Abstract
Background:
Use of smokeless tobacco in the form of moist snuff placed in the oral cavity is popular in rural India. The aim of the present cross-sectional study was to determine the effect of snuff on periodontitis by assessing interleukin (IL)-1 β and IL-8 levels in gingival crevicular fluid.
Materials and Methods:
A total of 60 subjects were selected for this study. 40 subjects presented with periodontitis, which included 20 snuff users (SP) and 20 nonsnuff users (NS). 20 periodontally healthy patients formed the controls (healthy control: HC). The clinical parameters recorded were gingival index (GI), plaque index, calculus index, bleeding on probing (BOP), probing depth (PD), recession (RC), and clinical attachment level (CAL). The IL-1 β and IL-8 levels were assessed through enzyme-linked immunosorbent assay (Quantikine®). Analysis of variance (ANOVA), post-hoc Tukey's, Kruskal-Walli's ANOVA and Mann-Whitney test was used for comparison among groups and P > 0.05 was considered statistically significant.
Results:
No significant difference was seen in levels of IL-1 β and IL-8 between SP and NS groups (P = 0.16, 0.97). However, both the periodontitis groups (SP and NS) had increased IL-β levels when compared to HC group (P = 0.01, 0.001). The snuff users showed significant increase in GI, BOP, RC, and CAL when compared with NS (P = 0.002, 0.001, 0.012, 0.002) whereas NS group had significant increase in PD (P = 0.003).
Conclusion:
Within the limitations of this study, use of snuff does not affect the host inflammatory response associated with periodontitis and leads to RC and increased CAL due to local irritant effect.
Keywords: Interleukin-8, interleukin-1 beta, periodontitis, smokeless tobacco, snuff
INTRODUCTION
India has the highest users of tobacco in both smoking and smokeless forms.[1,2] Smokeless tobacco (ST) use is more prevalent than smoking especially in rural parts of the country.[3] Global Adult Tobacco Survey (GATS) showed states in India such as Jharkhand, Bihar, and Uttar Pradesh having a very high prevalence of ST users.[4] ST can be used in two forms, chewable and inhalable. The common forms of chewing tobacco in India include moist snuff (powdered tobacco leaves), gutka (mixture of powdered tobacco, betul nut, and slaked lime), zarda (mixture of boiled tobacco leaves and slaked lime), mawa (combination on areca nut, tobacco and slaked lime), khaini (tobacco with slaked lime), and pan (areca nut and tobacco wrapped in betul leaf).[3,5]
Snuff is made from ground or pulverized tobacco leaves, which can either be used in dry or moist form. The dry snuff is a powdered form and is used by either sniffing or inhaling through the nose while the moist form is used by placing the tobacco called as pinch, dip, lipper or quid between the lower lip or cheek and gingiva, allowing absorption of nicotine through the oral tissues.[6,7] According to GATS 2009-2010, “creamy” snuff is most commonly used in the states of Chhattisgarh (28.3%), Maharashtra (8%), and Jharkhand (7.9%).[4] This habit is more popular among females (9.2%) than males (6.5%) and youths are more vulnerable to this habit. The creamy or moist snuff is routinely used by people of lower socioeconomic status and commonly known to local people as gul or gudaku available in tin cans or pouches.[4] Moist snuff contains high levels nitrosamine and nicotine and can lead to harmful health effects such as increased risk for hypertension, increased heart rate, type II diabetes, and pancreatic cancer[8,9] and possible association with myocardial infarction.[10] The use of moist snuff is detrimental for oral and periodontal health, causing oral cancer, mucosal lesions, gingival inflammation, gingival recession (RC), and attachment loss at sites adjacent to snuff placement and eventually tooth loss.[3,6,7,11]
Periodontitis is an infectious disease characterized by inflammation and loss of supporting tissues around teeth resulting in pocket formation, RC and loss of alveolar bone.[12] The two prominent inflammatory mediator associated with progressive periodontitis are an interleukin-1 beta (IL-1 β) and 8.[12] Nicotine is one of the active ingredients of ST which can directly modify the production of cytokines and inflammatory mediators by various cell types found in periodontal tissues.[13] Previous studies have shown an elevation in IL-1 β and IL-8 in nicotine stimulated gingival keratinocytes.[13,14] The rise in pro-inflammatory mediators could be an explanation for increased periodontal destruction among snuff consumers.
