Synopsis
This article reviews the most common therapeutic and neuroprosthetic applications of neuromuscular electrical stimulation (NMES) for upper and lower extremity stroke rehabilitation. Fundamental NMES principles and purposes in stroke rehabilitation are explained. NMES modalities used for upper and lower limb rehabilitation are described and efficacy studies are summarized. The evidence for peripheral and central mechanisms of action is also summarized.
Keywords: stroke rehabilitation, upper limb hemiplegia, neuroplasticity, medical device, electrical stimulation therapy
Introduction
Motor impairment is common after stroke and directly impacts the stroke survivor’s function and quality of life. Neuromuscular electrical stimulation (NMES) may reduce disability by improving recovery of volitional movement (therapeutic effect) or by assisting and replacing lost volitional movement (neuroprosthetic effect). This article describes NMES treatment modalities for upper and lower limb stroke rehabilitation and summarizes the research literature regarding the therapeutic and neuroprosthetic efficacy of those modalities. The scope of this article is limited to NMES interventions that produce limb movement by direct stimulation of the peripheral nerves or motor points of target muscles for the purpose of restoring motor function, and therefore does not cover somatosensory electrical stimulation,1 electrical stimulation for post-stroke shoulder pain,2 or brain stimulation modalities.3
NMES Fundamentals
NMES is the use of electrical current to produce contractions of paralyzed or paretic muscles. Lower motor neurons to target muscles must be intact for NMES to effectively produce muscle contractions; therefore, NMES is usually only applicable to patients whose paralysis or paresis is caused by upper motor neuron injury (e.g., stroke, spinal cord injury, etc.). NMES can be applied to paretic muscles with surface electrodes positioned on the skin over the motor points of target muscles, or with electrodes that are implanted near or on the muscle motor points or nerves that innervate target muscles. The electrical current generated by most NMES devices can be characterized as a waveform of pulses having a particular pulse frequency, width, and amplitude. The strength of evoked muscle contraction can be modulated by adjusting the pulse parameters. Typically, the stimulation frequency is set between 12 to 50 Hz, and the strength of muscle contraction is modulated by changing either the pulse amplitude (typically 0 to 100 mA) or pulse width (typically 0 to 300 μsec).
An NMES device fundamentally consists of electrodes that are connected to a stimulator, and a controller (Fig. 1). A pair of electrodes constitutes a stimulus channel. Surface (i.e., transcutaneous) electrodes, percutaneous intramuscular wire electrodes, and implanted epimysial, intramuscular, or nerve cuff electrodes may be used. The stimulator (i.e., pulse generator) may have a controller built into it or have a separate controller attached or wirelessly linked to it. The controller regulates the timing and intensity of stimulation delivered through one or multiple stimulus channels. Input to the stimulator’s controller may be via buttons, switches, and/or various types of external or implanted sensors or recording (e.g., electromyographic) electrodes.
Figure 1.
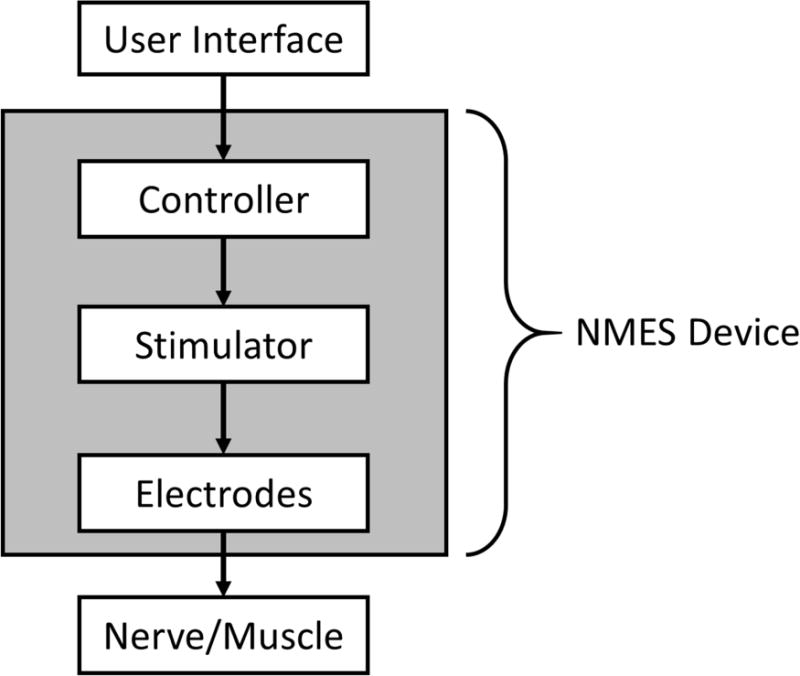
Diagram of a basic neuromuscular electrical stimulation (NMES) device.
Purposes of NMES for Upper and Lower Limb Rehabilitation after Stroke
Paresis is the inability or decreased ability to volitionally activate motor units and is one of the most common manifestations of stroke.4 Clinically, paresis presents as muscle weakness and reduced speed of activation, and the inability to generate functionally useful movement of the involved limb. Lang and associates studied the relative strengths of the associations between specific upper limb impairments and function, and concluded that paresis was the strongest contributor to the loss of function.5 In the upper limb, the combination of paresis, loss of fractionated movements, flexor hypertonia, and somatosensory abnormalities often manifests as difficulty extending the elbow and opening the hand in a functional manner, which severely limits the functional workspace. At six months post-stroke about 65% of patients still cannot incorporate the affected arm and hand into their daily activities.6 Therefore, NMES for upper limb stroke rehabilitation is usually applied to elbow, wrist, and/or hand extensor muscles.
In the lower limb, paresis, along with the inability to grade muscle contractions, poor motor coordination, poor endurance, spasticity, and impaired balance have significant consequences on ambulation.7 At six months post-stroke approximately 30% of stroke survivors are unable to walk unassisted.8 A major contributor to impaired ambulation is the inability to dorsiflex the ankle during the swing phase of gait. Diminished ankle dorsiflexion, knee flexion, or hip flexion can result in inability to clear the floor with the affected limb during the swing phase of gait, resulting in difficult and unsafe ambulation or nonambulation. Patients frequently use compensatory strategies such as circumduction, hip-hiking, or vaulting to clear the toes. An ankle-foot-orthosis (AFO) is the standard of care for footdrop, but because AFOs limit ankle mobility they may actually inhibit recovery of dorsiflexion. Therefore, NMES has been used to improve ankle dorsiflexion and a more normal gait pattern.
Various NMES modalities have been used for upper and lower limb motor relearning after stroke. Motor relearning is defined as the reacquisition of motor skills following central nervous system injury. NMES can be used as a motor relearning tool by enabling stroke survivors with significant paresis to participate in goal-oriented repetitive movement therapy. The NMES-mediated task must be repetitive, novel, volitionally controlled, and functionally relevant.9–11 While the stroke survivor may use an NMES motor relearning system to assist execution of daily activities, its primary intent is training, such that improved functional use of the hemiparetic limb is maintained when the system is not being used. Improved upper limb function or ambulation that remains after an NMES device has been used is called a therapeutic effect.
