Abstract
Background:
Azadirachta indica (Neem) is a medicinal plant, used in Ayurveda for treating various diseases, one of which is diabetes mellitus. It is known to possess antiinflammatory, antipyretic, antimicrobial, antidiabetic and diverse pharmacological properties. However, the molecular mechanism underlying the effect of A. indica on insulin signal transduction and glucose homeostasis is obscure.
Objective:
The aim was to study the effects of A. indica aqueous leaf extract on the expression of insulin signaling molecules and glucose oxidation in target tissue of high-fat and fructose-induced type-2 diabetic male rat.
Materials and Methods:
The oral effective dose of A. indica leaf extract (400 mg/kg body weight [b.wt]) was given once daily for 30 days to high-fat diet-induced diabetic rats. At the end of the experimental period, fasting blood glucose, oral glucose tolerance, serum lipid profile, and the levels of insulin signaling molecules, glycogen, glucose oxidation in gastrocnemius muscle were assessed.
Results:
Diabetic rats showed impaired glucose tolerance and impairment in insulin signaling molecules (insulin receptor, insulin receptor substrate-1, phospho-IRS-1Tyr632, phospho-IRS-1Ser636, phospho-AktSer473, and glucose transporter 4 [GLUT4] proteins), glycogen concentration and glucose oxidation. The treatment with A. indica leaf extract normalized the altered levels of blood glucose, serum insulin, lipid profile and insulin signaling molecules as well as GLUT4 proteins at 400 mg/kg b.wt dose.
Conclusion:
It is concluded from the present study that A. indica may play a significant role in the management of type-2 diabetes mellitus, by improving the insulin signaling molecules and glucose utilization in the skeletal muscle.
Keywords: Azadirachta indica, high-fat diet, insulin resistance, type-2 diabetes
INTRODUCTION
Type-2 diabetes is a chronic metabolic disorder that results from defects in both insulin action and secretion. Diabetes is increasing at pace universally, and its intensity can be inferred from International Diabetes Federation report that estimated number of adults living with diabetes has soared to 366 million and this number is projected to increase to 552 million people by 2030. With an estimated 50.8 million people living with diabetes, India has world's largest diabetes population, followed by China with 43.2 million.[1] Many oral antidiabetic drugs used today fail to give a long-term glycemic control.[2,3] Metformin remains the first line of drug therapy for patients with type-2 diabetes.[4] The need of new therapies for glycemic control is the fact that existing treatments have limitations because of their side effects.[5] The herbal extracts which are effective in lowering blood glucose level with minimal or no side effects are known to be used as antidiabetic remedies.[6,7] Recently, the natural products have become a vital are of research. Many Indian medicinal plants have been scientifically explored for their antidiabetic and antioxidant activities.[3,6,7,8]
Azadirachta indica A. Juss (Family Meliaceae) is well known in India and its neighboring countries as one of the most versatile medicinal plants having wide spectrum of biological activity. Each part of Neem tree has some medicinal property and thus commercially exploitable.[9] A. indica is known to possess antiinflammatory, antipyretic, antimicrobial, antidiabetic and diverse pharmacological properties.[10,11,12] During the last five decades, considerable progress has been achieved regarding the biological activity and medicinal application of A. indica A Juss (Neem). A. indica has been commonly used to treat diabetes in Indian system of medicine from time immemorial.[13] There are several reports that suggest the hypoglycemic potential of A. indica.[14,15] Leaf and bark extract of A. indica had been shown to reduce blood glucose level and lipid peroxidantion and increased the antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase in liver and kidney tissues of alloxan-diabetic female rats. Recently it has been reported that treatment with A. indica leaf aqueous extract to high-fat diet-induced diabetic Charles foster rats for 30 days, significantly increased the activities of enzymatic antioxidants in hepatic tissues suggesting that A. indica leaf extract has both antidiabetic and antioxidant potentials.[16] Moreover, chronic treatment with ethanolic extract of A. indica has been shown to reduce the blood glucose level and ameliorates lesions of pancreatic islets in streptozotocin-induced diabetic rats.[17,18] However, the molecular mechanisms underlying anti-diabetic potentials of A. indica on insulin signaling molecules and glucose oxidation have not been studied so far. Hence, the present study was undertaken to appraise the molecular events by which A. indica exhibits antidiabetic action in type-2 diabetic male Wistar rats.
MATERIALS AND METHODS
Preparation of extract
Fresh and matured A. indica leaves were collected from Bharath University Campus, Selaiyur, Chennai - 600 073, Tamil Nadu, India, and a voucher specimen was deposited in the herbarium of the department. The leaves were dried in the shade and grounded into fine powder. The powder (1 kg) was extracted thrice with double distilled water for 8 h in a percolator at room temperature, and the fraction was pooled and concentrated by rotavapour. The vacuum drying concentrated fraction yielded dried extracts of which was used for in vivo studies.
Chemicals
All chemicals and reagents used in this study were of molecular and analytical grade; and they were purchased from Sigma Chemical Company, St. Louis, MO, USA; and Sisco Research Laboratories, Mumbai, India. On-Call Plus Blood Glucose test strips were obtained from ACON Laboratories, Inc., San Diego, USA. Polyclonal insulin receptor β-subunit, insulin receptor substrate-1 (IRS-1), phospho IRS-1 (Tyrosine 632), phospho IRS-1 (Ser 636), Akt (1/2/3) and phospho Akt (p-Akt) (Thr 308) and glucose transporter 4 (GLUT4) antibodies were purchased from Santa Cruz Biotechnology (USA).
Animals
This study was approved by the Institutional Animal's Ethics Committee and by the regulatory body of the government (FN.41/2009). Adult male Wistar rats (180–200 g body weight [b.wt]) were obtained from the Laboratory of Animal Medicine, Centre for Animal Health Studies, Tamil Nadu Veterinary and Animal Sciences Studies, Madhavaram, Chennai, Tamil Nadu and kept at Department of Medical Biochemistry, Bharath University, Selaiyur, Chennai - 600 073, Tamil Nadu under laboratory conditions of temperature (22°C + 21°C), humidity (45% +5%) and 12 h day: 12 h night cycle, and were allowed free access to food (standard pellet diet- [Sai Durga Feeds and Foods, Bangalore, India]) and water ad libitum.
