input. Many VE therapies for stroke are now sold and attract intense interest.
Thus, this review focuses on VEs for stroke that are widely available outside of research programs. Those interested the broader academic field can refer to texts such as that by Dietz and Ward5. This article will begin with rationale for VE training along with potential mechanisms of action. It groups interventions by their targeted impairments, discusses their efficacy, and concludes with challenges for the field.
II. Features for Motor Learning in Virtual Environments
Human training was the first applications for VEs beyond their conception as entertainment in the form of stereoscopes and video arcades. Circa 1960, VEs enhanced military flight simulators with visual information that followed pilot head movements. Since 1990, the following features associated with promoting neuroplasticity6 were incorporated into effective VEs for stroke rehabilitation7.
Performance feedback
Repetitive, goal-oriented tasks with variability covering a range of conditions
Controlled environment where mistakes have minimal consequences
Task difficulty scaled to a stroke survivor’s capabilities and skill8
Assist9, resist10, or repel movement and exaggerate errors11
Focus on targeted skills by reducing contributions from unwanted movements12
Increase motivation and engagement using features from video games13
Facilitate remote social interaction with peers or therapists12
III. Potential Mechanisms of Action
A. Augmented Feedback’s Effect on Motor Learning
There is sufficient evidence that providing stroke survivors with information about movement quality and task outcome benefits motor skill acquisition and retention14. Delivering feedback only about task measures leads to immediate improvements the measures with no gain in movement quality. If feedback is provided only about motor performance (path deviations or compensatory behavior), participants immediately improved both task outcomes and movement quality.
B. Effect of Virtual Environments on Cortical Networks
Imaging reveals that visuomotor network activation occurs when both able-bodied and stroke survivors view hand motion from a virtual avatar. As the visual quality15 and sense of immersion16,17 increases, so does the recruitment of visuomotor networks – which is maximized when the avatar moves in synchrony with the physical hands17. In initial reports, recovery from VE training appears to also demonstrate similar patterns of cortical network change as observed in non-virtual therapy18.
Another method of assessing the state of cortical networks is to infer motor corticospinal excitability using motor evoked potentials induced by transcranial magnetic stimulation. In stroke survivors, lower conduction time19, higher baseline motor evoked potential amplitude20, and greater motor evoked potential amplitude21 may benefit motor performance and learning. However, very few studies investigated the effect of VE interventions on corticospinal excitability. One study found that skill learning increased corticospinal excitability, but not task performance22.
C. Effect of Immersion on Motor Performance
In the healthy, motor performance improves with increased VE immersion, but very few have investigated the effect of immersion on motor learning after stroke. Levels of VE immersion range from using typical PC monitors all the way to 3D goggles. Less immersion reduces movement accuracy, smoothness, and velocity, while increasing task performance time in the healthy23. Stroke survivors performing reaching tasks in an immersive, head-mounted VE with a robotic exoskeleton (vs. real world) had 35% longer completion time and increased elbow extension and horizontal shoulder abduction24.
IV. Impairments Targeted by VE Interventions
The following will focus on interventions commercially available in the US.
Upper Extremity is an Area of Focus
After the initial stroke, 80% experience upper limb impairment25. Though 15% may have full spontaneous recovery26 – after 6 months, up to 65% cannot use their hands for activities of daily living3. Unsurprisingly, a majority of VE interventions were developed for upper extremity motor training. Recent reviews consistently note that VEs are low risk and may be beneficial for motor relearning when administered as part of a physiotherapy program, but they also agree that the quality of current evidence is low and rigorous comparative studies are needed25,27. Costs range from $100-$200K.
i. Proximal Movement - Shoulder and Elbow
Commercial motion-controlled games made for the Wii (Nintendo of America Corp, Redmond, WA), Playstation Move (Sony Computer Entertainment Corp., San Mateo, CA), and Xbox Kinect (Microsoft Corp, Redmond, WA) use gesture-based shoulder-elbow motions as input to various sports simulations or motor coordination games. A recent review found high user acceptance, that 180 mins/wk can be safely tolerated, and no evidence for negative effects on motor function28.
However, evidence is weak that they are more beneficial than conventional care. Only 4 small controlled trials exist and 2 of them reported significantly improved outcomes over controls (3 points in Fugl-Myer29 and 5.5 in the Functional Independence Measure). The current consensus is that commercial games are likely beneficial as a supplement to conventional occupational therapy28,30. However, it is important to note that they do not train finger or wrist movements, difficulty levels may unsuitable for the severely impaired, and there is no guard against using compensatory body mechanics.