Most studies do not distinguish between the different types of ST used.[3,11,15,16,17] Each product should be studied separately due to their difference in content and use. There are few documented evidence on the effect of snuff on periodontal disease.[6,7,18,19,20,21] Some studies have shown snuff use being associated with severe forms of periodontal disease[18,19] while others failed to show such association except for gingival RC at the site of placement.[6,7,20,21] The pathogenesis of periodontitis related to moist snuff use is not well understood, and we hypothesized that snuff consumption would have an effect on the local immune response, causing destruction on periodontal tissues. We selected a population having a habit of moist snuff use and periodontitis as a common finding. Hence, the aim of the present study was to investigate the effect of moist snuff on the periodontal disease by assessing the local cytokine levels.
MATERIALS AND METHODS
The present cross-sectional was conducted in Department of Periodontology, Vananchal Dental College and Hospital, Jharkhand from November 2013 to April 2014. Informed consent was obtained from all the participants before enrolment in the study as required in the approval by the Institutional Ethics Committee (protocol number: VSET/05/SN). All the participants were selected from the rural population of Garhwa district, India.
Sample size calculation
To detect a minimum difference of 100 units at a standard deviation (SD) of 90 based on the result from a similar study by Jacob et al.,[22] the calculated sample size was 15 per group at an alpha error of 0.05 and power at 80%.
Study participants
Sixty subjects from the rural population of Garhwa, India were recruited for this study. The study included three groups, consisting of 40 patients with chronic periodontitis and 20 periodontally healthy controls (HCs). Among 40 periodontitis patients, 20 were snuff users (SP) and 20 were nonsnuff users (NS). Definition of moderate periodontitis proposed by CDC Periodontal Disease Surveillance Workgroup and American Academy of Periodontology (AAP) was used in our study.[23] Periodontitis was diagnosed when ≥2 interproximal sites with clinical attachment level (CAL) of ≥4 mm (not on same tooth) or ≥2 interproximal sites with probing depth (PD) of ≥5 mm (not on same tooth) was present.[23] Snuff users were selected if they were chronic users; average use of at least three cans of moist snuff per week for the past 2 years.[14] The moist snuff was available in tin cans weighing about 1.2 oz (34.02 gms). Nonsnuff users had no history of use of any ST product.
Exclusion criteria were:
Current or former smokers,
Current or former users of other forms of ST other than snuff,
Any systemic or general medical condition,
Undergone periodontal therapy in the last 3 months,
Under medication for antibiotics, steroids or nonsteroidal anti-inflammatory drugs.
All the participating subjects were required to answer a questionnaire concerning their use of snuff. Cumulative lifetime snuff use was assessed by can years, that is, the average number of cans consumed in a week multiplied by years of use.
Clinical examination
The clinical examination included assessment of plaque index (PI),[24] gingival index (GI),[24] calculus index (CI),[25] bleeding on probing (BOP), RC, CAL, and PD. All the parameters were measured by a single calibrated periodontist (V.P.) and recorded by another dentist (S.A.S.) and both were blinded to the subject group and interview recordings. Full mouth examination was carried out for PI, GI, CI, and BOP. Plaque Index (PI) was calculated from a score ranging from 0 (tooth surface clean) to 3 (abundant plaque). GI was calculated from a score ranging from 0 (gingival unit healthy) to 3 (ulcerating or spontaneous bleeding). CI simplified was used to determine the amount of calculus with score 0 representing the absence of calculus and score 3 indicating severe supra and subgingival calculus. BOP was recorded as present for sites which had BOP after 15 s and were given a score of 1 or 0 for the absence of bleeding. The periodontal parameters, PD, RC, and CAL were recorded at six sites of tooth (mesiobuccal, mid-buccal, distobuccal, distolingual/palatal, mid-lingual/palatal, and mesiolingual/palatal) from a selected quadrant in each group. In snuff users, the quadrant where the snuff dip was placed was selected, usually the mandibular quadrant. In nonsnuff users, the site affected most with periodontitis was selected and any quadrant from HC group. In case of healthy control (HC), the probing depth was measured till base of sulcus and in case of periodontitis it was measured till base of periodontal pocket. RC was measured as the distance from the cementoenamel junction to the gingival margin. The CAL was calculated from the PD and RC measurements. All the measurement were taken by the straight periodontal probe (UNC-15, Hu Friedy®, Chicago, IL, USA) and measured to the nearest mm.