Induction of type-2 diabetes
Type-2 diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (35 mg/kg b.wt) after 30 days of high-fat diet containing cholesterol 2 g, cholic acid 1 g, coconut oil 30 ml, standard rat feed 100 g, and 25% fructose feeding through drinking water and were continued until the end of the study (30 days).[19] The low dose of streptozotocin was given to generate a slight trauma to beta cells of the pancreas to mimic the condition of chronic hyperinsulinemic insulin resistance condition.[19]
Experimental design
The following experimental design was framed and accordingly rats were subjected to A. indica leaves aqueous extract treatment for 30 days. Rats were randomly divided into five groups of 6 rats each. The dose of A. indica leaf extract was selected by Shrivastava et al.[16] The authors have used 100, 200 and 400 mg A. indica leaf extract in high-fat diet-induced diabetic Charles foster rats for 30 days. Among three doses, the 400 mg showed significant improvement in glucose tolerance. Moreover, treatment with A. indica leaf extract significantly normalized the altered levels of lipid peroxidation and antioxidant status at 400 mg/kg. b.wt dose.[16] Therefore, in the present study, 400 mg dose was selected.
Group I: Control
Group II: Rats were made diabetic (type-2) by giving high fat diet and 25% fructose feeding through drinking water for 30 days
Group III: Diabetic (type-2) rats were treated with A. indica leaf aqueous extract (400mg/kg body weight/day), orally for 30 days after high fat diet and fructose feeding
Group IV: Diabetic (type-2) rats were treated with metformin (50 mg/kg body weight/day) orally for 30 days after high fat diet and fructose feeding
Group V: Control rats were treated with A. indica leaf aqueous extract (400mg/kg body weight/day), orally for 30 days.
Two days prior to sacrifice, control and experimental animals were subjected to oral glucose tolerance test after overnight fasting. At the end of 30 days, blood was collected and animals were perfused with normal saline under ether anesthesia. Gastrocnemius muscle was dissected out for the assessment of various parameters.
Fasting blood glucose
Blood glucose was estimated using On-Call Plus Blood Glucose test strips (ACON Laboratories, Inc., San Diego, USA) after overnight fasting. Blood was collected from rat tail tip and results are expressed as mg/dl.
Oral glucose tolerance test
Blood glucose was estimated using On-Call Plus Blood Glucose test strips at various time points (60, 120 and 180 min) after giving oral glucose load (10 ml/kg bodyweight; 50% w/v). Blood glucose value before giving glucose is considered as ’0’ min value. Results are expressed as mg/dl.
Insulin assay
Serum insulin was assayed using ultra-sensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit obtained from Crystal Chem Inc., USA. The sensitivity of assay was 0.005 ng/ml. The percentage reactivity of C-peptide was not detectable. Intra-assay coefficient of variation (CV) was ≤10%, and inter-assay CV was ≤10%. Results are expressed as μIU/ml.
Serum lipid profile
Serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), high-density lipoprotein cholesterol (HDL-C) and free fatty acids (FFA) were estimated using biochemical assay kits obtained from Spinreact, Spain according to manufacturer's instructions.
Glucose oxidation
Glucose oxidation in gastrocnemius muscle was estimated by the method of Kraft and Johnson[20] using 14C-glucose. Briefly, 10 mg tissue was weighed and placed in a 2 ml ampoule containing 170 μl Dulbecco's modified Eagle's medium (DMEM), pH 7.4, 10-IU penicillin and 0.5-μCi14 C-glucose. The ampoules were aerated with a gas mixture (5% CO2, 95% air) for 30 s and tightly covered with rubber cork containing CO2 trap (a piece of filter paper was inserted into the rubber cork and 0.1 ml of diethanolamine was applied to the filter paper before closing the ampoule). This closed system with CO2 trap was placed in an incubator at 37°C. CO2 trap was replaced every 2 h. After removing the second trap, 0.1 ml of 1N H2SO4 was added to halt further metabolism and release of any residual CO2 from the sample. The system was again closed for 1 h before the third and the final trap is removed. All the CO2 traps were placed in the scintillation vials containing 10 ml of scintillation fluid and counted in a beta counter. Results are expressed as CPM of 14CO2 released/10 mg tissue.
Estimation of glycogen content
Glycogen was estimated by the method of Hassid and Abraham.[21] Five milligrams of tissue was digested with 1 ml of 30% KOH for 20 min in a boiling water bath. The contents were cooled on an ice bath and 1.25 ml of 95% ethanol was added, thoroughly mixed and gently brought to boil in a hot water bath. Then cooled and centrifuged for 15 min at 3000 × g. The supernatant was decanted and the tubes were allowed to drain on a filter paper for few min. The precipitate was redissolved in 1 ml of distilled water, reprecipitated with 1 ml of 95% ethanol, centrifuged, and drained as stated before. The precipitate was dissolved in 5 ml distilled water and 10 ml of 0.2% anthrone reagent was added under ice cold conditions. Five milliliters of distilled water and series of standards with a final volume of 5 ml were treated with anthrone reagent and subjected to the same procedure. The tubes were covered with glass marbles and heated for 10 min, in boiling water bath. The contents were cooled immediately and the color developed was read at 680 nm. The amount of glycogen is expressed as mg/g of wet tissue.
Western blot analysis
Isolation of plasma membrane and cytosolic fractions
Plasma membrane and cytosolic fractions from skeletal muscle of control and experimental animals were prepared as described previously.[22,23] Tissues were homogenized in buffer A containing 10 mM/l NaHCO3 (pH 7.0), 250 mM/l sucrose, 5 mM/l NaN3, protease inhibitor cocktail (Sigma Chemical Company, USA), and 100 μM/l phenyl methyl sulfonyl fluoride (PMSF) using a Polytron-equipped homogenizer (Model PT 3000, Kinematica, Littau, Switzerland) at a precise low setting on ice. The resulting homogenate was clarified at 1300 × g for 10 min at 4°C. The resultant supernatant was centrifuged at 20,000 × g for 30 min at 4°C. The pellet was resuspended in buffer A, applied on discontinuous sucrose gradients (25, 32, and 35%, wt/wt), and centrifuged at 150,000 × g for 16 h at 4°C. Membranes at 25–32% (plasma membrane) and 32–35% (cytosolic fraction) interfaces were recovered, diluted with sucrose-free buffer A, and centrifuged at 190,000 × g for 1 h at 4°C. Pellets were resuspended in buffer A, and protein concentration was estimated using bovine serum albumin as a standard. The insulin receptor (IR) protein expression was evaluated in plasma membrane fraction. IRS-1, p-IRS-1Tyr632, p-IRS-1Ser636, Akt, p-AktSer473, protein levels were seen in cytosolic fraction. GLUT4 level was assessed both in cytosolic and plasma membrane fractions.