Rehabilitation-specific VE system such as IREX (GestureTek Corp., Toronto, CA), OmniVR (Accelerated Care Plus Corp., Reno, NV), and Jintronix (Jintronix Corp., Seattle WA) use custom VEs that integrate with 3D cameras. In addition, they offer task customization, movement analysis, and usage logs that game consoles cannot offer. However, meta-analysis did not find a significant advantage of clinical systems to game consoles and no trials have directly compared clinical systems to game consoles25.
The largest RCT using systems of this type used the Virtual Reality Rehabilitation System (EU only, Khymeia Group Ltd, Noventa Padovana, Italy) on 376 stroke survivors (< 12 mo post stroke) for 40 2-hr sessions (1 hr conventional care, 1 hr VE) over 4 wks and found a significant effect size of 2.5 ± 0.5 points (4.9 ± 0.9 for those 3–12 mo post stroke) for the Fugl-Meyer over controls that had conventional therapy31.
InMotion ARM (Interactive Motion Technologies Corp, Watertown, MA) is a robot that guides participants toward targets as they move its handle like a computer mouse in reaching tasks and games. A multi-center trial for those < 6 mo post stroke showed that 36 hrs of robot therapy over 12 wks significantly increased Fugl-Myer by 2 points over usual care, which was not clinically meaningful32. Other measures were no better than usual care or dose-matched therapy. Both groups improved, but a follow-up study questioned the robot’s cost-effectiveness due to a $5K premium per participant33.
Armeo (Hocoma Corp, Norwell, MA) Power, Spring, and Boom are exoskeletons that allow for assisted 3D arm movement in virtual tasks and games. Power uses motors to guide the arm, Spring uses passive springs to reduce arm movement effort, and Boom suspends the arm against gravity. Patients use Power first, then Spring, then Boom as they regain movement and require less assistance for virtual task practice – though this progression has not yet been tested in clinical trials. A multi-site RCT using Power on 77 chronic stroke survivors for 24 45-min sessions over 8 wks found a significant effect of 0.78 points on the Fugl-Meyer compared to conventional care. An uncontrolled trial of Spring on chronic stroke (N=23) for 36 hrs over 12 wks found a 5- point gain in Fugl-Myer and no change in secondary functional measures34.
ii. Distal Movement – Hand, Wrist, and Fingers
Music Glove (Flint Rehabilitation Devices LLC, Irvine, CA) trains finger motion by wearing a sensor glove and touching the thumb to the other fingertips to play music notes. A single-blinded crossover trial in 12 moderately-impaired chronic stroke survivors (3 treatments, each for 6 hrs over 2 wks) showed significant gain of 3.2 blocks on the Box and Blocks Test vs conventional care, but no gain over the game with an isometric force sensor that did not require finger motion35.
HandTutor (MediTouch Ltd, Israel) uses a sensor glove to control therapy games by finger or wrist flexion/extension. A controlled trial treated 31 chronic subacute (< 4 mo post stroke) participants by adding 20–30 min of VE training (experiment) or conventional care (control) to usual care36. Results showed significant effects for primary outcomes, but were not sustained at 10 d follow-up.
Amadeo (TyroMotion GmbH, Graz, Austria) is a hand exoskeleton that assists individual finger movement during VE training, but requires the arm to be strapped to a fixed base. A RCT with 20 acute inpatient, stroke survivors had 20 40-min treatment sessions over 4 wks added to standard care (3 hr/day). Both usual care and robot groups had significant gains (end and 3-mo follow-up), but no group effects were found.
B. Gait Training with VEs have Limited Effect
Lower extremity impairment affects 75% of all stroke survivors and only 15% regain full recovery26. Up to 25% will require assistive aids to walk for the rest of their lives26. A meta-analysis of 7 RCTs found that VE groups improved gait speed by 0.17 m/s over placebo groups and 0.15 m/s over non-VE usual walking therapy. Though promising, the majority of studies used custom systems and the same amount of evidence is not available for commercial systems costing $200K to $1M.
