Abstract
Objectives:
To assess alterations in the composition of peripheral immune cells in acute progressive multifocal leukoencephalopathy (PML).
Methods:
Fresh blood samples from 5 patients with acute PML and 10 healthy controls were analyzed by flow cytometry for naive, central memory and effector memory CD4 and CD8 T cells, B lymphocytes, plasma cells, memory B cells, plasma blasts, and natural killer (NK) cells. The frequency of central memory CD4 T cells was determined longitudinally during the course of PML in 2 patients.
Results:
The frequencies of naive, central memory and effector memory CD8 T cells, B cells, plasma cells, and NK cells were not altered in patients with PML. In contrast, the frequencies of naive CD4 T cells (p = 0.04) and central memory CD4 T cells (p < 0.00001) were reduced and the frequencies of effector memory CD4 T cells were increased (p = 0.01). Longitudinal analysis showed that this pattern was preserved in a patient with fatal PML outcome and restored in one patient who recovered from PML.
Conclusions:
These data indicate that PML is associated with reduced frequencies of peripheral central memory helper T cells but not with alterations in the frequencies of cytotoxic T cell populations, B lymphocytes, plasma cells, or NK cells.
Latent infection with the human polyomavirus John Cunningham virus (JCV) is common in healthy individuals. Under circumstances of reduced immunocompetence within the CNS, JCV, which was kept in a quiescent state, is reactivated and causes progressive multifocal leukoencephalopathy (PML).1 PML is a lytic infection of the brain that often leads to severe neurologic deficits or death. The immunologic characteristics that predispose to the development of PML are poorly defined.2
HIV infection accounts for about 85% of all PML cases,1 and up to 5% of patients with AIDS develop PML.3 In patients with HIV infection, PML typically develops before initiation of antiretroviral therapy, at a time when CD4 helper T cell numbers are low and their function is impaired. PML also develops in 3.7–5.2 in 1,000 patients with relapsing-remitting multiple sclerosis treated for more than 24 months with the VLA-4 antibody natalizumab, which prevents lymphocytes from entering the CNS. In addition, PML has been reported in patients treated with fumaric acid for psoriasis who were lymphopenic for at least several months.4 These combined observations indicate that persistent alterations in lymphocyte populations or their ability to enter the brain predispose to PML.
Herein, we provide direct evidence that a reduced frequency of peripheral central memory CD4 T cells is associated with the development of PML.
METHODS
Standard protocol approvals, registrations, and patient consents.
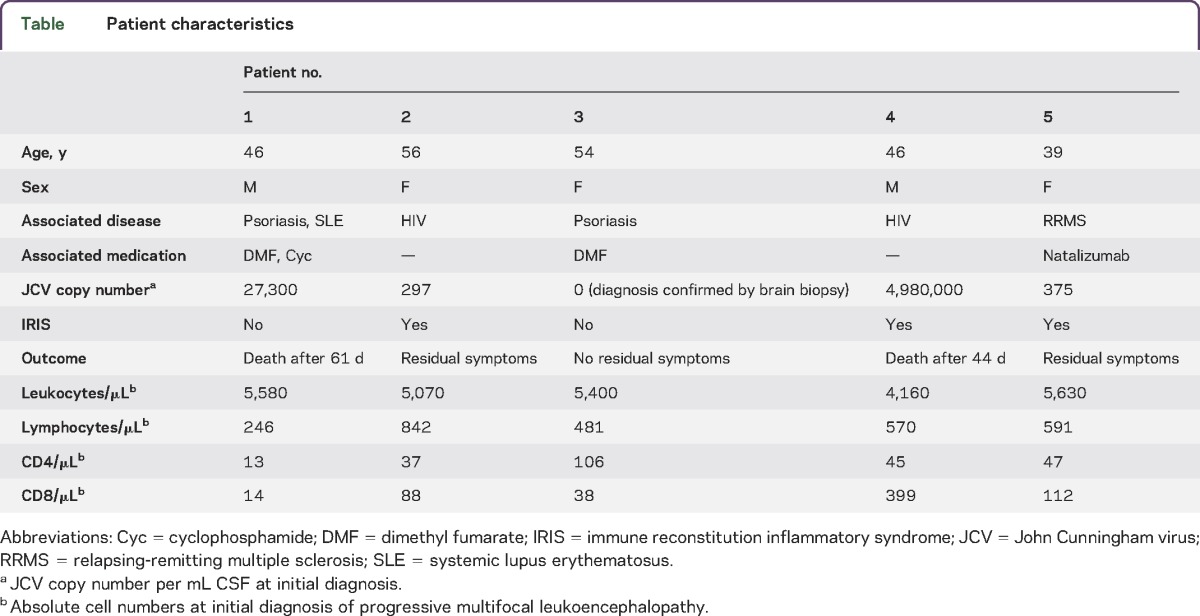
Patients were recruited at the Department of Neurology, University of Tübingen, Germany. All patients gave written informed consent for the provision of blood for the purpose of this research. The protocol under which these samples were obtained was approved by the ethics committee of the University of Tübingen, Germany. Baseline characteristics of the patients are depicted in the table.