The lysate proteins (50 μg/lane) were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (10% gel) and transferred by electroblotting to polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories Inc). The membranes were blocked with 5% non-fat dry milk and probed with the primary antibodies (which were diluted to 1:1000). Following incubation, the blot was washed for 3 times (5 min each) with Tris-buffered saline containing Tween-20 (TBS-T). After washing with TBS-T, the membranes were incubated for 1 h with horseradish peroxidase-conjugated rabbit-antimouse or goat-anti-rabbit antibodies (which were diluted to 1:5000, GeNei, Bangalore, India). The specific signals were detected with an enhanced chemiluminescence detection system (Thermo Fisher Scientific Inc., USA). The protein bands were captured using Chemidoc and quantified by Quantity One image analysis system (Bio-Rad Laboratories, CA). Later the membranes were incubated in stripping buffer at 50°C for 30 min. After this the membrane was reprobed using a β-actin antibody (1:5000). As the invariant control, the present study used rat β-actin.
Statistical analysis
The data were subjected to statistical analysis using one-way analysis of variance (ANOVA) and Duncan's multiple comparison test to assess the significance of individual variations between the control and treatment groups using a computer based software (SPSS 7.5 using Windows student version). The significance was considered at the level of P < 0.05.
RESULTS
Effect of Azadirachta indica on fasting blood glucose, serum insulin and glucose tolerance
Diabetic rats (Group II) showed a significant increase (P < 0.05) in fasting blood glucose (FBG) and insulin levels (2.3-fold) than the control rats [Group I, Figures 1a and 2]. However, A. indica leaf extract treatment to diabetic rats reduced blood glucose and insulin levels close to those seen in control (Group III) than metformin treated rats (Group IV). Glucose tolerance test [Figure 1b] was done for each group to investigate systemic insulin sensitivity. Group II showed significantly higher glucose levels on glucose challenge than the control group. However, oral administration of A. indica leaf aqueous extract to diabetic rats showed near normal glucose tolerance (Group III) than metformin treated rats. Extract treatment to control rats did not show any change in the glucose and insulin levels compared to control rats (Group V).
Figure 1.
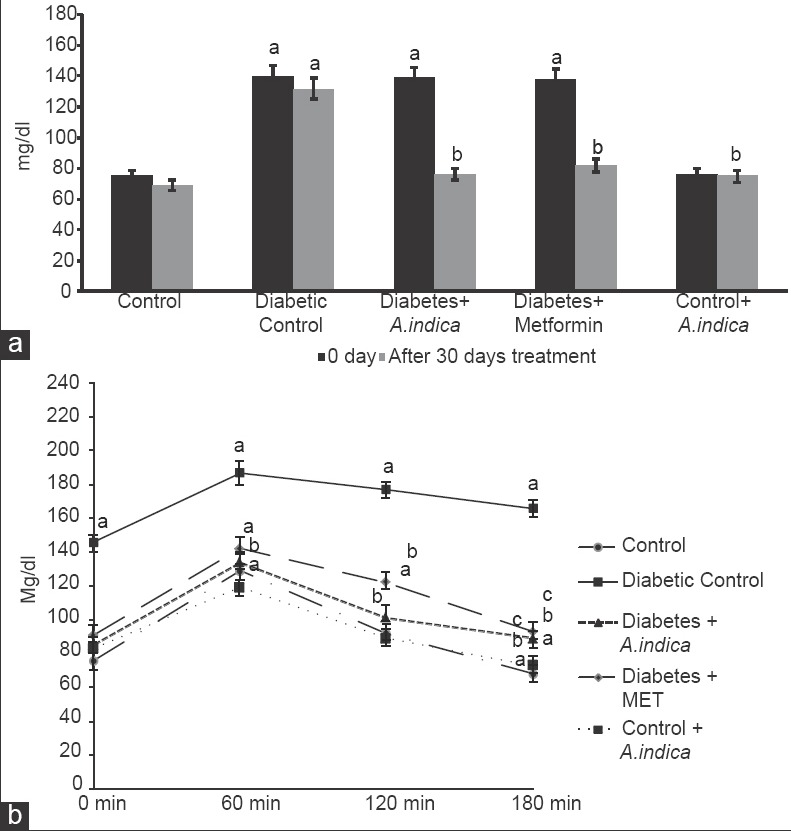
Effect of Azadirachta indica on fasting blood glucose (a) and oral glucose tolerance, (b) in type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of 6 animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control; c - Compared with diabetes + A. indica (400 mg/kg body weight [b.wt]); d - Compared with diabetes + metformin (50 mg/kg b.wt)
Figure 2.
