Abstract
Vital pulp therapy (VPT) is a biologic and conservative treatment modality to preserve the vitality and function of the coronal or remaining radicular pulp tissue in vital permanent teeth. A search was conducted via the Cochrane database, PubMed, MEDLINE, and Ovid for any articles with the criteria for “pulp-capping,” or “pulp-capping materials” and “VPT outcomes” from 1978 to mid 2014. All articles were evaluated and the valid papers were selected. The outcomes of various VPT techniques, including indirect pulp treatment, direct pulp treatment, partial pulpotomy, and complete pulpotomy in vital permanent teeth were extracted. Although various studies have different research approach, most studies noted a favorable treatment outcome. Mineral trioxide aggregate (MTA) appears to be more effective than calcium hydroxide (Ca(OH)2) for maintaining long-term pulp vitality after indirect and direct pulp-capping. However, it seems that the success rate for partial pulpotomy and pulpotomy with Ca(OH)2 is similar to MTA.
Keywords: Dental cements, calcium hydroxide, permanent dentition, mineral trioxide aggregate, root canal therapies
INTRODUCTION
The aim of treatment after pulp exposure is to promote the pulp tissue healing and facilitate the formation of reparative dentin in order to preserve the pulp vitality and health.[1] Vital pulp therapy (VPT) procedures involve removal of local irritants and placement of a protective material directly or indirectly over the pulp.[2] These treatments must be followed by an overlying tight-sealed restoration to decrease bacterial leakage from the restoration-dentin interface. VPT is performed to treat reversible pulpal injury in order to promote root development, apical closure and accomplish complete root canal therapy.[1,3,4,5]
There are controversies within the studies on VPT regarding judgment criteria and pulpal status at the time of treatment, optimal technique and treatment outcomes.[1,3,5] There is no consensus as to the best therapeutic technique and comprehensible diagnostic indications for the management of caries-exposed permanent teeth.[1,3,5] No long-term data regarding the outcome and the survival rate of VPT is available, either.[2] The purpose of this paper was to review the outcomes of various VPT techniques, including indirect pulp treatment (IPT), direct pulp treatment (DPT), partial pulpotomy, and full pulpotomy in vital permanent teeth.
A search was conducted via the Cochrane database, PubMed, MEDLINE and Ovid for any articles with the criteria for “pulp-capping,” or “pulp-capping materials” and “VPT outcomes” from 1978-2014. No specific inclusion or exclusion criteria were applied as to what articles would be included in this review. It was hoped that the extent of the literature reviewed would be as comprehensive as possible.
FEATURES OF SUCCESSFUL VITAL PULP THERAPY
In accordance with several authors,[1,2,3,5,6] the two major clinical and radiographic criteria below are the indicators for successful treatment: Maintenance of pulp vitality, minimum pulp inflammatory responses, formation of a continuous layer of reparative dentin, absence of postoperative clinical signs or symptoms of thermal or periapical and/or both sensitivity, as pain, or swelling, absence of radiographic evidence of internal or external root resorption, periapical and/or inter-radicular radiolucency, irregular calcification, or other pathologic changes, continuous root development and apexogenesis of teeth with incompletely formed roots.[2,4]
Various publications demonstrated that success rates decrease with time.[2,4,6,7] However, there is no clear explanation for this fact, and further investigations are needed. Unfavorable outcomes are caused by infection due to either remaining bacteria, or new bacteria from penetrating restoration margins. Thus, beside the immediate placement of a bacteria-tight restoration, the use of rubber dam and aseptical treatment conditions are strongly recommended.[6]
Although clinical studies point out that the VPT success rates of caries-exposed immature permanent teeth might be comparable with the success rate of root canal treatment,[8] the clinicians are less confident about the success of VPT.[5] Some complications may develop after VPT, so patients should be regularly followed up. It has been shown that a tooth with a favorable treatment outcome 5 years after VPT, the likelihood that it will stay vital in the following years is more than 95%. Consequently, the time for an adequate postoperative 1-2 years follow-up examination, as often recommended, may well be too short.[6]
FACTORS INFLUENCING TREATMENT OUTCOMES OF VITAL PULP THERAPY TECHNIQUES
The VPT success rate in caries-exposed permanent teeth is the subject of many debate fluctuating between 13%[9] and 100% after[10] [Tables 1-4]. The 10-year success rate was 13% in Barthel et al. study in which pulp-capping treatments were performed by students. After rubber dam placement and cleansing with 3% H2O2, a setting calcium hydroxide (Ca(OH)2) paste (Kerr Life, Kerr, Karlsruhe, Germany) was the wound and a base of either zinc phosphate cement or glass ionomer cement was applied. The teeth were then restored with amalgam fillings, composite fillings, gold cast restorations, or with temporary fillings within the first 2 days or more after pulp exposure.[9]
Table 1.
Treatment outcome of indirect pulp-capping in permanent teeth
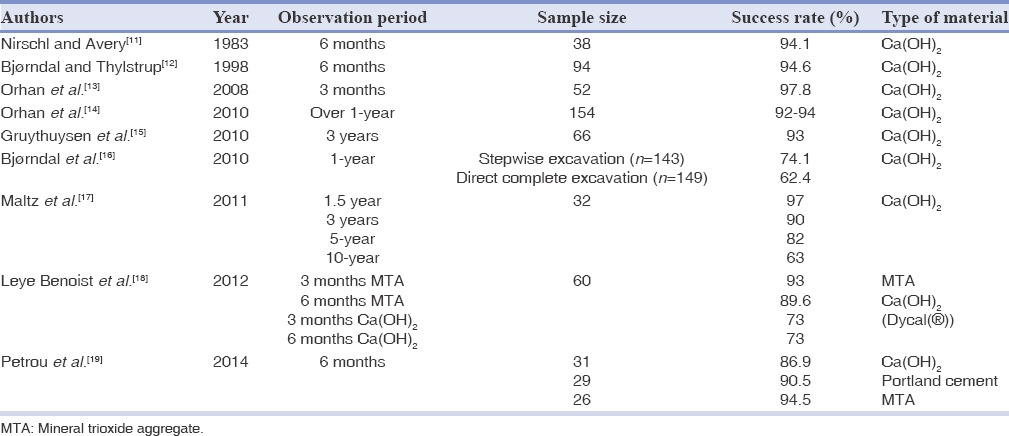
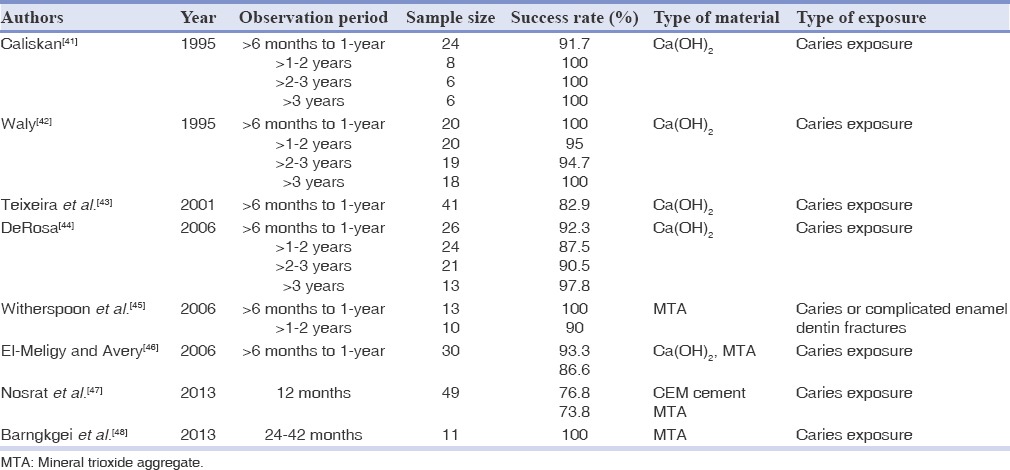
Table 4.