LOKOMAT (Hokoma Corp) is a lower extremity exoskeleton for body-weight supported treadmill training and can be equipped with a computer monitor for use with VE tasks to simulate walking and leg motion training. No studies examined the effect of adding VEs to this system for stroke rehabilitation, but a RCT in children with various neurological impairments found that a soccer ball kicking simulation increased motivation, but did not result in greater joint torques37.
Motek Medical BV (Amsterdam, Netherlands) has treadmill systems that are used with wall-sized computer projection screens for immersive VE gait training. CAREN is the most immersive with a treadmill that moves in 6 degrees of freedom, while GRAIL, and V-Gait are split belt treadmills with no moving bases. One study found that adding a VE led to treadmill walking mechanics that were closer to the over-ground condition, but the differences in the VE condition were clinically negligible38. No controlled studies for stroke exist on the CAREN system, but case studies used artificially slow optical flow to illicit faster walking39.
C. Balance Interventions Comparable to Conventional Care
Many VE balance interventions demonstrated positive effects, but they do not exceed controls treated with conventional care25,40. These interventions use a wide range of devices, including motion video games41, Xbox Kinect42, treadmills43, and reaching tasks44. Many studies used commercial games for Wii Fit balance board, which is a force plate accessory ($100, Nintendo Corp, Redmond, WA). The games (skiing, hoola-hooping, and yoga) are controlled by body weight shifting and were shown to be feasible for both inpatient and home use45. Although these VE methods may not be more effective than conventional care, they have been shown to reduce therapist costs without sacrificing efficacy if prescribed appropriately for home use42.
D. Cognitive Rehabilitation Interventions are Lacking
The efficacy of VEs for cognitive rehabilitation is a weak point in the literature, with few controlled trials and even fewer commercial interventions25. Those that exist cost $150–850. RehaCom (Hasomed GmbH, Berlin, Germany) PC software trains attention (alertness, vigilance, visual-spatial, selective and divided), memory, executive functions, and visuo-motor skills. A double-blind RCT on 36 stroke survivors (< 6 mo post stroke) showed significantly improved working memory and word fluency over conventional therapy after nine 30-min sessions46.
“Brain games” are popular in the consumer market, but the cognitive training designed for unimpaired individuals may be unsuitable for neurologic injury. Lumosity (Lumos Labs Corp., San Francisco, CA) is a website that trains cognitive functions such as memory, processing speed, attention, and problem solving. Though training transfers to long-term function in healthy adults47, an 8 wk uncontrolled trial on 5 stroke survivors (> 6 mo post stroke) found that 3 of the participants completed less than half of 40 30- min, self-administered home sessions and two did none of them. Reasons cited included fatigue and difficulty responding in the allotted time for certain tasks48.
E. Speech Rehabilitation Intervention Options are Few
Speech therapy VEs are also lacking in evidence from controlled trials, but have consistently positive case series and cost < $1K. AphasiaScripts (Rehabilitation Institute of Chicago, Chicago, IL) uses virtual avatars for script practice. The avatar’s mouth demonstrates proper speech articulation while it converses to the patient using predefined scripts (no speech recognition). An uncontrolled trial of 20 chronic, aphasic stroke survivors showed a clinically significant decrease of 6.67 points in Communication Difficulty of the Burden of Stroke Scale after 9 wks of 30 min/day home intervention49.
MossTalk Words 2 (Moss Rehabilitation Research Institute, Philadelphia, PA) is PC software that trains single word production using virtual flash cards and provides performance feedback of pronunciation accuracy using voice recognition. Four case studies spanning 17 stroke survivors all reported that 12 to 20 1-hr sessions improved untrained object naming ability when session frequency was at least 3–4 per wk50–53.
StepByStep (Steps Consulting Limited) is also a flash-card-like program, but has pre-recorded video of therapists pronouncing the words. It also can train spelling and sentence production, but does not have speech recognition. A single-blind controlled trial of 34 chronic stroke survivors compared 5 mo of the intervention (20 mins, 3 days per wk) against no therapy. The intervention group had 19.8% greater untrained object naming accuracy (10% was clinically significant) that did not persist at 3 mo follow-up54.
F. Spatial Neglect is an Area of Need
VEs have been developed for assessing hemispatial neglect that may be more sensitive than manual methods55, but there are no widely available VE-specific interventions. One uncontrolled study demonstrated short-term improvement of far field neglect in six stroke survivors (< 3 mo post stroke) by using a virtual reaching task with the patient’s hands altered to appear in the field of neglect56. Others used motor training to treat neglect with the rationale of providing arousal and attention to the neglected limb. An unblinded RCT of 24 stroke survivors (< 1 mo post stroke) compared reaching tasks using IREX to dose-matched conventional therapy. After 5 wks of therapy 30 min/day, 5 days/wk, the VE group reported greater effect over controls57.