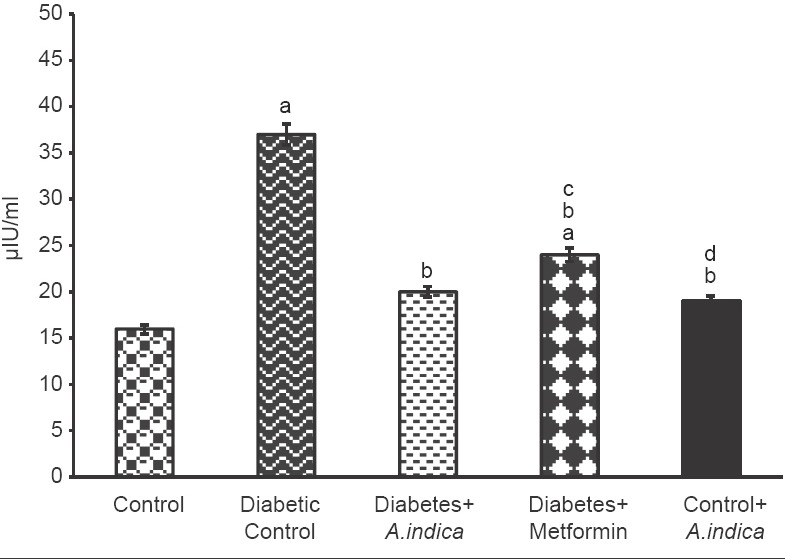
Effect of Azadirachta indica on serum insulin in type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of 6 animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control; c - Compared with diabetes + A. indica (400 mg/kg body weight [b.wt]); d - Compared with diabetes + metformin (50 mg/kg b.wt)
Effect of Azadirachta indica on lipid profile
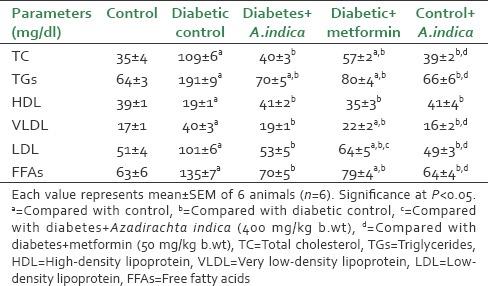
Diabetic rats showed a significant increase (P < 0.05) in the levels of serum TC (TC, 3.1-fold), TG (TG, 2.98-fold), LDL-C (LDL, 1.98-fold), VLDL-C (VLDL, 2.35-fold) and FFA (FFA, 2.1-fold) but a significant decrease in HDL (HDL, 2-fold) cholesterol levels compared control rats. However, diabetic rats treated with A. indica extract significantly decreased the TC (2.7-fold), TG (2.7-fold), LDL (1.9-fold), VLDL (2.1) and FFA (1.9-fold) levels and brought back to control levels. Serum HDL level was 2.1-fold increased in diabetic rats treated with A. indica reversed to control level [Table 1].
Table 1.
Effect of Azadirachta indica on serum lipid profile of type-2 diabetic adult male rats
Effect of Azadirachta indica on insulin receptor protein expression
Effect of A. indca leaf extract on IR protein expression in gastrocnemius muscle is depicted in Figure 3. In the present study, IR protein level was 2-fold reduced in diabetic rats. However, extract and metformin treatment, 1.6-fold increased the IR protein in diabetic rats.
Figure 3.
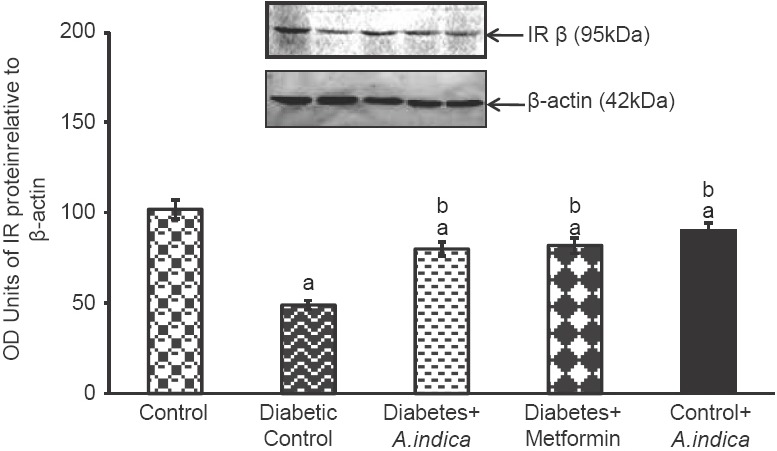
Effect of Azadirachta indica on insulin receptor protein expression in gastrocnemius muscle of type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of three observations representing six animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control
Effect of Azadirachta indica on insulin receptor substrate-1 protein expression and its phosphorylation
In diabetic rats, IRS-1 protein and its tyrosine phosphorylation (p-IRS-1Tyr632) were found to be reduced in to 2.1, 2.24-fold respectively [Figure 4a and b]. At the same time, 1.75-fold decreases in pIRS-1Ser636 level in diabetic rat [Figure 4c]. However, treatment with A. indica leaf extract and metformin, 2, 2.4-fold increased the IRS-1 and its tyrosine physposrylation. At the same time A. indica 1.9-fold decreased the serine phosphorylation and brought back to normal level. There was no significant difference between untreated and extracted non-diabetic groups.
Figure 4.
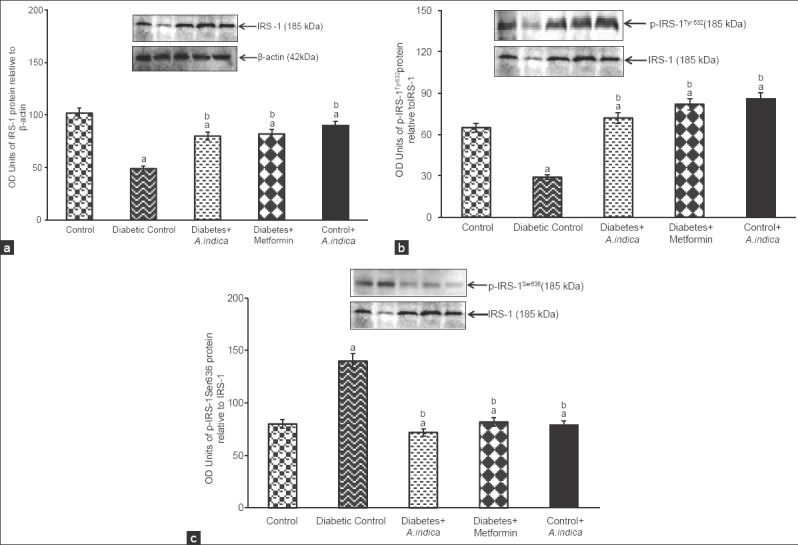
Effect of Azadirachta indica on insulin receptor substrate-1 (a), p-IRS-1Tyr632 (b) and p-IRS-1Ser636 (c) protein expression in gastrocnemius muscle of type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of three observations representing six animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control
Effect of Azadirachta indica on Akt protein and its serine phosphorylation
Figure 5 depicts the Akt protein expression in the gastrocnemius muscle of diabetic adult male rat. Akt protein expression was 0.9-fold decreased in diabetic rats compared control [Figure 5a]. Similarly to Akt, the p-AktSer473 protein level (b) was 1.8-fold reduced in diabetic rats compared to control. Interestingly, A. indica leaf ex tract increased 1, 1.8-fold Akt and p-AktSer473. No significance difference was observed in groups between III, IV and V. Treatment with extract to control rats had no significant influence.
