Abstract
Background:
Type 2 diabetes mellitus (T2DM) and periodontitis are inflammatory conditions with a bidirectional association. This pilot study aimed to evaluate whether T2DM and glycemic control interfere in inflammatory markers profiles in gingival crevicular fluid (GCF) in periodontitis patients.
Materials and Methods:
Fourteen diabetic periodontitis patients were enrolled in this study, seven with adequate glycemic control (glycated hemoglobin [HbA1c] <8.0%) (DMA + P) and seven with inadequate control (HbA1c ≥8.0%) (DMI + P). Seven chronic periodontitis patients without diabetes formed the control group (P). GCF was obtained from diseased sites (probing depth >6 mm) of an entirely hemiarch, pooled and cytokines levels determined using multiplex beads immunoassay. Clinical periodontal parameters were analyzed by Mann-Whitney test and levels of cytokines by Kruskal-Wallis and Dunn's multiple comparison tests with confidence level of 95% (P < 0.05).
Results:
Cytokines profile of GCF obtained from deep periodontal pockets presented high levels of inflammatory cytokines, and there were no statistical differences between levels of interleukin-6 (IL-6), IL-8 and tumor necrosis factor-α according to presence of diabetes or percentage of HbA1c among the groups, despite groups with T2DM and periodontitis exhibit higher levels of PD.
Conclusion:
Within the limitations of this study, inflammatory mediators in GCF are dependent to the local response and do not correlate with the diabetic status.
Keywords: Cytokines, diabetes mellitus, gingival crevicular fluid, inflammation, periodontitis
INTRODUCTION
According to World Health Organization,[1] the term “diabetes mellitus” (DM) describes a metabolic disorder of multiple etiology characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolisms due to defects in insulin secretion and/or activity. The condition is considered the major epidemic of the century affecting more than 340 million of people around the world.
It is associated with chronic inflammatory process and with a number of complications. Acute metabolic complications associated with mortality include diabetic ketoacidosis from exceptionally high blood glucose concentrations (hyperglycemia) and coma as the result of low blood glucose (hypoglycemia). However, the most devastating consequences of diabetes are long-term vascular complications, including kidney disease, blindness, heart attacks, strokes,[2] and amputation of extremities.[3]
Epidemiological studies[4,5,6,7,8] have found a high degree of association between DM and periodontal diseases (PDs), which were proposed as the sixth complication of DM.[9] According to recent studies,[10,11] this relationship is bidirectional, with periodontitis exerting an effect on DM and vice-versa. Over the years, various biologically plausible mechanisms have been established for a common inflammatory etiopathogenesis of DM and PDs.[12,13,14,15]
Chronic periodontitis, an inflammatory disease of tooth-supporting structures, results in the loss of periodontal support tissues, with increased tooth mobility and eventually tooth loss. Patients with chronic periodontitis present increased systemic inflammation and high levels of inflammatory markers when compared with healthy control subjects.[16]
The gingival crevicular fluid (GCF) is a serum exudate originated from the microcirculation in the gingival tissues which flows into periodontal pocket (gingival sulcus) carrying inflammatory mediators and products of tissue metabolism.[17] Several inflammatory markers such as interleukin (IL)-1β and C-reactive protein (CRP) are present in high levels in patients with periodontitis.[18]
Since the composition of GCF somewhat mirrors the plasma, it could be used as a less invasive medium to evaluate systemic conditions including DM.[17] However, the production of inflammatory mediators is increased locally at PDs sites.[19]
Biomarkers in GCF in diabetic patients with PDs have been evaluated in different groups of patients, exhibiting differences in periodontal treatment, diabetic status, sites characteristics which contributed to the biased results,[20,21] restricting the comparison among them.