Treatment outcome of pulpotomy in permanent teeth in literature
In Bogen et al. study with 97.96% success rate over an observation period of 9 years, one expert operator completed all direct pulp caps. Following placement of a dental dam, using a caries detector dye and caries excavation under magnification, NaOCl for hemostasis within 1 to 10 min, mineral trioxide aggregate (MTA) placed over the exposures and all surrounding dentin. The operator then restored the teeth provisionally with unbonded Clearfil Photocore (Kuraray Medical, Okayama, Japan). During a second visit, within 5 to 10 days the operator restored the teeth with bonded composite after sensibility testing and confirmed MTA curing.[10]
There is a controversy regarding the VPT procedures outcomes, with many methodological variations between the different studies. Therefore, it may be difficult to compare the various studies because the status of the pulp tissue at the time of treatment, the techniques, observation periods, examination methods, and examination criteria may noticeably differ. Although these studies have different research approach, most studies noted a favorable treatment outcome, which seems to be inconsistent with the clinical standpoint that the VPT outcome is uncertain.[6] Accurate diagnosis of the pulp status prior to treatment, removal of caries, prevention of leakage, and use of an aseptic technique are the main factors influencing treatment outcomes of VPT. The amount of crown destruction and the ability to restore a tooth are usually associated with the long-term prognosis of VPT.[1]
The presence of microorganisms with subsequent infection, the inflammatory status of the pulp tissue,[49] the size of the carious pulpal exposure,[50] the time of examination,[9] the final location and the quality of the dentin bridge, the type of pulp therapy material used,[51] the technique employed were the criteria used to determine success, play a role in determining VPT prognosis as well.[1] Furthermore, a distinction must be made between failure of the pulp therapy and failure of the overlying restoration.[52] There are some limitations in determining the long-term success of VPT in permanent teeth such as the difficulty of recalling patients regularly and the impossibility of determining histological success.[5] The careful decision should be made on the best treatment option of treatment, based on patient history, clinical/radiographic findings, the long-term prognosis, and the ability to restore a tooth.[52]
THE MATERIALS USED IN VITAL PULP THERAPY PROCEDURES
Materials used in VPT should have several properties, including the ability to eliminate bacteria, to create an adequate seal and to induce mineralization and normal root development.[53] At present, Ca(OH)2 and MTA are the materials of choice according to several studies.[45,53,54,55] Bone morphogenetic proteins (BMPs) and transforming growth factor-β (TGF-β), bioceramic, biodentine, enamel matrix derivative (EMD), propolis, calcium-enriched mixture (CEM) cement, tricalcium phosphate cement and some other bioactive materials has been also suggested for VPT.
Calcium hydroxide
Calcium hydroxide is a high-alkaline (pH = 11), white, crystalline, slightly soluble basic salt that dissociates into calcium and hydroxyl ions in solution. It is used in both hard-setting salicylate ester cements and paste (aqueous suspension) forms in VPT.[6] The advantages of Ca(OH)2 are its excellent antibacterial properties and the ability to induce reparative bridge formation when applied to pulp tissues.[54] However, it was unable to kill Enterococcus faecalis in the dentine.[55] Some disadvantages of Ca(OH)2 such as producing a dentinal bridge containing multiple defects and porosities, lack of inherent adhesive qualities, dissolution over time, and inability to provide a long-term seal against microleakage may account for its inability to suppress inflammation. However, in more human studies concerning VPT with Ca(OH)2, the dentinal defects are not a frequent finding[56,57] and as the bridge gets thicker, the quality of reparative dentin improves.[51,58]
Zones of obliteration and coagulation necrosis superficial to reparative dentin were detected as well due to very basic pH of some Ca(OH)2 inorganic substances. Stanley suggests that the internal resorption and the dystrophic calcification seen after VPT are less likely to occur with the Ca(OH)2 products at lower-pH such as Dycal (DeTrey Dentsply, Skarpnack, Sweden).[50] It is known that alkaline pH of Ca(OH)2 irritates the pulp cells and induces the release of bioactive molecules such as BMP and TGF-β1, which stimulate pulpal repair.[59,60]
Numerous studies have evaluated the outcomes of CH in vital pulp treatment [Tables 1-4].
Mineral trioxide aggregate
Mineral trioxide aggregate is composed of tricalcium silicate, tricalcium oxide, tricalcium aluminate, silicate oxide, and added bismuth oxides for radiopacity.[61] After hydration of the powder, colloidal gel forms, which is composed of calcium oxide crystals in an amorphous structure. The biocompatibility of MTA is due to the formation of Ca(OH)2 in reaction products.[62] Consequently, many of the advantages of MTA are comparable to those of Ca(OH)2, including high alkaline pH, its antibacterial and biocompatibility properties, radiopacity and its ability to stimulate the release of bioactive dentin matrix proteins.[61,62] There are some differences between MTA and Ca(OH)2, as well: MTA has demonstrated a superior ability to maintain the integrity of pulp tissue and produces a thicker and less porous dentinal bridge at a faster rate.[63] In addition, MTA is able to decrease pulp inflammation,[49] and presents significant less toxicity and pulpal necrosis which is statistically significant compared with Ca(OH)2 were detected in histological evaluations.[49,63,64]
However, more research studies are required to support this conclusion because optimal randomized clinical studies are lacking, and there are some limitations in the design of these studies. For instance, in one study, the following statement was noted: “In light of the results of the present and other relevant studies, MTA is superior to Ca(OH)2 for pulp-capping mechanically exposed human teeth.”[63] In that study 14 intentionally exposed pulps were divided into two groups. Half of them were covered with MTA and the other half with Ca(OH)2. Histological evaluation was carried out after 1-4 weeks and 6 months of teeth extraction. By the final assessment (6 months), only one tooth in each group was evaluated. This sample size was too small for statistical analysis. By the way, this study uses a gold standard which is histopathology and for such studies we cannot expect a high sample size due to the difficulties. Therefore, it seems that MTA has a superior property. Other researchers confirmed “the outcomes suggest that MTA is a more predictable pulp-capping material than Ca(OH)2.”[10] After approximately 4 years of follow-up of 49 teeth treated with MTA (direct pulp-capping [DPC]), a 98% success rate was reported. Although, there was no Ca(OH)2 control group in that study but in a study using the same procedure with a setting Ca(OH)2 paste, the 5-year success rate of 123 teeth was 37%.