Figure 5.
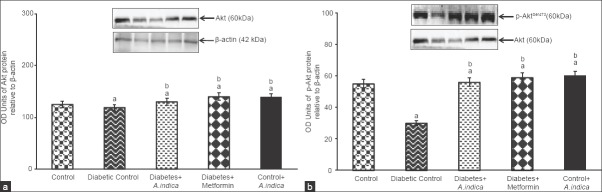
Effect of Azadirachta indica on protein kinase B (Akt) (a) and phosphor-AktSer473 (b) protein expression gastrocnemius muscle of type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of three observations representing six animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control
Effect of Azadirachta indica on plasma membrane and cytosolic GLUT4 protein expression
Figure 6 represents GLUT4 protein expression in cytosol (a) and plasma membrane (b) of gastrocnemius muscle. In diabetic rats, GLUT4 protein expression was 1.4-fold decreased whereas, in the A. indica leaf extract-treated diabetic rats cytosolic GLUT4 protein was 1.6-fold increased. Similar to that of cytosolic GLUT4 protein, in the plasma membrane also GLUT4 protein level was 1.6-fold decreased but extract treatment maintained the protein at near normal level by increasing 1.6 and 1.8-fold. There was no significant difference in GLUT4 protein levels in groups between the untreated and extract-treated non diabetic controls.
Figure 6.
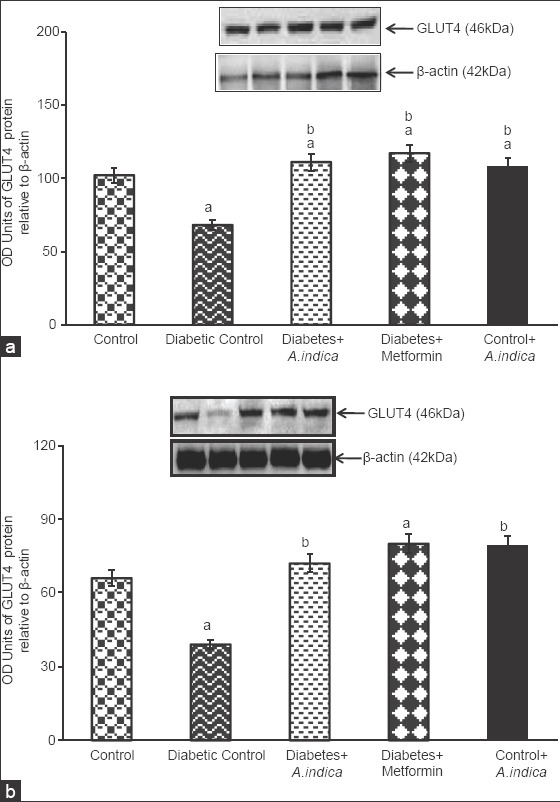
Effect of Azadirachta indica on cytosolic glucose transporter 4 (GLUT4) (a) and plasma membrane GLUT4 (b) protein expression gastrocnemius muscle of type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of three observations representing six animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control
Effect of Azadirachta indica leaf aqueous extract on 14C-glucose oxidation and glycogen
The rate of glucose oxidation depends on the rate of entry of glucose into the cells. Type-2 diabetic brought down glucose oxidation to 1.75-fold [Figure 7]. However, A. indica extract 1.8-fold increased oxidation glucose and brought back to normal level. It was found to be more effective than metformin. Diabetic rats 2.2-fold decreased glycogen concentration in gastrocnemius muscle control. However, treatment with A. indica leaf extract 2.3-fold increased the same to control level [Figure 8]. No statistical difference was observed in glycogen level between control and extract-treated control rats.
Figure 7.
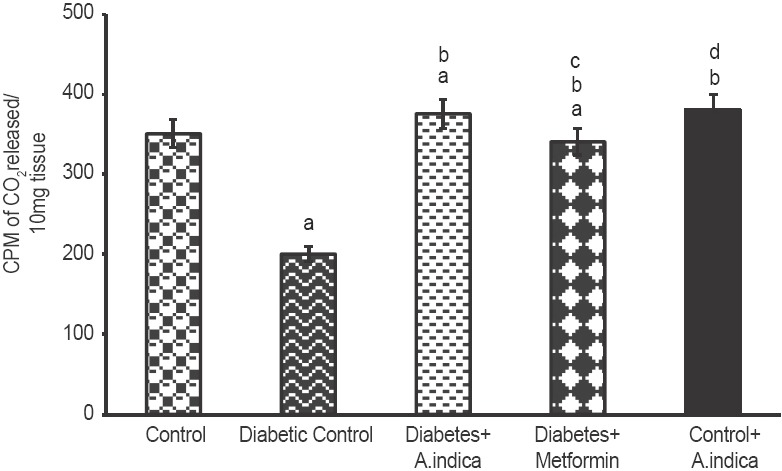
Effect of Azadirachta indica on glucose oxidation in gastrocnemius muscle of type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of six animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control; c - Compared with diabetes + A. indica (400 mg/kg body weight [b.wt]); d-compared with diabetes + metformin (50 mg/kg b.wt)
Figure 8.