Glycemic status in type 2 diabetes mellitus (T2DM) patients seems to modulate osteo-immune inflammatory mediators.[22] Furthermore, diabetes affects GCF levels of cytokines, by increasing levels of IL-6,[23] CRP[16] and resistin[24] and reducing the levels of β-glucuronidase and IL-8[25] in patients with periodontitis. However, there is still no consensus on the differences induced by DM and glycemic control in GCF cytokine profile in patients with PDs.[26]
Thus, in order to evaluate the effect of diabetes and glycemic control on the cytokines profile of periodontitis patients, we evaluated the levels of IL-6, IL-8 and tumor necrosis factor-α (TNF-α) in the GCF of sites with periodontal destruction (probing depth, PD >6 mm) in patients with periodontitis and type 2 diabetes mellitus with adequate glycemic contorl (DMA + P, glycated hemoglobin (HbA1c) <8.0%) and in patients with periodontits and type 2 diabetes mellitus with inadequate glycemic control (DMI + P, HbA1c ≥8.0%) glycemic control and these data were compared with nondiabetic subjects with periodontitis (P).
MATERIALS AND METHODS
Participants
This study was conducted between January 2012 and March 2013 in accordance with Declaration of Helsinki, and it was approved by Biomedical Sciences Institute Committee (#011/CEP) from University of São Paulo (SP, Brazil). Fourteen diabetic subjects and seven nondiabetic subjects, all with chronic periodontitis, attending the Diabetes Center, Federal University of São Paulo and School of Dentistry, University of São Paulo were enrolled in this study. Subjects who fulfilled the inclusion/exclusion criteria were invited to participate. Eligible subjects were informed of the nature, potential risks and benefits of their participation in the study and signed an informed consent.
Patients presented at least 15 teeth (excluding third molars), with at least ≥30% of sites showed probing depth (PD) >4 mm, clinical attachment level (CAL) ≥4 mm and bleeding on probing (BOP). Diabetic subjects should have type 2 DM diagnosed for at least 3 years and should be under hypoglycemic medication. The glycated haemoglobin (HbA1c) level was measured by Clinical Analysis Laboratory from University of São Paulo Hospital and based on the results participants were assigned as inadequate controlled (HbA1c ≥8.0%) (DMI + P), adequate controlled (HbA1c <8.0%) (DMA + P) type 2 diabetic subjects, and nondiabetic subjects (HbA1c ≤6.0%) (P). Exclusion criteria included pregnancy, lactation, current cigarette smoking and cigarette smoking within the past 5 years, periodontal and antibiotic therapies in the previous 12 months, continuous use of antimicrobial mouthrinses, systemic condition other than DM (e.g., immunological disorders, osteoporosis, heart diseases, rheumatoid arthritis, tumors) that could affect periodontal status, continued use of anti-inflammatory and immunosuppressive medications and morbid obesity (i.e., body mass index [BMI] ≥40 kg/m2).
All volunteers received full-mouth periodontal clinical examination performed at six sites per tooth (excluding third molars) from a calibrated examiner intra-examiner reliability for detecting PD within 1 mm was >90%. The presence of supragingival biofilm was recorded as plaque index (PL). Marginal gingival bleeding (GB), bleeding on probing (BOP), probing depth (PD) and attachment level (AL) were also evaluated using North Caroline probe (Hu-Friedy Mfg. Co., Chicago, IL, USA). PL, GB and BOP were recorded as absent (0) or present (1).
Samples
GCF was sampled 2 weeks after the clinical examination in order not to alter its nature. All mesio-vestibular sites with PD >6 mm from two half arches (one upper and other lower) were collected. After removal of the supragingival biofilm with sterile cotton pellets, the sites were isolated with cotton rolls and gently dried with an air syringe to eliminate the possibility of contamination with saliva. GCF was collected through inserting standard paper strips (Periopaper; Oraflow Inc., Smithtown, NY, USA) into the sulcus/pocket for 20s. Strips that were visually contaminated with blood were discarded. The GCF volume was measured in a calibrated device (Periotron 8000; Proflow Inc., Amityville, NY, USA) and the readings were converted to an actual volume by reference to a standard curve. Strips were immediately placed into microcentrifuge tubes and stored at −80°C until assays.