Examiner calibration
All the measurements were performed by a single periodontist (V.P.) who was blinded to the patient's group and interview recording. A calibration process was carried out for PI, GI, CI, PD, RC, and CAL to eliminate the intra-examiner error. Before the start of the study, 20 sites were examined twice from 10 patients, once at baseline and after 24 h. Calibration would be accepted if both the measurements were similar to 1 mm at the 95% level (correlation coefficients between duplicate measurements; r = 0.95).
Collection of gingival crevicular fluid
A total of 45 gingival crevicular fluid (GCF) samples, 15 from each group were collected for analysis. GCF collection was done at least 1-day and not more than 3 days after a clinical examination, which included probing to avoid its effect on the volume and content of the exudate. In the selected quadrant supragingival plaque was removed without touching the marginal gingiva and was air-dried and isolated with cotton rolls. A white color coded 1-5 μL calibrated volumetric microcapillary pipette (Sigma-Aldrich Chemicals Company Limited. St. Louis, MO, USA) was used to obtain GCF sample. From the selected quadrant, for each sample GCF was collected from five nonadjacent sites having the deepest PD. The micropipettes were kept at five sites for not more than 5 min at each site or until 3 μL of GCF was collected. A pooled volume for each sample was calculated. The sites from which no GCF could be obtained or at which the microcapillary pipettes were contaminated with blood or saliva were discarded and excluded from the study. Immediately after collection the samples were transferred into a plastic vial and frozen at −70°C until the time of assay.
Assessment of interleukin-1 β and interleukin-8
IL-1 β and IL-8 levels were determined using enzyme-linked immunoassays (ELISA). All the GCF sampling and ELISA assays (Quantikine®, RandD Systems, Minneapolis, MN) were performed by one examiner (P.M.) who was blinded to the clinical result. The concentrations of ILs were reported in picogram per microliter (pg/μL) of sample. The samples were diluted and the procedure was carried out according to the manufacturer instruction. Each sample in both the assays was performed in duplicate (two separate culture wells for each sample).
Statistical analysis
SPSS software (SPSS Version 17.0, SPSS Inc., Chicago, IL, USA) was used for data entry and analysis. Mean and SD were calculated for all the variables. Parametric and nonparametric tests were used based on the normality and distribution of the data. Analysis of variance (ANOVA) was done for comparison among all the three groups followed by post-hoc Tukey's test for group-wise comparison. Kruskal-Walli's ANOVA test was used for comparison among three groups and Mann-Whitney test was used for intergroup comparison of IL-1 β and IL-8. Pearson's correlation coefficient was calculated to evaluate the correlation between duration of snuff placement and RC, CAL, PD, IL-1 β, and IL-8. For all the comparisons P < 0.05 was accepted as statistically significant. The power of the present study was 84%.
RESULTS
Study population
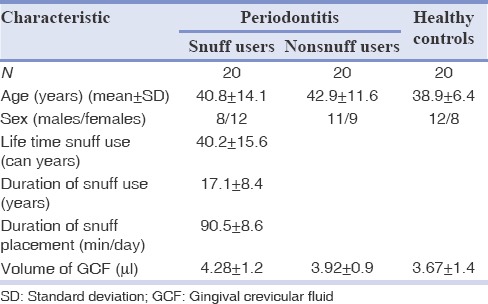
Table 1 describes the characteristic of the study population. A total of 60 subjects were examined for this study with 20 subjects in each group. The mean ages were 40.8 ± 14.1, 42.9 ± 11.6, and 38.9 ± 6.4 among snuff user, nonsnuff user, and HCs. There were more number of females than males among snuff users (12:8) compared to nonsnuff users (9:11) and HCs (12:8). The average consumption of snuff was 17.1 ± 8.4 years and the average duration of snuff placement was 90.5 ± 8.6 min/day. The average can-year was calculated to 40.2 ± 15.6.
