Abstract
Glutathione, considered to be the master antioxidant (AO), is the most-important redox regulator that controls inflammatory processes, and thus damage to the periodontium. Periodontitis patients have reduced total AO capacity in whole saliva, and lower concentrations of reduced glutathione (GSH) in serum and gingival crevicular fluid, and periodontal therapy restores the redox balance. Therapeutic considerations for the adjunctive use of glutathione in management of periodontitis, in limiting the tissue damage associated with oxidative stress, and enhancing wound healing cannot be underestimated, but need to be evaluated further through multi-centered randomized controlled trials.
Keywords: Antioxidants, gingival crevicular fluids, glutathione, periodontitis, saliva
INTRODUCTION
Oxidation is defined as a “chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent.” Although, it is essential for survival of an organism, oxidation can produce reactive oxygen species (ROS). These include both true or oxygen derived free radicals such as superoxide radical (O2−),[1] hydroxyl radical (OH) and nitric oxide (NO) radical (NO−) as well as non-oxygen derived radicals such as hydrogen peroxide (H2O2) and hypochlorous acid (HOCl);[2] and can start chain reactions resulting in cell damage or death.
“Antioxidants (AOs) are molecules, which when present at low concentrations compared to those of an oxidizable substrate, will significantly delay or inhibit oxidation of that substance.”[3,4] They cease destructive chain reactions started by ROS.[1] Living organism cells have developed numerous AO defense systems to contain ROS mediated damage, viz enzymatic AOs such as peroxidases,[5] thiol-containing peptides such as glutathione and thioredoxine, and sophisticated transcriptional programs such as ones controlled by Forkhead box O.[6] Melatonin, which is N-acetyl-5-methoxy-tryptamine, mainly produced by pineal gland, has also been shown to encompass powerful free-radical scavenger activity.[7,8] Currently, AOs have been categorized according to their location of action (intracellular, extracellular or membrane associated); solubility (water soluble or fat soluble); origin (exogenous, endogenous or synthetic); structures they protect (DNA protective, protein-protective or lipid protective); mode of action (preventive or scavenging by chain breaking);[4] Major exogenous sources of AOs are naturally obtained from the diet such as phytonutrients. Recently, manganese-based superoxide dismutase like synthetic AO synzyme (M40403), and azymes[9] have also been added to synthetic AOs group of N-acetylcysteine, pencillinamine and tetracyclines.[4]
Oxidative stress (OS) leads to molecular damage resulting from disturbances in redox signaling and control due to an imbalance between oxidants and AOs oxidants ROS.[10,11,12] OS is considered to play an important role in the pathogenesis of chronic inflammatory conditions like non-insulin dependent diabetes mellitus, cardiovascular disease, metabolic syndrome,[11,13,14,15] acute respiratory distress syndrome,[5,16] and periodontal disease.[5,17,18,19,20,21,22] Recently, it has been proposed as a central link between systemic diseases and periodontitis.[23]
Ubiquitous AO master, glutathione is a low-molecular weight, major non-protein cellular thiol, present in both eukaryotic as well as prokaryotic cells,[24,25,26] and exist in every single cell of the human body.[24] It is a potent AO that prevents ROS mediated damage to essential cellular components and acts as a cofactor for enzymes in the destruction of ROS. It serves as a reservoir for cysteine, participates in detoxification reactions for xenobiotics and metabolism of numerous cellular compounds (e.g., NO), and is required for synthesis of some prostaglandins (PGs) and thermotolerance.[25] It also has a significant role in nutrient metabolism and regulating cellular events as cell apoptosis, cell proliferation, cytokine production, immune response, gene expression, DNA and protein synthesis, signal transduction and protein glutathionylation.[27]
Periodontitis is a chronic inflammatory condition initiated in response to plaque biofilm, characterized by exaggerated inflammation and loss of periodontal and bone support, with the excessive production of ROS and proteolytic enzymes.[28] Periodontitis patients reported having reduced total AO capacity in whole saliva,[5] and lower concentrations of reduced glutathione (GSH) in serum and gingival crevicular fluid (GCF).[29,30] This paper thus aims to review and discuss the basic mechanism and properties of glutathione homeostasis for the clinicians and researchers along with updating the current knowledge and investigations implicit in periodontal health.
MATERIALS AND METHODS
Related literature was searched using the MedLine/PubMed database, online Cochrane library and Google scholar, with an emphasis on peer-reviewed dental journal until October 2013. Appropriate information was also obtained from Kyoto Encyclopedia of Genes and Genomes and BioProject online database. The medical subject headings (MeSH) terms used for search were “glutathione and periodontal disease,” “glutathione and periodontitis” and “glutathione and periapical health.” Search engine MedLine/PubMed when searched for MeSH word “glutathione” revealed 109509 papers, and search when was further filtered for MeSH word “glutathione and periodontal disease,” and “glutathione and periodontitis” shown 85, and 71 papers respectively. Online Cochrane library database revealed one controlled clinical trial when searched for “glutathione and periodontal.” Pertinent articles in English language on the topic and abstracts of relevant papers were scrutinized thoroughly, and finally papers pertaining to the topic were included after excluding the duplicates [Tables 1-5].[4,5,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111] Relevant literature in common textbooks, bibliographies of papers and review article together with appropriate peer-reviewed print journals, were also analyzed for additional information.
Table 1.
List of relevant studies in search engine PubMed/Medline and the Cochrane library till October 2013
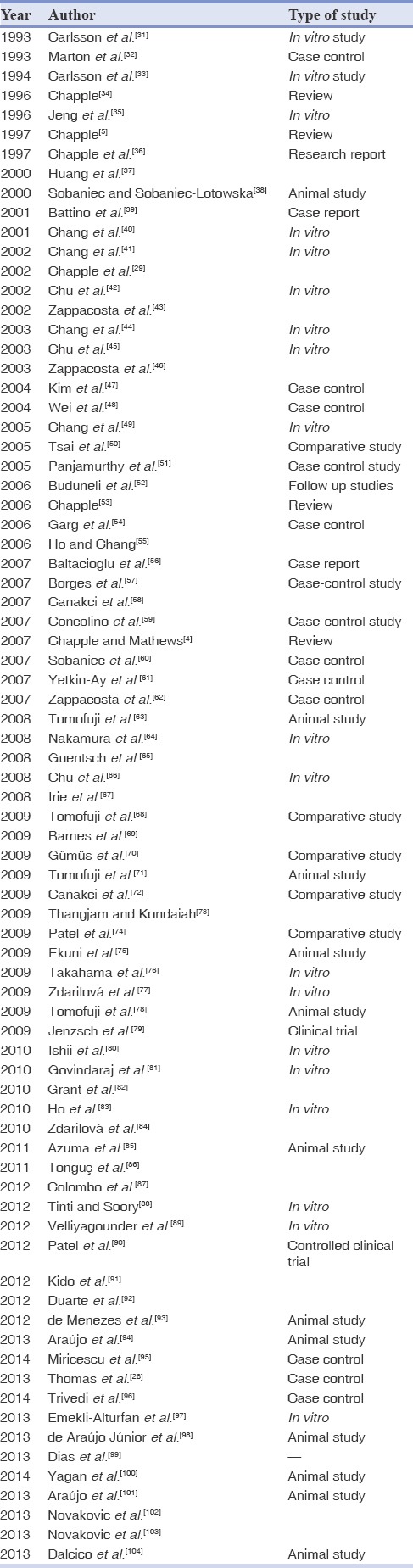
Table 5.
Mean values of antioxidant markers in health and disease in blood/plasma/serum/gingival tissues
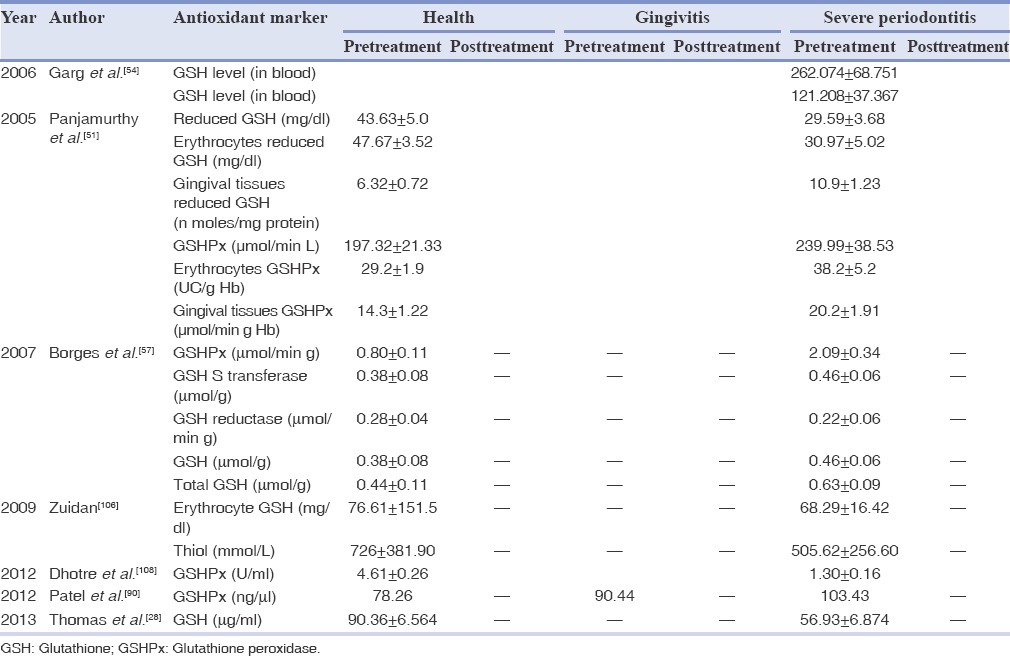
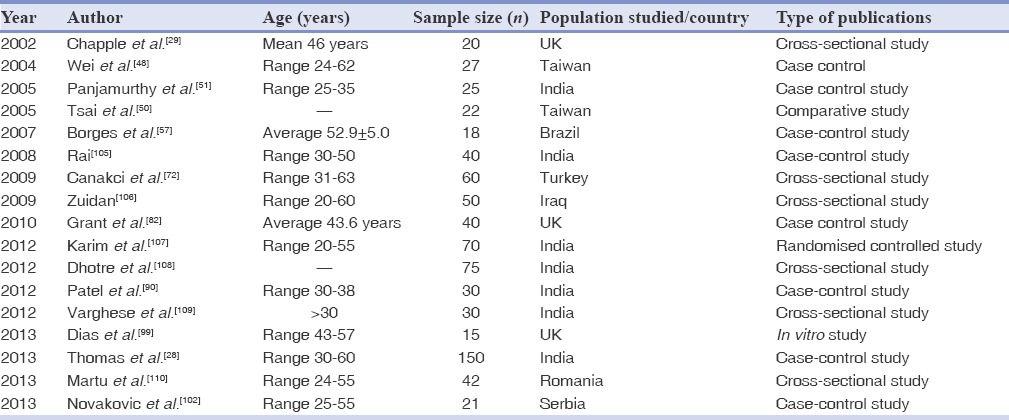
Table 2.