Assays
Periopapers from different tooth of the same participant were pooled, and 300 μL of phosphate-buffered saline (pH 7.4) with bovine serum albumin (0.5%) was added. After mixing and centrifugation for 10 min at 13000 rpm at 4°C, the levels of cytokines in the supernatant were analyzed in duplicate using a multi-analyte method according to the manufacturer's recommendations (Bio-Plex Pro Human Cytokine Standard, Bio-Rad, Hercules, CA, USA) in the multiplex platform MagPix System (Luminex Corporation, Austin, TX, USA). The amount of biomarkers in each sample was estimated by comparison with a standard curve using appropriate software (Beadview; Merck Millipore, Darmstadt, Germany).
Data analysis
Periodontal clinical data were analyzed using a statistical packet (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL, USA) considering the individual as a study unit. Data were analyzed by nonparametric statistical methods. Intragroup and intergroup comparisons were determined using Mann–Whitney test. Data were tested for normality before applying nonparametric tests. Tests were based on average values with variability measures (standard deviation [SD]). Results were considered as statistically significant at P < 0.05. For GB, PL and BOP data, percentages of positive sites were obtained per patient and thereafter, mean values were calculated for the groups. For PD and AL, measured by millimeters, percent frequency was first obtained per patient and thereafter, as a mean value for the group. Data from MagPix were analyzed by Kruskal-Wallis and Dunn's multiple comparison tests (Prisma 5 Project, GraphPad Software Inc., La Jolla, CA, USA). Values were obtained per patient, and a median value was calculated for each group.
RESULTS
Analysis of demographic data (average ± SD) showed age in years of 47.4 ± 4.9 for P group, 58.4 ± 8.1 for DMA + P group and 51.7 ± 6.15 for DMI + P group (Kruskal–Wallis test, P = 0.03). There was no significant difference of BMI (kg/m2) among groups (P = 26.33 ± 3.07; DMA + P = 28.97 ± 3.83; DMI + P = 29.28 ± 5.94). Also, there was no difference among GCF average volume (mL) measured per teeth in each group (P = 0.48 ± 0.23; DMA + P = 0.53 ± 0.20; DMI + P = 0.49 ± 0.27). As expected, there was significant difference in HbA1c levels (%) between DMI + P (11.63 ± 2.22) and the other two groups (P = 5.40 ± 0.48 and DMA + P = 6.98 ± 0.65) (Kruskal-Wallis test, P < 0.001).
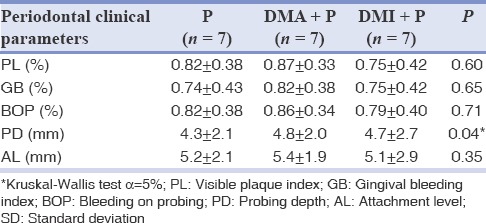
Table 1 presents the average of periodontal clinical parameters of patients in each group. Subgingival clinical parameters in all groups were similar, with the exception of PD, which was higher in the diabetics groups (Kruskal–Wallis test, P = 0.048).
Table 1.
Average ± SD of clinical parameters for patients of P, DMA + P and DMI + P groups
The GCF levels of IL-6, IL-8 and TNF-α did not differ among any of the periodontitis groups (P, DMA + P and DMI + P) (Kruskal–Wallis test, P > 0.05) as shown in Figure 1.
Figure 1.