For patients who are in the chronic phase of stroke and in whom motor relearning strategies have been exhausted, NMES may be used as a neuroprosthesis. The primary intent of a neuroprosthesis is to enable patients to execute functional tasks with the affected upper limb or walk while using the device as part of routine daily living. Improved function that is realized while using an NMES device is called a neuroprosthetic effect.
NMES Modalities for Upper Limb Rehabilitation
Cyclic NMES uses a one- or two-channel stimulator to activate the wrist and/or finger and thumb extensors in a repetitive (cyclic) fashion via surface electrodes placed on the forearm over the motor points of those muscles. Cyclic NMES devices typically have a menu of on/off cycle settings from which to choose. Once the device is set up and switched on, the stimulation automatically ramps on and off according to a selected duty cycle, with the patient not having to exert any simultaneous effort. The patient does not control the timing or intensity of cyclic stimulation (Table 1); therefore, this modality is not typically used to mediate functional task practice.
Table 1.
Degree of Patient Control for Different Upper Limb NMES Modalities
| Patient has real-time control of: | Cyclic NMES | EMG-triggered NMES | Switch-triggered NMES | Contralaterally Controlled NMES |
|---|---|---|---|---|
| Timing of NMES | No | Onset only | Yes | Yes |
| Intensity of NMES | No | No | No | Yes |
Cyclic NMES has been shown in several randomized controlled trials (RCTs) of acute and subacute hemiplegic patients to reduce upper limb motor impairment (e.g., increase in strength, upper limb Fugl-Meyer score, etc.) relative to controls.12–16 Some studies reported an enduring effect over 2 to 6 months,12,13,15,16 while others found that the effect was not sustained beyond the treatment period.14 Some studies found that the positive effects on impairment did not translate to significant improvements in basic self-care tasks or upper limb function (i.e., functional independent measure (FIM) score, action research arm test (ARAT)) relative to controls,13,14 while other studies did show significant, though sometimes transient, improvements in function relative to controls.12,17 The beneficial effects of cyclic NMES seem to be more apparent in patients who have some residual movement at baseline.12,18 In a study of 95 subacute patients, initial motor severity (i.e., baseline Fugl-Meyer score) was identified as the most significant predictor of improvement in upper limb function after 4 weeks of cyclic NMES.17 Studies of cyclic NMES in chronic hemiplegia have typically been relatively small case series designs (i.e., no control group), but have also demonstrated improvements in various upper limb motor impairment measures.18,19
EMG (electromyographic)-triggered NMES attempts to make stimulated hand opening coincide with the patient’s own effort to open the hand. Surface EMG recording electrodes are placed over the wrist and/or finger extensors of the paretic side to detect EMG signals when the patient attempts to open the hand. When the processed EMG signal surpasses a pre-set threshold, electrical stimulation ramps on to a pre-set stimulation intensity that produces full hand opening. After several seconds the stimulation turns off and the patient is prompted with visual and/or audio cues to try to open the hand again, repeating the EMG-triggered NMES cycle. Thus, EMG-triggered stimulation facilitates repetitive and volitionally initiated exercises of the hemiparetic upper extremity and provides cutaneous and proprioceptive feedback time-locked to each attempted movement,20 which may be important for motor relearning.21 Like cyclic NMES, EMG-triggered NMES is not typically used to mediate functional task practice because the intensity and duration of stimulation are not controlled by the patient (Table 1). And because EMG-triggered NMES requires the patient to be able to produce discernable EMG signals consistently, it may not be applicable to the most severely impaired patients.21
EMG-triggered NMES has been shown to improve upper limb motor impairment. An early case series study of 69 chronic patients reported improvement in wrist active range of motion and extensor EMG activity in response to EMG-triggered NMES integrated with conventional therapy.21 The participants who received a greater dosage (i.e., sessions per week) of EMG-triggered NMES had greater increases in voluntary extensor EMG amplitude. RCTs in chronic hemiplegia also show that EMG-triggered NMES improved performance on one or more measures of motor impairment (e.g., Fugl-Meyer score, Box and Blocks score, extensor and grip strength) as compared to conventional therapy, though not all studies agree on which outcomes improve relative to controls.22–25 Most of the trials in chronic patients did not assess upper limb function (i.e. activity limitation) or the persistence of effect. In acute and subacute patients, a RCT showed greater improvement on impairment measures but not on upper limb function relative to conventional therapy,20 but another study showed the opposite – improvement on function (i.e., ARAT) but not on impairment measures relative to usual care.26 Nearly all of the RCTs of EMG-triggered NMES have had small sample sizes (i.e., < 10 per group), and like cyclic NMES, the improvements relative to controls are generally modest and of questionable clinical relevance.
Although EMG-triggered NMES might be expected to improve upper limb movement and function more than cyclic NMES,27 several RCTs that directly compared the two treatments showed no significant difference in the outcomes of cyclic and EMG-triggered NMES, whether in chronic28,29 or subacute30,31 subjects. Explanations for why no differences in outcomes were found between cyclic and EMG-triggered NMES include: 1) EMG-triggered NMES may not require enough active involvement (i.e., patients only trigger stimulation, not control duration or intensity) to create a large enough contrast with cyclic NMES,28 2) the cyclic NMES group may have also been exerting effort during stimulation, further reducing the contrast between the two treatments,28 3) with EMG-triggered NMES, any time delays between the attempt to extend the wrist and fingers and the initiation of stimulation may negate any neurophysiological advantage the treatment might have had over cyclic NMES.
Switch-triggered NMES is a modality intended to facilitate functional task practice. Switches (or button presses) allow the patient32 or therapist33 to control both the initiation and termination of stimulation sequences (i.e., the timing of the stimulated movement, Table 1) with button presses so that the device can be used in assisting task practice during therapy sessions.34 The intensity of stimulation is not controlled by the patient, but is pre-set. The Bioness H200 (Bioness Inc., Valencia, CA) is an example of a switch-triggered device that stimulates finger and thumb extensors and flexors through surface electrodes that are mounted inside a wrist-forearm orthosis, which also houses the stimulator. The patient turns stimulation on and off to the extensors and flexors by pressing buttons on a separate control unit with their unaffected hand. Stimulation sequences that produce different hand opening and closing postures can be programmed and selected to match the task to be performed. Significant therapeutic effects were reported on several measures of motor impairment (e.g., Box and Blocks score, Ashworth score) and function (e.g., timed Jebsen-Taylor Hand Function tasks) in chronic patients after 5 weeks of home exercise and task practice with the Bioness H200.35 Several follow-up RCTs in acute34,36 and subacute32 patients found that switch-triggered NMES with therapy had greater improvements than therapy alone on measures of spasticity, wrist extension, Box and Blocks score, Fugl-Meyer score, and timed tasks. Although the Bioness H200 can be used as a neuroprosthesis and has been shown to have a significant neuroprosthetic effect,37 it is typically used and studied as a motor relearning tool.