However, negative aspects of MTA exist, such as its prolonged setting time of approximately 2 h and 45 min and the handling difficulty of the powder-liquid MTA compared to the paste formulations of Ca(OH)2. The presence of iron in the gray MTA formulation may discolor the tooth.[51] In addition, it has been reported that the discoloration is seen with white MTA as a result of the chemical interaction of bismuth oxide with dentin collagen.[65,66] The outcomes of various techniques of VPT using MTA would be discussed later.
Calcium enriched mixture cement
An endodontic cement referred to as CEM cement has been developed, which predominantly consists of different calcium compounds.[67]
The main components of CEM cement powder are CaO, SO3, P2O5, SiO2, and minor components are Al2O3, Na2O, MgO, and Cl as necessary ingredients, which provide a bioactive calcium — and phosphate-enriched material when mixed with a water-base solution.[66,67] Calcium and phosphate ions released from this material form hydroxyapatite not only in simulated body tissue fluid, like MTA, but also in normal saline.[68]
Some characteristics have been demonstrated for CEM cement, e.g., similar pH, shorter setting time, good handling characteristics, superior film thickness and flow, and a lower estimated price in comparison with MTA.[69] The antibacterial effect of CEM cement is comparable to that of CH and better than that of MTA or Portland cement.[69,70]
In addition, CEM cement stimulates hard tissue healing similar to MTA[71] and provides an effective seal; similar to MTA and superior to IRM.[67]
Calcium enriched mixture cement has shown favorable results in pulpotomy of permanent molar teeth with irreversible pulpitis, in the management of internal root resorption, in pulp-capping and furcation perforation repair.[71,72] However, it should be noted that almost all the papers regarding CEM cement are from the same authors who have produced the material and the reports from other researchers are needed.
Adhesive technique
Contemporary researches have stated that healthy pulps without dentine bridges in teeth treated with an etch and composite-resin technique were observed. The theory is that this adhesive system effectively seal the exposed pulp against bacterial microleakage, thus a dentine bridge is not necessary. However, the long-term success of this seal has not been ascertained, and conflicting findings exist.[73] Sixty days after direct capping of exposed noninflamed pulp with the bonding technique, a persistent inflammatory reaction without any evidence of pulpal repair was found compared to pulp repair and complete dentine bridging with Ca(OH)2.[73] It has been claimed that the moisture and exudation from the exposed pulp may prevent the adhering of resin materials to peripheral dentine, as a result oral bacteria eventually access the pulp through microleakage.[74] In addition, the potential cytotoxicity of adhesive technique and induction of immune-based hypersensitivity reactions has been reported.[75] At present, there is little long-term information to support this technique and its use is not recommended.
Resin modified glass ionomers
Although successful results of IPT with resin-modified glass ionomers (RMGIs) have been found, direct contact with the pulp tissue results in inflammation, necrosis and lack of dentin bridge formation. Therefore, it is not recommended to use RMGIs directly on the pulp tissue.[76,77]
FUTURE ADVANCES IN MATERIALS AND BIOLOGICAL SCIENCES
Recent advances in the field of growth factors offer new therapeutic approaches. BMPs and TGF-β have been reported to induce reparative dentin formation.[78] At present, commercially available recombinant human BMP-2, -4, and -7 are available for experimentation and clinical trials., bioceramic, biodentine, EMD (STRAUMANN, USA), propolis, tricalcium phosphate cement, and some other bioactive materials has been also suggested for VPT.[31,79,80,81,82] These bioactive molecules have been shown to be inductive by direct or indirect contact with the pulp tissue, and the formed reparative dentin thickness depends on the dose of the biologic agent.[83] Further evaluation of these factors as new modalities for VPT should be performed. Future development of more efficient wound dressings containing growth factors, proper carrier vehicles with most favorable clinical handling characteristics and delivery, advanced local antimicrobial and anti-inflammatory materials will combine with the improved sealing ability of restorative materials, leading to more definite and predictable results.[80] At some point, pulpotomies for primary and permanent teeth may be handled with growth factors and predictably induce sound dentin bridges, leaving the remaining radicular tissue entirely surrounded by healthy tissue, eliminating the need for root canal therapy.[53]
THE TECHNIQUE EMPLOYED
Vital pulp therapy techniques for treatment of exposed immature permanent teeth can be classified into four categories:
Indirect pulp-capping;
DPC;
Partial pulpotomy;
Indirect pulp treatment.
Indirect pulp treatment is recommended for pulp preservation in asymptomatic teeth with a deep carious lesion adjacent to the pulp, as well as in teeth with a diagnosis of reversible pulpitis. A medicament is then placed over the carious dentin to stimulate and encourage pulp healing.[4] The placement of a restorative material with adequate seal against the microorganisms is necessary and more important to success than the type of medicament.[4,6]
Historically several materials have been used for this procedure, including resin-modified glass-ionomer cements, tricalcium phosphates, hydrophilic resins, zinc oxide-eugenol (ZOE), and Ca(OH)2. The latter two were the most commonly used materials. Within the theory of maintaining pulp vitality, IPT showed no differences in symptoms at 12 months using different formulations of Ca(OH)2.[84]
However, unlike ZOE mineral content of the residual dentin will increase in contact with Ca(OH)2.[85] A minimum indirect pulp postoperative time period of 6-8 weeks is essential to produce sufficient remineralization of the cavity floor. This favorable outcome is fundamentally dependent on the preservation of a hermetic seal against microleakage by the provisional and final restorations.[84]
Today treating the dentin with various bioactive molecules, such as enamel matrix protein (emdogain) or TGF-β, is recommended to promote a reactive response in the underlying odontoblasts, stimulate reparative dentin formation and decrease dentin permeability. However, these strategies are being investigated and cannot yet be applied in the clinic.[86]
One- or two-visit approach IPT in both primary and young permanent teeth can result in a high survival rate and be successful with a one- or two-visit approach. The two-visit treatment method involves the stepwise excavation of deep caries in two steps. The outmost layer of the infected dentin will be removed in the first step, leaving a carious mass above the pulp. The removal of the remaining caries and placement of a final restoration is performed in the second step. Formation of reparative dentin and a definitive pulpal diagnosis will be assessed after 6-8 weeks. An adequately sealed restoration is essential to both steps.[84]
Partial caries removal significantly diminishes the viable microorganisms, especially during the treatment stages of two-visit IPT, and reduces the risk of pulp exposure during caries excavation by 98%, compared to complete caries excavation in teeth with deep caries.[84] The IPT success rate with Ca(OH)2 in permanent teeth is 93%[15] and 63%[17] after 3 and 10 years, respectively. Regarding a new study, MTA appears to be more effective than Ca(OH)2 6 months after indirect pulp-capping[18] [Table 1].