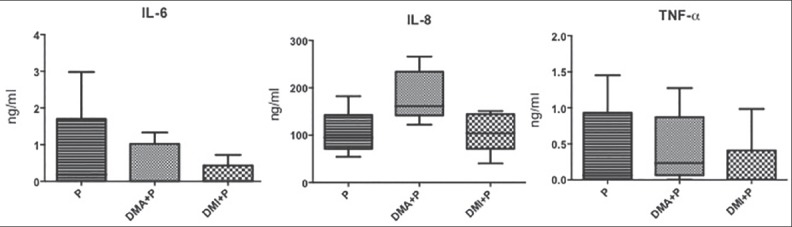
Interleukin-6 (IL-6), IL-8 and tumor necrosis factor-α gingival crevicular levels in patients with periodontitis (P) and without type 2 diabetes mellitus (T2DM), patients with T2DM with adequate glycemic control and periodontitis (DMA + P) and patients with T2DM with inadequate glycemic control and periodontitis (DMI + P).
DISCUSSION
The mechanisms underlying the association between DM and PDs are still a matter of controversial discussion.[24] DM patients may exhibit an exhacerbated response to subgingival bacteria and or altered microbiota. Furthermore, GCF composition may be altered by DM since it comprises the sum of components produced locally in the gingival tissues and those of other sources, brought with the plasma. Thus, comparison of GCF composition analysis may provide information not only on the gingival inflammatory status but also on the systemic inflammatory response.
The availability of methods to measure cytokines and other inflammatory mediators with high sensitivity and specificity is critically important. The conventional method enzyme-linked immunosorbant assay (ELISA) is the most widely used and best- validated method, but it is limited by its ability to measure only a single biomarker in each sample what is a problem especially in samples with minimum volume such as GCF. On the other hand, Multiplex Arrays technique offers the potential of better evaluating the complexity and dynamic nature of inflammatory responses with reduced cost and sample savings over traditional ELISA measurements. Despite the potential advantages of this new technology, experience with these techniques is limited.[27]
All patients enrolled in this study presented periodontitis (P), but differed on their metabolic condition and/or glycemic status. The only clinical parameter that differed significantly between diabetic and nondiabetic groups was the mean PD, since diabetic subjects (DMA + P and DMI + P) showed a higher mean full-mouth PD than nondiabetic subjects (P), as observed by others.[28]
Our study did not aim to compare epidemiological clinical data between the studied groups, not only due to the low number of enrolled subjects, but also due to the exclusion criteria used for selection. GCF volume is usually high in periodontitis sites and tends to decrease after the periodontal treatment.[29] Since periodontitis is associated with diabetes, it is reasonable to expect higher GCF volume in diabetes than in the systemically health subjects, as reported.[28,30,31,32] However, the GCF volume was similar when periodontitis patients with and without diabetes were compared, which is in agreement with other data[21] reporting similar GCF volumes in well-controlled diabetic chronic periodontitis and systemically healthy chronic periodontitis patients.
Certain systemic conditions such as diabetes and obesity are characterized by pro-inflammatory state,[33] exerting a profound effect on the levels of inflammatory markers, both locally at sites of periodontal disease, and elsewhere[19] and components of GCF may be affected by its systemic levels. For instance, it was recently shown that there was correlation in levels of IL-6 but not of TNF-α between serum and GCF samples.[34]
Many studies have been performed to define biomarkers in saliva and GCF associated to periodontal diseases. IL-1β levels are usually higher in the saliva and/or CGF of patients with gingivitis,[35,36] but there is no clear consensus among the authors on the levels of other cytokines in the oral fluids of periodontitis patients.[37]
A recent review[26] analyzing 10 studies on the GCF cytokines profile in patients with periodontitis with poorly controlled T2DM and medically healthy individuals showed that there is no consensus on this topic, with contradictory results among studies on the association of IL-6, IL-8, IL-17, IL-23, IL-1α and IF-γ and T2DM.