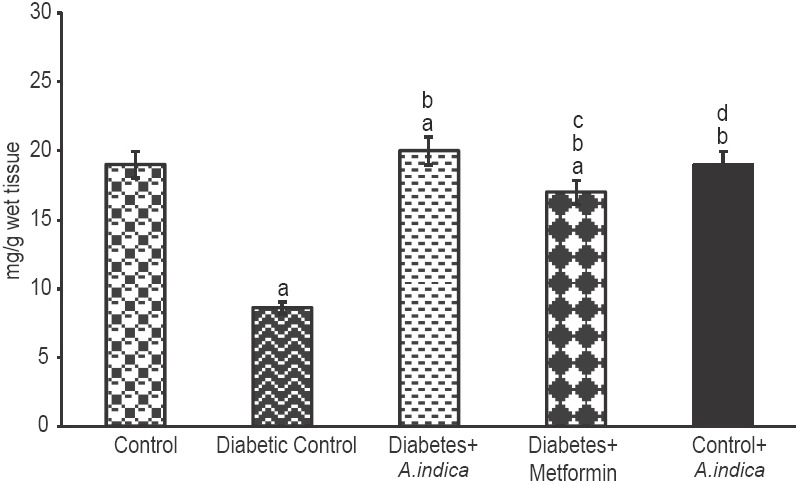
Effect of Azadirachta indica on glycogen concentration in gastrocnemius muscle of type-2 diabetic adult male rats. Each value is mean ± standard error of the mean of six animals (n = 6). Significance at P < 0.05, a - Compared with control; b - Compared with diabetic control; c - Compared with diabetes + A. indica (400 mg/kg body weight [b.wt]); d - Compared with diabetes + metformin (50 mg/kg b.wt)
DISCUSSION
Blood glucose and serum insulin
Skeletal muscle is the predominant site for glucose disposal in the postprandial state, and insulin-stimulated glucose uptake in this tissue represents the most important process for maintaining glucose homeostasis.[24] In the present study, diabetic rats showed higher fasting blood glucose and insulin levels than that of control rats. Studies have shown that high-fat diet and sucrose feeding in rodents leads to the development of whole body insulin resistance and impaired ability of insulin to stimulate glucose uptake in the skeletal muscle.[25,26] Several kinases including c-Jun NH2-terminal kinase,[27] inhibitor of nuclear factor kappa B kinase complex,[28] extracellular signal-regulated kinases,[29] protein kinase C-ζ,[30] and protein kinase C-θ[31] have been shown to be elevated in high-fat fed rats[32,33] and cause serine phosphorylation of IRS-1, resulting in impaired insulin-stimulated glucose transport activity as a result of insulin resistance, which in turn causes hyperglycaemia.[34] This might be the reason for significant increase in blood glucose and serum insulin levels in high-fat and fructose-induced type-2 diabetic rats. However, A. indica leaf aqueous extract administration decreased the levels of insulin and fasting blood glucose to normal range probably by improving insulin sensitivity and thus glucose tolerance. In accordance with the present study, it has been reported that high-fat diet-induced diabetic Charles foster rats showed increase in blood glucose level and the same has been brought back to control level in treatment with A. indica leaf extract suggesting that A. indica leaf extract has antidiabetic potential.[16]
Insulin receptor protein expression
Insulin receptor is a member of the receptor tyrosine kinase (RTK) family,[35] which includes the receptors for insulin, insulin-like growth factors (IGFs) and many other growth factors. In our study, IR protein expression was found to be decreased in gastrocnemius muscle of diabetic rats whereas treatment with A. indica leaf extract significantly improved the IR protein in diabetic rats. It has been reported that increased FFA diminishes IR gene expression and contributes to a reduced amount of IR protein in insulin target cells.[36] A. indica, being a potent antioxidant and anti-hyperlipidemic agent,[16,37] it was able to increase the IR level notably by decreasing the lipid levels in type-2 diabetic rats to that of metformin. In this regard, it has been reported by Chattopadhyay and Bandyopadhyay[37] that administration of A. indica leaf extract exhibited antihyperlipidemic activity due to presence of active compounds such as Quercetin-3-O-B-D-glucoside, Myricetin-3-Orutinoside, Quercetin-3-O-rutinoside, Kaempferol-3-O-rutinoside, Kaempferol-3-O-B-D-glucoside, Quercetin-3-O-L-rhamnoside isolated from the A. indica leaf extract.[38]
Insulin receptor substrate-1 protein and its phosphorylation
In the present study, IRS-1 protein and its phosphorylation at Tyr632 (essential for appropriate insulin signaling) were decreased in diabetic rats. At the same time, phosphorylation of IRS-1 at Ser636 was considerably elevated in the diabetic group. High-fat diet was shown to increase intracellular fatty acyl-CoA and diacylglycerol (DAG) concentrations, which in turn result in activation of PKC-θ leading to increased IRS-1Ser307 and Ser636 phosphorylation.[39,40,41] Further, high-fat diet[32,33] has been shown to elevate the levels of c-Jun NH2-terminal kinase,[27] inhibitor of nuclear factor kappa B kinase complex,[28] extracellular signal-regulated kinases[29] and cause serine phosphorylation (Ser636) of IRS-1, resulting in impaired insulin-stimulated tyrosine phosphorylation (Tyr632) in IRS-1.[34] A. indica leaf extract-treated type-2 diabetic rats showed an improved tyrosine phosphorylation (Tyr632) of IRS-1 along with reduced serine phosphorylation (Ser636) in the IRS-1. This may be attributed to increase in IR protein expression recorded in the present study.
Akt protein and its phosphorylation
The serine/threonine kinase Akt, triggers insulin effects on the skeletal muscle, such as glycogen synthesis and activates GLUT4 translocation by phosphorylating Akt substrate 160 kDa (AS160), and hence the Akt is considered to be one of the important downstream signaling molecules.[42] In the present study, Akt protein was unaltered but there was a significant decrease in the phosphorylation of Akt protein at Ser473 in diabetic rats and this may be due to defects in the IR and IR substrate-1 in the gastrocnemius muscle of high-fat and fructose-induced type-2 diabetic rats. Whereas, A. indica treatment significantly improved the IR and IRS-1 phosphorylation thereby improves the activity of Akt.