Direct pulp treatment
Direct pulp treatment is defined as wound dressing of an exposed healthy pulp tissue with a biocompatible material to promote pulp healing and generate reparative dentin.[2] The two key points to the success of Ca(OH)2 DPC have been emphasized; pulp-capping should only be performed in asymptomatic teeth and a well-sealed restoration should be placed immediately after DPT.[39]
A variety of materials have been recommended for use in DPT: Antibiotics, calcitonin, collagen, corticosteroids, cyanoacrylate, resorbable tricalcium phosphate ceramic, resin-based adhesive composite systems, bioceramic, biodentine, CEM cement, tricalcium phosphate cement,[31,76,87] ZOE, Ca(OH)2 and MTA. The three last ones have been used more frequently.[88]
Some disadvantages for ZOE formulations have been known: Eugenol released from ZOE is extremely cytotoxic, and the interfacial microleakage with ZOE increases over time.[89] In a human clinical study using ZOE as a DPC agent, up to 12 weeks after DPT, chronic inflammation, without any evidence of pulp healing or reparative dentin formation, was observed compared to CH control group in which all the teeth demonstrated healing within 4 weeks.[90]
Calcium hydroxide has been regarded as the “gold standard” for DPC for several decades. To date, the literature on the treatment outcomes of DPT with Ca(OH)2 after iatrogenic pulp exposure has been inconsistent. The success rates of Ca(OH)2 pulp-capping in a review of 19 clinical studies, including over 2,400 cases, were about 60% to almost 100% if carried out by an experienced clinician [Table 2]. Despite this fact, Barthel et al. found only 13% success rate 10-year after capping caries-exposed asymptomatic vital pulps in which dental students were the operators.[9] However, it should be kept in mind that the short-term success of DPT in immature teeth that leads to the apical closure is valuable. The knowledge of pulpal physiology has progressed so much since then, and better understanding of the pulp healing potential is obtained. In addition, the wide-open apices and high vascularity of immature permanent teeth enhance the successful outcome of direct capping techniques.[1] Furthermore, even under suboptimal conditions, Ca(OH)2 has shown a clinical success. Thirty-four traumatically exposed teeth with an approximately 4-h delay before Ca(OH)2 pulp-capping showed a 97% success rate for periods of up to 17 years.[91]
Table 2.
Treatment outcome of direct pulp-capping in permanent teeth in literature
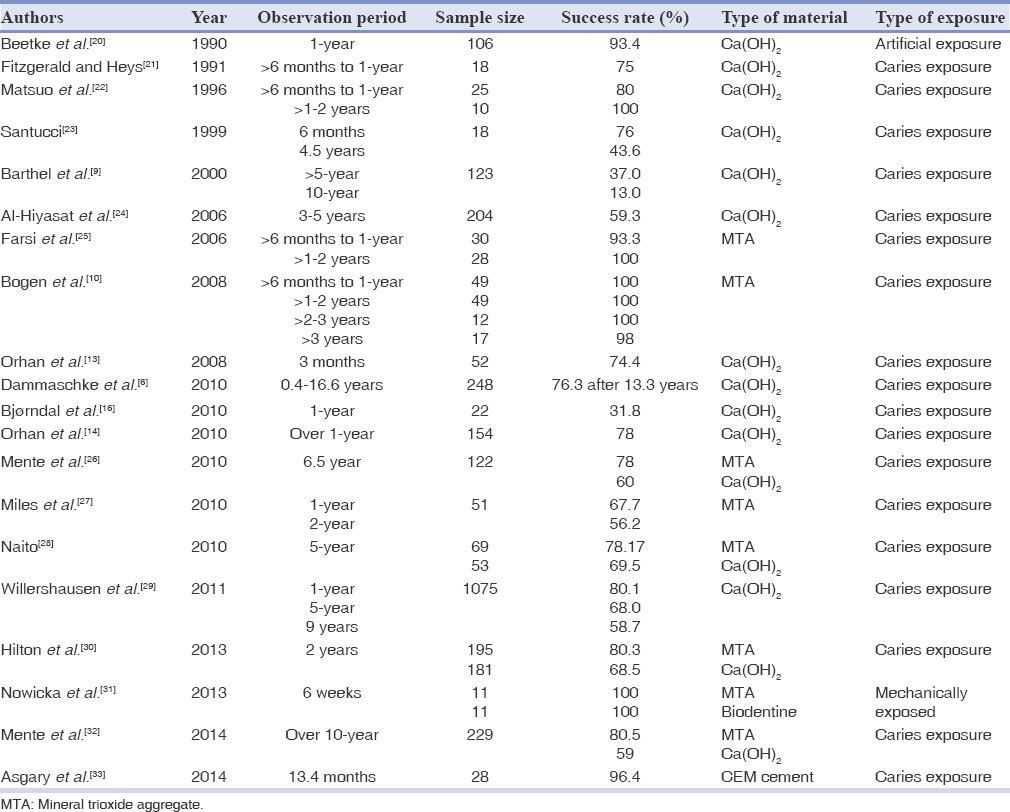
Animal DPT studies comparing MTA to Ca(OH)2 generally exhibit better-quality pulp healing with MTA.[92] Most human studies illustrated comparable pulp cap outcomes of MTA and Ca(OH)2. In a study using 11 pairs of third molars with DPT was possible to observe better pulp healing was demonstrated in MTA groups versus Ca(OH)2 groups. Pulps of these teeth were mechanically exposed and capped with either MTA or Ca(OH)2, covered with ZOE, and restored with amalgam. After extraction of teeth histological evaluation at 1-week and 2-, 3-, 4-, and 6-month intervals revealed less pulpal hyperemia, less inflammation and necrosis, and more predictable and consistent dentinal bridge formation in the MTA-treated teeth.[63] Similar results have been reported by other investigators.[16,18,28,26,31] Long-term studies in large-scale and prospective clinical trials are highly desirable to elucidate the treatment outcome of DPT. The outcomes of studies on DPT with Ca(OH)2 or MTA in permanent teeth are summarized in Table 2. The 5-year success rate DPT with Ca(OH)2 in permanent teeth was about 59-69%[28,24] and for MTA was 78-98%.[10,28] MTA appears to be more effective than Ca(OH)2 for maintaining long-term pulp vitality after DPC.[26,28]
Partial pulpotomy
Partial pulpotomy is indicated in a young permanent tooth for a small (<2 mm) pulp exposure in which the pulpal bleeding is controlled in 1-2 min.[4] Partial pulpotomy has been shown to be more successful in traumatically exposed rather than caries-exposed young permanent molars.[8,39,40,93] In immature teeth, this treatment is significantly more successful amongst the VPT techniques as well.