Studies in humans published till October 2013
LITERATURE REVIEW
In 1888, de Ray Pailhade gave the first hint of “organic stuff” related to the metabolism of sulfur.[112] In 1922, Sir Frederick Gowland Hopkins[113] discovered and characterized glutathione, and erroneously described it as dipeptide of glutamic acid and cysteine in a classic paper published in Journal of Biological Chemistry. In 1927, Hunter and Eagles doubted Hopkin's hypothesis, and reported tripeptide structure of glutathione;[114] which was later on accepted by Hopkins[115] in 1929, who then indeed described it as a tripeptide of glutamic acid, glycine and cysteine (Glu-Cys-Gly).[116]
Biochemistry
Tripeptide, glutathione (chemical formula, C10H17N3O6S; structural formula, γ-L-glutamyl-L-cysteinylglycine, International Union of Pure and Applied Chemistry name 2-amino-5-{[2-carboxymethyl)-amino]-1-(mercaptomethyl)-2-oxoethyl]-amino}-5-oxopentanoic acid) consists of a unique gamma (γ) peptide linkage among the amine group of cysteine with the carboxyl group of the glutamate side-chain.[117] It is water soluble in nature, having molecular mass of 307.3235 g/mol and melting point of 383°F/195°C. Glutathione is the predominant cellular thiol present in mammalian cells at concentration 0.5-10 mmol/L. Eighty-five to ninety percent of cellular glutathione is present in the cytosol, and rest 10-15% is distributed in many intracellular organelles like mitochondria, nuclear matrix, endoplasmic reticulum (ER), peroximes and nucleus.[26,27,118] Concentration of mitochondrial glutathione is similar to that of the cytosol (10-14 mM) by the volume of the mitochondrial matrix.[118] Its concentration in the human liver tissue is evaluated as 6,400 mmol/kg.[117] Relatively low (2-20 μmol/L in plasma) concentration of glutathione is observed in the extracellular environment, whereas, bile acids may contain up to 10 mmol/L glutathione.[27] Glutathione concentration is found to be greatest in the liver, the organ involved in detoxification and elimination of toxins, and in the lung epithelial lining fluid (ELF) (of about 400 μM), which is raised in the case of chronic smokers and lung cancer patients,[34,117] and decreased in patients with pulmonary fibrosis and acute respiratory distress syndrome.[34]
The AO properties of glutathione are mediated by redox-active thiol group that becomes oxidised when glutathione reduces target molecules, which may be attributed to the fact that glutathione displays a low redox potential (E’0 = −240 mV), and is found in high concentration in the cells (approximately 10-15 Mm).[118] In the presence of electrophilic substances, e.g., free radicals and ROS/nitrogen sps, glutathione readily oxidizes non-enzymatically to glutathione disulfide (GSSG).[27] As glutathione acts as electron carriers, so when electrons are lost by oxidation, glutathione becomes oxidized (GS−), and two such molecules dimerized by a disulfide bridge to form disulfide glutathione or oxidized glutathione (GSSG). Total glutathione pool consist of more than 90% of “the reduced-form (GSH)”, and <10% exists in “the disulfide or oxidized form (GSSG)” in healthy cells and tissues,[117] and a significant amount (15%) of total glutathione of cell (GSH + 2GSSH) concentration may be bound to protein.[27] Under normal physiological conditions, the GSH: GSSH ratio (that is an indicator of cellular redox state) is >10. Antioxidative capacity of cells is determined majorly by redox couple GSH/GSSG, however, other redox couples as nicotinamide adenine dinucleotide phosphate (NADPH)/NADP+ and thioredoxinred/thioredoxinox could also affect its value. Many pathological conditions, OS, protein malnutrition, can markedly reduce the cellular glutathione concentrations.[27] The process of regulation of relative levels of glutathione and GSSG, constitute glutathione peroxidase (GSHPX) reaction by which glutathione reduces H2O2 or lipid peroxides producing GSSG. NADPH is used to reduce the GSSG to glutathione, which is catalyzed through glutathione reductase (GR), a flavoprotein enzyme.[119] NADPH is resupplied by a reduction of NADP+ via pentose-phosphate pathway [Figure 1].[118] In cases of rapid production of GSSG, or impaired GR activities, GSSG accumulation occurs, that is either transported from the cells or reacts with protein sulfhydryls (SHs), via a mixed disulfide reaction, potentially resulting in impaired protein function, and net loss of intracellular glutathione.[25]
Figure 1.
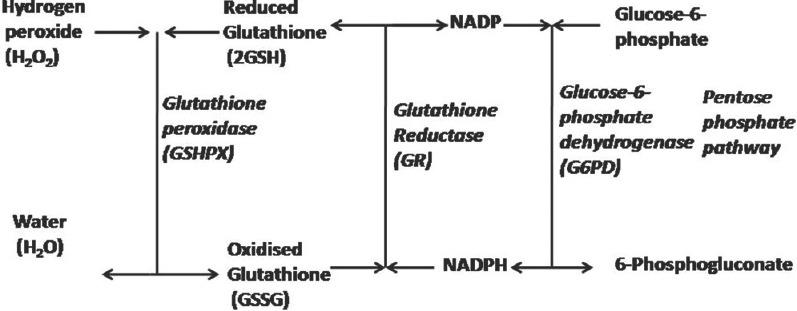
Oxidation and reduction of glutathione.[118]
Intracellular compartmentalization of glutathione includes separate redox pools that are distinct from the cytoplasmic pools in terms of GSH/GSSG ratio, their redox potential, and their control of cellular activities. In the nucleus, critical protein sulfhydryl necessary for DNA repair and expression is maintained by glutathione. Further, it plays a contributory role in DNA synthesis by donating hydrogen in ribonucleotide reductase-catalyzed reduction of ribonucleotides to deoxyribonucleotides. In the ER, glutathione mainly exist as GSSG, which is the main source of oxidizing equivalents to provide a suitable environment for favoring disulfide bond formation and proper folding of nascent proteins. However, in mitochondria, glutathione is found mainly in reduced form (GSH) and represents a minor fraction of the total glutathione pool (10-15%). Because of the lack of catalase in mitochondria, H2O2 metabolism is mediated through glutathione, with participation of GSHPX or peroxiredoxin. In mitochondria matrix, cytosolic GSHPX (cGSHPX or GSHX-1), which is the major isoform of GSHPX localized in cytoplasm, is found only in a small fraction.[118]
Glutathione biosynthesis
Glutathione is synthesized intracellularly, by two enzymes namely glutamate-cysteine ligase (GCL) or γ-glutamate-cysteinyl-synthetase (GCS), which is the rate-limiting step and glutathione synthetase, and is regenerated by six enzyme-catalyzed reactions known as γ-glutamyl cycle.[34] Of the three building blocks of glutathione, viz., cysteine, glutamic acid and glycine, the sulphydryl or thiol (SH) group in cysteine serves as a proton donor, and acts as a limiting factor in glutathione synthesis. In the absence of reduced cysteine, the addition of an acetyl group to cysteine (N-acetyl-cysteine [NAC]) provides the ability for a molecule to cross the cell membrane and promotes intracellular glutathione synthesis.[117]
It is synthesized in cytosol outside the ribosomes that requires ATP, but does not require the participation of RNA. L-glutamate condenses with L-cysteine to form γ-L-glutamyl-L-cysteine, which requires GCS in presence of ATP, K+ and Mg++ with the formation of the enzyme bound γ-glutamyl-(P) as an intermediate.[9] γ-carboxyl group of glutamate form a peptide γ-linkage after reaction with the amino group of cysteine, which protects glutathione from hydrolysis by intracellular peptidase. γ-L-glutamyl-L-cysteine can be a substrate for γ-glutamylcyclotransferase (GCT), however, due to higher affinity and activity of glutathione synthetase (GS), glutathione synthesis is favored in animal cells.[118] In the presence of GS and ATP, γ-L-glutamyl-L-cysteine is phosphorylated to form enzyme bound γ-glutamyl-cysteinyl-(P), which reacts with Gly to liberate free glutathione and Pi.[9] This pathway occurs virtually in all cell types (except epithelial cells), with liver being the major producer and exporter of glutathione.
γ-glutamate-cysteinyl-synthetase or GCL is in part regulated by glutathione feedback inhibition,[26] which can be partially prevented by an excess of glutamic acid that blocks the regulatory site on the enzyme.[25] Thus, if glutathione is exhausted due to OS, exposure to xenobiotics or inflammation, de novo synthesis of glutathione is up-regulated.[26] Inhibition of GS results in the accumulation of γ-L-glutamyl-L-cysteine that is converted to cysteine and oxoproline by GCT, and then oxoproline is converted to glutamic acid via 5-oxoprolinase reaction by oxoproplinase. Life-threatening acidosis occurs due to the accumulation of oxoproline in the absence of GS [Figure 2].[25] Two amino acid transport systems namely; sodium-dependent system for cysteine transports, and sodium-independent cysteine and glutamate transport system, participate in primary regulation of substrates for glutathione synthesis. Transpeptidase activity at the cell surface is accountable for recovering amino acids from circulating glutathione for reuse in the intracellular glutathione synthesis.[25]
Figure 2.