G. Proprioception and Sensory Deficits are Gaining Attention
Between 17–50% of stroke survivors experience impaired proprioception or sensation, but there are very few interventions available, as treatments are passive and VEs are only used to assess deficits58. VEs revealed that motor recovery is strongly associated with the return of proprioception59. One uncontrolled study in (N=7, >1 yr post stroke) showed that recovery of proprioception after may be facilitated by a virtual reaching task with a custom built planar robot providing guidance for 5 1-hr sessions over 2 wks32. The robot used force pulses to guide arms toward target elbow angles. All participants showed gains in perceptual acuity, but three of the more impaired participants did not have sustained effects at follow-up.
Sensory deficits occur in 50% of stroke survivors60, but few treatments exist and no VE interventions were published. There lacks evidence for treatments that use electrical cutaneous stimulation and discrimination task training has little effect61.
V. Current Challenges
The field has advanced in the last 25 years, but there are still many questions. To start, the effect of VE immersion, dose, time after stroke, and severity on outcomes needs to be unraveled62. Also, the use of VE-facilitated social interaction to boost motor recovery has only begun to be explored. Furthermore, it is important to advocate technology companies to design technologies to be suitable for neurologic impairment. Proffit and Lange recently challenged the field to also investigate the effect of presence and immersion on motor recovery as immersive displays become more accessible63.
The greatest challenge is the trend of large comparative trials of treatments successful in smaller studies showing little difference compared to conventional care64. It is possible that larger sample sizes increase heterogeneity in impairments, demographics, and pathophysiology65. The National Center for Medical Rehabilitation Research recently advocated for defining therapies’ active ingredients and integrating them into treatment packages to maximize efficacy in diverse populations66.
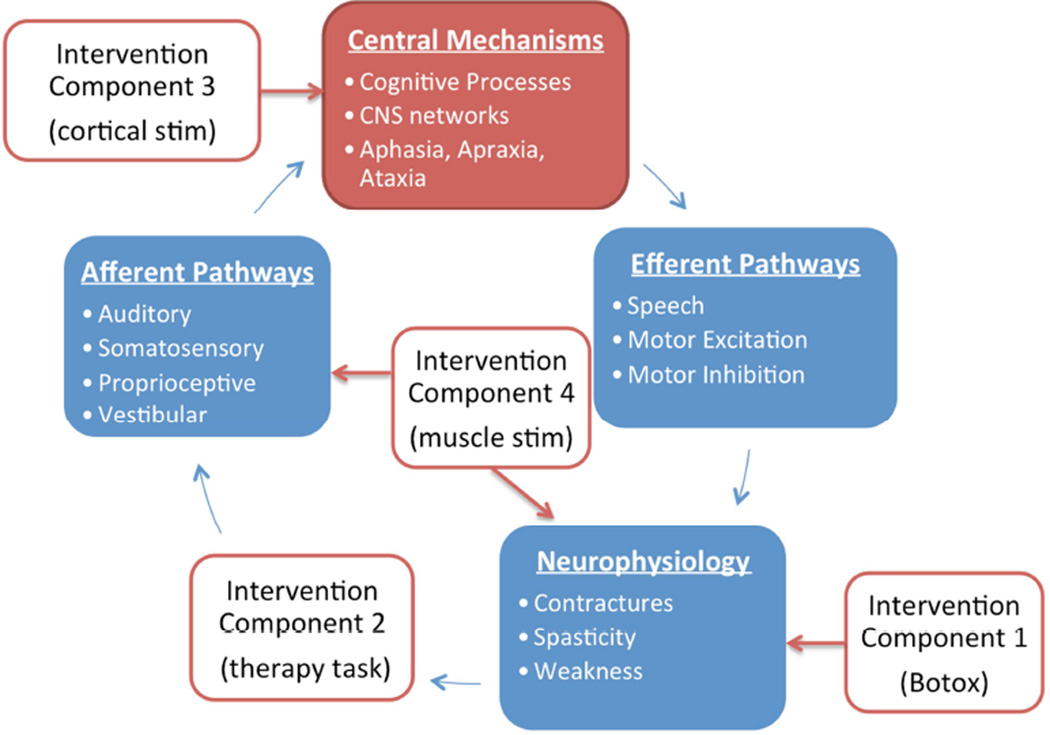
This is a complicated task that may benefit from a framework that can expand as mechanisms are defined. Figure 1 is therefore introduced as a potential framework. It is adapted from the close-loop feedback model of motor learning67 to include placeholders for processes that are affected by stroke and can be targeted by interventions. This illustrates how the framework can describe an intervention package that incorporates 1) cortical stimulation to increase neuronal excitability, 2) Botox to relieve hypertonia, 3) electrical stimulation to assist hand opening, and 4) a VE to perform therapy task practice. This may help define the roles and relationships that belong to each part of a treatment package. As mechanisms are found, new hypotheses may also be generated.