Another switch-triggered NMES approach uses stimulation only as needed to assist first with repetitive reaching tasks (stimulating shoulder and elbow muscles), and then with grasping tasks (stimulating wrist, finger, and thumb muscles), progressively decreasing the use of NMES as the patient improves.38 The treating therapist uses button switches to activate the stimulation sequences that are needed to perform tasks. Greater therapeutic effects were measured in acute patients who had 12–16 weeks of this switch-triggered NMES approach as compared to patients who received conventional task-specific occupational therapy.33
Sensor- and EMG-controlled NMES modalities use controllers that are designed to let the patient control the timing and intensity of stimulation to their hand in a way that can be fluid with task practice, which may result in greater sensorimotor integration and superior motor relearning (i.e., therapeutic effects). Such systems may also be suitable as neuroprostheses to assist with activities of daily living. Indeed, the earliest NMES devices for upper limb stroke rehabilitation used a sensor mounted to the contralateral shoulder to let the patient proportionally control the intensity of stimulation to the forearm extensors as they practiced tasks.39 Electrogoniometers, bend sensors, touch-sensitive mats, and accelerometers are among the external sensors that have been incorporated into NMES systems for upper limb stroke rehabilitation.40–43 Researchers also continue to explore the use of EMG signals from the impaired upper limb to not merely trigger the onset of a pre-set intensity and duration of stimulation, but to control the intensity and timing of stimulation.44,45 A challenge for EMG-controlled NMES modalities is that the effort required from the patient to contract the muscle that operates the controller may induce flexor synergies or hypertonia, which can overpower the electrical stimulation of extensors and result in reduced degrees of stimulated hand opening.44,46
Contralaterally controlled NMES is a unique version of sensor-controlled stimulation that uses movement from the unimpaired side to control the timing and intensity of stimulation to the paretic side (Table 1).43 The hand system consists of a glove with bend sensors worn on the non-paretic hand and a multi-channel stimulator that delivers stimulation to the paretic hand extensors with an intensity that is proportional to the degree of opening of the glove (Fig. 2). This modality enables repetitive hand opening exercise and functional task practice with the paretic hand. The control strategy gives the user intimate proportional control of the stimulation intensity without requiring any residual movement or EMG signals from the paretic hand. Therefore, the likelihood of triggering flexor synergy patterns may be less than sensor-controlled or EMG-controlled stimulation devices that require control signals from the paretic limb. Contralaterally controlled NMES produced larger improvements in maximum voluntary finger extension and other measures of upper extremity impairment and activity limitation than cyclic NMES in a RCT of subacute patients.47
Figure 2.
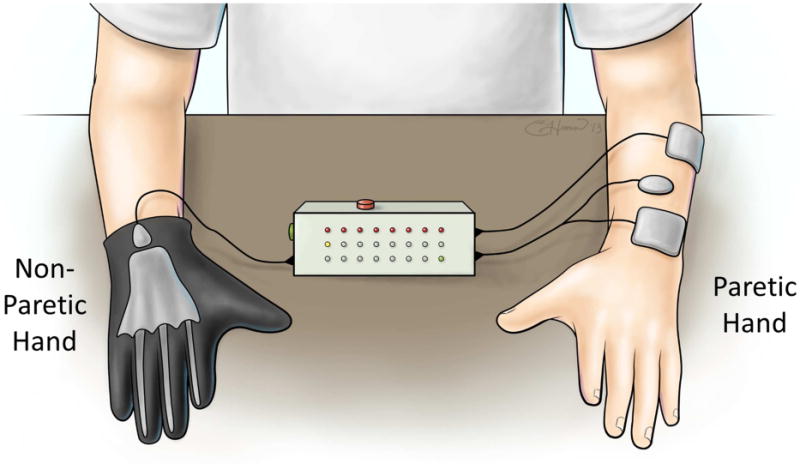
Contralaterally Controlled NMES System, an example of a sensor-controlled NMES modality. Volitional opening of the non-paretic hand wearing an instrumented glove produces a proportional intensity of stimulation to the paretic hand, giving patients control of the timing and intensity of NMES.
NMES Modalities for Lower Limb Rehabilitation
Cyclic, EMG-triggered, and contralaterally controlled NMES applied to paretic lower limb muscles while the subject is seated or side-lying have been evaluated for therapeutic effects. In a randomized placebo-controlled trial of 46 acute hemiplegic subjects, cyclic NMES was applied to the quadriceps, hamstring, tibialis anterior, and medial gastrocnemius in an activation sequence that mimicked normal gait while the subjects were side-lying with their lower extremity supported by a sling.48 Significantly greater improvement in ankle dorsiflexion torque and EMG activity and significantly less spasticity and co-contraction were demonstrated after 3 weeks of multi-channel cyclic NMES as compared to the control group. Also, a significantly greater percentage of subjects in the cyclic NMES group were able to complete a timed walking task by the end of the 3-week treatment and 5 weeks later as compared to the control group. EMG-triggered NMES of paretic ankle dorsiflexors has been shown to have positive effects on ankle strength, range of motion, balance, and ambulation in chronic patients.21,49,50 Contralaterally controlled NMES, where the patient controlled the intensity of stimulation to the paretic ankle dorsiflexors by dorsiflexing their non-paretic ankle while seated, was first tested in a case series51 and later in an RCT.52 Contralaterally controlled NMES was shown to increase lower extremity Fugl-Meyer score, maximum dorsiflexion angle and moment while seated, and performance on the modified Emory Functional Ambulation Profile in chronic patients, but not more than cyclic NMES.
Applying NMES to the paretic ankle dorsiflexors (i.e., peroneal nerve) during the swing phase of gait was first described by Liberson and associates in 1961.53 The common peroneal nerve was stimulated with a pair of electrodes, one placed just below the head of the fibula and the other over the tibialis anterior. A heel switch worn in the shoe of the paretic side turned the stimulation on when the foot was lifted off the ground, and turned the stimulation off at heel strike and during the stance phase of gait. Currently there are 3 FDA approved surface electrode NMES systems for preventing footdrop during gait: the Odstock Dropped-Foot Stimulator (Odstock Medical Limited, Salisbury, UK), WalkAide (Innovative Neurotronics Inc., Austin, TX), and Bioness L300 Footdrop System (Bioness Inc., Valencia, CA). These devices utilize either a heel switch or a tilt sensor below the knee to synchronize the timing of stimulation to the swing phase of gait (Fig. 3).54,55 Two multi-channel footdrop systems with implanted electrodes and stimulator have the CE mark in Europe. One is a dual-channel device developed by the University of Twente (Netherlands) that stimulates the deep and superficial branches of the common peroneal nerve for better control of ankle dorsiflexion, eversion, and inversion.56 The other is a fourchannel device, developed at Aalborg University (Denmark) and utilizes a 4-channel nerve cuff electrode surgically placed around the common peroneal nerve.57
Figure 3.