It was previously shown that uncontrolled T2DM (HbA1c ≥7.5%) modulates local levels of several cyto/chemokines at both healthy and diseased periodontal sites in favor of a proinflammatory profile, which may partially explain the greater susceptibility of diabetic subjects to periodontal breakdown.[28]
In the present study, there are no statistical differences among IL-6 GCF levels in deep pockets among P, DMA + P and DMI + P groups. This interleukin is considered the major mediator of the host response to tissue injury, infection, and bone resorption.[23] Although IL-6 levels decrease after periodontal treatment,[30] this result is not so evident in diabetic patients.[31] Despite the increased PD in diabetes groups, our data indicated no differences in IL-6 levels between diabetes and control (P), in accordance with previous data.[23] However, the lack of difference in IL-6 levels in GCF between diabetes patients with inadequate glycemic control (DMI + P) and control (P) is in disagreement with another study using multiplex analysis, which had shown that IL-6 levels were higher in healthy and diseases sites of uncontrolled T2DM (HbA1c >7.5%) than in nondiabetic patient.[28]
These data highlighted that the effect of diabetes and glycemic control on IL-6 GCF levels are still controversial. Differences in parameters to define periodontitis and controlled/uncontrolled glycemic status, use of drugs and others such as the method to access cytokines levels should account for these different results.
Interleukin-8 is a potent neutrophils chemoattractive, aiming to eliminate the invasive microorganisms. However, red complex bacteria associated with periodontitis present an effective immune-evasive strategy by suppressing the IL-8 production.[38] The periodontopathogen Porphyromonas gingivalis down-regulates the expression of this chemokine at both the mRNA and protein levels, leading to a so-called “chemokine paralysis”.[39,40]
In the present study, there were no differences in IL-8 CGF levels between diabetes (DMA + P and DMI + P) and nondiabetes periodontitis (P) groups. Our data contradict a previous report who showed that GCF IL-8 levels were lower in T2DM periodontitis patients than in periodontitis nondiabetic patients.[25] This observation is further reinforced by the demonstration that IL-8 levels did not change according to the glycemic control, despite the high differences in mean levels of HbA1c between both groups (DMA + P and DMI + P), which is in accordance with the same authors[25] who reported that IL-8 and HbA1c levels were not correlated.
Regardless of the higher disease severity in diabetes patients when compared to controls in the present study, represent by significant higher PD measurements, there were no statistical differences in TNF-α levels in GCF among the studied groups. A wide range of cells in periodontal tissues secretes inflammatory cytokines, such as TNF-α recognized by Toll-Like receptors.[38] This result agrees with others[22,41] who showed no difference between TNF-α GCF levels according to diabetes or glycemic control.
Despite the limitation of the present study due to the low number of subjects, the patients enrolled in each group were very homogenous regarding diabetes status and periodontitis severity. Even in such groups, the production of inflammatory mediators in gingival tissues were similar, indicating that, possibly, the local challenge overwhelm the potential of gingival tissues to produce the studied factors in periodontitis patients, independently of the diabetes status, and other mediators may be involved with the increased severity of periodontitis in diabetes patients.
CONCLUSION
Our data indicate that GCF levels of IL-6, IL-8 and TNF-α were not correlated with glycemic status and T2DM in periodontitis patients.