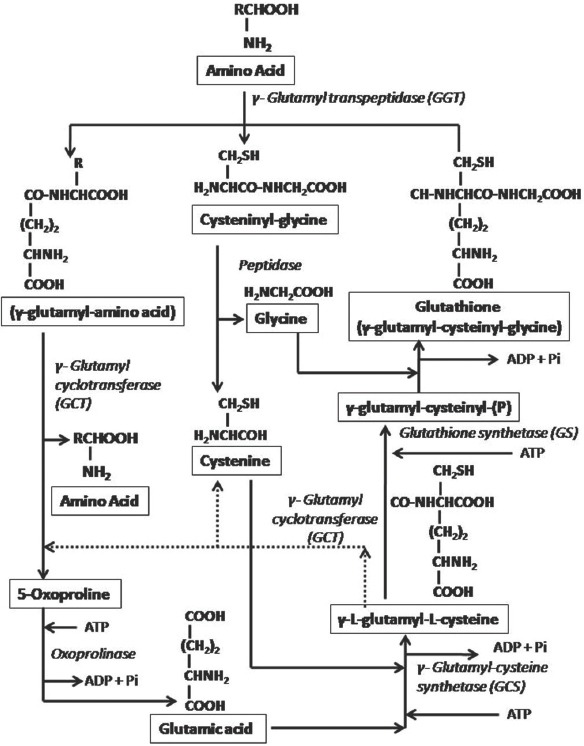
Glutathione Synthesis (γ-glutamyl cycle). Dotted line show mechanism of life threatening acidosis in case of inhibition of glutathione synthetase.[25]
After synthesis, intracellular glutathione is exported from most cells. Outside the cell, γ-glutamyl-cysteine bond of glutathione may be hydrolyzed by γ-glutamyl transpeptidase (GGT)[24,26] and transfers a γ-glutamyl moiety from extracellular glutathione to an amino acid.[25] The activity of GGT is important in glutathione cycle and serves as a salvage pathway for cellular glutathione.[26] Glutathione and other γ-glutamyl containing compounds (e.g. GSSG and γ-glutamyl glutathione) react with the transpeptidase at the outer cell surface. The γ-glutamyl moiety is transferred to a suitable amino acid acceptor, and both the γ-glutamyl amino acid (dipeptide) and the cysteinylglycine are transported into the cell, and reused for glutathione synthesis. Peptidase cleaves cysteineglycine, and if the dipeptide is also γ-glutamylcysteine, they can be utilized directly for glutathione synthesis; otherwise dipeptide is converted to glutamate by GCT and oxoprolinase.[25]
Glutathione functions in systemic health
Glutathione has been considered as a true protagonist in cellular regulation.[112] Interrelations linking the antitoxic, AO, prooxidant, and modulator roles of glutathione in cellular homeostasis have been outlined by Pompella et al., in 2003.[112] Glutathione is involved in the formation and maintenance of protein disulfide bonds, as well as in the amino acids transport across cell membranes. Glutathione efficiently scavenges free radicals and other ROS (OH, lipid peroxyl radical, peroxynitrites and H2O2), directly or indirectly, while oxidizing glutathione to GSSG.[27] By donating H2, it helps to destroy H2O2 and other peroxides in cells, which are catalyzed by containing GSHPX.[9,27] The reaction given below is catalyzed by GSHPX as follows:[120,121]
2GSH + H2O2 → 2GSSH + 2H2O
Rotruck et al.[122] recognized that the selenium (as an active site selenocysteine residue)[119] is a structural constituent of the active center of the animal enzyme GSHPX-1. Analysis of selenoproteome characterized the functions and sequence of six types of GSHPX in mammals as; cytosolic (cGSHPX or GSHPX-1), gastrointestinal (GI-GSHPX or GSHPX-2), plasma (pGSHPX or GSHPX3), phospholipid hydroperoxide (phGSHPX or GSHPX-4), epididymal androgen related protein or secretory (GSHPX-5) and olfactory (GSHPX-6).[123]
Glutathione peroxidase-1 prevents cytotoxic peroxide-induced oxidative damage, lipid peroxidation and protein degeneration. GSHPX-1 counteracts hydroperoxide-modulated events, such as cytokine signaling and cluster of differentiation-95-triggered apoptosis, to eliminate neoplastic cells and plays an important role in HIV infection. Studies report that reduction in glutathione and GSHPX-1 activity prior to infection can result in a decrease in virus spread because of apoptosis initiated by oxidative microenvironment. In HIV patients, the glutathione levels are the sole predictor of death, and there is also a hypothesis that, “the mechanism responsible for AIDS could be reversed by the administration of reducing agents, especially those containing SH groups”.[117] GSHPX-2 might be an anti-inflammatory and anti-carcinogenic enzyme. GSHPX-4 is required for embryogenesis and male fertility.[123]
For AO actions, glutathione acts as a cofactor for GSHPX and other enzymes (as glutathione-dependent dehydroascorbate reductase) active in cell defense against prooxidants. Further, glutathione-dependent dehydroascorbate reductase is involved in the reduction of the oxidized product, dehydroascorbate to ascorbic acid by using glutathione as an electron donor (ascorbate recycling). It is thus a primary cellular tool in order to maintain steady state concentrations of ascorbic acid (a perfect AO) in accelerated oxidation conditions.[112]
It acts as a coenzyme with liver enzyme glutathione-insulin transhydrolase which helps in the catabolism and degradation of the protein hormone inulin.[9] Targeting endogenous NO, intracellular glutathione, conjugates with NO to form an S-nitrosoglutathione (GSNO) adduct that in-turn is cleaved by the thioredoxin system to produce glutathione and NO. Both glutathione and NO are necessary for the hepatic action of insulin-sensitizing agents, thus have a critical role in regulating glucose, amino acid and lipid utilization.[27] Glutathione and the enzyme glutathione transhydrogenase help to cause reductive cleavage of S-S linkages in thyroglobulin glycoprotein. Many-SH group contacting enzymes (e.g. glyceraldehydes-3-P-dehydrogenase) are also protected by glutathione against their - SH groups oxidation.[9]
Glutathione plays an important role for vital functions as it is required for the proliferation of cells (lymphocytes and interstitial epithelial cells); spermatogenesis and sperm maturation; activation of T-lymphocytes and polymorphonuclear leukocytes; for cytokine production and for inhibiting influenza virus infection as reported by in vivo studies.[27] It is required as coenzyme/cofactor with prostaglandin-synthetase system (cyclooxegenase) used for formation of endoperoxides from arachidonate in prostaglandin synthesis,[9] and for the conversion of prostaglandin H2 into prostaglandin D2 and E2 by endoperoxide isomerase.[27] It is required as a coenzyme with maleylacetoacetate isomerase that catalyzes Cis-trans isomerization of the maleylacetoacetate to fumarylacetoacetate, and for the enzyme glyoxylase which converts methylglyxol to lactic acid through intramolecular oxidation-reduction.[9,27] It also takes part in γ-glutamyl cycle for absorption of amino acid from the gut.[9]
It function as a coenzyme with formaldehyde dehydrogenase which catalyzes the oxidation of formaldehyde, a carcinogen, produced during metabolism of methionine, choline, methanol, sarcosine and xenobiotics (by the cytochrome P450-dependent monooxygenase system of the ER), to formic acid.[9,27] Glutathione-S-transferase (GST), a family of phase II detoxification enzymes, initiates reaction of glutathione with various electrophiles, physiological metabolites (e.g. estrogen, PGs, melanins and leukotrienes), and xenobiotics (e.g. drugs [bromobenzenes and acetaminophen], pollutants, carcinogens) to form mercaptures.[27] Beside conjugation of xenobiotics, GST-Pi act as Jun N-terminal kinase (JNK)-inhibitor, thus preventing c-Jun phosphorylation, by forming macromolecular complexes with JNK in vitro.[124]
It is capable of forming disulfide bonds with cysteine residues of protein known as S-glutathionylation.[112] S-glutathionylation of proteins (ubiquitin-conjugating enzyme, cytochrome c oxidase and thioredoxin) plays an important role in cell physiology,[27] affecting members of signal transduction chains involved in cell proliferation.[112] Increase in apoptosis and reduction in cellular proliferation by the activation of several signaling pathways like protein kinase B, H-ras, T-cell p59, c-JNK, apoptosis signal-regulated kinase 1, protein phosphatase 1 and 2A, calcineurin, nuclear factor-kB (NF-kB)/p50, and mitogen-activated protein kinase, is evident by the reduction in GSH/GSSG ratio.[27,112]
Glutathione is needed for the conversion of Dopa-quinone to glutathione-Dopa synthesis of pheomelanin and trichochromes, as well as a coenzyme for liver enzyme for activation of methionine to form active form “S-adenosyl methionine.”[9] GSSG is harmful to red blood cell (RBC) membranes and lens proteins and is converted to reduced glutathione, which is required for the integrity of RBCs membrane and lens proteins.[9]
Recent studies have shown the pro-oxidant properties of glutathione in which glutathione and its catabolites (e.g. Cys-Gly) promote oxidative process, by participating in metal ion-mediated reactions membrane-bound by GGT activity, eventually leading to formation of ROS and free radicals.[112] Extracellular breakdown of glutathione by GGT, and the consequent release of the highly reactive dipeptide thiol Cys-Gly is likely to be a major determinant of protection exhibited by glutathione cisplastin nephrotoxicity.[125] Studies have further identified membrane-bound GGT activity as a factor of drug resistance, in normal and cancer cells.[126] Cells expressing sufficient GGT activity at their surface might be able to effect ‘extracellular detoxification’ of electrophilic drugs.[112]
Transcription factor “NF (erythroid-derived 2)-like 2 (NFE2-L2 or NFE2-related-factor-2 [Nrf2])” is a master regulator of the AO response that modulate the expression of hundreds of genes controlling various immune and inflammatory responses, carcinogenesis and metastasis, tissue remodeling and fibrosis, and cognitive dysfunction and addictive behavior.[126] Nrf2, a positive regulator of the human AO response element (ARE), develops expression of AO enzyme such as NADPH: Quinone oxidoreductase 1.[127] Itoh et al.[128] described the mechanism of Nrf2 activation that involve a protein Keap1 which is a suppressor protein anchored in the cytoplasm that physically binds Nrf2, thus preventing its translocation to the nucleus and its access to ARE containing promoters. Wild et al.[129] have validated that Nrf2 is positive controller of GCS gene expression, involved in glutathione synthesis. Fan et al.[130] hypothesized that “HIV-1-related proteins inhibit Nrf2 mediated AOs defences and thereby disrupt the normally tight alveolar epithelial barrier”. They suggested that “activating Nrf2/ARE pathway with the dietary supplement sulforaphane (SFN) could augment AO defenses and lung health in HIV-1 infected individuals.”[130] Dietary supplement Protandim, a potent composition of highly synergistic phytochemical Nrf2 activators, shows significant modulation not only of AO enzymes, but also those related to colon cancer, cardiovascular disease and Alzheimer disease.[126]