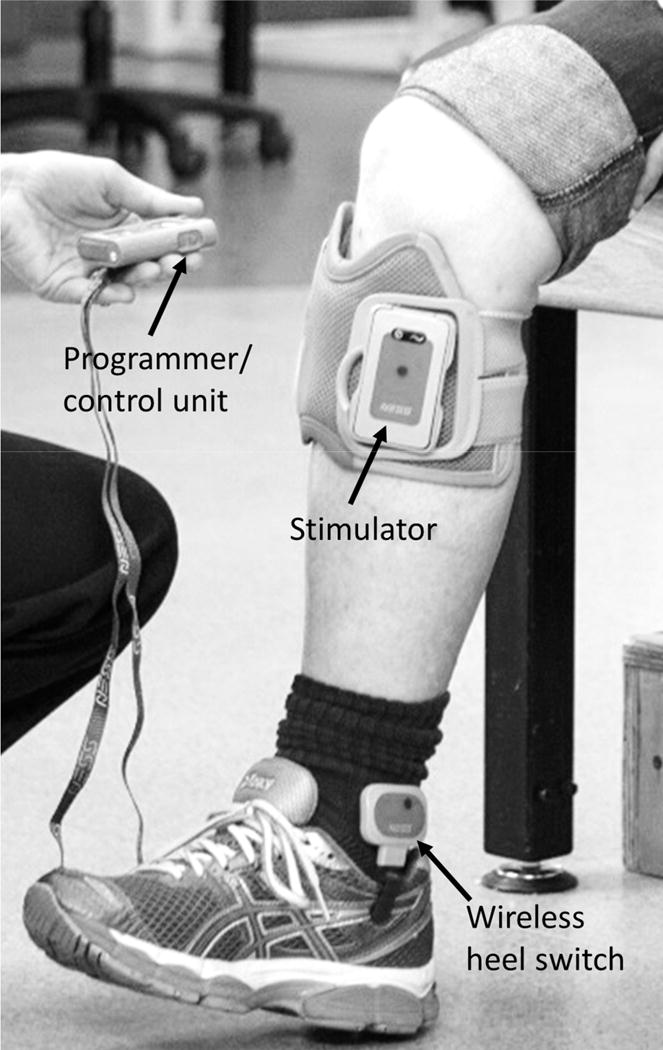
Example of a peroneal nerve stimulator with a wireless heel switch for dorsiflexing the paretic ankle during gait. (Photograph shows the Bioness L300® Foot Drop System).
Peroneal nerve stimulation (PNS) during gait has positive neuroprosthetic and therapeutic effects on ambulation. Neuroprosthetic effects have been shown in a number of case series studies and several RCTs, with outcome measures ranging from gait kinematic and spatio-temporal parameters to metabolic cost indices.54,55,58–60 According to a systematic review, there is a positive neuroprosthetic effect of PNS on walking speed.61 A recent multicenter clinical trial of 99 chronic patients showed that after 42 weeks of PNS during gait, 67% of participants had a gain of ≥ 0.1 m/sec (the minimal clinically important difference) in comfortable gait speed when walking with PNS.62 Therapeutic effects associated with PNS during gait have also been observed since the earliest studies. That is, PNS during gait produces not only positive neuroprosthetic effects (i.e., the effects on gait observed when the stimulator is on), but also “carry-over” or therapeutic effects after the device has been turned off. Such effects have been observed in multiple case series studies, and include improvements in ambulation function, normalization of EMG muscle activation patterns, emergence of EMG signals in previously silent muscles, and decreased co-contraction of antagonist muscles.53–55,63–68 After 30 weeks of PNS during gait, 29% of 99 chronic stroke patients had a therapeutic effect on comfortable walking speed of ≥ 0.1 m/sec.62
The research on PNS during gait has progressed to RCTs comparing the effects of PNS to standard of care, which is an ankle-foot-orthosis (AFO). In order for PNS to challenge standard of care practice, definitive evidence would be necessary to show that PNS provides either an equivalent neuroprosthetic effect on walking or a superior therapeutic effect restoring volitional gait. Four large clinical trials have recently been published comparing PNS to AFO. Sheffler et al. compared the therapeutic effects of 12 weeks of PNS and AFO on lower limb impairment, ambulation, and quality of life in 110 chronic patients and found significant but similar therapeutic effects on ambulation and quality of life from both PNS and AFO.69 Kluding et al. compared the effects of 30 weeks of PNS and AFO in 197 chronic patients and found both groups to improve similarly on walking speed with their assigned device (neuroprosthetic effect). They also noted significant but similar therapeutic effects on walking speed for both groups.70 Everaert et al. enrolled 121 patients who were less than 1 year post-stroke and found similar improvements in walking speed between PNS and AFO groups.71 Bethoux et al. reported a 30-site study that enrolled 495 chronic stroke patients who wore a PNS device or an AFO for 6 months.72 Both groups had significant improvements in gait velocity while wearing their device (AFO or PNS), but no between-group differences were found.
Based on these four large RCTs, PNS during gait for 12–30 weeks can have significant therapeutic effects on functional mobility and walking speed. Wearing the PNS device can further increase walking speed and walking endurance beyond the therapeutic effects. However, no significant differences were found between PNS and AFO on walking speed or functional ambulation, although questionnaires showed that patients preferred PNS over AFO with respect to long-term use, all-day use, confidence on inclines, and ease of donning/doffing.71
Since gait deviation in hemiplegia is not limited to ankle dysfunction, multichannel stimulation systems have been investigated for therapeutic effects. Early work used surface electrodes and demonstrated improvements in qualitative and quantitative measures of gait after training with a 6-channel surface system that activated ankle dorsiflexion and plantarflexion, knee flexion and extension, and hip extension.73,74 However, as the number of electrodes increases, surface systems become increasingly difficult to implement due to difficulty of donning and doffing of multiple electrodes, pain of stimulation, and poor repeatability of electrode placement and muscle contractions. Therefore, multichannel percutaneous systems have also been explored for motor relearning.75 A single-blinded RCT of 32 chronic stroke patients demonstrated that multi-channel percutaneous NMES-mediated ambulation training in combination with body-weight supported treadmill training (BWSTT) improved gait components and knee flexion coordination more than BWSTT without NMES,76 and that the gains were maintained at 6 months post-treatment.77
Peripheral and Central Effects of NMES in Stroke Rehabilitation
The mechanisms by which NMES reduces motor impairment and activity limitation have not been fully elucidated, but therapeutic effects are probably due to a combination of peripheral and central effects. Peripheral effects of NMES include increase in contractile force and fatigue resistance,78,79 increase in muscle mass,80 reduction of edema,81 conversion of fast-twitch fast-fatiguing glycolytic type II muscle fibers to slow-twitch fatigue-resistant oxidative type I muscle fibers,79 and enhanced hyperemic arterial response and endothelium-dependent cutaneous vasodilation.82 These peripheral effects can reverse disuse atrophy and may explain in part some improvements stroke patients experience after various NMES treatments.