GLUT 4 protein expression in plasma membrane and cytosol
GLUT4, which is highly expressed in skeletal muscle and adipose tissue, is responsible for the insulin-stimulated increase in glucose uptake.[43] GLUT4 mRNA expression has been reported to be reduced in type-2 diabetic subjects because of defective transcription of GLUT4 gene and alteration in the stability of its mRNA transcript.[44] GLUT4 protein level was significantly reduced in high-fat and fructose-induced type-2 diabetic rats. High level of FFA has been shown to reduce the GLUT4 gene expression and translocation of GLUT4 from cytosol to plasma membrane.[45] Excess fatty acid (FA) in the diabetic rats was restored to normal after treatment with A. indica leaf extract and this might have been contributed for the restoration of GLUT4 gene expression. Restoration of GLUT4 protein level in the plasma membrane may be ascribed to the lipid lowering effects of the extract. In this regards it has shown that troxerutin administration reduced the lipid accumulation, restricted obesity and improved whole body insulin sensitivity.[46] Further, it up-regulates FA oxidation and down-regulates FA transport and lipogenesis.[46] This amelioration in the translocation following treatment with A. indica aqueous leaf extract may also be due to enhancement in the activity of insulin signaling molecules and thus the improved insulin sensitivity.
Lipid profile
Elevated levels of TG and FFA, TC in serum and altered lipoprotein profile (increased LDL-C and VLDL-C and decreased HDL-C) were observed in high-fat and fructose-fed diabetic rats. In the present study administration of A. indica leaf extract restored the changes in lipid profile to normal rats through its antihyperlipidmic activity. In this regard, Geetha et al.[46] have observed that troxerutin exhibits antihyperlipidmic activity by increasing the AMP activated protein kinase phosphorylation, which, in turn, facilitates FA oxidation and reduces the expression of cluster of differentiation 36 protein and fatty acid transport protein-1 responsible for FA transport in high-fat and fructose fed mice. In this study also high-fat diet could have enhanced the same through the same mechanism.
Glucose oxidation and tissue glycogen
Glucose oxidation in gastrocnemius muscle was clearly impaired in the high-fat diet and fructose fed diabetic rat, whereas A. indica leaf extract was able to successfully restore it to the normal range. The decreased rate of glucose oxidation in skeletal muscle might be due to reduced levels of GLUT4 in the plasma membrane which are likely to contribute for elevated blood glucose. In accordance with the present study, Turner et al.[47] reported that high-fat feeding in mice resulted in significant impairment in skeletal muscle glucose uptake. Glycogen concentration was also reduced in the gastrocnemius muscle of high-fat and fructose fed rats which may be the result of impairment in glycogenesis due to diminished levels of Akt phosphorylation at Ser473 that is essential for the activation of glycogen synthase.[48] It has been shown a reduction in hepatic glycogen concentration of high-fat fed rats.[19,49] Enhanced glucose uptake and oxidation as well as glycogen level observed in the A. indica administered diabetic rats may be attributed to the improved activity of insulin signaling molecules and increased expression of GLUT4 in the gastrocnemius muscle. Having demonstrated the potent effect of A. indica leaf extract extract in combating type-2 diabetic manifestations, our further focus is on isolation of principles/compounds from the extract to test the bioactive molecule (s) for development as an alternative therapeutic agent against type 2 diabetes [Figure 9].
Figure 9.
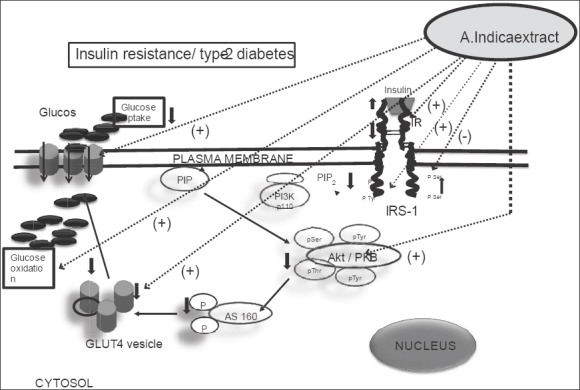
Varied actions of Azadirachta indica leaf aqueous extract (dark dotted arrows with [+] and [−] sign) on insulin signaling molecules in gastrocnemius muscle of high-fat diet-induce type-2 diabetic rats. In addition, A. indica extract demonstrated increased the muscle glucose oxidation through the activation of glucose transporter 4 protein from cytosolic compartment to the plasma membrane of gastrocnemius (in vivo). Dark arrows indicate insulin signal transduction in insulin resistance/type-2 condition
CONCLUSION
A. indica leaf extract may play a significant role in the management of type-2 diabetes mellitus by improving the expression of insulin signaling molecules and GLUT4 protein to enhance and oxidation in the skeletal muscle. The beneficial effects of A. indica leaf extract on these parameters could be significant considering the therapeutic potential of the A. indica leaf extract.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.IDF Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. International Diabetes Federation. Available from: Online version of IDF Diabetes Atlas: www.idf.org/diabetesatlas . ISBN: 2-930229-85-3. [PubMed] [Google Scholar]
- 2.Valiathan MS. Healing plants. Curr Sci. 1998;75:1122–7. [Google Scholar]
- 3.Singh SK, Rai PK, Jaiswal D, Watal G. Evidence-based Critical evaluation of glycemic potential of Cynodon dactylon. Evid Based Complement Alternat Med. 2008;5:415–20. doi: 10.1093/ecam/nem044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Home P, Mant J, Diaz J, Turner C. Guideline Development Group. Management of type 2 diabetes: Summary of updated NICE guidance. BMJ. 2008;336:1306–8. doi: 10.1136/bmj.39560.442095.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365:1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 6.Venkateswaran S, Pari L. Effect of Coccinia indica leaves on antioxidant status in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2003;84:163–8. doi: 10.1016/s0378-8741(02)00294-5. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2007;30:4–41. [Google Scholar]
- 8.Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127:457–62. doi: 10.1016/j.jep.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay V. Biological and medicinal properties of Neem (Azadirachta indica) Curr Sci. 2002;82:1136–45. [Google Scholar]
- 10.Chopra RN, Chopra IC. A review of work on Indian Medicinal plants including indigenous drugs and poisonous plants. Special Research Series No. 30 Location: UniM BioMed ST, ICMR. 1955:27. [Google Scholar]
- 11.Satyanarayana MK, Narayana RD, Krishna RD, Gopalakrishna ML. A preliminary study on hypoglycaemic and antihyperglycaemic effects of Azadirachta indica. Indian J Pharmacol. 1978;10:247–50. [Google Scholar]