Table 1.
Characteristics of study population
Clinical parameters
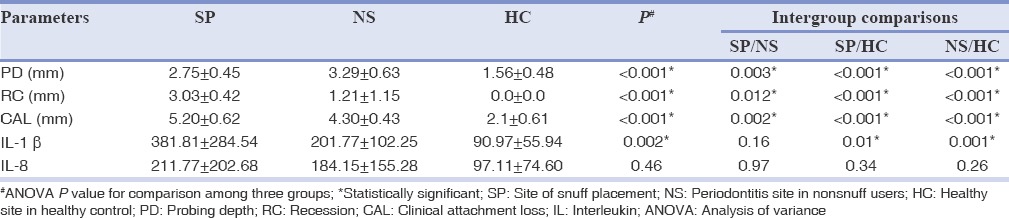
Table 2 shows the comparison of clinical parameter taken full mouth among the three groups and Table 3 describes the periodontal parameters such as PD, RC, and CAL of the selected quadrant for each group. All the parameters such as PI, GI, CI, and BOP showed significant difference when compared group wise (<0.001) and this significance was present only when HCs were compared with snuff users and nonsnuff user. This difference became nonsignificant when snuff users were compared with nonsnuff users, except for GI (P = 0.002) and BOP (P = 0.001) which showed a higher score for snuff users. When HCs were compared with snuff users and nonsnuff users’ significant difference was present for PD, RC, and CAL. Increased RC and CAL was seen in snuff placement site (RC = 3.03 ± 0.42, CAL = 5.20 ± 0.62) which reached statistical significance (P = 0.012, 0.002) when compared with periodontitis site among nonsnuff users (RC = 1.21 ± 1.15, CAL = 4.30 ± 0.43). PD was significantly higher for nonsnuff users (P = 0.003) than snuff users.
Table 2.
Comparison of clinical parameters among all the three groups
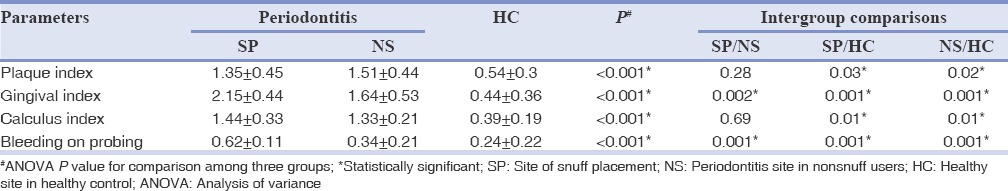
Table 3.
Comparison of periodontal parameters and cytokine levels (pg/μL) among all the three groups
Cytokine assessment
The mean volume of GCF was 4.28 ± 1.2 μl, 3.92 ± 0.9 μl, and 3.67 ± 1.4 μl in all the three groups [Table 1]. The intergroup and intragroup comparison of IL-1 β and IL-8 are described in Table 3. When group wises comparison was done significant difference was seen for IL-1 β (P = 0.002). Among snuff users (IL-1 β =381.81 ± 284.54, IL-8 = 211.77 ± 202.68) and nonsnuff users (IL-1 β = 201.77 ± 102.25, IL-8 = 184.15 ± 155.28) no significant difference was seen for both the cytokine (P = 0.16, 0.97). When HCs (90.97 ± 55.94, 97.11 ± 74.60) were compared with snuff users and nonsnuff user significant difference was present only for IL-1 β (P = 0.01, 0.001). A correlation analysis revealed no significant correlation between the clinical parameters and cytokine levels and snuff usage, and the results are not shown.