Generally, the most common material used in partial pulpotomy of immature permanent teeth is Ca(OH)2.[83] MTA has been confirmed as a pulp dressing material for partial pulpotomy in caries-exposed permanent molars with the success rate comparable to that of Ca(OH)2.[5] Fuks et al. reported a success rate of 87.5% for partial pulpotomies in complicated crown fractures in permanent incisors for 7.5-11 years. In general, as the duration of the follow-up period increased, the success rate diminished.[94]
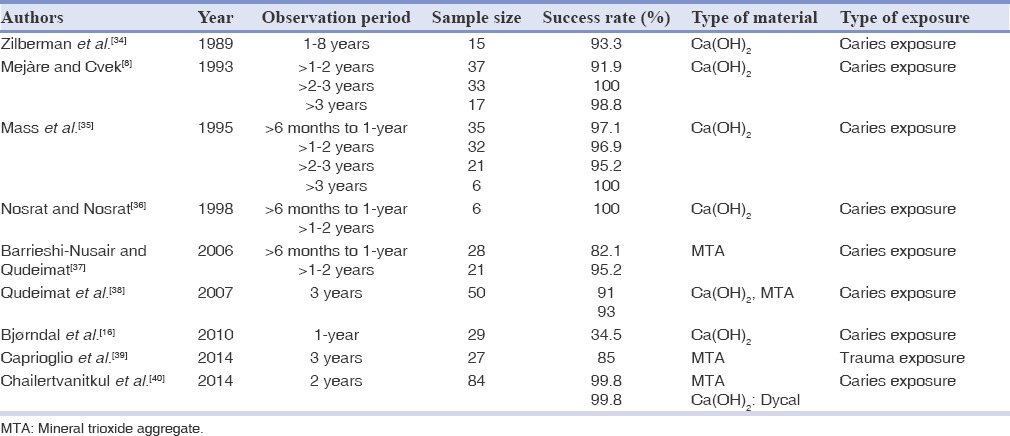
The outcomes of studies on partial pulpotomy with Ca(OH)2 or MTA are summarized in Table 3. The success rate with Ca(OH)2 in permanent teeth was about 91-100%[8,36] after 2 years and for MTA was 95.2-99.8%.[40,37] The difference between MTA and Ca(OH)2 for partial pulpotomy was not significant.
Table 3.
Treatment outcome of partial pulpotomy in permanent teeth in literature
Full pulpotomy
The pulpotomy procedure aims to preserve pulp vitality of young permanent teeth to promote normal root development. The necessity for root canal treatment following pulpotomy is to avoid a possible pulpal necrosis, continuous calcification or internal resorption.[5] After complete coronal pulpotomy with Ca(OH)2 dressing, later root canal treatment must be considered because of the dangers of dystrophic calcification of root canals.
Calcium hydroxide is the recommended pulpotomy material due to improved clinical outcomes. Dystrophic calcification in the root canals will be created following Ca(OH)2 pulpotomy procedure, which may prevent future endodontic treatments.[83] Besides, this calcification may reduce blood supply, which could lead to pulp necrosis.[95] The rationale is that a small amount of remaining pulp tissue is confined with a large amount of calcium in full coronal pulpotomy procedures. Therefore, the root canal treatment is performed after complete apical closure.[83] The success rate of Ca(OH)2 pulpotomies in cariously exposed teeth ranged from approximately 50-92%[41,43,78] and 72-96% in teeth with traumatic exposures.[96,97,98,99] Similar to DPC, the formation of dentin bridge is not the only indicator of treatment success since this bridge may be incomplete and filled with the tissue remnants. It is also possible that a fibrous tissue without evidence of dentinal bridge covers up the remaining pulp. Studies have shown that MTA may be useful as a substitute for Ca(OH)2 as the material of choice for pulpotomy procedures.[45] The calcification of the root canal entrances can occur after long periods of exposure to Ca(OH)2 or MTA. The outcomes of studies on pulp therapy with Ca(OH)2 or MTA in permanent teeth are summarized in Table 4.
The success rate with Ca(OH)2 in permanent teeth was about 87.5-100%[41,44] after 2 years and for MTA was 90-100%.[45,48] The difference between MTA and Ca(OH)2 for pulpotomy was not significant. MTA has clinical success rate comparable to CH as a pulp dressing material for full pulpotomy in permanent molars with carious exposures.
As a final point, there are still controversies about the treatment outcomes and it is suggested that further randomized controlled clinical trials regarding the VPT long-term outcomes be conducted.
CONCLUSION
There is a controversy regarding the VPT procedures outcomes, with many methodological variations between the different studies. Although various studies have different research approach, most studies noted a favorable treatment outcome. The type of coronal restoration and clinical status of the pulp tissue have a significant influence on the results.
Mineral trioxide aggregate appears to be more effective than Ca(OH)2 for maintaining long-term pulp vitality after indirect and DPC. However, it seems that the success rate for partial pulpotomy and pulpotomy with Ca(OH)2 is similar to MTA.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
ACKNOWLEDGMENTS
The authors wish to acknowledge Vise Chancellery for Research (IUMS), the staff of Pediatric Dentistry and Endodontic Department, Dental School of Isfahan University of Medical Sciences (IUMS) for their supports.