Table.
Patient characteristics
Flow cytometric analysis.
White blood cell counts were determined by routine testing at the central laboratory of our hospital. Flow cytometric analysis was performed with fresh whole blood specimens after staining with fluorochrome-labeled antibodies. The following antibodies were used: anti-human CD3-APC (1/100) clone UCHT1, anti-human CD4-FITC (1/100) clone SK3, anti-human CD8-PerCP (1/100) clone SK1, anti-human CD19-FITC (1/100) clone HIB19, anti-human CD27-PE (1/50) clone L128, anti-human CD38-APC (1/50) clone HIT2, anti-human CD56-PE (1/50) clone B159, anti-human CD197(CCR7)-PE (1/50) clone 3D12, all from BD Biosciences, and anti-human CD45RA-APC (1/50) clone HI100 from BioLegend. As a reference, these analyses were performed in 10 healthy controls. The mean age of healthy controls was 45 years. Absolute numbers of CD4 and CD8 T cells were calculated by multiplying lymphocyte numbers obtained from the white blood cell count with the frequency of CD4 and CD8 cells in flow cytometric analysis.
Quantitative JCV-PCR.
JCV copy numbers were determined by quantitative PCR at the Institute for Virology, Heinrich Heine University, Düsseldorf, Germany. This assay is able to detect less then 10 viral copies/mL CSF.
Statistical analysis.
Statistical analysis was performed by unpaired t test using SPSS 22 (IBM Corp., Armonk, NY).
RESULTS
Patient characteristics.
Three female and 2 male patients between 39 and 56 years of age were recruited at our institution at the initial presentation of PML between 2012 and 2015 (table). Two patients had HIV-associated PML, 2 patients were on treatment with fumaric acid (Fumaderm) for psoriasis for more than 6 months, and one patient with relapsing-remitting multiple sclerosis was on natalizumab for more than 24 months. PML outcome was related to the JCV copy numbers within the CSF at the time of PML diagnosis. In one patient with negative JCV-PCR, PML diagnosis was established by brain biopsy. This patient was completely free of neurologic deficits after resolution of PML. An immune reconstitution inflammatory syndrome (IRIS) developed in both patients with HIV-PML following initiation of combined antiretroviral therapy and in the natalizumab-treated patient with multiple sclerosis following plasma exchange. IRIS was characterized by the development of new neurologic symptoms and new contrast-enhancing lesions on cerebral MRI. Two patients with less than 500 JCV copies per mL CSF had only moderate residual symptoms and 2 patients who displayed >10,000 copies of the JCV DNA per mL of CSF died of PML within 44 and 61 days, respectively. All patients had reduced lymphocyte numbers at the initial diagnosis of PML (table).
Reduced frequency of central memory CD4 T cells in patients with PML.