DISCUSSION
The present study tried to assess the effect of the use of snuff on the pathogenesis of periodontitis by evaluating the levels of two prominent inflammatory cytokine in GCF. We selected the participants from a population in which the use of ST was common, unlike other studies which were carried among young adolescent, sportsmen especially baseball players who had short exposure to ST use.[6,20,21] Most studies failed to differentiate the type of ST[11,26] used and have been conducted on population of high socioeconomic or either in adolescent population with brief exposure to ST where the prevalence of ST use is low.[11,20,21] The use of snuff is a culturally ingrained habit and a part of daily routine and lifestyle among the rural population of Garhwa. The women believe that it is a less harmful habit than the smoking form and is popular among them. In our study, we found snuff placement was more common among females than males. The variety of toxic components present in ST is nicotine, carcinogenic nitrosamines, toxic metals such as lead, mercury, arsenic, cadmium, alkaloids, and polycyclic aromatic hydrocarbons.[6,7]
In this study, we used the CDC-AAP classification for moderate periodontitis.[23] Snuff use leads to RC and hence increased attachment loss, which could have occurred even in the absence of periodontitis. Hence, it was imperative for us to use a definition, which could identify periodontitis more specifically.[23,27] The selected definition had the feature of identifying patients with attachment loss due to periodontitis only. The definition relies on the PD and attachment loss in interproximal areas, as these sites are affected only by periodontitis.[22,23,27] The use of snuff shows increased attachment loss in the form of gingival RC than periodontal pocketing. Snuff users in our study had periodontitis based on the selection criteria adopted. Hence, it can be deduced that consumption of snuff has a detrimental effect on the gingival wall of periodontal pocket leading to its destruction and hence causes gingival RC. Ground tobacco can cause mechanical trauma by its physical texture and the chemical content causes damage of the soft tissues.[17,22] In addition, the presence of thin alveolar bone especially the lower anteriors, which is a common site for snuff placement, has less resistance to trauma further causing destruction of gingival tissues.[14] This could explain the effect of increased gingival RC and hence attachment loss in the snuff group compared to other groups. This is consistent with other reports on snuff use.[6,7,20,21] Jacob et al.[22] similarly found that ST in the form of gutka leads to gingival RC due to the physical effect than any biological action. A study in south India conducted by Nagarajapa and Prasad[28] reported a higher incidence of periodontal disease, although not statistically significant. Fisher et al.[26] reported that ST users are twice more likely to have the severe active disease than adults who had never used ST.
Plaque levels were found higher in periodontitis patients (SP and NS) than the healthy subjects (HC) which contributes to the periodontal destruction. Plaque and calculus scores were similar in the two periodontitis groups (SP and NS) and indicates that the use of snuff does not lead to an increase in plaque and hence calculus too. Similar findings were seen in studies by Jacob et al.,[22] Singh et al.[17] and Montén et al.[6] among ST users. However, David et al.[29] and Rolandsson et al.[30] found less plaque and calculus formation among ST users. Even though the plaque and calculus levels did not vary, there was higher gingival inflammation and BOP among snuff users. This is due to the irritational properties of snuff either chemical or mechanical which may contribute to the increased inflammation and bleeding in placement sites. Several authors have reflected similar results.[14,17,31,32] Several studies[6,21,20,18] failed to find any relationship between snuff use and gingivitis as nicotine might have a vasoconstrictive effect causing a reduction in blood flow.[33]
The GCF is a transudate or inflammatory exudate that is found in the gingival sulcus. Secretion of GCF increases in gingival inflammation and periodontitis.[12,14] Our study also showed that increased the volume of GCF per unit time was collected in both the periodontitis groups than the healthy group. In our study, collection of GCF was done by using micropipettes. Collection by micropipettes is noninvasive, relatively simple, and easy to elute its constituents. Microcapillary pipettes were preferred over paper strips as the volume collected by micropipettes can be easily determined and it allows analysis of an undiluted sample of native GCF.[12] A similar method was adopted by Faizuddin et al.[34] and Jacob et al.[12,22]