Some NMES treatments may also affect the central nervous system and how it controls movement. For example, NMES may promote motor relearning by uniquely providing an artificial way of ensuring synchronized presynaptic and postsynaptic activity (Hebbian plasticity), especially if the electrical stimulation is paired with simultaneous voluntary effort that activates the residual upper motor neurons.83 Indeed, cortical excitability, as assessed by measuring motor evoked potentials in response to transcranial magnetic stimulation, has been shown to increase more when NMES is paired with voluntary muscle contraction than with NMES alone.84 This finding suggests that the effect of NMES on cortical excitability is improved by concurrent voluntary cortical drive. Whether the increase in cortical excitability is due to changes at the spinal level, cortical reorganization, or both is unclear. Several researchers have hypothesized that EMG-triggered NMES may produce functional cortical reorganization by inducing long-term potentiation in sensorimotor cortex caused by proprioceptive and cutaneous afferent feedback occurring concurrently with attempted movements.20,21,23 A regimen of EMG-triggered NMES to the upper extremity has been shown to increase metabolic activity (measured by positron emission tomography) in the contralesional supplementary motor area, primary motor cortex, and primary somatosensory cortex,85 and to increase the intensity of hand related cortical activity (measured by fMRI) in contralesional somatosensory cortex.24 In contrast, a shift in the laterality index toward the ipsilesional sensorimotor cortex was shown after EMG-triggered NMES,22 and brain cortical perfusion (measured by near-infrared spectroscopy) was greater in the ipsilesional sensorimotor cortex during EMG-controlled NMES than during cyclic NMES or voluntary attempts to extend the wrist and fingers.86
There is also evidence that NMES when unpaired with voluntary effort may produce changes in the brain. For example, progressively increasing the intensity of surface NMES of the quadriceps muscle from sensory threshold to maximum motor response produced proportional increases in cortical activity in specific areas of interest, including primary somatosensory and motor cortices, as shown by fMRI.87 Another study showed that stimulation of the common peroneal nerve at 25Hz with intensities above motor threshold for 30 minutes while seated at rest increased the motor-evoked potential (MEP) in the tibialis anterior by 50% at a transcranial magnetic stimulation (TMS) intensity that initially gave a half-maximum MEP. This effect was evident after 10 minutes of stimulation and persisted for at least 30 minutes after stimulation ended.88 Follow-up experiments provided evidence that the increase in excitability did not occur at the level of motorneurons, but rather at the cortical level.88,89 Long-term use of a footdrop stimulator has been found to increase both MEPs elicited by TMS and maximum voluntary contraction of the tibialis anterior in stroke patients, evidence that regular use of a PNS device strengthens activation of motor cortical areas and their residual descending connections.90
These and other studies provide mounting evidence that there is a cortical component to NMES, but more studies are needed to elucidate the precise mechanisms at work under specific NMES modalities and patient characteristics.
Emerging Directions for NMES in Stroke Rehabilitation
New NMES techniques for upper and lower limb stroke rehabilitation continue to be developed, especially those that use sensors to trigger stimulation when patients achieve some minimum volitional movement.42,91,92 It is highly doubtful that any single NMES modality used in isolation from other motor rehabilitation therapies will lead to substantial motor recovery. Therefore, there is a growing trend toward combining NMES with other emerging therapeutic strategies that have shown promise. Examples include combining NMES with mirror therapy,93 repetitive transcranial magnetic stimulation,94 constraint-induced movement therapy,95 robot-assisted movement therapy,96 motor imagery,30,85 bilateral movement training,97 virtual reality games,98 transcranial direct current stimulation,99 and body-weight-supported treadmill training.77,100 Perhaps the best stroke rehabilitation program would have a defined sequence of therapies and combination therapies that become suitable for stroke patients as they progress from severe impairment to complete motor recovery, NMES being an important component in the slate of rehabilitation therapies and techniques.
At the present time, a clinically viable upper extremity neuroprosthesis for daily long-term use as an assistive device is not available for persons with hemiparesis. Implantable microstimulator40,101 or multi-channel implantable pulse generator44 approaches may be suitable for stroke patients who have been carefully screened for prohibitive flexor hypertonia. But most patients will not be able to realize a robust neuroprosthetic effect unless a means of suppressing flexor hypertonia is incorporated. Emerging technology that uses nerve cuff electrodes to deliver high-frequency stimulus waveforms to block action potentials in nerves may prove capable of suppressing hypertonia.102 Adding such spasticity suppressing stimulation to an NMES system could considerably improve its neuroprosthetic effect and widen its applicability.
As more NMES modalities and technology continue to emerge, more clinical research studies will be needed. With some exceptions, most of the NMES efficacy studies to date have been relatively small and therefore limited in power to make strong conclusions. Large RCTs comparing different NMES modalities as well as comparing NMES to standard of care are still needed. Studies aimed at elucidating the mechanisms of NMES-mediated recovery (i.e., specific effects on the CNS) could lead to treatment optimization. Also studies are needed to define optimum treatment dose and the most likely responders for any given NMES modality.
Key Points.
Hemiparesis following stroke is associated with significant upper and lower limb impairment, activity limitation, and reduced quality of life
Neuromuscular electrical stimulation as a motor relearning tool reduces upper and lower limb motor impairment following stroke
Neuromuscular electrical stimulation as a neuroprosthesis improves ambulation function of stroke survivors, but not more than standard of care ankle-foot-orthoses.
Research is needed to more firmly establish the effects of electrical stimulation on upper limb activity limitations and quality of life.
The benefit of upper limb neuromuscular electrical stimulation modalities relative to alternative therapies or standard of care remains to be fully elucidated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Knutson and Dr. Chae are co-inventors on the contralaterally controlled neuromuscular electrical stimulation patent assigned to Case Western Reserve University, Patent 8,165,685: System and Method for Therapeutic Neuromuscular Electrical Stimulation.
Contributor Information
Michael J. Fu, Email: mjf24@case.edu.
Lynne R. Sheffler, Email: lsheffler@metrohealth.org.
John Chae, Email: jchae@metrohealth.org.