Glutathione in periodontal health and disease
Role of reduced glutathione in the regulation of pro-inflammatory cytokines is of great importance in periodontal disease. Studies revealed that by increasing the concentrations of the cytosolic cysteine, and thus glutathione of monocytes and macrophages using a synthetic form of cysteine called NAC, blocks H2O2 mediated activation of NF-kB, and thus pro-inflammatory cytokines (tumor necrosis factors [TNF]-α, interleukin [IL]-1 β and IL-6) by this route. TNF-α, IL-1 β and IL-6 are associated with activation of bone-resorbing processes, and IL-8 is reported to polymorphonuclear leukocyte activity.[5,34]
More recently, Dias et al.,[99] first time revealed that significant redox disturbances exist in neutrophils of patients with periodontitis, which are associated with deregulation of anti-inflammatory transcription factor Nrf2 pathway resulting in neutrophil hyperactivity/hyper-reactivity. Further, activation of the redox-sensitive protein acid sphingomyelinase to promote lipid raft formation, and therefore assembly of the NADPH oxidase enzyme was also mediated by a disturbance in redox balance. Both mechanisms, result in persistent hyperactivity in neutrophils of periodontitis patients.[99]
Activities of certain pathogens and cigarette smoking have shown to lower glutathione levels within periodontal cells, and OS depletes glutathione, resulting in activation of redox-sensitive transcription factors and thus in the creation of a pro-inflammatory state.[4] Certain putative periodontopathogens, e.g., Fusobacterium nucleatum vincentii, F. nucleatum polymorphum, F. nucleatum nucleatum, F. nucleatum fusiforme, Treponema denticola, Bacteroides Loescheii, Porphyromonas gingivalis, Bacteroides inermedius and Peptostreptococcus micros, are capable of metabolizing L-cysteine or degrading glutathione to form hydrogen sulfide (H2S) within the periodontal pocket, which is toxic to mammalian cells by inactivating cytochrome oxidase, and is also reported to inhibit the catalase.[5,34]
Mäkinen and Mäkinen[131] reported the presence of enzyme GGT (that breaks down glutathione to Cys-Gly in human cell membrane) in the outer cell envelope of T. denticola that may play an important role in its propagation in inflamed periodontal tissues. Three-step pathways have been proposed for the glutathione metabolism in T. denticola. In the first step, glutathione is cleaved into glutamate or glutamine and dipeptide Cys-Gly, catalyzed by GGT. Second step include cleavage of Cys-Gly into Gly and L-cysteine, in the presence of key enzyme cysteinyl-glycinase (52-kDa leucyl aminopeptidase);[66] and finally, L-cysteine is degraded into pyruvate, ammonia and H2S, in the presence of L-cysteine desulfhydrase (cystalysin, 46-kDa protein).[42,45,66,132] Chu et al.,[42,45] ascribed virulence activity of T. denticola to the synthesis of H2S and puryvate from glutathione. Former is crucial for hemoxidative, hemolytic and other toxic activities that could occur in vivo, and pyruvate promotes bacterial growth.
Two sub-species of P. micros and five Fusobacteria species have also been reported to utilize glutathione to produce H2S.[31,34] Within the periodontal pocket, H2S formation is toxic to mammalian cells that inactivate cytochrome oxidase and inhibit the catalase. The latter activity could positively feedback into NF-kB mediated transcription of proinflammatory cytokines.[29,133] Peptostreptococcus, Eubacterium, Selenomonas, Centipede, Bacteriodes, and Fusobacterium also metabolized cysteine to produce volatile sulfur compounds.[134] Moreover, Chapple[5,34] advocated that degradation of cysteine by oral microorganisms may also prevent the inhibition of NF-kB mediated production of pro-inflammatory cytokines in the periodontal environment, and hence cytokine related tissue destruction.
A series of studies have shown association linking cigarette smoking and nicotine toxicity to glutathione depletion within periodontal tissues.[4] Studies of betel nut chewing, and periodontitis have shown that arcea nut alkaloid, arecoline-induced thiol depletion in periodontal ligament fibroblasts may render them more susceptible to the effects of nicotine;[57,58] glutathione protection negated nicotine toxicity;[41] and that cigarette smoke decreased periodontal ligament fibroblast glutathione levels in a dose-dependent manner and stimulated stress-specific genes.[44]
As already discussed, GST is xenobiotic metabolizing enzyme required for the detoxification reactions and utilizes free glutathione. Thus, inducers of GST gene expression generate ROS by metabolic processes resulting in depletion of free intracellular glutathione. Kim et al.,[47] reported that subjects with polymorphic GST-M1 allele have significantly increased risk of periodontitis.
Glutathione and saliva
Periodontal diseases are associated with disturbances in the balance between the oxidants and AOs, in favor of oxidants due to both an increase in ROS as well as decreased AO activity in saliva. Salivary glutathione levels, evidence of the neutralization (scavenging) of ROS were low in patients from periodontal diseases in contrast to individuals with healthy gums.[111] Studies have demonstrated reduced amounts of salivary glutathione, GSH peroxides and thiol levels in the gingivitis and periodontitis patients as compared to the healthy group [Table 3].[72,107,111]
Table 3.
Mean values of antioxidant markers in health and disease in saliva
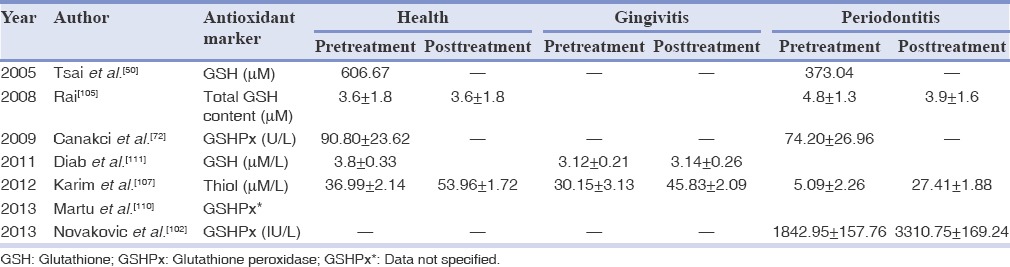
Studies reveal that the periodontal treatment result in improvement in salivary concentrations of glutathione and thiol levels in gingivitis and periodontitis subgroups, which was significant within groups, but not between groups.[50,102,107] Diab et al.,[111] reported improvement in salivary glutathione concentrations from 3.12 ± 0.21 μM/L to 3.6 ± 0.22 μM/L, collected in the patients with periodontal disease before and after treatment with mouthwash with extracts of Solanum melongena (egg-plant) peduncles against placebo during 3 months. They further reported that S. melongena extracts may protect the oral cavity by enhancing its AO properties, and consequently the adverse effect of smoking was attenuated.[111]
Glutathione and gingival crevicular fluid
Data from the high-performance liquid chromatography analyses indicate that glutathione present within the GCF is responsible for the stepped AO response. In GCF concentrations of glutathione (range 0.5-2.5 mM) were reported to be 1000 times greater than that normally detected within extracellular tissue compartments (0.5-5 μM in human plasma).[29] The high levels of glutathione within GCF may be the result of increased synthesis by cells of the periodontal tissues, or of active release mechanisms or passive release secondary to protease activity on gingival epithelial cells.[4] Neutrophils and/or junctional/crevicular epithelium (JE) are significant contributors for the high concentrations of glutathione in GCF. Glutathione may be added to the list of defense factors in gingival crevice and lung known to be epithelial products (e.g., β-defensins and migration inhibiting factor-8). The relative position and function of JE of the gingival crevice are similar to the alveolar epithelium in the lungs; however, in contrast, lung epithelium is bathed and protected by alveolar ELF rather than GCF. Neutrophilic inflammation occurs in both lung and GCF, where activating cytokines are produced by the underlying fibroblasts, lining the epithelium and inflammatory cells in response to microbial insult.
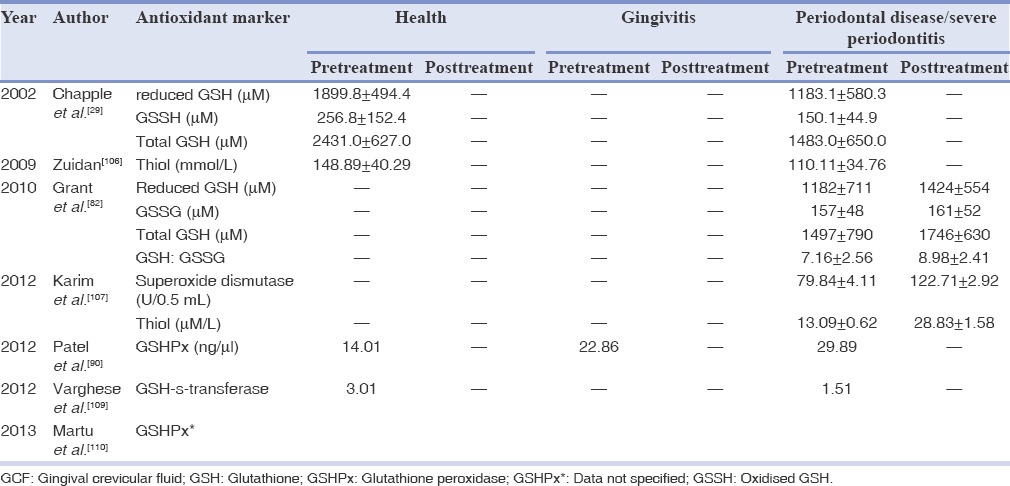
The high concentrations of glutathione in GCF in health and extracellularly in the lung are similar, and therefore may represent an important AO and anti-inflammatory (anti-proinflammatory cytokines) defense strategy common to exposed epithelial cells.[29] Both sites consist of fluid containing high concentrations of glutathione, and in chronic inflammatory conditions, glutathione concentrations are reduced in both sites, owing to the failure of normal tissue homeostatic mechanisms to protect against the host-mediated tissue damage. Chapple et al.,[29] were first to investigate the possible differences in GCF AO capacity between periodontal health and disease. They observed that glutathione concentrations are reduced in chronic periodontal diseases to about 1183.1 ± 580.3 μM as compared to 1899.8 ± 494 μM in control GCF. The lower concentrations of glutathione in GCF of periodontitis patients could be because of various factors resulting in decreased synthesis and/or enhanced local degradation. It may involve an inborn defect in γ-glutamyl pathway of glutathione synthesis (controlled by cellular factor) or other exogenous or endogenous agents such as the local microbes (already explained).[29] More recently, Grant et al.,[82] reported improvement in glutathione concentrations in GCF following periodontal therapy from 1182 ± 711 μM to 1424 ± 554 μM [Table 4].