Figure 1.
General system-level framework to identify mechanistic targets for multimodal approaches to stroke rehabilitation. The unfilled boxes are example interventions that point to the processes they directly affect, which are represented by filled boxes. The contents of each process are examples and not comprehensive. Red arrows represent intervention effects and blue arrows represent outputs from each process and input into the next process.
VI. Summary
This review focused on rationale, mechanisms, and availability of commercial VE stroke rehabilitation interventions. It also identified that cognitive, proprioceptive, and sensory dysfunction are under-addressed by the field. The consensus from over 30 RCTs is that VEs are low risk and recommended as supplements to conventional therapy. Questions to answer going forward include what mechanisms of action drive recovery and which participant groups will respond. Finally, a framework was introduced to define therapy combinations necessary to impact the heterogeneity of stroke and move the field out of the current stagnation caused by small effects in large clinical trials.
Key Points.
Virtual environment interventions for motor relearning are very popular and well received, but they have small positive effect over conventional therapy.
Common consensus is that virtual environment interventions are low risk and are likely beneficial if used as an adjunct to conventional therapy.
There is a lack of effective and widely available virtual environment treatments for non-motor deficits such as speech, cognitive function, and sensory dysfunction.
Future approaches may need to strategically combine multiple interventions to address the multi-faceted nature of stroke rehabilitation.
Acknowledgments
Disclosures: M. Fu. is supported by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 (NIH NCATS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feigin VL, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, et al. Heart Disease and Stroke Statistics--2014 Update: A Report From the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobkin BH. Rehabilitation after Stroke. N. Engl. J. Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 5.Dietz V, Ward N. Oxford Textbook of Neurorehabilitation. Vol. 418. Oxford University Press; 2015. [Google Scholar]
- 6.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. JSLHR. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 7.Rizzo A, Skip, Kim GJ. A SWOT Analysis of the Field of Virtual Reality Rehabilitation and Therapy. Presence Teleoperators Virtual Environ. 2005;14:119–146. [Google Scholar]
- 8.Da Silva Cameirão M, Bermúdez I, Badia S, Duarte E, Verschure PFMJ. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the rehabilitation gaming system. Restor. Neurol. Neurosci. 2011;29:287–298. doi: 10.3233/RNN-2011-0599. [DOI] [PubMed] [Google Scholar]
- 9.Krebs HI, et al. Overview of clinical trials with MIT-MANUS: a robot-aided neuro-rehabilitation facility. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 1999;7:419–423. [PubMed] [Google Scholar]
- 10.Wu M, et al. Robotic resistance/assistance training improves locomotor function in individuals poststroke: a randomized controlled study. Arch. Phys. Med. Rehabil. 2014;95:799–806. doi: 10.1016/j.apmr.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdollahi F, et al. Error augmentation enhancing arm recovery in individuals with chronic stroke: a randomized crossover design. Neurorehabil. Neural Repair. 2014;28:120–128. doi: 10.1177/1545968313498649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novak D, Nagle A, Keller U, Riener R. Increasing motivation in robot-aided arm rehabilitation with competitive and cooperative gameplay. J. Neuroengineering Rehabil. 2014;11:64. doi: 10.1186/1743-0003-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putrino D. Telerehabilitation and emerging virtual reality approaches to stroke rehabilitation: Curr. Opin. Neurol. 2014;27:631–636. doi: 10.1097/WCO.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian SK, Massie CL, Malcolm MP, Levin MF. Does Provision of Extrinsic Feedback Result in Improved Motor Learning in the Upper Limb Poststroke? A Systematic Review of the Evidence. Neurorehabil. Neural Repair. 2010;24:113–124. doi: 10.1177/1545968309349941. [DOI] [PubMed] [Google Scholar]