References
- 1.Conforto AB, Cohen LG, dos Santos RL, Scaff M, Marie SK. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254:333–339. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93:17–28. doi: 10.1097/PHM.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey RL, Stinear JW. Cortical Stimulation as an Adjuvant to Upper Limb Rehabilitation After Stroke. Pm&R. 2010;2:S269–S278. doi: 10.1016/j.pmrj.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Sathian K, Buxbaum LJ, Cohen LG, et al. Neurological principles and rehabilitation of action disorders: common clinical deficits. Neurorehabil Neural Repair. 2011;25:21S–32S. doi: 10.1177/1545968311410941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther. 2013;26:104–114. doi: 10.1016/j.jht.2012.06.005. quiz 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobkin BH. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert review of neurotherapeutics. 2007;7:1417–1436. doi: 10.1586/14737175.7.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 9.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 10.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 11.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 12.Powell J, Pandyan AD, Granat M, Cameron M, Stott DJ. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30:1384–1389. doi: 10.1161/01.str.30.7.1384. [DOI] [PubMed] [Google Scholar]
- 13.Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29:975–979. doi: 10.1161/01.str.29.5.975. [DOI] [PubMed] [Google Scholar]
- 14.Rosewilliam S, Malhotra S, Roffe C, Jones P, Pandyan AD. Can surface neuromuscular electrical stimulation of the wrist and hand combined with routine therapy facilitate recovery of arm function in patients with stroke? Arch Phys Med Rehabil. 2012;93:1715–1721 e1711. doi: 10.1016/j.apmr.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Hsu SS, Hu MH, Wang YH, Yip PK, Chiu JW, Hsieh CL. Dose-response relation between neuromuscular electrical stimulation and upper-extremity function in patients with stroke. Stroke. 2010;41:821–824. doi: 10.1161/STROKEAHA.109.574160. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Yan T. Long-term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. J Rehabil Med. 2011;43:506–510. doi: 10.2340/16501977-0807. [DOI] [PubMed] [Google Scholar]
- 17.Hsu SS, Hu MH, Luh JJ, Wang YH, Yip PK, Hsieh CL. Dosage of neuromuscular electrical stimulation: is it a determinant of upper limb functional improvement in stroke patients? J Rehabil Med. 2012;44:125–130. doi: 10.2340/16501977-0917. [DOI] [PubMed] [Google Scholar]
- 18.Hendricks HT, MJ IJ, de Kroon JR, in ’t Groen FA, Zilvold G. Functional electrical stimulation by means of the ‘Ness Handmaster Orthosis’ in chronic stroke patients: an exploratory study. Clin Rehabil. 2001;15:217–220. doi: 10.1191/026921501672937235. [DOI] [PubMed] [Google Scholar]
- 19.Santos M, Zahner LH, McKiernan BJ, Mahnken JD, Quaney B. Neuromuscular electrical stimulation improves severe hand dysfunction for individuals with chronic stroke: a pilot study. J Neurol Phys Ther. 2006;30:175–183. doi: 10.1097/01.npt.0000281254.33045.e4. [DOI] [PubMed] [Google Scholar]
- 20.Francisco G, Chae J, Chawla H, et al. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79:570–575. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- 21.Fields RW. Electromyographically triggered electric muscle stimulation for chronic hemiplegia. Arch Phys Med Rehabil. 1987;68:407–414. [PubMed] [Google Scholar]
- 22.Shin HK, Cho SH, Jeon HS, et al. Cortical effect and functional recovery by the electromyography-triggered neuromuscular stimulation in chronic stroke patients. Neurosci Lett. 2008;442:174–179. doi: 10.1016/j.neulet.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000;31:1360–1364. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 24.Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res. 2004;154:450–460. doi: 10.1007/s00221-003-1695-y. [DOI] [PubMed] [Google Scholar]
- 25.Kraft GH, Fitts SS, Hammond MC. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch Phys Med Rehabil. 1992;73:220–227. [PubMed] [Google Scholar]
- 26.Bello AI, Rockson BE, Olaogun MO. The effects of electromyographic-triggered neuromuscular electrical muscle stimulation on the functional hand recovery among stroke survivors. African journal of medicine and medical sciences. 2009;38:185–191. [PubMed] [Google Scholar]
- 27.de Kroon JR, Ijzerman MJ, Chae J, Lankhorst GJ, Zilvold G. Relation between stimulation characteristics and clinical outcome in studies using electrical stimulation to improve motor control of the upper extremity in stroke. J Rehabil Med. 2005;37:65–74. doi: 10.1080/16501970410024190. [DOI] [PubMed] [Google Scholar]
- 28.de Kroon JR, Ijzerman MJ. Electrical stimulation of the upper extremity in stroke: cyclic versus EMG-triggered stimulation. Clin Rehabil. 2008;22:690–697. doi: 10.1177/0269215508088984. [DOI] [PubMed] [Google Scholar]
- 29.Boyaci A, Topuz O, Alkan H, et al. Comparison of the effectiveness of active and passive neuromuscular electrical stimulation of hemiplegic upper extremities: a randomized, controlled trial. International journal of rehabilitation research Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. 2013;36:315–322. doi: 10.1097/MRR.0b013e328360e541. [DOI] [PubMed] [Google Scholar]
- 30.Hemmen B, Seelen HA. Effects of movement imagery and electromyography-triggered feedback on arm hand function in stroke patients in the subacute phase. Clin Rehabil. 2007;21:587–594. doi: 10.1177/0269215507075502. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RD, Page SJ, Delahanty M, et al. Electrical stimulation for upper limb recovery in stroke: a randomized controlled trial comparing EMG-triggered, cyclic, and sensory electrical stimulation. doi: 10.1177/1545968316650278. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37:32–36. doi: 10.1080/16501970410035387. [DOI] [PubMed] [Google Scholar]
- 33.Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22:706–714. doi: 10.1177/1545968308317436. [DOI] [PubMed] [Google Scholar]
- 34.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21:207–215. doi: 10.1177/1545968306297871. [DOI] [PubMed] [Google Scholar]
- 35.Alon G, Sunnerhagen KS, Geurts AC, Ohry A. A home-based, self-administered stimulation program to improve selected hand functions of chronic stroke. NeuroRehabilitation. 2003;18:215–225. [PubMed] [Google Scholar]
- 36.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation (FES) may modify the poor prognosis of stroke survivors with severe motor loss of the upper extremity: a preliminary study. Am J Phys Med Rehabil. 2008;87:627–636. doi: 10.1097/PHM.0b013e31817fabc1. [DOI] [PubMed] [Google Scholar]
- 37.Alon G, McBride K, Ring H. Improving selected hand functions using a noninvasive neuroprosthesis in persons with chronic stroke. Journal of Stroke and Cerebrovascular Disease. 2002;11:99–106. doi: 10.1053/jscd.2002.127107. [DOI] [PubMed] [Google Scholar]
- 38.Popovic MR, Thrasher TA, Zivanovic V, Takaki J, Hajek V. Neuroprosthesis for retraining reaching and grasping functions in severe hemiplegic patients. Neuromodulation : journal of the International Neuromodulation Society. 2005;8:58–72. doi: 10.1111/j.1094-7159.2005.05221.x. [DOI] [PubMed] [Google Scholar]