Table 4.
Mean values of antioxidant markers in health and disease in GCF
Glutathione levels in serum, plasma, gingival tissues and other cells
Gingival tissue analysis revealed that the AO activity in patients with periodontitis was decreased when compared to control the group. Various studies reported significant increase in GSHPX and GST, whereas decrease in GSH and total glutathione levels in gingival tissues and serum from an experimental group (periodontitis patients) when compared to the control group (periodontally healthy patients) [Table 5].[28,57,90,92,103] Peripheral blood neutrophils from chronic periodontitis patients have more GSSG level relative to GSH as compared to neutrophils from healthy control subjects. Normally, the kelch-like erythroid-cell-derived protein with CNC homology (ECH)-Associated protein-1-Nrf2-(Keap-1-Nrf2-ARE) signaling pathway elicits an adaptive response for cell survival under OS, but they did not appear to operate effectively in periodontitis patients.[99]
Therapeutic considerations of glutathione
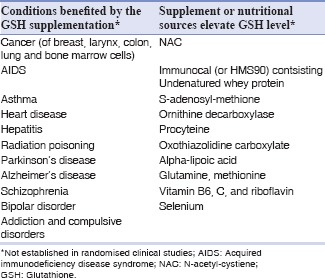
Glutathione supplementation may help to prevent or treat many conditions associated with impaired immune function due to elevated OS or aging [Table 6].[117] It has been postulated that raising glutathione levels in cells have reduced cancer growth by suppressing the activity of certain chemically-reactive oxygen molecules in the test tubes. Although, glutathione is readily available in fresh fruits, vegetables and meat (e.g. apple, carrot, grapes, spinach, tomato, asparagus, avocado and purslane), but consuming them would transfer only small amount of reduced glutathione into the blood stream because most of it is lost in the digestive tract. However, studies revealed that blood glutathione levels increased by supplementing its precursors or other dietary products.[117]
Table 6.
Therapeutic role of glutathione[117]
Hence, with the growing evidences that link reduced glutathione levels with periodontal inflammation, Chapple and Matthews[4] postulated that increasing or preserving intracellular glutathione levels within the cells of the periodontal tissues is likely to provide a novel adjunctive AO and anti-inflammatory strategy to traditional periodontal therapies. P. gingivalis induces excessive activation of the innate immune response and induces melanization which leads to the generation of ROS, and apoptotic cell death in the host tissue. AOs (N-acetyl-L-cysteine, glutathione and catalase) have therapeutic effects in delaying P. gingivalis induced death of silk-worms.[80] Further, the stress-inducible protein heme oxygenase-1 expressed in human gingival fibroblasts exposed to nicotine has been reported to be overcome by the addition of the glutathione precursor N-acetyl-L-cysteine.[49,88] Minor antioxidative effects have been documented after the application of exogenous glutathione and cysteine, however, whether exogenously applied substances elevated the intracellular glutathione concentrations, still need to be investigated.[135]
Improvement in the rate of wound healing has been observed with topical NO donor, GSNO. Improved rates of decreased inflammation, re-epithelialization, wound contraction, increased collagen fiber density and organization as well as decreased neovascularization when applied to ischemic wounds have been observed as compared with control groups. Thus, topical GSNO containing hydrogel has therapeutic potential to improve wound healing in an environment driven by OS by re-establishment of matrix metalloproteinase-1/tissue inhibitor of matrix metalloproteinase-1 (MMP-1/TIMP-1) ratio, resulted in extracellular matrix production and re-epithelialization, which could be beneficial to periodontitis patients.[88] Both exercise particularly moderate exercise (vs. vigorous exercise) and dietary interventions such as consumption of cruciferous vegetables have also been shown to increase glutathione levels.[26]
Recent evidence of the increase in glutathione level and reduction in activity of GSHPX has been observed in experimental-periodontitis rats treated with proanthocyanidins (PC), a type of flavanoid extracted generally from grape seeds, suggests enhanced host resistance and inhibited OS by the dietary supplementation of PC.[81] SFN, a natural product found in cruciferous vegetables, induces Nrf2 dependent AO gene expression by binding to cys151 on Keap-1. SFN have been reported to reinstate the intracellular redox state (GSH:GSSG) in neutrophils from periodontitis. Recent evidences highlighted the use of SFN to control Nrf2 dysregulation to restore neutrophil redox balance along with reducing hyper-reactivity.[99] Chapple et al.[136] also reported statistically significant clinical benefits of daily dietary supplements of capsules containing AO juice powder phytonutrients juice plus fruit-vegetable and fruit-vegetable-berry as adjunct to conventional scaling root planing in a double-blind, placebo-controlled randomized clinical trial.
DISCUSSION
Oxidative stress in the pathogenesis of periodontitis is the result of an alteration in redox balance of the cells and tissues of innate immunity system. Glutathione, considered to be the master AO, is the most important redox regulator that controls inflammatory processes, and thus damage to the periodontium. Glutathione acts directly as a generic ROS scavenger or as a cofactor of GSHPX and GST, either by the conjugation/excretion processes of the Phase II reactions or by catalyzing the reduction of lipid hydroperoxides and H2O2.
Review of literature reported the utilization of different AO diagnostic biomarkers for periodontal diseases associated with glutathione. These include levels of reduced (GSH) and oxidized (GSSH) glutathione level, ratio of GSH: GSSH,[82] thio levels, glutaredoxin or N-acetyl cysteine,[107] GST,[109] GSHPX, GR,[57] and cysteine level, in patients’ GCF, saliva, serum or plasma, erythrocytes and gingival tissue. Studies have demonstrated high concentrations of glutathione in GCF, saliva, serum and gingival tissues which are compromised in the diseased state, due to abnormal redox balance secondary to OS resulting from periodontal inflammation. Periodontal pathogens may deplete glutathione and shift GSH: GSSG ratio toward oxidants, representing a clear biomarker of OS in periodontitis. Further, successful periodontal therapy has proven to restore the redox balance.[82]
Increased GSHPX in the gingival tissue in periodontal diseases has been reported by many.[57] This increase may signify possible AO recompense in detoxification reactions of organic peroxides created due to OS in gingival tissues. Similarly, the significant increase in GST concentrations in periodontitis patients is probably related to their catalyzing role in detoxification of xenobiotics derived from periopathogens, and neutralization of hydroperoxides derived from the lipoperoxidation process related to OS caused by the periodontal inflammatory process, in conjunction with glutathione. GR has a significant auxiliary AO function related to GSHPX and GST. It intervenes continuously to regenerate glutathione from GSSG in the presence of NADPH, therefore, prevents cellular loss of glutathione.[57] Patel et al.,[90] reported statistically significant reduction in GSHPX concentrations of 19.41 ng/μl and 85.21 ng/μl in GCF and serum after non-surgical periodontal therapy, respectively.
Antioxidant as an adjunct to root surface debridement have improved the periodontal therapy outcome in periodontitis patients by overcoming delayed wound healing induced by imbalance in redox status. Zhang et al.[137] in a clinical survey demonstrated that lower dietary AOs intake leads to destruction of the periodontium and a higher incidence of periodontal diseases. AO epigallocatechin-3-gallate, a most abundant catechin in green tea, reduces gingival inflammation, prevent bone resorption and limit the growth of periodontal pathogens, thus promoting periodontal health.[138] Organosulfur-compound diallyl sulfide enhances AO activities by scavenging superoxide ions and enhancing GSHPX levels, thus unveiling a significant antimicrobial effect against the Aggregatibacter actinomycetemcomitans.[139] Recent reports suggest that the topical use of esterified glutathione could potentially minimize effects of OS with raised intracellular levels of glutathione and acceleration of wound healing by improving the capacity of fibroblasts and keratinocytes.[88]
Oxidative stress is one of the underlying factors explaining the systemic pathophysiological mechanisms associated with the chronic inflammatory conditions as periodontitis.[99] A decrease in salivary reduced-glutathione levels in type 1 diabetes mellitus patients may have a role in the destruction of the periodontium by predisposing the periodontal tissues to OS.[70] It can be postulated that patients with reduced or depleted glutathione content, as in systemic disorders with compromised AO defenses,[82] may be more prone to further increase in OS, and worsening of the condition when superimposed with periodontal infection. Hence, future studies exploring this interrelationship of worsening of redox balance in systemically compromised patients and periodontitis, are further needed.