REFERENCES
- 1.Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: A systematic review. J Endod. 2011;37:581–7. doi: 10.1016/j.joen.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves KM, Cohen S, Berman LH. 10th ed. St. Louis: Mosby Year Book; 2011. Cohen's Pathways of the Pulp. [Google Scholar]
- 3.Bergenholtz G, Spångberg L. Controversies in endodontics. Crit Rev Oral Biol Med. 2004;15:99–114. doi: 10.1177/154411130401500204. [DOI] [PubMed] [Google Scholar]
- 4.Cohenca N, Paranjpe A, Berg J. Vital pulp therapy. Dent Clin North Am. 2013;57:59–73. doi: 10.1016/j.cden.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Ward J. Vital pulp therapy in cariously exposed permanent teeth and its limitations. Aust Endod J. 2002;28:29–37. doi: 10.1111/j.1747-4477.2002.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 6.Dammaschke T, Leidinger J, Schäfer E. Long-term evaluation of direct pulp capping — Treatment outcomes over an average period of 6.1 years. Clin Oral Investig. 2010;14:559–67. doi: 10.1007/s00784-009-0326-9. [DOI] [PubMed] [Google Scholar]
- 7.Amini P, Parirokh M. The importance of long time follow-up after vital pulp therapy: A case report. Iran Endod J. 2008;3:90–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Mejàre I, Cvek M. Partial pulpotomy in young permanent teeth with deep carious lesions. Endod Dent Traumatol. 1993;9:238–42. doi: 10.1111/j.1600-9657.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 9.Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: Treatment outcome after 5 and 10 years: A retrospective study. J Endod. 2000;26:525–8. doi: 10.1097/00004770-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: An observational study. J Am Dent Assoc. 2008;139:305–15. doi: 10.14219/jada.archive.2008.0160. [DOI] [PubMed] [Google Scholar]
- 11.Nirschl RF, Avery DR. Evaluation of a new pulp capping agent in indirect pulp therapy. ASDC J Dent Child. 1983;50:25–30. [PubMed] [Google Scholar]
- 12.Bjørndal L, Thylstrup A. A practice-based study on stepwise excavation of deep carious lesions in permanent teeth: A 1-year follow-up study. Community Dent Oral Epidemiol. 1998;26:122–8. doi: 10.1111/j.1600-0528.1998.tb01938.x. [DOI] [PubMed] [Google Scholar]
- 13.Orhan AI, Oz FT, Ozcelik B, Orhan K. A clinical and microbiological comparative study of deep carious lesion treatment in deciduous and young permanent molars. Clin Oral Investig. 2008;12:369–78. doi: 10.1007/s00784-008-0208-6. [DOI] [PubMed] [Google Scholar]
- 14.Orhan AI, Oz FT, Orhan K. Pulp exposure occurrence and outcomes after 1-or 2-visit indirect pulp therapy vs complete caries removal in primary and permanent molars. Pediatr Dent. 2010;32:347–55. [PubMed] [Google Scholar]
- 15.Gruythuysen RJ, van Strijp AJ, Wu MK. Long-term survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep carious lesions. J Endod. 2010;36:1490–3. doi: 10.1016/j.joen.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Bjørndal L, Reit C, Bruun G, Markvart M, Kjaeldgaard M, Näsman P, et al. Treatment of deep caries lesions in adults: Randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci. 2010;118:290–7. doi: 10.1111/j.1600-0722.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 17.Maltz M, Alves LS, Jardim JJ, Moura Mdos S, de Oliveira EF. Incomplete caries removal in deep lesions: A 10-year prospective study. Am J Dent. 2011;24:211–4. [PubMed] [Google Scholar]
- 18.Leye Benoist F, Gaye Ndiaye F, Kane AW, Benoist HM, Farge P. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal(®)) in the formation of a dentine bridge: A randomised controlled trial. Int Dent J. 2012;62:33–9. doi: 10.1111/j.1875-595X.2011.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrou MA, Alhamoui FA, Welk A, Altarabulsi MB, Alkilzy M, H Splieth C. A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin Oral Investig. 2014;18:1383–9. doi: 10.1007/s00784-013-1107-z. [DOI] [PubMed] [Google Scholar]
- 20.Beetke E, Wenzel B, Lau B, Bienengräber V. Direct capping of the artificial exposed pulp in teeth with deep caries. Stomatol DDR. 1990;40:246–9. [PubMed] [Google Scholar]
- 21.Fitzgerald M, Heys RJ. A clinical and histological evaluation of conservative pulpal therapy in human teeth. Oper Dent. 1991;16:101–12. [PubMed] [Google Scholar]
- 22.Matsuo T, Nakanishi T, Shimizu H, Ebisu S. A clinical study of direct pulp capping applied to carious-exposed pulps. J Endod. 1996;22:551–6. doi: 10.1016/S0099-2399(96)80017-3. [DOI] [PubMed] [Google Scholar]
- 23.Santucci PJ. Dycal versus Nd:YAG laser and Vitrebond for direct pulp capping in permanent teeth. J Clin Laser Med Surg. 1999;17:69–75. doi: 10.1089/clm.1999.17.69. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hiyasat AS, Barrieshi-Nusair KM, Al-Omari MA. The radiographic outcomes of direct pulp-capping procedures performed by dental students: A retrospective study. J Am Dent Assoc. 2006;137:1699–705. doi: 10.14219/jada.archive.2006.0116. [DOI] [PubMed] [Google Scholar]
- 25.Farsi N, Alamoudi N, Balto K, Al Mushayt A. Clinical assessment of mineral trioxide aggregate (MTA) as direct pulp capping in young permanent teeth. J Clin Pediatr Dent. 2006;31:72–6. doi: 10.17796/jcpd.31.2.n462281458372u64. [DOI] [PubMed] [Google Scholar]
- 26.Mente J, Geletneky B, Ohle M, Koch MJ, Friedrich Ding PG, Wolff D, et al. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: An analysis of the clinical treatment outcome. J Endod. 2010;36:806–13. doi: 10.1016/j.joen.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Miles JP, Gluskin AH, Chambers D, Peters OA. Pulp capping with mineral trioxide aggregate (MTA): A retrospective analysis of carious pulp exposures treated by undergraduate dental students. Oper Dent. 2010;35:20–8. doi: 10.2341/09-038CR1. [DOI] [PubMed] [Google Scholar]
- 28.Naito T. Uncertainty remains regarding long-term success of mineral trioxide aggregate for direct pulp capping. J Evid Based Dent Pract. 2010;10:250–1. doi: 10.1016/j.jebdp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Willershausen B, Willershausen I, Ross A, Velikonja S, Kasaj A, Blettner M. Retrospective study on direct pulp capping with calcium hydroxide. Quintessence Int. 2011;42:165–71. [PubMed] [Google Scholar]
- 30.Hilton TJ, Ferracane JL, Mancl L. Northwest Practice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp capping: A PBRN randomized clinical trial. J Dent Res. 2013;92:16S–22. doi: 10.1177/0022034513484336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–7. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Mente J, Hufnagel S, Leo M, Michel A, Gehrig H, Panagidis D, et al. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J Endod. 2014;40:1746–51. doi: 10.1016/j.joen.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Asgary S, Fazlyab M, Sabbagh S, Eghbal MJ. Outcomes of different vital pulp therapy techniques on symptomatic permanent teeth: a case series. Iran Endod J 2014 Fall. 9:295–300. [PMC free article] [PubMed] [Google Scholar]