Financial support and sponsorship
This study was supported by Foundation for Research Financial Support in the State of Saão Paulo (FAPESP), São Paulo, Brazil, under protocol numbers 2011/18618-5; 10057-4; 06982-4.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Diabetes-Fact Sheet No 312. World Health Organization, Media Centre. 2013. [Last access on 2014 Aug 18]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/
- 2.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Alvarsson A, Sandgren B, Wendel C, Alvarsson M, Brismar K. A retrospective analysis of amputation rates in diabetic patients: Can lower extremity amputations be further prevented? Cardiovasc Diabetol. 2012;11:18. doi: 10.1186/1475-2840-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: A meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–53. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 6.Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: Update on associations and risks. J Clin Periodontol. 2008;35:398–409. doi: 10.1111/j.1600-051X.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 7.Chávarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: A meta-analysis. Oral Health Prev Dent. 2009;7:107–27. [PubMed] [Google Scholar]
- 8.Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ. Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res Clin Pract. 2012;98:494–500. doi: 10.1016/j.diabres.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 10.Grover HS, Luthra S. Molecular mechanisms involved in the bidirectional relationship between diabetes mellitus and periodontal disease. J Indian Soc Periodontol. 2013;17:292–301. doi: 10.4103/0972-124X.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bascones-Martínez A, González-Febles J, Sanz-Esporrín J. Diabetes and periodontal disease. Review of the literature. Am J Dent. 2014;27:63–7. [PubMed] [Google Scholar]
- 12.Iacopino AM, Cutler CW. Pathophysiological relationships between periodontitis and systemic disease: recent concepts involving serum lipids. J Periodontol. 2000;71:1375–84. doi: 10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Bhat KM. Diabetes mellitus — A modifier of periodontal disease expression. J Int Acad Periodontol. 2004;6:13–20. [PubMed] [Google Scholar]
- 14.Galli C, Passeri G, Macaluso GM. FoxOs, Wnts and oxidative stress-induced bone loss: New players in the periodontitis arena? J Periodontal Res. 2011;46:397–406. doi: 10.1111/j.1600-0765.2011.01354.x. [DOI] [PubMed] [Google Scholar]
- 15.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–48. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 16.Mohan M, Jhingran R, Bains VK, Gupta V, Madan R, Rizvi I, et al. Impact of scaling and root planing on C-reactive protein levels in gingival crevicular fluid and serum in chronic periodontitis patients with or without diabetes mellitus. J Periodontal Implant Sci. 2014;44:158–68. doi: 10.5051/jpis.2014.44.4.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanes PJ, Krishna R. Characteristics of inflammation common to both diabetes and periodontitis: Are predictive diagnosis and targeted preventive measures possible? EPMA J. 2010;1:101–16. doi: 10.1007/s13167-010-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzsimmons TR, Sanders AE, Bartold PM, Slade GD. Local and systemic biomarkers in gingival crevicular fluid increase odds of periodontitis. J Clin Periodontol. 2010;37:30–6. doi: 10.1111/j.1600-051X.2009.01506.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalra N, Pradeep AR, Priyanka N, Kumari M. Association of stem cell factor and high-sensitivity C reactive protein concentrations in crevicular fluid and serum in patients with chronic periodontitis with and without type 2 diabetes. J Oral Sci. 2013;55:57–62. doi: 10.2334/josnusd.55.57. [DOI] [PubMed] [Google Scholar]
- 20.Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–29. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 21.Sakallioglu EE, Lütfioglu M, Sakallioglu U, Diraman E, Keskiner I. Fluid dynamics of gingiva in diabetic and systemically healthy periodontitis patients. Arch Oral Biol. 2008;53:646–51. doi: 10.1016/j.archoralbio.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro FV, de Mendonça AC, Santos VR, Bastos MF, Figueiredo LC, Duarte PM. Cytokines and bone-related factors in systemically healthy patients with chronic periodontitis and patients with type 2 diabetes and chronic periodontitis. J Periodontol. 2011;82:1187–96. doi: 10.1902/jop.2011.100643. [DOI] [PubMed] [Google Scholar]