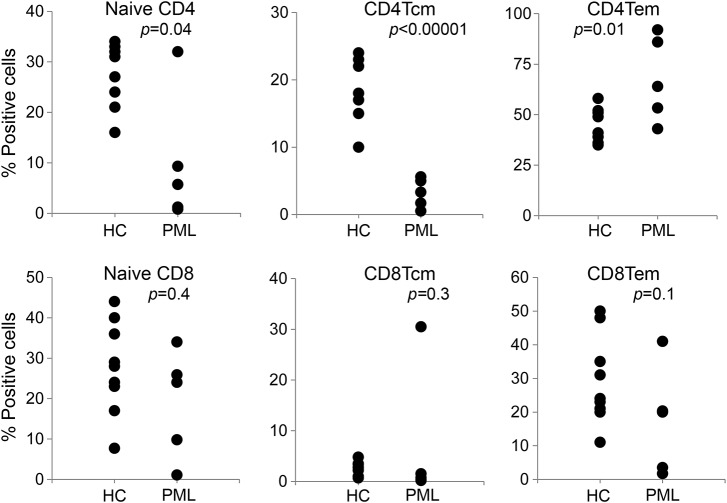
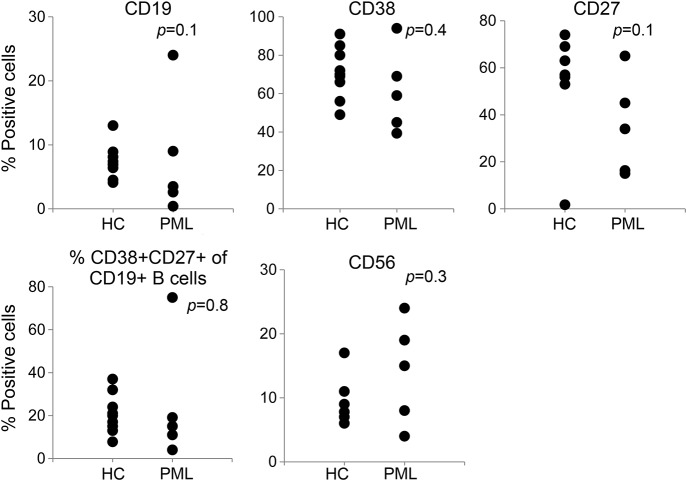
At the initial diagnosis of PML, peripheral blood samples were obtained from all patients and cells were directly analyzed by flow cytometry after staining with the indicated fluorochrome-labeled antibodies (figure 1). As compared with healthy controls, the frequency of central memory CD4 T cells was highly significantly reduced (figure 2; p < 0.00001), as was the frequency of naive CD4 T cells (p = 0.04). The proportion of effector memory CD4 T cells was increased (p = 0.01). In contrast, the frequencies of naive, central memory and effector memory CD8+ T cells (figure 2), CD19+ B lymphocytes, CD19+CD38+ plasma cells, CD19+CD27+ memory B cells, and CD56+ natural killer cells (figure 3) were not significantly different from healthy controls.
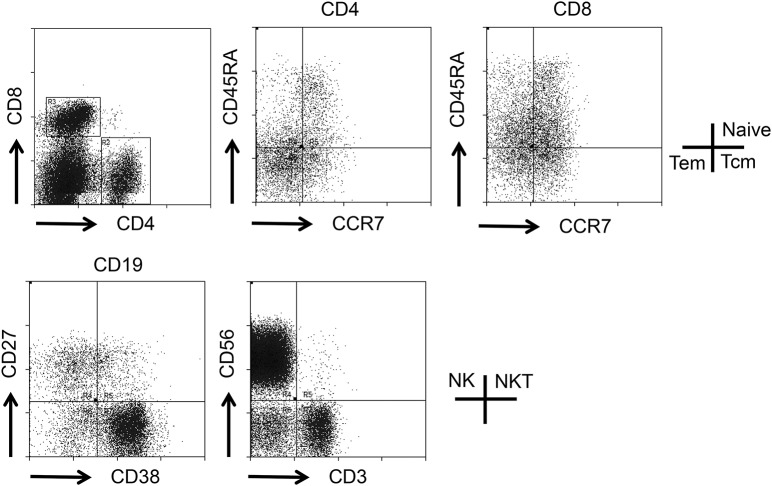
Figure 1. Schematic overview of flow cytometry.
Schematic overview on the analysis of peripheral immune cells by flow cytometry. Expression of CCR7 and CD45RA was determined on live CD4 and CD8 cells, and expression of CD38 and CD27 was determined on live CD19 cells as indicated. NK = natural killer cells; NKT = natural killer T cells.