- 39.Merletti R, Acimovic R, Grobelnik S, Cvilak G. Electrophysiological orthosis for the upper extremity in hemiplegia: feasibility study. Arch Phys Med Rehabil. 1975;56:507–513. [PubMed] [Google Scholar]
- 40.Burridge JH, Turk R, Merrill D, et al. A personalized sensor-controlled microstimulator system for arm rehabilitation poststroke. Part 2: Objective outcomes and patients’ perspectives. Neuromodulation : journal of the International Neuromodulation Society. 2011;14:80–88. doi: 10.1111/j.1525-1403.2010.00310.x. discussion 88. [DOI] [PubMed] [Google Scholar]
- 41.Merrill DR, Davis R, Turk R, Burridge JH. A personalized sensor-controlled microstimulator system for arm rehabilitation poststroke. Part 1: System architecture. Neuromodulation : journal of the International Neuromodulation Society. 2011;14:72–79. doi: 10.1111/j.1525-1403.2010.00309.x. discussion 79. [DOI] [PubMed] [Google Scholar]
- 42.Mann G, Taylor P, Lane R. Accelerometer-triggered electrical stimulation for reach and grasp in chronic stroke patients: a pilot study. Neurorehabil Neural Repair. 2011;25:774–780. doi: 10.1177/1545968310397200. [DOI] [PubMed] [Google Scholar]
- 43.Knutson JS, Harley MY, Hisel TZ, Chae J. Improving hand function in stroke survivors: a pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Arch Phys Med Rehabil. 2007;88:513–520. doi: 10.1016/j.apmr.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knutson JS, Chae J, Hart RL, et al. Implanted neuroprosthesis for assisting arm and hand function after stroke: A case study. J Rehabil Res Dev. 2012;49:1505–1516. doi: 10.1682/jrrd.2011.09.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorsen R, Cortesi M, Jonsdottir J, et al. Myoelectrically driven functional electrical stimulation may increase motor recovery of upper limb in poststroke subjects: a randomized controlled pilot study. J Rehabil Res Dev. 2013;50:785–794. doi: 10.1682/JRRD.2012.07.0123. [DOI] [PubMed] [Google Scholar]
- 46.Makowski N, Knutson J, Chae J, Crago P. Interaction of poststroke voluntary effort and functional neuromuscular electrical stimulation. J Rehabil Res Dev. 2013;50:85–98. doi: 10.1682/jrrd.2011.04.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson JS, Harley MY, Hisel TZ, Hogan SD, Maloney MM, Chae J. Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: an early-phase randomized clinical trial in subacute stroke patients. Neurorehabil Neural Repair. 2012;26:239–246. doi: 10.1177/1545968311419301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36:80–85. doi: 10.1161/01.STR.0000149623.24906.63. [DOI] [PubMed] [Google Scholar]
- 49.Chae J, Fang ZP, Walker M, Pourmehdi S, Knutson J. Intramuscular electromyographically controlled neuromuscular electrical stimulation for ankle dorsiflexion recovery in chronic hemiplegia. Am J Phys Med Rehabil. 2001;80:842–847. doi: 10.1097/00002060-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Barth E, Herrman V, Levine P, Dunning K, Page SJ. Low-dose, EMG-triggered electrical stimulation for balance and gait in chronic stroke. Top Stroke Rehabil. 2008;15:451–455. doi: 10.1310/tsr1505-451. [DOI] [PubMed] [Google Scholar]
- 51.Knutson JS, Chae J. A novel neuromuscular electrical stimulation treatment for recovery of ankle dorsiflexion in chronic hemiplegia: a case series pilot study. Am J Phys Med Rehabil. 2010;89:672–682. doi: 10.1097/PHM.0b013e3181e29bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knutson JS, Hansen K, Nagy J, et al. Contralaterally controlled neuromuscular electrical stimulation for recovery of ankle dorsiflexion: a pilot randomized controlled trial in patients with chronic post-stroke hemiplegia. Am J Phys Med Rehabil. 2013;92:656–665. doi: 10.1097/PHM.0b013e31829b4c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liberson WT, Holmquest HJ, Scot D, Dow M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961;42:101–105. [PubMed] [Google Scholar]
- 54.Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11:201–210. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 55.Stein RB, Chong S, Everaert DG, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006;20:371–379. doi: 10.1177/1545968306289292. [DOI] [PubMed] [Google Scholar]
- 56.Kottink AI, Hermens HJ, Nene AV, et al. A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil. 2007;88:971–978. doi: 10.1016/j.apmr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Burridge JH, Haugland M, Larsen B, et al. Phase II trial to evaluate the ActiGait implanted drop-foot stimulator in established hemiplegia. J Rehabil Med. 2007;39:212–218. doi: 10.2340/16501977-0039. [DOI] [PubMed] [Google Scholar]
- 58.Merletti R, Andina A, Galante M, Furlan I. Clinical experience of electronic peroneal stimulators in 50 hemiparetic patients. Scand J Rehabil Med. 1979;11:111–121. [PubMed] [Google Scholar]
- 59.Granat MH, Maxwell DJ, Ferguson AC, Lees KR, Barbenel JC. Peroneal stimulator; evaluation for the correction of spastic drop foot in hemiplegia. Arch Phys Med Rehabil. 1996;77:19–24. doi: 10.1016/s0003-9993(96)90214-2. [DOI] [PubMed] [Google Scholar]
- 60.Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010;32:1594–1603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- 61.Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, MJ IJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004;28:577–586. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- 62.O’Dell MW, Dunning K, Kluding P, et al. Response and prediction of improvement in gait speed from functional electrical stimulation in persons with poststroke drop foot. PM & R : the journal of injury, function, and rehabilitation. 2014;6:587–601. doi: 10.1016/j.pmrj.2014.01.001. quiz 601. [DOI] [PubMed] [Google Scholar]
- 63.Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–1583. doi: 10.1016/s0003-9993(99)90333-7. [DOI] [PubMed] [Google Scholar]
- 64.Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. 2006;87:853–859. doi: 10.1016/j.apmr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 65.Stefancic M, Rebersek M, Merletti R. The therapeutic effects of the Ljublijana functional electrical brace. Eur Medicophys. 1976;12:1–9. [Google Scholar]
- 66.Carnstam B, Larsson LE, Prevec TS. Improvement of gait following functional electrical stimulation. I. Investigations on changes in voluntary strength and proprioceptive reflexes. Scand J Rehabil Med. 1977;9:7–13. [PubMed] [Google Scholar]
- 67.Kljajic M, Malezic M, Acimovic R, et al. Gait evaluation in hemiparetic patients using subcutaneous peroneal electrical stimulation. Scand J Rehabil Med. 1992;24:121–126. [PubMed] [Google Scholar]
- 68.Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–167. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- 69.Sheffler LR, Taylor PN, Gunzler DD, Buurke JH, Ijzerman MJ, Chae J. Randomized controlled trial of surface peroneal nerve stimulation for motor relearning in lower limb hemiparesis. Arch Phys Med Rehabil. 2013;94:1007–1014. doi: 10.1016/j.apmr.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kluding PM, Dunning K, O’Dell MW, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke. 2013;44:1660–1669. doi: 10.1161/STROKEAHA.111.000334. [DOI] [PubMed] [Google Scholar]
- 71.Everaert DG, Stein RB, Abrams GM, et al. Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabil Neural Repair. 2013;27:579–591. doi: 10.1177/1545968313481278. [DOI] [PubMed] [Google Scholar]