- 12.Rochanakij S, Thebtaranonth Y, Yenjai C, Yuthavong Y. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66–72. [PubMed] [Google Scholar]
- 13.Shukia R, Sharma SB, Puri D, Prabhu KM, Murthy PS. Medicinal plants for treatment of diabetes mellitus. Indian J Clin Biochem. 2000;15:169–77. doi: 10.1007/BF02867556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Hawary ZM, Kholeif TS. Biochemical studies on hypoglycemic agents (1) Effect of Azadirachta indica leaf extract. Arch Phytother Res. 1990;13:108–12. [Google Scholar]
- 15.Mahdi AA, Chandra A, Singh RK, Shukla S, Mishra LC, Ahmad S. Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian J Clin Biochem. 2003;18:8–15. doi: 10.1007/BF02867361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrivastava A, Chaturvedi U, Sonkar R, Khanna AK, Saxena JK, Bhatia G. Antioxidant effect of Azadirachta indica on high fat diet induced diabetic Charles Foster rats. Appl Biochem Biotechnol. 2012;167:229–36. doi: 10.1007/s12010-012-9681-0. [DOI] [PubMed] [Google Scholar]
- 17.Akinola O, Ezekiel A, Dini CM. Diabetes-induced prefrontal nissl substance deficit and the effects of Neem-Bitter leaf extract treatment. J Morphol. 2010;28:291–302. [Google Scholar]
- 18.Akpan HD, Ekaidem IS, Usoh IF, Ebong PE, Isong NB. Effect of aqueous extract of Azadirachta indica (Neem) leaves on some indices of pancreatic function in alloxan-induced diabetic wistar rats. Pharmacologia. 2012;3:420–5. [Google Scholar]
- 19.Balaji V, Selvaraj J, Sathish S, Mayilvanan C, Balasubramanian K. Molecular mechanism underlying the antidiabetic effects of a siddha polyherbal preparation in the liver of type-2 diabetic adult male rats. J Evid Based Complementary Altern Med. 2012;00:1–14. [Google Scholar]
- 20.Kraft LA, Johnson AD. Epididymal carbohydrate metabolism. II. Substrates and pathway utilization of caput and cauda epididymal tissue from the rabbit, rat and mouse. Comp Biochem Physiol B. 1972;42:451–61. doi: 10.1016/0305-0491(72)90261-1. [DOI] [PubMed] [Google Scholar]
- 21.Hassid WZ, Abraham S. Determination of glycogen with anthrone reagent. Methods Enzymol. 1975;3:34–7. [Google Scholar]
- 22.Dombrowski L, Roy D, Marcotte B, Marette A. A new procedure for the isolation of plasma membranes, T tubules, and internal membranes from skeletal muscle. Am J Physiol. 1996;270:E667–76. doi: 10.1152/ajpendo.1996.270.4.E667. [DOI] [PubMed] [Google Scholar]
- 23.Nevado C, Valverde AM, Benito M. Role of insulin receptor in the regulation of glucose uptake in neonatal hepatocytes. Endocrinology. 2006;147:3709–18. doi: 10.1210/en.2005-1663. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–55. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Z, Xue J, Zhang Y, Wang H, Xie M. Osthole ameliorates insulin resistance by increment of adiponectin release in high-fat and high-sucrose-induced fatty liver rats. Planta Med. 2011;77:231–5. doi: 10.1055/s-0030-1250268. [DOI] [PubMed] [Google Scholar]
- 26.Kandasamy AD, Sung MM, Boisvenue JJ, Barr AJ, Dyck JR. Adiponectin gene therapy ameliorates high-fat, high-sucrose diet-induced metabolic perturbations in mice. Nutr Diabetes. 2012;2:e45. doi: 10.1038/nutd.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser (307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 28.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 29.De Fea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J Biol Chem. 1997;272:31400–6. doi: 10.1074/jbc.272.50.31400. [DOI] [PubMed] [Google Scholar]
- 30.Liu YF, Paz K, Herschkovitz A, Alt A, Tennenbaum T, Sampson SR, et al. Insulin stimulates PKCzeta -mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J Biol Chem. 2001;276:14459–65. doi: 10.1074/jbc.M007281200. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, et al. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser (1101) J Biol Chem. 2004;279:45304–7. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XF, Tan BK. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Med J. 2000;41:9–13. [PubMed] [Google Scholar]
- 33.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–83. doi: 10.1046/j.1365-201X.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 34.Taniguchi CM, Ueki K, Kahn R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest. 2005;115:718–27. doi: 10.1172/JCI23187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Petruzzelli LM, Ganguly S, Smith CJ, Cobb MH, Rubin CS, Rosen OM. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982;79:6792–6. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya S, Dey D, Roy SS. Molecular mechanism of insulin resistance. J Biosci. 2007;32:405–13. doi: 10.1007/s12038-007-0038-8. [DOI] [PubMed] [Google Scholar]
- 37.Chattopadhyay RR, Bandyopadhyay M. Effect of Azadirachta indica leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr J Biomed Res. 2005;8:101–4. [Google Scholar]
- 38.Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: part V. J Ethnopharmacol. 1999;67:373–6. doi: 10.1016/s0378-8741(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 39.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 40.Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J Nutr. 2006;136:582–7. doi: 10.1093/jn/136.3.582. [DOI] [PubMed] [Google Scholar]
- 41.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet. 2010;375:2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes. 2010;1:68–75. doi: 10.4239/wjd.v1.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 44.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–15. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- 45.Karnieli E, Armoni M. Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: From physiology to pathology. Am J Physiol Endocrinol Metab. 2008;295:E38–45. doi: 10.1152/ajpendo.90306.2008. [DOI] [PubMed] [Google Scholar]
- 46.Geetha R, Yogalakshmi B, Sreeja S, Bhavani K, Anuradha CV. Troxerutin suppresses lipid abnormalities in the heart of high-fat-high-fructose diet-fed mice. Mol Cell Biochem. 2014;387:123–34. doi: 10.1007/s11010-013-1877-2. [DOI] [PubMed] [Google Scholar]
- 47.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–92. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 48.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;28(378):785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 49.Anne AW, Akilavalli N, Mangalapriya V, Balasubramanian Evaluation of antidiabetic property of Andrographis paniculata powder in high fat and sucrose-induced type-2 diabetic adult male rat. Asian Pac J Trop Dis. 2014;4:930–7. [Google Scholar]