The two markers chosen by our study to assess periodontal inflammation were IL-1 β and IL-8. In periodontitis patients both IL-1 β and IL-8 have been reported in GCF and periodontal tissues. The amount of these ILs has been closely associated with periodontal disease severity.[12,22,34] The concentration of these pro-inflammatory cytokines gives us a measure of local inflammation.[12,13,22] IL-1 β acts on endothelial cells to promote adhesion and migration of leukocytes into inflamed tissue sites inducing periodontal inflammation. Over production of IL-1 causes periodontal tissue destruction and blocking by receptor antagonist promotes healing and regeneration.[35] IL-8 is known as leukocyte chemotactic cytokine and plays a multifunctional role in the pathogenesis of periodontal disease. Il-8 regulates neutrophil chemotaxis, angiogenesis, and epithelial proliferation by specific receptors.[35]
The physical placement of raw or processed tobacco leads to the release of nicotine into the oral mucosa.[13,14] Nicotine is the primary addictive substance in snuff and has shown to affect the levels of cytokine and inflammatory mediators.[36,37] Johnson et al.[13,36] found nicotine increasing levels of PGE2, IL-1 β, IL-8 in gingival keratinocyte cultures and Payne et al.[38] have shown the deleterious effect on fibroblasts. This local effect can affect the inflammatory profile of the gingival and periodontal tissues supplementing the periodontal destruction. However, our results revealed that there are no enhanced IL-1 β and IL-8 levels from snuff use. Both the periodontitis groups showed similar levels of the tested cytokines proving that the use of snuff does not affect the inflammatory pathway of periodontitis. Similar observations were seen in the study on gutka by Jacob et al.,[22] Poore et al.,[14] and Bernzweig et al.[37] on ST users. In vitro study by Payne et al.[37] on ST and cigarette smoke extract showed similar results. Few studies on ST have shown an increase in IL levels.[13,36,39] Nicotine, the common ingredient, failed to show any effect on IL-1 β secretion in blood monocytes, lymphocytes or gingival mono-nuclear cells.[14,37,38] One of the reasons could be the inclusion of patient with periodontitis, which could have caused maximal stimulation of inflammatory cells residing in gingival tissues, and this would have prevented further increase even after the use of snuff.[37]
Increased levels of IL-1 β was observed in both the periodontitis groups (SP, NS) when compared to healthy group of our study. Increased secretion of IL-1 β occurs in periodontitis.[3,37] However, we found that the levels of IL-8 in periodontitis groups were similar to the healthy group as seen in other studies.[40] Though there was a similar increase in periodontitis groups over the healthy, it did not reach any statistical significance. Arimilli et al.[41] however, found nicotine to suppress the activity of mononuclear immune cells leading to decreased IL-8 levels. IL-8 has been detected in the healthy gingiva, as it plays a role in the constant migration of neutrophils through the gingival tissues and establishes a balance between continuous bacterial challenge and host defense.[35]
One of the limitations of our study was the cross-sectional nature, which precludes us in making any temporal association between periodontal inflammation and snuff use. As well, there was more heterogeneity in the dose of snuff use in our study patients, which could have affected our results.
The authors would like to conclude, that snuff (pulverized tobacco) use leads to periodontal attachment loss by its mechanical and chemical effects. There is no effect of snuff on the inflammatory pathway in the pathogenesis of periodontitis. The findings are based on only two inflammatory mediators and periodontal disease involves a complex network of inflammatory mediators, where the immune system also plays an important role. Therefore, further studies involving other mediators should be carried out if there is consistency to our present findings. This will elucidate the role of snuff in periodontitis.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Rooban T, Joshua E, Rao UK, Ranganathan K. Prevalence and correlates of tobacco use among urban adult men in India: A comparison of slum dwellers vs non-slum dwellers. Indian J Dent Res. 2012;23:31–8. doi: 10.4103/0970-9290.99034. [DOI] [PubMed] [Google Scholar]
- 2.Joshi U, Modi B, Yadav S. A study on prevalence of chewing form of tobacco and existing quitting patterns in urban population of Jamnagar, Gujarat. Indian J Community Med. 2010;35:105–8. doi: 10.4103/0970-0218.62560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand PS, Kamath KP, Shekar BR, Anil S. Relationship of smoking and smokeless tobacco use to tooth loss in a central Indian population. Oral Health Prev Dent. 2012;10:243–52. [PubMed] [Google Scholar]
- 4.Global Adult Tobacco Survey, India 2009-2010. Ministry of Health & Family Welfare Government of India; c2010. [Last accessed on 2014 Aug 15]. Available from: http://www.mohfw.nic.in/WriteReadData/l892s/1455618937GATS%20India.pdf .