CONCLUSION
Although emerging evidences mostly in the form of case-control and animal studies have shown the role of glutathione in both periodontal pathogenesis as well as controlling periodontal disease, however limited number of randomized clinical trials have been published. Nevertheless, therapeutic considerations for the adjunctive use of glutathione in management of periodontitis, in limiting the tissue damage associated with OS and enhancing wound healing cannot be underestimated; however, it needs to be evaluated further through multi-centered randomized controlled trials.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 2.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. 2nd ed. Oxford: Clarendon Press; 1989. Free Radicals in Biology and Medicine. [DOI] [PubMed] [Google Scholar]
- 4.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 6.Galli C, Passeri G, Macaluso GM. FoxOs, Wnts and oxidative stress-induced bone loss: New players in the periodontitis arena? J Periodontal Res. 2011;46:397–406. doi: 10.1111/j.1600-0765.2011.01354.x. [DOI] [PubMed] [Google Scholar]
- 7.Cutando A, Galindo P, Gómez-Moreno G, Arana C, Bolaños J, Acuña-Castroviejo D, et al. Relationship between salivary melatonin and severity of periodontal disease. J Periodontol. 2006;77:1533–8. doi: 10.1902/jop.2006.050287. [DOI] [PubMed] [Google Scholar]
- 8.Cutando A, Gómez-Moreno G, Arana C, Acuña-Castroviejo D, Reiter RJ. Melatonin: Potential functions in the oral cavity. J Periodontol. 2007;78:1094–102. doi: 10.1902/jop.2007.060396. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjea MN, Shinde R. 7th ed. New Delhi: Jaypee Brothers Medical Publishers Pvt Ltd; 2007. Textbook of Medical Biochemistry; p. 478. [Google Scholar]
- 10.Sies H, Jones DP. San Diego: Elsevier; 2007. Oxidative Stress. Encyclopedia of Stress. [Google Scholar]
- 11.D’Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89:1241–6. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camera A, Hopps E, Caimi G. Diabetic microangiopathy: Physiopathological, clinical and therapeutic aspects. Minerva Endocrinol. 2007;32:209–29. [PubMed] [Google Scholar]
- 14.Di Filippo C, Verza M, Coppola L, Rossi F, D’Amico M, Marfella R. Insulin resistance and postprandial hyperglycemia the bad companions in natural history of diabetes: Effects on health of vascular tree. Curr Diabetes Rev. 2007;3:268–73. doi: 10.2174/157339907782330012. [DOI] [PubMed] [Google Scholar]
- 15.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 16.Tate RM, Repine JE. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983;128:552–9. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- 17.Asman B, Engström PE, Olsson T, Bergström K. Increased luminol enhanced chemiluminescence from peripheral granulocytes in juvenile periodontitis. Scand J Dent Res. 1984;92:218–23. doi: 10.1111/j.1600-0722.1984.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 18.Henry CA, Winford TE, Laohapund P, Yotnuengnit P. Neutrophil chemi-luminescence and opsonic activities of young people with periodontitis in Thailand. Arch Oral Biol. 1984;29:623–7. doi: 10.1016/0003-9969(84)90132-8. [DOI] [PubMed] [Google Scholar]
- 19.Hoffeld JT. Oxygen radicals in inflammation and immunity. In: Genco RJ, Mergenhagen SE, editors. Host-Parasite Interaction in Periodontal Disease. Washington, DC: American Society for Microbiology; 1982. pp. 343–53. [Google Scholar]
- 20.Kimura S, Yonemura T, Kaya H. Increased oxidative product formation by peripheral blood polymorphonuclear leukocytes in human periodontal diseases. J Periodontal Res. 1993;28:197–203. doi: 10.1111/j.1600-0765.1993.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 21.Shapira L, Borinski R, Sela MN, Soskolne A. Superoxide formation and chemiluminescence of peripheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J Clin Periodontol. 1991;18:44–8. doi: 10.1111/j.1600-051x.1991.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 22.Whyte GJ, Seymour GJ, Cheung K, Robinson MF. Chemiluminescence of peripheral polymorphonuclear leukocytes from adult periodontitis patients. J Clin Periodontol. 1989;16:69–74. doi: 10.1111/j.1600-051x.1989.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.Chapple IL. Potential mechanisms underpinning the nutritional modulation of periodontal inflammation. J Am Dent Assoc. 2009;140:178–84. doi: 10.14219/jada.archive.2009.0131. [DOI] [PubMed] [Google Scholar]
- 24.Pavarino EC, Russo A, Galbiatti AL, Almeida WP, Bertollo EM. Bosynthesis and mechanism of action. In: Labrou N, Flemetakis E, editors. Biochemistry and Mechanism of Action. New York: Nova Science Publishers, Inc; 2013. pp. 1–31. [Google Scholar]
- 25.Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–73. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 26.Pizzorno JE, Katzinger JJ. Glutathione: Physiological and clinical relevance. J Restor Med. 2012;1:24–37. [Google Scholar]
- 27.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 28.Thomas B, Ramesh A, Suresh S, Prasad BR. A comparative evaluation of antioxidant enzymes and selenium in the serum of periodontitis patients with diabetes mellitus type 2. Contemp Clin Dent. 2013;4:176–80. doi: 10.4103/0976-237X.114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapple IL, Brock G, Eftimiadi C, Matthews JB. Glutathione in gingival crevicular fluid and its relation to local antioxidant capacity in periodontal health and disease. Mol Pathol. 2002;55:367–73. doi: 10.1136/mp.55.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 2003;105:167–72. doi: 10.1042/CS20030031. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson J, Larsen JT, Edlund MB. Peptostreptococcus micros has a uniquely high capacity to form hydrogen sulfide from glutathione. Oral Microbiol Immunol. 1993;8:42–5. doi: 10.1111/j.1399-302x.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 32.Marton IJ, Balla G, Hegedus C, Redi P, Szilagyi Z, Karmazsin L, et al. The role of reactive oxygen intermediates in the pathogenesis of chronic apical periodontitis. Oral Microbiol Immunol. 1993;8:254–7. doi: 10.1111/j.1399-302x.1993.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson J, Larsen JT, Edlund MB. Utilization of glutathione (L-gamma-glutamyl-L-cysteinylglycine) by Fusobacterium nucleatum subspecies nucleatum. Oral Microbiol Immunol. 1994;9:297–300. doi: 10.1111/j.1399-302x.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 34.Chapple IL. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin Mol Pathol. 1996;49:M247–55. doi: 10.1136/mp.49.5.m247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeng JH, Lan WH, Hahn LJ, Hsieh CC, Kuo MY. Inhibition of the migration, attachment, spreading, growth and collagen synthesis of human gingival fibroblasts by arecoline, a major areca alkaloid, in vitro. J Oral Pathol Med. 1996;25:371–5. doi: 10.1111/j.1600-0714.1996.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 36.Chapple IL, Mason GI, Garner I, Matthews JB, Thorpe GH, Maxwell SR, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem. 1997;34(Pt 4):412–21. doi: 10.1177/000456329703400413. [DOI] [PubMed] [Google Scholar]
- 37.Huang P, Su T, Wang H. The relationship between GPx activity in gingival fluid and clinical parameters of adult periodontitis. Hua Xi Kou Qiang Yi Xue Za Zhi. 2000;18:106–8. [PubMed] [Google Scholar]
- 38.Sobaniec H, Sobaniec-Lotowska ME. Morphological examinations of hard tissues of periodontium and evaluation of selected processes of lipid peroxidation in blood serum of rats in the course of experimental periodontitis. Med Sci Monit. 2000;6:875–81. [PubMed] [Google Scholar]
- 39.Battino M, Ferreiro MS, Bompadre S, Leone L, Mosca F, Bullon P. Elevated hydroperoxide levels and antioxidant patterns in Papillon-Lefèvre syndrome. J Periodontol. 2001;72:1760–6. doi: 10.1902/jop.2001.72.12.1760. [DOI] [PubMed] [Google Scholar]
- 40.Chang YC, Lii CK, Tai KW, Chou MY. Adverse effects of arecoline and nicotine on human periodontal ligament fibroblasts in vitro. J Clin Periodontol. 2001;28:277–82. doi: 10.1034/j.1600-051x.2001.028003277.x. [DOI] [PubMed] [Google Scholar]
- 41.Chang YC, Huang FM, Tai KW, Yang LC, Chou MY. Mechanisms of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J Periodontal Res. 2002;37:279–85. doi: 10.1034/j.1600-0765.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- 42.Chu L, Dong Z, Xu X, Cochran DL, Ebersole JL. Role of glutathione metabolism of Treponema denticola in bacterial growth and virulence expression. Infect Immun. 2002;70:1113–20. doi: 10.1128/IAI.70.3.1113-1120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zappacosta B, Persichilli S, Mordente A, Minucci A, Lazzaro D, Meucci E, et al. Inhibition of salivary enzymes by cigarette smoke and the protective role of glutathione. Hum Exp Toxicol. 2002;21:7–11. doi: 10.1191/0960327102ht202oa. [DOI] [PubMed] [Google Scholar]
- 44.Chang YC, Hsieh YS, Lii CK, Huang FM, Tai KW, Chou MY. Induction of c-fos expression by nicotine in human periodontal ligament fibroblasts is related to cellular thiol levels. J Periodontal Res. 2003;38:44–50. doi: 10.1034/j.1600-0765.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 45.Chu L, Xu X, Dong Z, Cappelli D, Ebersole JL. Role for recombinant gamma-glutamyltransferase from Treponema denticola in glutathione metabolism. Infect Immun. 2003;71:335–42. doi: 10.1128/IAI.71.1.335-342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zappacosta B, Manni A, Persichilli S, Scribano D, Minucci A, Lazzaro D, et al. HPLC analysis of some sulphur compounds in saliva: Comparison between healthy subjects and periodontopathic patients. Clin Chim Acta. 2003;338:57–60. doi: 10.1016/j.cccn.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Park JY, Chung WY, Choi MA, Cho KS, Park KK. Polymorphisms in genes coding for enzymes metabolizing smoking-derived substances and the risk of periodontitis. J Clin Periodontol. 2004;31:959–64. doi: 10.1111/j.1600-051X.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 48.Wei PF, Ho KY, Ho YP, Wu YM, Yang YH, Tsai CC. The investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1beta in gingival crevicular fluid: Implications for oxidative stress in human periodontal diseases. J Periodontal Res. 2004;39:287–93. doi: 10.1111/j.1600-0765.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 49.Chang YC, Lai CC, Lin LF, Ni WF, Tsai CH. The up-regulation of heme oxygenase-1 expression in human gingival fibroblasts stimulated with nicotine. J Periodontal Res. 2005;40:252–7. doi: 10.1111/j.1600-0765.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40:378–84. doi: 10.1111/j.1600-0765.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 51.Panjamurthy K, Manoharan S, Ramachandran CR. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol Biol Lett. 2005;10:255–64. [PubMed] [Google Scholar]
