Abstract
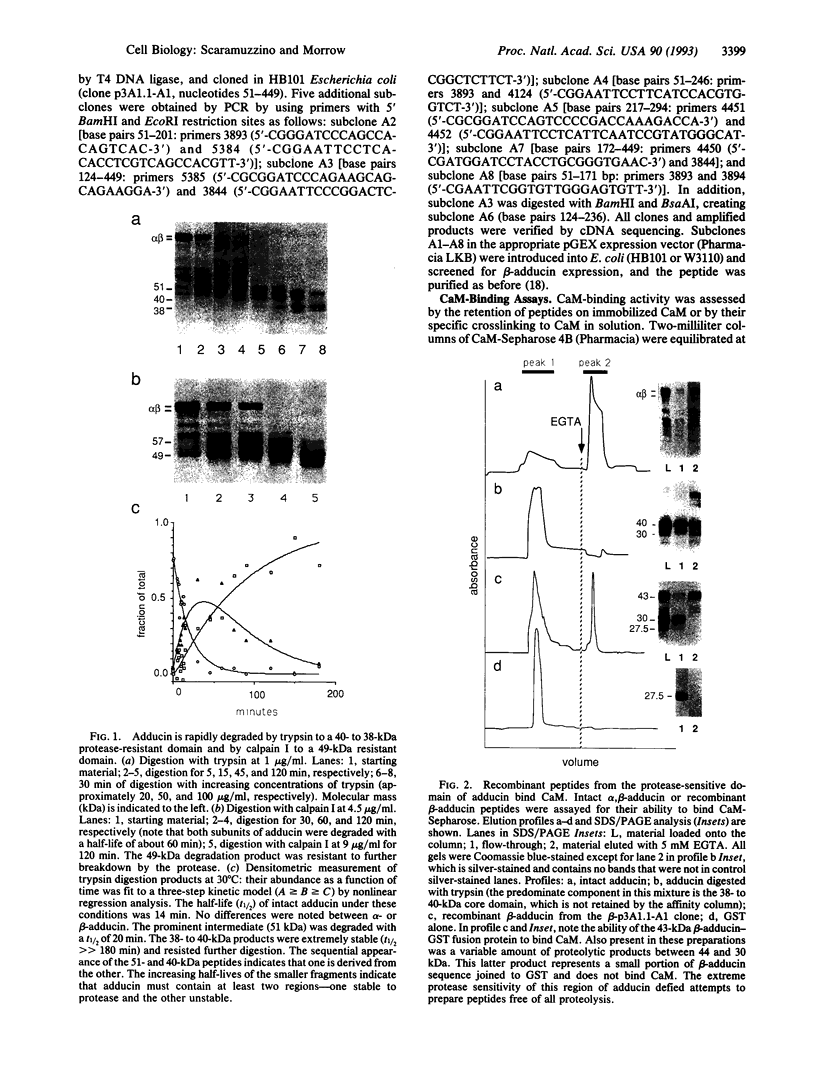
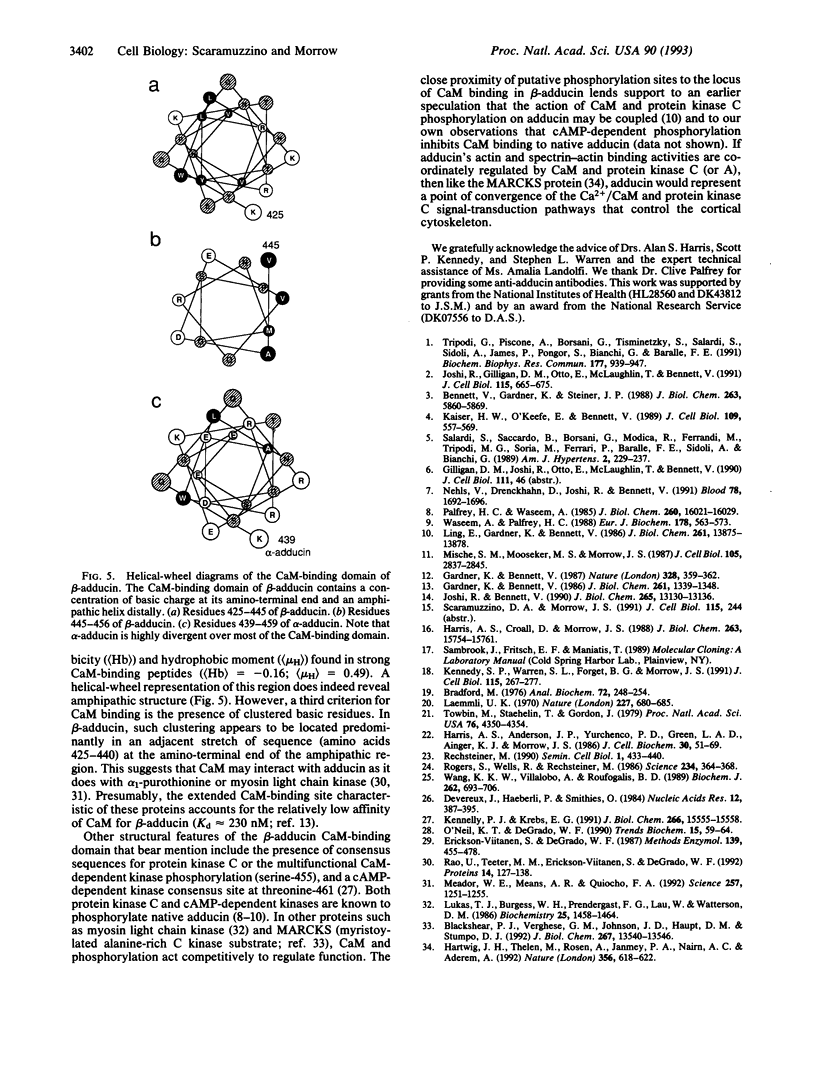
Adducin is a 200-kDa heterodimeric protein of the cortical cytoskeleton of mammalian erythrocytes. Analogs are also abundant in brain and several other tissues. In vitro, adducin bundles F-actin and enhances the binding of spectrin to actin. Previous studies have established that the beta subunit of adducin binds calmodulin (CaM) in a Ca(2+)-dependent fashion with intermediate affinity (approximately 200 nM) and that this activity is destroyed by proteolysis. We have confirmed the trypsin sensitivity of CaM binding by beta-adducin and the existence of a 38- to 39-kDa protease-resistant core. Calpain I digestion generates a larger core fragment (49 kDa) that is also devoid of CaM-binding activity. Use of recombinant beta-adducin peptides generated from partial cDNA clones identified strong CaM-binding activity within the protease-sensitive domain in residues 425-461: KQQKEKTRWLNTPNTYLRVNVADEVQRNMGSPRPKTT in single-letter amino acid codes. This region of the molecule is highly conserved between mouse, rat, and human and shares structural features with CaM-binding sequences in other proteins. Multiple flanking PEST sequences (sequences rich in proline, glutamic acid, serine, and threonine residues that enhance proteolytic sensitivity) may contribute to the protease sensitivity of this region. Consensus sequences for phosphorylation by cAMP-dependent kinases and by protein kinase C (or CaM-dependent kinase) are also found within or near this CaM-binding domain. Collectively, these data suggest a structural basis for the regulation of adducin by Ca(2+)-dependent CaM binding and possibly by covalent phosphorylation and calpain I-mediated proteolysis as well.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Gardner K., Steiner J. P. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J Biol Chem. 1988 Apr 25;263(12):5860–5869. [PubMed] [Google Scholar]
- Blackshear P. J., Verghese G. M., Johnson J. D., Haupt D. M., Stumpo D. J. Characteristics of the F52 protein, a MARCKS homologue. J Biol Chem. 1992 Jul 5;267(19):13540–13546. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Viitanen S., DeGrado W. F. Recognition and characterization of calmodulin-binding sequences in peptides and proteins. Methods Enzymol. 1987;139:455–478. doi: 10.1016/0076-6879(87)39106-2. [DOI] [PubMed] [Google Scholar]