- 5.Rani M, Bonu S, Jha P, Nguyen SN, Jamjoum L. Tobacco use in India: Prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control. 2003;12:e4. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montén U, Wennström JL, Ramberg P. Periodontal conditions in male adolescents using smokeless tobacco (moist snuff) J Clin Periodontol. 2006;33:863–8. doi: 10.1111/j.1600-051X.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergström J, Keilani H, Lundholm C, Rådestad U. Smokeless tobacco (snuff) use and periodontal bone loss. J Clin Periodontol. 2006;33:549–54. doi: 10.1111/j.1600-051X.2006.00945.x. [DOI] [PubMed] [Google Scholar]
- 8.Persson PG, Carlsson S, Svanström L, Ostenson CG, Efendic S, Grill V. Cigarette smoking, oral moist snuff use and glucose intolerance. J Intern Med. 2000;248:103–10. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Ye W, Zendehdel K, Adami J, Adami HO, Boffetta P, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: A retrospective cohort study. Lancet. 2007;369:2015–20. doi: 10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- 10.Hergens MP, Alfredsson L, Bolinder G, Lambe M, Pershagen G, Ye W. Long-term use of Swedish moist snuff and the risk of myocardial infarction amongst men. J Intern Med. 2007;262:351–9. doi: 10.1111/j.1365-2796.2007.01816.x. [DOI] [PubMed] [Google Scholar]
- 11.Chu YH, Tatakis DN, Wee AG. Smokeless tobacco use and periodontal health in a rural male population. J Periodontol. 2010;81:848–54. doi: 10.1902/jop.2010.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulikkotil SJ, Nath S. Effect on interleukin-1ß and interleukin-8 levels following use of fibrin sealant for periodontal surgery. Aust Dent J. 2014;59:156–64. doi: 10.1111/adj.12178. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GK, Guthmiller JM, Joly S, Organ CC, Dawson DV. Interleukin-1 and interleukin-8 in nicotine- and lipopolysaccharide-exposed gingival keratinocyte cultures. J Periodontal Res. 2010;45:583–8. doi: 10.1111/j.1600-0765.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 14.Poore TK, Johnson GK, Reinhardt RA, Organ CC. The effects of smokeless tobacco on clinical parameters of inflammation and gingival crevicular fluid prostaglandin E2, interleukin-1 alpha, and interleukin-1 beta. J Periodontol. 1995;66:177–83. doi: 10.1902/jop.1995.66.3.177. [DOI] [PubMed] [Google Scholar]
- 15.Anand PS, Kamath KP, Bansal A, Dwivedi S, Anil S. Comparison of periodontal destruction patterns among patients with and without the habit of smokeless tobacco use — A retrospective study. J Periodontal Res. 2013;48:623–31. doi: 10.1111/jre.12048. [DOI] [PubMed] [Google Scholar]
- 16.Wiener RC. Association of smokeless tobacco use and smoking in adolescents in the United States: An analysis of data from the Youth Risk Behavior Surveillance System Survey, 2011. J Am Dent Assoc. 2013;144:930–8. doi: 10.14219/jada.archive.2013.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh GP, Rizvi I, Gupta V, Bains VK. Influence of smokeless tobacco on periodontal health status in local population of north India: A cross-sectional study. Dent Res J (Isfahan) 2011;8:211–20. doi: 10.4103/1735-3327.86045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugoson A, Rolandsson M. Periodontal disease in relation to smoking and the use of Swedish snus: Epidemiological studies covering 20 years (1983-2003) J Clin Periodontol. 2011;38:809–16. doi: 10.1111/j.1600-051X.2011.01749.x. [DOI] [PubMed] [Google Scholar]