- 15.Perani D, et al. Different Brain Correlates for Watching Real and Virtual Hand Actions. NeuroImage. 2001;14:749–758. doi: 10.1006/nimg.2001.0872. [DOI] [PubMed] [Google Scholar]
- 16.Jäncke L, Cheetham M, Baumgartner T. Virtual Reality and the Role of the Prefrontal Cortex in Adults and Children. Front. Neurosci. 2009;3:52–59. doi: 10.3389/neuro.01.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh S, Adamovich SV, Tunik E. Mirrored Feedback in Chronic Stroke: Recruitment and Effective Connectivity of Ipsilesional Sensorimotor Networks. Neurorehabil. Neural Repair. 2014;28:344–354. doi: 10.1177/1545968313513074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orihuela-Espina F, et al. Neural Reorganization Accompanying Upper Limb Motor Rehabilitation from Stroke with Virtual Reality-Based Gesture Therapy. Top. Stroke Rehabil. 2013;20:197–209. doi: 10.1310/tsr2003-197. [DOI] [PubMed] [Google Scholar]
- 19.Vang C, Dunbabin D, Kilpatrick D. Correlation Between Functional and Electrophysiological Recovery in Acute Ischemic. Stroke. 1999;30:2126–2130. doi: 10.1161/01.str.30.10.2126. [DOI] [PubMed] [Google Scholar]
- 20.Rapisarda G, Bastings E, Noordhout AMde, Pennisi G, Delwaide PJ. Can Motor Recovery in Stroke Patients Be Predicted by Early Transcranial Magnetic Stimulation? Stroke. 1996;27:2191–2196. doi: 10.1161/01.str.27.12.2191. [DOI] [PubMed] [Google Scholar]
- 21.Koski L, Mernar TJ, Dobkin BH. Immediate and Long-Term Changes in Corticomotor Output in Response to Rehabilitation: Correlation with Functional Improvements in Chronic Stroke. Neurorehabil. Neural Repair. 2004;18:230–249. doi: 10.1177/1545968304269210. [DOI] [PubMed] [Google Scholar]
- 22.Bagce HF, Saleh S, Adamovich SV, Krakauer JW, Tunik E. Corticospinal excitability is enhanced after visuomotor adaptation and depends on learning rather than performance or error. J. Neurophysiol. 2013;109:1097–1106. doi: 10.1152/jn.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu MJ, Hershberger AD, Sano K, Çavuşoğlu MC. Effect of Visuo-Motor Co-location on 3D Fitts’ Task Performance in Physical and Virtual Environments. Presence Camb. Mass. 2012;21:305–320. doi: 10.1162/pres_a_00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin MF, Knaut LAM, Magdalon EC, Subramanian S. Virtual reality environments to enhance upper limb functional recovery in patients with hemiparesis. Stud. Health Technol. Inform. 2009;145:94–108. [PubMed] [Google Scholar]
- 25.Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2015;2:CD008349. doi: 10.1002/14651858.CD008349.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002;83:1629–1637. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 27.Lohse KR, Hilderman CGE, Cheung KL, Tatla S, Van der Loos HFM. Virtual Reality Therapy for Adults Post-Stroke: A Systematic Review and Meta-Analysis Exploring Virtual Environments and Commercial Games in Therapy. PLoS ONE. 2014;9:e93318. doi: 10.1371/journal.pone.0093318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson K, Pollock A, Bugge C, Brady M. Commercial gaming devices for stroke upper limb rehabilitation: a systematic review. Int. J. Stroke Off. J. Int. Stroke Soc. 2014;9:479–488. doi: 10.1111/ijs.12263. [DOI] [PubMed] [Google Scholar]
- 29.Manlapaz DG, et al. Effectiveness of Using Nintendo Wii in Rehabilitation of Chronic Stroke Patients with Upper Limb Hemiparesis. Hong Kong Physiother. J. 2010;28:25. [Google Scholar]
- 30.Thomson K, Pollock A, Bugge C, Brady MC. Commercial gaming devices for stroke upper limb rehabilitation: a survey of current practice. Disabil. Rehabil. Assist. Technol. 2015:1–8. doi: 10.3109/17483107.2015.1005031. [DOI] [PubMed] [Google Scholar]