- 52.Buduneli N, Kardesler L, Isik H, Willis CS, 3rd, Hawkins SI, Kinane DF, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–64. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 53.Chapple IL. Oxidative stress, nutrition and neutrogenomics in periodontal health and disease. Int J Dent Hyg. 2006;4(Suppl 1):15–21. doi: 10.1111/j.1601-5037.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 54.Garg N, Singh R, Dixit J, Jain A, Tewari V. Levels of lipid peroxides and antioxidants in smokers and nonsmokers. J Periodontal Res. 2006;41:405–10. doi: 10.1111/j.1600-0765.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 55.Ho YC, Chang YC. Regulation of nicotine-induced cyclooxygenase-2 protein expression in human gingival fibroblasts. Acta Pharmacol Sin. 2006;27:409–13. doi: 10.1111/j.1745-7254.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 56.Baltacioglu E, Akalin FA, Topaloglu E, Süküroglu E, Cobanoglu U. Ligneous periodontitis and gingival antioxidant status: Report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:803–8. doi: 10.1016/j.tripleo.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Borges I, Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm 2007. 2007:45794. doi: 10.1155/2007/45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canakci V, Yildirim A, Canakci CF, Eltas A, Cicek Y, Canakci H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J Periodontol. 2007;78:1602–11. doi: 10.1902/jop.2007.060469. [DOI] [PubMed] [Google Scholar]
- 59.Concolino P, Cecchetti F, D’Autilia C, Santonocito C, Di Stasio E, Zuppi C, et al. Association of periodontitis with GSTM1/GSTT1-null variants — A pilot study. Clin Biochem. 2007;40:939–45. doi: 10.1016/j.clinbiochem.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Sobaniec H, Sobaniec W, Sendrowski K, Sobaniec S, Pietruska M. Antioxidant activity of blood serum and saliva in patients with periodontal disease treated due to epilepsy. Adv Med Sci. 2007;52(Suppl 1):204–6. [PubMed] [Google Scholar]
- 61.Yetkin-Ay Z, Cadir B, Uskun E, Bozkurt FY, Delibas N, Gültepe FM, et al. The periodontal status of indirectly lead-exposed apprentices working in autorepair workshops. Toxicol Ind Health. 2007;23:599–606. doi: 10.1177/0748233708090906. [DOI] [PubMed] [Google Scholar]
- 62.Zappacosta B, Manni A, Persichilli S, Boari A, Scribano D, Minucci A, et al. Salivary thiols and enzyme markers of cell damage in periodontal disease. Clin Biochem. 2007;40:661–5. doi: 10.1016/j.clinbiochem.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Tomofuji T, Sanbe T, Ekuni D, Azuma T, Irie K, Maruyama T, et al. Oxidative damage of rat liver induced by ligature-induced periodontitis and chronic ethanol consumption. Arch Oral Biol. 2008;53:1113–8. doi: 10.1016/j.archoralbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura Y, Shimetani A, Fujii H, Amano O, Sakagami H, Takahashi K. Glutathione can efficiently prevent direct current-induced cytotoxicity. J Endod. 2008;34:693–7. doi: 10.1016/j.joen.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Guentsch A, Preshaw PM, Bremer-Streck S, Klinger G, Glockmann E, Sigusch BW. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: Effect of smoking and periodontal treatment. Clin Oral Investig. 2008;12:345–52. doi: 10.1007/s00784-008-0202-z. [DOI] [PubMed] [Google Scholar]
- 66.Chu L, Lai Y, Xu X, Eddy S, Yang S, Song L, et al. A 52-kDa leucyl aminopeptidase from Treponema denticola is a cysteinylglycinase that mediates the second step of glutathione metabolism. J Biol Chem. 2008;283:19351–8. doi: 10.1074/jbc.M801034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irie K, Tomofuji T, Tamaki N, Sanbe T, Ekuni D, Azuma T, et al. Effects of ethanol consumption on periodontal inflammation in rats. J Dent Res. 2008;87:456–60. doi: 10.1177/154405910808700511. [DOI] [PubMed] [Google Scholar]
- 68.Tomofuji T, Ekuni D, Irie K, Azuma T, Endo Y, Tamaki N, et al. Preventive effects of a cocoa-enriched diet on gingival oxidative stress in experimental periodontitis. J Periodontol. 2009;80:1799–808. doi: 10.1902/jop.2009.090270. [DOI] [PubMed] [Google Scholar]
- 69.Barnes VM, Teles R, Trivedi HM, Devizio W, Xu T, Mitchell MW, et al. Acceleration of purine degradation by periodontal diseases. J Dent Res. 2009;88:851–5. doi: 10.1177/0022034509341967. [DOI] [PubMed] [Google Scholar]
- 70.Gümüs P, Buduneli N, Cetinkalp S, Hawkins SI, Renaud D, Kinane DF, et al. Salivary antioxidants in patients with type 1 or 2 diabetes and inflammatory periodontal disease: A case-control study. J Periodontol. 2009;80:1440–6. doi: 10.1902/jop.2009.090159. [DOI] [PubMed] [Google Scholar]
- 71.Tomofuji T, Ekuni D, Sanbe T, Irie K, Azuma T, Maruyama T, et al. Effects of vitamin C intake on gingival oxidative stress in rat periodontitis. Free Radic Biol Med. 2009;46:163–8. doi: 10.1016/j.freeradbiomed.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 72.Canakci CF, Cicek Y, Yildirim A, Sezer U, Canakci V. Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur J Dent. 2009;3:100–6. [PMC free article] [PubMed] [Google Scholar]
- 73.Thangjam GS, Kondaiah P. Regulation of oxidative-stress responsive genes by arecoline in human keratinocytes. J Periodontal Res. 2009;44:673–82. doi: 10.1111/j.1600-0765.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 74.Patel SP, Pradeep AR, Chowdhry S. Crevicular fluid levels of plasma glutathione peroxidase (eGPx) in periodontal health and disease. Arch Oral Biol. 2009;54:543–8. doi: 10.1016/j.archoralbio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Ekuni D, Tomofuji T, Sanbe T, Irie K, Azuma T, Maruyama T, et al. Vitamin C intake attenuates the degree of experimental atherosclerosis induced by periodontitis in the rat by decreasing oxidative stress. Arch Oral Biol. 2009;54:495–502. doi: 10.1016/j.archoralbio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Takahama U, Imamura H, Hirota S. Nitration of the salivary component 4-hydroxyphenylacetic acid in the human oral cavity: Enhancement of nitration under acidic conditions. Eur J Oral Sci. 2009;117:555–62. doi: 10.1111/j.1600-0722.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 77.Zdarilová A, Svobodová A, Simánek V, Ulrichová J. Prunella vulgaris extract and rosmarinic acid suppress lipopolysaccharide-induced alteration in human gingival fibroblasts. Toxicol In Vitro. 2009;23:386–92. doi: 10.1016/j.tiv.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 78.Tomofuji T, Yamamoto T, Tamaki N, Ekuni D, Azuma T, Sanbe T, et al. Effects of obesity on gingival oxidative stress in a rat model. J Periodontol. 2009;80:1324–9. doi: 10.1902/jop.2009.080621. [DOI] [PubMed] [Google Scholar]
- 79.Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H. Nutritional intervention in patients with periodontal disease: Clinical, immunological and microbiological variables during 12 months. Br J Nutr. 2009;101:879–85. doi: 10.1017/S0007114508047776. [DOI] [PubMed] [Google Scholar]
- 80.Ishii K, Hamamoto H, Imamura K, Adachi T, Shoji M, Nakayama K, et al. Porphyromonas gingivalis peptidoglycans induce excessive activation of the innate immune system in silkworm larvae. J Biol Chem. 2010;285:33338–47. doi: 10.1074/jbc.M110.112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Govindaraj J, Emmadi P, Deepalakshmi, Rajaram V, Prakash G, Puvanakrishnan R. Protective effect of proanthocyanidins on endotoxin induced experimental periodontitis in rats. Indian J Exp Biol. 2010;48:133–42. [PubMed] [Google Scholar]
- 82.Grant MM, Brock GR, Matthews JB, Chapple IL. Crevicular fluid glutathione levels in periodontitis and the effect of non-surgical therapy. J Clin Periodontol. 2010;37:17–23. doi: 10.1111/j.1600-051X.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 83.Ho YC, Lin HJ, Tsai CH, Chang YC. Regulation of type I plasminogen activator inhibitor in human gingival fibroblasts with cyclosporine A. Oral Dis. 2010;16:396–401. doi: 10.1111/j.1601-0825.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 84.Zdarilová A, Rajnochová Svobodová A, Chytilová K, Simánek V, Ulrichová J. Polyphenolic fraction of Lonicera caerulea L. fruits reduces oxidative stress and inflammatory markers induced by lipopolysaccharide in gingival fibroblasts. Food Chem Toxicol. 2010;48:1555–61. doi: 10.1016/j.fct.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 85.Azuma T, Tomofuji T, Endo Y, Tamaki N, Ekuni D, Irie K, et al. Effects of exercise training on gingival oxidative stress in obese rats. Arch Oral Biol. 2011;56:768–74. doi: 10.1016/j.archoralbio.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 86.Tonguç MÖ, Öztürk O, Sütçü R, Ceyhan BM, Kilinç G, Sönmez Y, et al. The impact of smoking status on antioxidant enzyme activity and malondialdehyde levels in chronic periodontitis. J Periodontol. 2011;82:1320–8. doi: 10.1902/jop.2011.100618. [DOI] [PubMed] [Google Scholar]