Figure 2. Reduced frequency of central memory CD4 T cells in patients with PML.
Reduced frequency of central memory CD4 T cells (CD4Tcm) (p < 0.00001), naive CD4 T cells (p = 0.04), and effector memory CD4 T cells (CD4Tem) (p = 0.01) in patients with PML. Healthy controls (HC) are depicted in the first column of each dotplot, patients with PML in the second. PML = progressive multifocal leukoencephalopathy.
Figure 3. Frequency of B lymphocytes, plasma cells, and NK cells.
Frequency of CD19+ B lymphocytes, CD19+CD38+ plasma cells, CD19+CD27+ memory B cells, and CD56+ NK cells does not significantly differ from healthy controls (HC). Healthy controls are depicted in the first column of each dotplot, patients with PML in the second. NK = natural killer; PML = progressive multifocal leukoencephalopathy.
Longitudinal analysis of peripheral immune cell composition.
Longitudinal analysis of peripheral immune cells was performed during the course of PML in patients 1 and 2. Patient 1 displayed a high JCV viral load within the CSF (27,300 copies/mL CSF) and died 61 days after initial PML diagnosis. He displayed highly reduced frequencies of naive CD4 T cells and central memory CD4 T cells and an increased proportion of effector memory CD4 T cells throughout the progressive course of the disease (figure 4). In patient 2, the reduced frequencies of naive CD4 T cells and central memory CD4 T cells returned almost to normal levels after initiation of combined antiretroviral therapy and during subsequent resolution of PML. Patient 1 neither clinically nor radiologically showed signs of IRIS whereas patient 2 already showed radiologic signs (edema, gadolinium enhancement) when diagnosed with PML.
Figure 4. Longitudinal analysis of peripheral immune cell composition.
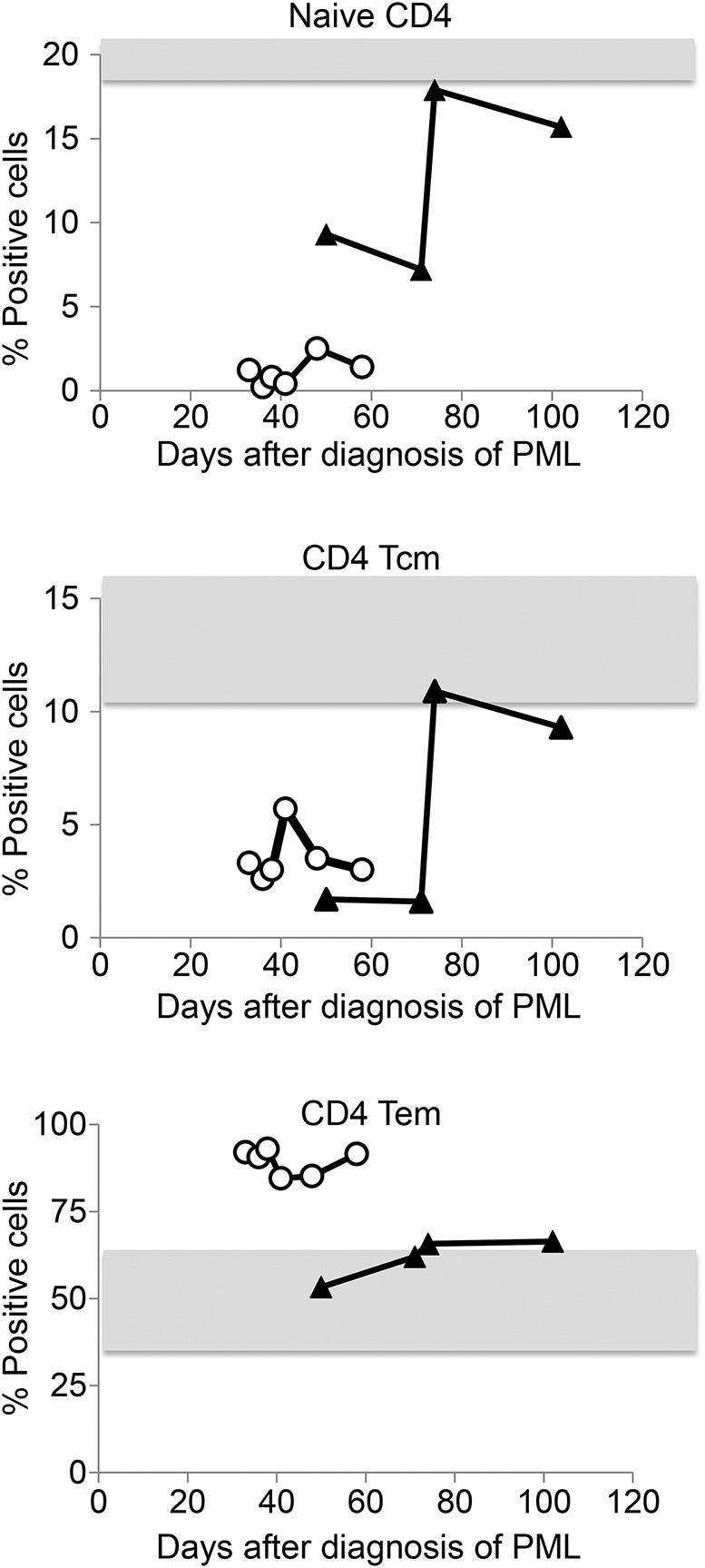
Frequency of naive, central memory (Tcm), and effector memory (Tem) CD4 T cells over the course of PML in patient 1 (open circles) and patient 2 (closed triangles). Gray areas indicate normal values (mean values ± 2 SDs of healthy controls). Timeline indicates days after diagnosis of PML. PML = progressive multifocal leukoencephalopathy.
DISCUSSION
Herein, we provide data indicating that alterations in the composition of peripheral CD4 helper T cell subpopulations are associated with development of PML. CD4 T cells have a pivotal role in the defense against viral infections as has been shown in numerous human diseases including HIV, hepatitis C virus, and JCV.5–8 The development of PML is known to be associated with low total numbers of CD4 T cells in patients with late-stage HIV infections and patients with idiopathic CD4 T cell lymphopenia.9 Our patients with PML did not display any significant alterations in the frequencies of naive, central memory and effector memory CD8 T cells, B lymphocyte populations, and