- 31.Turolla A, et al. Virtual reality for the rehabilitation of the upper limb motor function after stroke: a prospective controlled trial. J. NeuroEngineering Rehabil. 2013;10:85. doi: 10.1186/1743-0003-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Santis D, et al. Robot-assisted training of the kinesthetic sense: enhancing proprioception after stroke. Front. Hum. Neurosci. 2014;8:1037. doi: 10.3389/fnhum.2014.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner TH, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke J. Cereb. Circ. 2011;42:2630–2632. doi: 10.1161/STROKEAHA.110.606442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colomer C, et al. Efficacy of Armeo® Spring during the chronic phase of stroke. Study in mild to moderate cases of hemiparesis. Neurol. Barc. Spain. 2013;28:261–267. doi: 10.1016/j.nrl.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Friedman N, et al. Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training. J. NeuroEngineering Rehabil. 2014;11:76. doi: 10.1186/1743-0003-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmeli E, Peleg S, Bartur G, Elbo E, Vatine J-J. HandTutorTM enhanced hand rehabilitation after stroke — a pilot study. Physiother. Res. Int. 2011;16:191–200. doi: 10.1002/pri.485. [DOI] [PubMed] [Google Scholar]
- 37.Brütsch K, et al. Influence of virtual reality soccer game on walking performance in robotic assisted gait training for children. J. NeuroEngineering Rehabil. 2010;7:15. doi: 10.1186/1743-0003-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloot LH, van der Krogt MM, Harlaar J. Effects of adding a virtual reality environment to different modes of treadmill walking. Gait Posture. 2014;39:939–945. doi: 10.1016/j.gaitpost.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Lamontagne A, Fung J, McFadyen BJ, Faubert J. Modulation of walking speed by changing optic flow in persons with stroke. J. Neuroengineering Rehabil. 2007;4:22. doi: 10.1186/1743-0003-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatica-Rojas V, Méndez-Rebolledo G. Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases. Neural Regen. Res. 2014;9:888–896. doi: 10.4103/1673-5374.131612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laufer Y, Dar G, Kodesh E. Does a Wii-based exercise program enhance balance control of independently functioning older adults? A systematic review. Clin. Interv. Aging. 2014;9:1803–1813. doi: 10.2147/CIA.S69673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloréns R, Noé E, Colomer C, Alcañiz M. Effectiveness, usability, and costbenefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015;96:418–425.e2. doi: 10.1016/j.apmr.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, et al. Improving balance skills in patients who had stroke through virtual reality treadmill training. Am. J. Phys. Med. Rehabil. Assoc. Acad. Physiatr. 2011;90:969–978. doi: 10.1097/PHM.0b013e3182389fae. [DOI] [PubMed] [Google Scholar]
- 44.McEwen D, Taillon-Hobson A, Bilodeau M, Sveistrup H, Finestone H. Virtual Reality Exercise Improves Mobility After Stroke An Inpatient Randomized Controlled Trial. Stroke. 2014;45:1853–1855. doi: 10.1161/STROKEAHA.114.005362. [DOI] [PubMed] [Google Scholar]
- 45.Morone G, et al. The efficacy of balance training with video game-based therapy in subacute stroke patients: a randomized controlled trial. BioMed Res. Int. 2014;2014:580861. doi: 10.1155/2014/580861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter KM, Modden C, Eling P, Hildebrandt H. Working Memory Training and Semantic Structuring Improves Remembering Future Events, Not Past Events. Neurorehabil. Neural Repair. 2015;29:33–40. doi: 10.1177/1545968314527352. [DOI] [PubMed] [Google Scholar]
- 47.Willis SL, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zickefoose S, Hux K, Brown J, Wulf K. Let the games begin: a preliminary study using attention process training-3 and Lumosity™ brain games to remediate attention deficits following traumatic brain injury. Brain Inj. 2013;27:707–716. doi: 10.3109/02699052.2013.775484. [DOI] [PubMed] [Google Scholar]