- 34.Zilberman U, Mass E, Sarnat H. Partial pulpotomy in carious permanent molars. Am J Dent. 1989;2:147–50. [PubMed] [Google Scholar]
- 35.Mass E, Zilberman U, Fuks AB. Partial pulpotomy: Another treatment option for cariously exposed permanent molars. ASDC J Dent Child. 1995;62:342–5. [PubMed] [Google Scholar]
- 36.Nosrat IV, Nosrat CA. Reparative hard tissue formation following calcium hydroxide application after partial pulpotomy in cariously exposed pulps of permanent teeth. Int Endod J. 1998;31:221–6. doi: 10.1046/j.1365-2591.1998.00147.x. [DOI] [PubMed] [Google Scholar]
- 37.Barrieshi-Nusair KM, Qudeimat MA. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod. 2006;32:731–5. doi: 10.1016/j.joen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Qudeimat MA, Barrieshi-Nusair KM, Owais AI. Calcium hydroxide vs mineral trioxide aggregates for partial pulpotomy of permanent molars with deep caries. Eur Arch Paediatr Dent. 2007;8:99–104. doi: 10.1007/BF03262577. [DOI] [PubMed] [Google Scholar]
- 39.Caprioglio A, Conti V, Caprioglio C, Caprioglio D. A long-term retrospective clinical study on MTA pulpotomies in immature permanent incisors with complicated crown fractures. Eur J Paediatr Dent. 2014;15:29–34. [PubMed] [Google Scholar]
- 40.Chailertvanitkul P, Paphangkorakit J, Sooksantisakoonchai N, Pumas N, Pairojamornyoot W, Leela-Apiradee N, et al. Randomized control trial comparing calcium hydroxide and mineral trioxide aggregate for partial pulpotomies in cariously exposed pulps of permanent molars. Int Endod J. 2014;47:835–42. doi: 10.1111/iej.12225. [DOI] [PubMed] [Google Scholar]
- 41.Caliskan MK. Pulpotomy of carious vital teeth with periapical involvement. Int Endod J. 1995;28:172–6. doi: 10.1111/j.1365-2591.1995.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 42.Waly NG. A five-year comparative study of calcium hydroxide-glutaraldehyde pulpotomies versus calcium hydroxide pulpotomies in young permanent molars. Egypt Dent J. 1995;41:993–1000. [PubMed] [Google Scholar]
- 43.Teixeira LS, Demarco FF, Coppola MC, Bonow ML. Clinical and radiographic evaluation of pulpotomies performed under intrapulpal injection of anaesthetic solution. Int Endod J. 2001;34:440–6. doi: 10.1046/j.1365-2591.2001.00414.x. [DOI] [PubMed] [Google Scholar]
- 44.DeRosa TA. A retrospective evaluation of pulpotomy as an alternative to extraction. Gen Dent. 2006;54:37–40. [PubMed] [Google Scholar]
- 45.Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: A case series outcomes assessment. J Am Dent Assoc. 2006;137:610–8. doi: 10.14219/jada.archive.2006.0256. [DOI] [PubMed] [Google Scholar]
- 46.El-Meligy OA, Avery DR. Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis) Pediatr Dent. 2006;28:399–404. [PubMed] [Google Scholar]
- 47.Nosrat A, Seifi A, Asgary S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: A randomized clinical trial. Int J Paediatr Dent. 2013;23:56–63. doi: 10.1111/j.1365-263X.2012.01224.x. [DOI] [PubMed] [Google Scholar]
- 48.Barngkgei IH, Halboub ES, Alboni RS. Pulpotomy of symptomatic permanent teeth with carious exposure using mineral trioxide aggregate. Iran Endod J. 2013;8:65–8. [PMC free article] [PubMed] [Google Scholar]
- 49.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with Mineral Trioxide Aggregate: A randomized controlled trial 2008. Int Endod J. 2009;42:422–44. doi: 10.1111/j.1365-2591.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- 50.Stanley HR. Pulp capping: Conserving the dental pulp — Can it be done?. Is it worth it. Oral Surg Oral Med Oral Pathol. 1989;68:628–39. doi: 10.1016/0030-4220(89)90252-1. [DOI] [PubMed] [Google Scholar]
- 51.Aljandan B, AlHassan H, Saghah A, Rasheed M, Ali AA. The effectiveness of using different pulp-capping agents on the healing response of the pulp. Indian J Dent Res. 2012;23:633–7. doi: 10.4103/0970-9290.107381. [DOI] [PubMed] [Google Scholar]
- 52.Bjørndal L, Mjör IA. Pulp-dentin biology in restorative dentistry. Part 4: Dental caries — Characteristics of lesions and pulpal reactions. Quintessence Int. 2001;32:717–36. [PubMed] [Google Scholar]
- 53.Witherspoon DE. Vital pulp therapy with new materials: New directions and treatment perspectives — Permanent teeth. Pediatr Dent. 2008;30:220–4. [PubMed] [Google Scholar]
- 54.Sawicki L, Pameijer CH, Emerich K, Adamowicz-Klepalska B. Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am J Dent. 2008;21:262–6. [PubMed] [Google Scholar]
- 55.Sirén EK, Haapasalo MP, Waltimo TM, Ørstavik D. In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodide on Enterococcus faecalis. Eur J Oral Sci. 2004;112:326–31. doi: 10.1111/j.1600-0722.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 56.Hörsted-Bindslev P, Vilkinis V, Sidlauskas A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:591–600. doi: 10.1016/s1079-2104(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 57.Eskandarizadeh A, Parirokh M, Eslami B, Asgary S, Eghbal MJ. A Comparative Study between Mineral Trioxide Aggregate and Calcium Hydroxide as Pulp Capping Agents in Dog's Teeth. Dent Res J (Isfahan) 2006;2:1–9. [Google Scholar]
- 58.Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003;29:324–33. doi: 10.1097/00004770-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006;27:2865–73. doi: 10.1016/j.biomaterials.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Walboomers XF, Jansen JA. The formation of tertiary dentin after pulp capping with a calcium phosphate cement, loaded with PLGA microparticles containing TGF-beta1. J Biomed Mater Res A. 2008;85:439–44. doi: 10.1002/jbm.a.31558. [DOI] [PubMed] [Google Scholar]
- 61.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41:408–17. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 62.Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: A review of the constituents and biological properties of the material. Int Endod J. 2006;39:747–54. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 63.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: A preliminary report. Int Endod J. 2003;36:225–31. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 64.Jabbarifar E, Razavi SM, Ahmadi N. Histopathologic responses of dog's dental pulp to mineral trioxide aggregate, bio active glass, formocresol, hydroxyapatite. Dent Res J (Isfahan) 2007;4:83–7. [Google Scholar]