- Gardner K., Bennett V. A new erythrocyte membrane-associated protein with calmodulin binding activity. Identification and purification. J Biol Chem. 1986 Jan 25;261(3):1339–1348. [PubMed] [Google Scholar]
- Gardner K., Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature. 1987 Jul 23;328(6128):359–362. doi: 10.1038/328359a0. [DOI] [PubMed] [Google Scholar]
- Harris A. S., Anderson J. P., Yurchenco P. D., Green L. A., Ainger K. J., Morrow J. S. Mechanisms of cytoskeletal regulation: functional and antigenic diversity in human erythrocyte and brain beta spectrin. J Cell Biochem. 1986;30(1):51–69. doi: 10.1002/jcb.240300107. [DOI] [PubMed] [Google Scholar]
- Harris A. S., Croall D. E., Morrow J. S. The calmodulin-binding site in alpha-fodrin is near the calcium-dependent protease-I cleavage site. J Biol Chem. 1988 Oct 25;263(30):15754–15761. [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Joshi R., Bennett V. Mapping the domain structure of human erythrocyte adducin. J Biol Chem. 1990 Aug 5;265(22):13130–13136. [PubMed] [Google Scholar]
- Joshi R., Gilligan D. M., Otto E., McLaughlin T., Bennett V. Primary structure and domain organization of human alpha and beta adducin. J Cell Biol. 1991 Nov;115(3):665–675. doi: 10.1083/jcb.115.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H. W., O'Keefe E., Bennett V. Adducin: Ca++-dependent association with sites of cell-cell contact. J Cell Biol. 1989 Aug;109(2):557–569. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. P., Warren S. L., Forget B. G., Morrow J. S. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991 Oct;115(1):267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling E., Gardner K., Bennett V. Protein kinase C phosphorylates a recently identified membrane skeleton-associated calmodulin-binding protein in human erythrocytes. J Biol Chem. 1986 Oct 25;261(30):13875–13878. [PubMed] [Google Scholar]
- Lukas T. J., Burgess W. H., Prendergast F. G., Lau W., Watterson D. M. Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry. 1986 Mar 25;25(6):1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- Meador W. E., Means A. R., Quiocho F. A. Target enzyme recognition by calmodulin: 2.4 A structure of a calmodulin-peptide complex. Science. 1992 Aug 28;257(5074):1251–1255. doi: 10.1126/science.1519061. [DOI] [PubMed] [Google Scholar]