- 23.Kurtis B, Develioglu H, Taner IL, Balos K, Tekin IO. IL-6 levels in gingival crevicular fluid (GCF) from patients with non-insulin dependent diabetes mellitus (NIDDM), adult periodontitis and healthy subjects. J Oral Sci. 1999;41:163–7. doi: 10.2334/josnusd.41.163. [DOI] [PubMed] [Google Scholar]
- 24.Patel SP, Raju PA. Resistin in serum and gingival crevicular fluid as a marker of periodontal inflammation and its correlation with single-nucleotide polymorphism in human resistin gene at-420. Contemp Clin Dent. 2013;4:192–7. doi: 10.4103/0976-237X.114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engebretson SP, Vossughi F, Hey-Hadavi J, Emingil G, Grbic JT. The influence of diabetes on gingival crevicular fluid beta-glucuronidase and interleukin-8. J Clin Periodontol. 2006;33:784–90. doi: 10.1111/j.1600-051X.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 26.Javed F, Al-Askar M, Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: A literature review. J Periodontol. 2012;83:156–61. doi: 10.1902/jop.2011.110207. [DOI] [PubMed] [Google Scholar]
- 27.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–84. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duarte PM, Bezerra JP, Miranda TS, Feres M, Chambrone L, Shaddox LM. Local levels of inflammatory mediators in uncontrolled type 2 diabetic subjects with chronic periodontitis. J Clin Periodontol. 2014;41:11–8. doi: 10.1111/jcpe.12179. [DOI] [PubMed] [Google Scholar]
- 29.Navarro-Sanchez AB, Faria-Almeida R, Bascones-Martinez A. Effect of non-surgical periodontal therapy on clinical and immunological response and glycaemic control in type 2 diabetic patients with moderate periodontitis. J Clin Periodontol. 2007;34:835–43. doi: 10.1111/j.1600-051X.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 30.da Silva HA, Euzebio Alves VT, Spolidório LC, César Neto JB, Eichler RS, de Carvalho MH, et al. Expression of protease activated receptor-1 in chronic periodontitis. J Periodontol. 2014;85:1763–9. doi: 10.1902/jop.2014.140172. [DOI] [PubMed] [Google Scholar]
- 31.Kardesler L, Buduneli N, Çetinkalp S, Lappin D, Kinane DF. Gingival crevicular fluid IL-6, tPA, PAI-2, albumin levels following initial periodontal treatment in chronic periodontitis patients with or without type 2 diabetes. Inflamm Res. 2011;60:143–51. doi: 10.1007/s00011-010-0248-7. [DOI] [PubMed] [Google Scholar]
- 32.Pradeep AR, Agarwal E, Bajaj P, Rao NS. 4-Hydroxy-2-nonenal, an oxidative stress marker in crevicular fluid and serum in type 2 diabetes with chronic periodontitis. Contemp Clin Dent. 2013;4:281–5. doi: 10.4103/0976-237X.118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J Clin Endocrinol Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 34.Fell RA, Zee KY, Arora M. The correlation of serum and gingival crevicular fluid cytokines in obese subjects. J Int Acad Periodontol. 2013;15:20–8. [PubMed] [Google Scholar]
- 35.Zhou M, Meng HX, Zhao YB, Chen ZB. Changes of four proinflammatory proteins in whole saliva during experimental gingivitis. Chin J Dent Res. 2012;15:121–7. [PubMed] [Google Scholar]
- 36.Trombelli L, Scapoli C, Carrieri A, Giovannini G, Calura G, Farina R. Interleukin-1 beta levels in gingival crevicular fluid and serum under naturally occurring and experimentally induced gingivitis. J Clin Periodontol. 2010;37:697–704. doi: 10.1111/j.1600-051X.2010.01573.x. [DOI] [PubMed] [Google Scholar]
- 37.Boronat-Catalá M, Catalá-Pizarro M, Bagán Sebastián JV. Salivary and crevicular fluid interleukins in gingivitis. J Clin Exp Dent. 2014;6:e175–9. doi: 10.4317/jced.51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji S, Choi Y. Innate immune response to oral bacteria and the immune evasive characteristics of periodontal pathogens. J Periodontal Implant Sci. 2013;43:3–11. doi: 10.5051/jpis.2013.43.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998;66:1660–5. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang GT, Kim D, Lee JK, Kuramitsu HK, Haake SK. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: Multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun. 2001;69:1364–72. doi: 10.1128/IAI.69.3.1364-1372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos VR, Ribeiro FV, Lima JA, Napimoga MH, Bastos MF, Duarte PM. Cytokine levels in sites of chronic periodontitis of poorly controlled and well-controlled type 2 diabetic subjects. J Clin Periodontol. 2010;37:1049–58. doi: 10.1111/j.1600-051X.2010.01624.x. [DOI] [PubMed] [Google Scholar]