- 65.Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimarães BM, Duarte MA. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod. 2014;40:1235–40. doi: 10.1016/j.joen.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 66.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009;35:243–50. doi: 10.1016/j.joen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 67.Asgary S, Eghbal MJ, Parirokh M. Sealing ability of a novel endodontic cement as a root-end filling material. J Biomed Mater Res A. 2008;87:706–9. doi: 10.1002/jbm.a.31678. [DOI] [PubMed] [Google Scholar]
- 68.Asgary S, Parirokh M, Eghbal MJ, Ghoddusi J. SEM evaluation of pulp reaction to different pulp capping materials in dog's teeth. Iran Endod J. 2006;1:117–23. [PMC free article] [PubMed] [Google Scholar]
- 69.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 70.Asgary S, Akbari Kamrani F, Taheri S. Evaluation of antimicrobial effect of MTA, calcium hydroxide, and CEM cement. Iran Endod J. 2007;2:105–9. [PMC free article] [PubMed] [Google Scholar]
- 71.Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:609–14. doi: 10.1016/j.tripleo.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Asgary S, Ehsani S. Permanent molar pulpotomy with a new endodontic cement: A case series. J Conserv Dent. 2009;12:31–6. doi: 10.4103/0972-0707.53340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schuurs AH, Gruythuysen RJ, Wesselink PR. Pulp capping with adhesive resin-based composite vs. calcium hydroxide: A review. Endod Dent Traumatol. 2000;16:240–50. doi: 10.1034/j.1600-9657.2000.016006240.x. [DOI] [PubMed] [Google Scholar]
- 74.Accorinte ML, Loguercio AD, Reis A, Costa CA. Response of human pulps capped with different self-etch adhesive systems. Clin Oral Investig. 2008;12:119–27. doi: 10.1007/s00784-007-0161-9. [DOI] [PubMed] [Google Scholar]
- 75.Paranjpe A, Bordador LC, Wang MY, Hume WR, Jewett A. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J Dent Res. 2005;84:172–7. doi: 10.1177/154405910508400212. [DOI] [PubMed] [Google Scholar]
- 76.Ghoddusi J, Forghani M, Parisay I. New approaches in vital pulp therapy in permanent teeth. Iran Endod J. 2014;9:15–22. [PMC free article] [PubMed] [Google Scholar]
- 77.Khoroushi M, Keshani F. A review of glass-ionomers: From conventional glass-ionomer to bioactive glass-ionomer. Dent Res J (Isfahan) 2013;10:411–20. [PMC free article] [PubMed] [Google Scholar]
- 78.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 79.Nakamura Y, Hammarström L, Matsumoto K, Lyngstadaas SP. The induction of reparative dentine by enamel proteins. Int Endod J. 2002;35:407–17. doi: 10.1046/j.1365-2591.2002.00556.x. [DOI] [PubMed] [Google Scholar]
- 80.Guven EP, Yalvac ME, Sahin F, Yazici MM, Rizvanov AA, Bayirli G. Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod. 2011;37:650–6. doi: 10.1016/j.joen.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Min KS, Yang SH, Kim EC. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endod. 2009;35:847–51. doi: 10.1016/j.joen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 82.Hirschman WR, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endod. 2012;38:385–8. doi: 10.1016/j.joen.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Ranly DM, Garcia-Godoy F. Current and potential pulp therapies for primary and young permanent teeth. J Dent. 2000;28:153–61. doi: 10.1016/s0300-5712(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 84.Bjørndal L. Indirect pulp therapy and stepwise excavation. Pediatr Dent. 2008;30:225–9. [PubMed] [Google Scholar]
- 85.King M. Preserving the vital pulp in operative dentistry. Dent Update. 2003;30:159. [PubMed] [Google Scholar]
- 86.Fransson H. On the repair of the dentine barrier. Swed Dent J Suppl. 2012;226:9–84. [PubMed] [Google Scholar]
- 87.Asgary S, Ahmadyar M. Vital pulp therapy using calcium-enriched mixture: An evidence-based review. J Conserv Dent. 2013;16:92–8. doi: 10.4103/0972-0707.108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tziafas D, Kalyva M, Papadimitriou S. Experimental dentin-based approaches to tissue regeneration in vital pulp therapy. Connect Tissue Res. 2002;43:391–5. doi: 10.1080/03008200290001014. [DOI] [PubMed] [Google Scholar]
- 89.Ho YC, Huang FM, Chang YC. Mechanisms of cytotoxicity of eugenol in human osteoblastic cells in vitro. Int Endod J. 2006;39:389–93. doi: 10.1111/j.1365-2591.2006.01091.x. [DOI] [PubMed] [Google Scholar]
- 90.Ghoddusi J, Shahrami F, Alizadeh M, Kianoush K, Forghani M. Clinical and radiographic evaluation of vital pulp therapy in open apex teeth with MTA and ZOE. N Y State Dent J. 2012;78:34–8. [PubMed] [Google Scholar]
- 91.Robertson A, Andreasen FM, Andreasen JO, Norén JG. Long-term prognosis of crown-fractured permanent incisors. The effect of stage of root development and associated luxation injury. Int J Paediatr Dent. 2000;10:191–9. doi: 10.1046/j.1365-263x.2000.00191.x. [DOI] [PubMed] [Google Scholar]
- 92.de Souza Costa CA, Duarte PT, de Souza PP, Giro EM, Hebling J. Cytotoxic effects and pulpal response caused by a mineral trioxide aggregate formulation and calcium hydroxide. Am J Dent. 2008;21:255–61. [PubMed] [Google Scholar]
- 93.Akhlaghi N, Nourbakhsh N, Khademi A, Karimi L. General Dental Practitioners’ Knowledge about the Emergency Management of Dental Trauma. Iran Endod J. 2014;9:251–6. [PMC free article] [PubMed] [Google Scholar]
- 94.Fuks AB, Cosack A, Klein H, Eidelman E. Partial pulpotomy as a treatment alternative for exposed pulps in crown-fractured permanent incisors. Endod Dent Traumatol. 1987;3:100–2. doi: 10.1111/j.1600-9657.1987.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 95.Fuks AB. Pulp therapy for the primary and young permanent dentitions. Dent Clin North Am. 2000;44:571–96. [PubMed] [Google Scholar]
- 96.Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod. 1978;4:232–7. doi: 10.1016/S0099-2399(78)80153-8. [DOI] [PubMed] [Google Scholar]
- 97.Cox CF, Bergenholtz G, Fitzgerald M, Heys DR, Heys RJ, Avery JK, et al. Capping of the dental pulp mechanically exposed to the oral microflora — A 5 week observation of wound healing in the monkey. J Oral Pathol. 1982;11:327–39. doi: 10.1111/j.1600-0714.1982.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 98.Gelbier MJ, Winter GB. Traumatised incisors treated by vital pulpotomy: A retrospective study. Br Dent J. 1988;164:319–23. doi: 10.1038/sj.bdj.4806440. [DOI] [PubMed] [Google Scholar]
- 99.Ravn JJ. Follow-up study of permanent incisors with complicated crown fractures after acute trauma. Scand J Dent Res. 1982;90:363–72. doi: 10.1111/j.1600-0722.1982.tb00749.x. [DOI] [PubMed] [Google Scholar]