natural killer cells. In contrast, the proportions of CD4 central memory and naive T cells were highly significantly decreased and the proportion of effector memory CD4 T cells was increased. Central memory T cells express the chemokine receptor CRR7 and CD62L and recirculate through secondary lymphoid organs. Effector memory CD4 T cells do not express CCR7 and CD62L, molecules required for entry into lymph nodes, but express chemokine receptors such as CCR5, which direct their migration to the sites of inflammation. They are preferentially located within nonlymphoid tissues or remain within the blood and rapidly exhibit effector functions after a new antigen encounter.10,11 The fact that the proportion of CD4 effector memory T cells was high in the peripheral blood of patients with PML thus indicates that a large proportion of peripheral CD4 T cells is actively engaged in viral defense. This is consistent with the previously observed prominent infiltration of CD4 T cells into inflammatory CNS lesions in PML12 and further supports the central role of CD4 rather than CD8 T cells in immune defense against JCV.
Before the diagnosis of PML, 2 patients had been on immunomodulatory treatment with fumaric acid and one patient with natalizumab. These treatments are known to induce alterations in peripheral immune cells and may thus have influenced our results. Dimethyl fumarate has been shown to both reduce the frequency of lymphocytes in peripheral blood and to disproportionally reduce the frequency of CD8 T cells,13 while natalizumab increases peripheral lymphocyte counts and the proportion of peripheral B lymphocytes.14
The fact that the reduction of naive and central memory CD4 T cells was partially restored in patient 2 after resolution of PML and not in patient 1 who displayed a fatal course of PML may further indicate that the frequencies of these cells negatively correlate to PML disease activity. Collectively, these observations are in line with previous reports that found reduced frequencies of CD62L-expressing CD4 T cells in natalizumab-treated patients with relapsing-remitting multiple sclerosis who developed PML.15 In our cohort, the frequency of central memory CD4 T cells was reduced in patients with HIV-associated PML as well as in patients who developed PML under immunomodulatory treatment. This further supports the notion that the reduced frequency of central memory CD4 T cells represents a state of the immune system with reduced immunocompetence against JCV. Furthermore, these data indicate that additional in-depth analysis of the role of CD4 helper T cell populations during the course of PML could provide important insights into the immune conditions that predispose to this disease. Reconstitution of CD4 T cells in longitudinal analysis might be a predictor for clinical outcome.