- 72.Bethoux F, Rogers HL, Nolan KJ, et al. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2014;28:688–697. doi: 10.1177/1545968314521007. [DOI] [PubMed] [Google Scholar]
- 73.Bogataj U, Gros N, Kljajic M, Acimovic R, Malezic M. The rehabilitation of gait in patients with hemiplegia: a comparison between conventional therapy and multichannel functional electrical stimulation therapy. Physical Therapy. 1995;75:490–502. doi: 10.1093/ptj/75.6.490. [DOI] [PubMed] [Google Scholar]
- 74.Stanic U, Acimovic-Janezic R, Gros N, Trnkoczy A, Bajd T, Kljajic M. Multichannel electrical stimulation for correction of hemiplegic gait. Scand J Rehab Med. 1978;10:75–92. [PubMed] [Google Scholar]
- 75.Daly JJ, Barnicle K, Kobetic R, Marsolais EB. Electrically induced gait changes post stroke, using an FNS system with intramuscular electrodes and multiple channels. J Neuro Rehab. 1993;7:17–25. [Google Scholar]
- 76.Daly JJ, Roenigk K, Holcomb J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–178. doi: 10.1161/01.STR.0000195129.95220.77. Epub 2005 Dec 2001. [DOI] [PubMed] [Google Scholar]
- 77.Daly JJ, Zimbelman J, Roenigk KL, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair. 2011;25:588–596. doi: 10.1177/1545968311400092. [DOI] [PubMed] [Google Scholar]
- 78.Peckham PH, Mortimer JT, Marsolais EB. Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin Orthop Relat Res. 1976:326–333. [PubMed] [Google Scholar]
- 79.Gondin J, Brocca L, Bellinzona E, et al. Neuromuscular electrical stimulation training induces atypical adaptations of the human skeletal muscle phenotype: a functional and proteomic analysis. Journal of applied physiology. 2011;110:433–450. doi: 10.1152/japplphysiol.00914.2010. [DOI] [PubMed] [Google Scholar]
- 80.Arija-Blazquez A, Ceruelo-Abajo S, Diaz-Merino MS, et al. Effects of electromyostimulation on muscle and bone in men with acute traumatic spinal cord injury: A randomized clinical trial. J Spinal Cord Med. 2013 doi: 10.1179/2045772313Y.0000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meijer JW, Voerman GE, Santegoets KM, Geurts AC. Short-term effects and long-term use of a hybrid orthosis for neuromuscular electrical stimulation of the upper extremity in patients after chronic stroke. J Rehabil Med. 2009;41:157–161. doi: 10.2340/16501977-0299. [DOI] [PubMed] [Google Scholar]
- 82.Wang JS, Chen SY, Lan C, Wong MK, Lai JS. Neuromuscular electric stimulation enhances endothelial vascular control and hemodynamic function in paretic upper extremities of patients with stroke. Arch Phys Med Rehabil. 2004;85:1112–1116. doi: 10.1016/j.apmr.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 83.Rushton DN. Functional electrical stimulation and rehabilitation–an hypothesis. Med Eng Phys. 2003;25:75–78. doi: 10.1016/s1350-4533(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 84.Khaslavskaia S, Sinkjaer T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res. 2005;162:497–502. doi: 10.1007/s00221-004-2153-1. [DOI] [PubMed] [Google Scholar]
- 85.Hong IK, Choi JB, Lee JH. Cortical changes after mental imagery training combined with electromyography-triggered electrical stimulation in patients with chronic stroke. Stroke. 2012;43:2506–2509. doi: 10.1161/STROKEAHA.112.663641. [DOI] [PubMed] [Google Scholar]
- 86.Hara Y, Obayashi S, Tsujiuchi K, Muraoka Y. The effects of electromyography-controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clin Neurophysiol. 2013;124:2008–2015. doi: 10.1016/j.clinph.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 87.Smith GV, Alon G, Roys SR, Gullapalli RP. Functional MRI determination of a dose-response relationship to lower extremity neuromuscular electrical stimulation in healthy subjects. Exp Brain Res. 2003;150:33–39. doi: 10.1007/s00221-003-1405-9. [DOI] [PubMed] [Google Scholar]
- 88.Knash ME, Kido A, Gorassini M, Chan KM, Stein RB. Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res. 2003;153:366–377. doi: 10.1007/s00221-003-1628-9. [DOI] [PubMed] [Google Scholar]
- 89.Thompson AK, Stein RB, Chen XY, Wolpaw JR. Modulation in spinal circuits and corticospinal connections following nerve stimulation and operant conditioning. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2138–2141. doi: 10.1109/IEMBS.2006.259544. [DOI] [PubMed] [Google Scholar]
- 90.Everaert DG, Thompson AK, Chong SL, Stein RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010;24:168–177. doi: 10.1177/1545968309349939. [DOI] [PubMed] [Google Scholar]
- 91.Hayward KS, Barker RN, Brauer SG, Lloyd D, Horsley SA, Carson RG. SMART Arm with outcome-triggered electrical stimulation: a pilot randomized clinical trial. Top Stroke Rehabil. 2013;20:289–298. doi: 10.1310/tsr2004-289. [DOI] [PubMed] [Google Scholar]
- 92.Chung Y, Kim JH, Cha Y, Hwang S. Therapeutic effect of functional electrical stimulation-triggered gait training corresponding gait cycle for stroke. Gait Posture. 2014;40:471–475. doi: 10.1016/j.gaitpost.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 93.Kim H, Lee G, Song C. Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients. J Stroke Cerebrovasc Dis. 2014;23:655–661. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Theilig S, Podubecka J, Bosl K, Wiederer R, Nowak DA. Functional neuromuscular stimulation to improve severe hand dysfunction after stroke: does inhibitory rTMS enhance therapeutic efficiency? Exp Neurol. 2011;230:149–155. doi: 10.1016/j.expneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 95.Page SJ, Levine P. Back from the brink: electromyography-triggered stimulation combined with modified constraint-induced movement therapy in chronic stroke. Arch Phys Med Rehabil. 2006;87:27–31. doi: 10.1016/j.apmr.2005.07.307. [DOI] [PubMed] [Google Scholar]
- 96.Meadmore KL, Hughes AM, Freeman CT, et al. Functional electrical stimulation mediated by iterative learning control and 3D robotics reduces motor impairment in chronic stroke. J Neuroeng Rehabil. 2012;9:32. doi: 10.1186/1743-0003-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 98.Knutson JS, Harley MY, Hisel TZ, Makowski NS, Fu MJ, Chae J. Contralaterally controlled functional electrical stimulation for stroke rehabilitation. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:314–317. doi: 10.1109/EMBC.2012.6345932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schabrun SM, Chipchase LS, Zipf N, Thickbroom GW, Hodges PW. Interaction between simultaneously applied neuromodulatory interventions in humans. Brain stimulation. 2013;6:624–630. doi: 10.1016/j.brs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 100.Lee HJ, Cho KH, Lee WH. The effects of body weight support treadmill training with power-assisted functional electrical stimulation on functional movement and gait in stroke patients. Am J Phys Med Rehabil. 2013;92:1051–1059. doi: 10.1097/PHM.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 101.Turk R, Burridge JH, Davis R, et al. Therapeutic effectiveness of electric stimulation of the upper-limb poststroke using implanted microstimulators. Arch Phys Med Rehabil. 2008;89:1913–1922. doi: 10.1016/j.apmr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 102.Kilgore KL, Bhadra N. Reversible Nerve Conduction Block Using Kilohertz Frequency Alternating Current. Neuromodulation : journal of the International Neuromodulation Society. 2013 doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]