- 49.Manheim LM, Halper AS, Cherney L. Patient-Reported Changes in Communication After Computer-Based Script Training for Aphasia. Arch. Phys. Med. Rehabil. 2009;90:623–627. doi: 10.1016/j.apmr.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Raymer AM, Kohen FP, Saffell D. Computerised training for impairments of word comprehension and retrieval in aphasia. Aphasiology. 2006;20:257–268. [Google Scholar]
- 51.Jokel R, Cupit J, Rochon E, Leonard C. Relearning lost vocabulary in nonfluent progressive aphasia with MossTalk Words®. Aphasiology. 2009;23:175–191. [Google Scholar]
- 52.Fink RB, Brecher A, Schwartz MF, Robey RR. A computer-implemented protocol for treatment of naming disorders: Evaluation of clinician-guided and partially self-guided instruction. Aphasiology. 2002;16:1061–1086. [Google Scholar]
- 53.Ramsberger G, Marie B. Self-administered cued naming therapy: a single-participant investigation of a computer-based therapy program replicated in four cases. Am. J. Speech-Lang. Pathol. Am. Speech-Lang.-Hear. Assoc. 2007;16:343–358. doi: 10.1044/1058-0360(2007/038). [DOI] [PubMed] [Google Scholar]
- 54.Palmer R, et al. Computer Therapy Compared With Usual Care for People With Long-Standing Aphasia Poststroke: A Pilot Randomized Controlled Trial. Stroke. 2012;43:1904–1911. doi: 10.1161/STROKEAHA.112.650671. [DOI] [PubMed] [Google Scholar]
- 55.Barrett AM, et al. Cognitive Rehabilitation Interventions for Neglect and Related Disorders: Moving from Bench to Bedside in Stroke Patients. J. Cogn. Neurosci. 2006;18:1223–1236. doi: 10.1162/jocn.2006.18.7.1223. [DOI] [PubMed] [Google Scholar]
- 56.Castiello U, Lusher D, Burton C, Glover S, Disler P. Improving left hemispatial neglect using virtual reality. Neurology. 2004;62:1958–1962. doi: 10.1212/01.wnl.0000128183.63917.02. [DOI] [PubMed] [Google Scholar]
- 57.Kim YM, Chun MH, Yun GJ, Song YJ, Young HE. The Effect of Virtual Reality Training on Unilateral Spatial Neglect in Stroke Patients. Ann. Rehabil. Med. 2011;35:309. doi: 10.5535/arm.2011.35.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dukelow SP, Herter TM, Bagg SD, Scott SH. The independence of deficits in position sense and visually guided reaching following stroke. J. Neuroengineering Rehabil. 2012;9:72. doi: 10.1186/1743-0003-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semrau JA, Herter TM, Scott SH, Dukelow SP. Characterizing proprioceptive recovery after stroke using robotics. Proc. of the Translational and Computational Motor Control. 2014;2014 at < https://sites.google.com/site/acmcconference/2014/12.pdf>. [Google Scholar]
- 60.Sullivan JE, Hedman LD. Sensory dysfunction following stroke: incidence, significance, examination, and intervention. Top. Stroke Rehabil. 2008;15:200–217. doi: 10.1310/tsr1503-200. [DOI] [PubMed] [Google Scholar]
- 61.Schabrun SM, Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clin. Rehabil. 2009;23:27–39. doi: 10.1177/0269215508098897. [DOI] [PubMed] [Google Scholar]
- 62.Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 1996. at < http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008349.pub3/abstract>. [Google Scholar]
- 63.Proffitt R, Lange B. Considerations in the Efficacy and Effectiveness of Virtual Reality Interventions for Stroke Rehabilitation: Moving the Field Forward. Phys. Ther. 2014 doi: 10.2522/ptj.20130571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winstein C. Translating the Science into Neurorehabilitation Practice: Challenges and Opportunities. 2014 at < http://www.asnr.com/files/ASNR_Viste%20Award_Winstein.pptx.pdf>. [Google Scholar]
- 65.Muir KW. Heterogeneity of stroke pathophysiology and neuroprotective clinical trial design. Stroke J. Cereb. Circ. 2002;33:1545–1550. doi: 10.1161/01.str.0000018684.86293.ab. [DOI] [PubMed] [Google Scholar]
- 66.Nitkin R. Support for Clinical Trials at the National Center for Medical Rehabilitation Research and the NIH. 2014 at < http://www.asnr.com/files/NitkinHandouts.pdf>. [Google Scholar]
- 67.Magill RA. Motor learning and control: concepts and applications. Vol. 86. McGraw-Hill; 2007. [Google Scholar]