- 87.Colombo G, DalleDonne I, Orioli M, Giustarini D, Rossi R, Clerici M, et al. Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic Biol Med. 2012;52:1584–96. doi: 10.1016/j.freeradbiomed.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 88.Tinti F, Soory M. Mechanisms for redox actions of nicotine and glutathione in cell culture, relevant to periodontitis. Sci Rep. 2012;2:566. doi: 10.1038/srep00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velliyagounder K, Ganeshnarayan K, Velusamy SK, Fine DH. In vitro efficacy of diallyl sulfides against the periodontopathogen Aggregatibacter actinomycetemcomitans. Antimicrob Agents Chemother. 2012;56:2397–407. doi: 10.1128/AAC.00020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patel SP, Rao NS, Pradeep AR. Effect of nonsurgical periodontal therapy on crevicular fluid and serum glutathione peroxidase levels. Dis Markers. 2012;32:1–7. doi: 10.3233/DMA-2012-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kido J, Bando M, Hiroshima Y, Iwasaka H, Yamada K, Ohgami N, et al. Analysis of proteins in human gingival crevicular fluid by mass spectrometry. J Periodontal Res. 2012;47:488–99. doi: 10.1111/j.1600-0765.2011.01458.x. [DOI] [PubMed] [Google Scholar]
- 92.Duarte PM, Napimoga MH, Fagnani EC, Santos VR, Bastos MF, Ribeiro FV, et al. The expression of antioxidant enzymes in the gingivae of type 2 diabetics with chronic periodontitis. Arch Oral Biol. 2012;57:161–8. doi: 10.1016/j.archoralbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 93.de Menezes AM, de Souza GF, Gomes AS, de Carvalho Leitão RF, Ribeiro Rde A, de Oliveira MG, et al. S-nitrosoglutathione decreases inflammation and bone resorption in experimental periodontitis in rats. J Periodontol. 2012;83:514–21. doi: 10.1902/jop.2011.110332. [DOI] [PubMed] [Google Scholar]
- 94.Araújo AA, Souza TO, Moura LM, Brito GA, Aragão KS, Araújo LS, et al. Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J Clin Periodontol. 2013;40:1104–11. doi: 10.1111/jcpe.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miricescu D, Totan A, Calenic B, Mocanu B, Didilescu A, Mohora M, et al. Salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol Scand. 2014;72:42–7. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 96.Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85:713–20. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 97.Emekli-Alturfan E, Yarat A, Çaliskan-Ak E, Pisiriciler R, Kuru B, Noyan Ü. Determination of storage time of saliva samples obtained from patients with and without chronic periodontitis for the comparison of some biochemical and cytological parameters. J Clin Lab Anal. 2013;27:261–6. doi: 10.1002/jcla.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Araújo Júnior RF, Souza TO, de Medeiros CA, de Souza LB, Freitas Mde L, de Lucena HF, et al. Carvedilol decrease IL-1β and TNF-α, inhibits MMP-2, MMP-9, COX-2, and RANKL expression, and up-regulates OPG in a rat model of periodontitis. PLoS One. 2013;8:e66391. doi: 10.1371/journal.pone.0066391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dias IH, Chapple IL, Milward M, Grant MM, Hill E, Brown J, et al. Sulforaphane restores cellular glutathione levels and reduces chronic periodontitis neutrophil hyperactivity in vitro. PLoS One. 2013;8:e66407. doi: 10.1371/journal.pone.0066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yagan A, Kesim S, Liman N. Effect of low-dose doxycycline on serum oxidative status, gingival antioxidant levels, and alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2014;85:478–89. doi: 10.1902/jop.2013.130138. [DOI] [PubMed] [Google Scholar]
- 101.Araújo AA, Lopes de Souza G, Souza TO, de Castro Brito GA, Sabóia Aragão K, Xavier de Medeiros CA, et al. Olmesartan decreases IL-1β and TNF-α levels; downregulates MMP-2, MMP-9, COX-2, and RANKL; and upregulates OPG in experimental periodontitis. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:875–84. doi: 10.1007/s00210-013-0886-8. [DOI] [PubMed] [Google Scholar]
- 102.Novakovic N, Cakic S, Todorovic T, Raicevic BA, Dozic I, Petrovic V, et al. Antioxidative status of saliva before and after non-surgical periodontal treatment. Srp Arh Celok Lek. 2013;141:163–8. doi: 10.2298/sarh1304163n. [DOI] [PubMed] [Google Scholar]
- 103.Novakovic N, Todorovic T, Rakic M, Milinkovic I, Dozic I, Jankovic S, et al. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment outcome. J Periodontal Res. 2014;49:129–36. doi: 10.1111/jre.12088. [DOI] [PubMed] [Google Scholar]
- 104.Dalcico R, de Menezes AM, Deocleciano OB, Oriá RB, Vale ML, Ribeiro RA, et al. Protective mechanisms of simvastatin in experimental periodontal disease. J Periodontol. 2013;84:1145–57. doi: 10.1902/jop.2012.120114. [DOI] [PubMed] [Google Scholar]
- 105.Rai B. Total salivary glutathione levels: Periodontitis in smoker and non-smoker. Adv Med Dent Sci. 2008;2:47–9. [Google Scholar]
- 106.Zuidan TF. The role of lipid peroxidation in the induction and progression of chronic periodontitis. J Baghdad Coll Dent. 2009;21:70–3. [Google Scholar]
- 107.Karim S, Pratibha PK, Kamath S, Bhat GS, Kamath U, Dutta B, et al. Superoxide dismutase enzyme and thiol antioxidants in gingival crevicular fluid and saliva. Dent Res J (Isfahan) 2012;9:266–72. [PMC free article] [PubMed] [Google Scholar]
- 108.Dhotre PS, Suryakar AN, Bhogade RB. Oxidative stress in periodontitis. Eur J Gen Med. 2012;9:81–4. [Google Scholar]
- 109.Varghese JM, Bhat V, Bhat GS, Rao N. Evaluation of glutathione-S-transferase and ceruloplasmin levels in gingival crevicular fluid and gingival tissue as diagnostic markers for chronic periodontitis. Adv Biosci Biotechol. 2012;3:437–41. [Google Scholar]
- 110.Martu I, Luchian I, Goriuc A, Checherita L, Martu S, Forna N. Correlations between the periodontal modifications and lipid peroxidation in periodontal modifications and lipid peroxidation in periodontal disease patients. Rom J Oral Rehabil. 2013;5:26–31. [Google Scholar]
- 111.Diab R, Mounayar A, Maalouf E, Chahine R. Beneficial effects of Solanum melongena (Solanaceae) peduncles extracts, in periodontal diseases. J Med Plant Res. 2011;5:2309–15. [Google Scholar]
- 112.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 113.Hopkins FG, Dixon M. On glutathione II. Athermostable oxidation-reduction system. J Biol Chem. 1922;54:527–63. [PubMed] [Google Scholar]
- 114.Hunter G, Eagles BA. Non-protein sulfur compounds in blood. II. Glutathione. J Biol Chem. 1927;72:133–46. [Google Scholar]
- 115.Hopkins FG. On glutathione: a reinvestigation. J Biol Chem. 1929;84:269–320. [Google Scholar]
- 116.Simoni RD, Hill RL, Vaughan M. The discovery of glutathione by Frederick Gowlan Hopkins and the beginning of biochemistry at Cambridge University. J Biol Chem. 2002;277:e13. [Google Scholar]
- 117.Dhivya H. Glutathione: A master antioxidant and an immune system modulator. J Bio Inf Sci. 2012;1:28–30. [Google Scholar]
- 118.Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Halliwell B, Cross CE. Oxygen-derived species: Their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–76. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]
- 121.Sree SL, Mythili R. Antioxidants in periodontal diseases: A review. Indian J Multidici Dent. 2011;1:140–6. [Google Scholar]
- 122.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 123.Jurkovic S, Osredkar J, Marc J. Molecular impact of glutathione peroxidases in antioxidant processes. Biochem Med. 2008;18:162–74. [Google Scholar]
- 124.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paolicchi A, Sotiropuolou M, Perego P, Daubeuf S, Visvikis A, Lorenzini E, et al. gamma-Glutamyl transpeptidase catalyses the extracellular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney. Eur J Cancer. 2003;39:996–1003. doi: 10.1016/s0959-8049(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 126.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–46. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 127.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–5. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–36. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 130.Fan X, Staitieh BS, Jensen JS, Mould KJ, Greenberg JA, Joshi PC, et al. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am J Physiol Lung Cell Mol Physiol. 2013;305:L267–77. doi: 10.1152/ajplung.00288.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mäkinen PL, Mäkinen KK. gamma-Glutamyltransferase from the outer cell envelope of Treponema denticola ATCC 35405. Infect Immun. 1997;65:685–91. doi: 10.1128/iai.65.2.685-691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai Y, Chu L. Novel mechanism for conditional aerobic growth of the anaerobic bacterium Treponema denticola. Appl Environ Microbiol. 2008;74:73–9. doi: 10.1128/AEM.01972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Claesson R, Granlund-Edstedt M, Persson S, Carlsson J. Activity of polymorphonuclear leukocytes in the presence of sulfide. Infect Immun. 1989;57:2776–81. doi: 10.1128/iai.57.9.2776-2781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5:195–201. doi: 10.1111/j.1399-302x.1990.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 135.Martins CA, Leyhausen G, Geurtsen W, Volk J. Intracellular glutathione: A main factor in TEGDMA-induced cytotoxicity? Dent Mater. 2012;28:442–8. doi: 10.1016/j.dental.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 136.Chapple IL, Milward MR, Ling-Mountford N, Weston P, Carter K, Askey K, et al. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: A double-blind RCT. J Clin Periodontol. 2012;39:62–72. doi: 10.1111/j.1600-051X.2011.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang MF, Chen RJ, Tang W, Zhang HF. The relationship between dietary factors and susceptibility of periodontal disease. Shanghai Kou Qiang Yi Xue. 2012;21:99–103. [PubMed] [Google Scholar]
- 138.Chatterjee A, Saluja M, Agarwal G, Alam M. Green tea: A boon for periodontal and general health. J Indian Soc Periodontol. 2012;16:161–7. doi: 10.4103/0972-124X.99256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Velliyagounder K, Ganeshnarayan K, Velusamy SK, Fine DH. In vitro efficacy of diallyl sulfides against the periodontopathogen Aggregatibacter actinomycetemcomitans. Antimicrob Agents Chemother. 2012;56:2397–407. doi: 10.1128/AAC.00020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