- Mische S. M., Mooseker M. S., Morrow J. S. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987 Dec;105(6 Pt 1):2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Waseem A. Protein kinase C in the human erythrocyte. Translocation to the plasma membrane and phosphorylation of bands 4.1 and 4.9 and other membrane proteins. J Biol Chem. 1985 Dec 15;260(29):16021–16029. [PubMed] [Google Scholar]
- Rao U., Teeter M. M., Erickson-Viitanen S., DeGrado W. F. Calmodulin binding to alpha 1-purothionin: solution binding and modeling of the complex. Proteins. 1992 Oct;14(2):127–138. doi: 10.1002/prot.340140202. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. PEST sequences are signals for rapid intracellular proteolysis. Semin Cell Biol. 1990 Dec;1(6):433–440. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Salardi S., Saccardo B., Borsani G., Modica R., Ferrandi M., Tripodi M. G., Soria M., Ferrari P., Baralle F. E., Sidoli A. Erythrocyte adducin differential properties in the normotensive and hypertensive rats of the Milan strain. Characterization of spleen adducin m-RNA. Am J Hypertens. 1989 Apr;2(4):229–237. doi: 10.1093/ajh/2.4.229. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi G., Piscone A., Borsani G., Tisminetzky S., Salardi S., Sidoli A., James P., Pongor S., Bianchi G., Baralle F. E. Molecular cloning of an adducin-like protein: evidence of a polymorphism in the normotensive and hypertensive rats of the Milan strain. Biochem Biophys Res Commun. 1991 Jun 28;177(3):939–947. doi: 10.1016/0006-291x(91)90629-l. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Villalobo A., Roufogalis B. D. Calmodulin-binding proteins as calpain substrates. Biochem J. 1989 Sep 15;262(3):693–706. doi: 10.1042/bj2620693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem A., Palfrey H. C. Erythrocyte adducin. Comparison of the alpha- and beta-subunits and multiple-site phosphorylation by protein kinase C and cAMP-dependent protein kinase. Eur J Biochem. 1988 Dec 15;178(2):563–573. doi: 10.1111/j.1432-1033.1988.tb14483.x. [DOI] [PubMed] [Google Scholar]