Obviously, this investigation is limited by the small number of patients, by the different conditions and medications under which PML developed, and the different courses of disease (i.e., with and without development of IRIS). If confirmed, the observed alterations in CD4 T cell subpopulations could provide new insights into the pathogenesis of PML and may ultimately represent a biomarker for PML disease activity or to detect patients at risk of PML development. This would be urgently needed as JCV infections of the nervous system including PML, JCV meningitis,16 and cerebellar granule cell neuronopathy17 become more clinically prominent.
ACKNOWLEDGMENT
The authors are grateful to the patients and their relatives for agreeing to participate in this study.
GLOSSARY
- IRIS
immune reconstitution inflammatory syndrome
- JCV
John Cunningham virus
- NK
natural killer
- PML
progressive multifocal leukoencephalopathy
AUTHOR CONTRIBUTIONS
Study concept and design: F.B. Acquisition of data: E.D. Analysis and interpretation: E.D., C.R., F.B. Critical revision of the manuscript for important intellectual content: E.D., C.R., F.B. Study supervision: F.B. Statistical analysis: F.B.
STUDY FUNDING
No targeted funding.
DISCLOSURE
E. Dubois reports no disclosures. C. Ruschil is on the scientific advisory board for Novartis Pharma GmbH. F. Bischof served on the scientific advisory board for Genzyme, Novartis, and Roche, received speaker honoraria and travel funding from Biogen Idec, Genzyme, and Novartis, and received research support from Novartis. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol 2010;6:667–679. [DOI] [PubMed] [Google Scholar]
- 2.Jelcic I, Jelcic I, Faigle W, Sospedra M, Martin R. Immunology of progressive multifocal leukoencephalopathy. J Neurovirol Epub 2015 Mar 5. [DOI] [PubMed]
- 3.Power C, Gladden JG, Halliday W, et al. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology 2000;54:743–746. [DOI] [PubMed] [Google Scholar]
- 4.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med 2013;368:1657–1658. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, Lauer GM, Robbins GK, et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol 2002;76:12584–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasnault J, Kahraman M, de Goer de Herve MG, Durali D, Delfraissy JF, Taoufik Y. Critical role of JC virus-specific CD4 T-cell responses in preventing progressive multifocal leukoencephalopathy. AIDS 2003;17:1443–1449. [DOI] [PubMed] [Google Scholar]
- 7.McNeil AC, Shupert WL, Iyasere CA, et al. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc Natl Acad Sci USA 2001;98:13878–13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature 2000;407:523–526. [DOI] [PubMed] [Google Scholar]
- 9.Puri V, Chaudhry N, Gulati P, Patel N, Tatke M, Sinha S. Progressive multifocal leukoencephalopathy in a patient with idiopathic CD4+T lymphocytopenia. Neurol India 2010;58:118–121. [DOI] [PubMed] [Google Scholar]
- 10.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 2012;188:5811–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol 2011;12:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousef S, Planas R, Chakroun K, et al. TCR bias and HLA cross-restriction are strategies of human brain-infiltrating JC virus-specific CD4+ T cells during viral infection. J Immunol 2012;189:3618–3630. [DOI] [PubMed] [Google Scholar]
- 13.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumbholz M, Meinl I, Kumpfel T, Hohlfeld R, Meinl E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008;71:1350–1354. [DOI] [PubMed] [Google Scholar]
- 15.Schwab N, Schneider-Hohendorf T, Posevitz V, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology 2013;81:865–871. [DOI] [PubMed] [Google Scholar]
- 16.Agnihotri SP, Wuthrich C, Dang X, et al. A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient. Ann Neurol 2014;76:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agnihotri SP, Dang X, Carter JL, et al. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology 2014;83:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]