- 19.Wickholm S, Söder PO, Galanti MR, Söder B, Klinge B. Periodontal disease in a group of Swedish adult snuff and cigarette users. Acta Odontol Scand. 2004;62:333–8. doi: 10.1080/00016350410001801. [DOI] [PubMed] [Google Scholar]
- 20.Ernster VL, Grady DG, Greene JC, Walsh M, Robertson P, Daniels TE, et al. Smokeless tobacco use and health effects among baseball players. JAMA. 1990;264:218–24. [PubMed] [Google Scholar]
- 21.Robertson PB, Walsh M, Greene J, Ernster V, Grady D, Hauck W. Periodontal effects associated with the use of smokeless tobacco. J Periodontol. 1990;61:438–43. doi: 10.1902/jop.1990.61.7.438. [DOI] [PubMed] [Google Scholar]
- 22.Jacob PS, Nath S, Patel RP. Evaluation of interleukin-1ß and 8 in gutka chewers with periodontitis among a rural Indian population. J Periodontal Implant Sci. 2014;44:126–33. doi: 10.5051/jpis.2014.44.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 Suppl):1387–99. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 24.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38 Suppl:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 25.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 26.Fisher MA, Taylor GW, Tilashalski KR. Smokeless tobacco and severe active periodontal disease, NHANES III. J Dent Res. 2005;84:705–10. doi: 10.1177/154405910508400804. [DOI] [PubMed] [Google Scholar]
- 27.Costa FO, Guimarães AN, Cota LO, Pataro AL, Segundo TK, Cortelli SC, et al. Impact of different periodontitis case definitions on periodontal research. J Oral Sci. 2009;51:199–206. doi: 10.2334/josnusd.51.199. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajappa S, Prasad KV. Oral microbiota, dental caries and periodontal status in smokeless tobacco chewers in Karnataka, India: A case-control study. Oral Health Prev Dent. 2010;8:211–9. [PubMed] [Google Scholar]
- 29.David J, Yee R, Lama D. The periodontal health of adult Nepalese. Oral Health Prev Dent. 2011;9:67–81. [PubMed] [Google Scholar]
- 30.Rolandsson M, Hellqvist L, Lindqvist L, Hugoson A. Effects of snuff on the oral health status of adolescent males: A comparative study. Oral Health Prev Dent. 2005;3:77–85. [PubMed] [Google Scholar]
- 31.Offenbacher S, Weathers DR. Effects of smokeless tobacco on the periodontal, mucosal and caries status of adolescent males. J Oral Pathol. 1985;14:169–81. doi: 10.1111/j.1600-0714.1985.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 32.Modéer T, Lavstedt S, Ahlund C. Relation between tobacco consumption and oral health in Swedish schoolchildren. Acta Odontol Scand. 1980;38:223–7. doi: 10.3109/00016358009003493. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Ono K, Honda E, Yokota M, Inenaga K. Central nicotinic stimulation reduces vascular conductance in the gingiva in anesthetized rats. J Periodontal Res. 2005;40:67–72. doi: 10.1111/j.1600-0765.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 34.Faizuddin M, Bharathi SH, Rohini NV. Estimation of interleukin-1beta levels in the gingival crevicular fluid in health and in inflammatory periodontal disease. J Periodontal Res. 2003;38:111–4. doi: 10.1034/j.1600-0765.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995;66:852–9. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- 36.Johnson GK, Poore TK, Payne JB, Organ CC. Effect of smokeless tobacco extract on human gingival keratinocyte levels of prostaglandin E2 and interleukin-1. J Periodontol. 1996;67:116–24. doi: 10.1902/jop.1996.67.2.116. [DOI] [PubMed] [Google Scholar]
- 37.Bernzweig E, Payne JB, Reinhardt RA, Dyer JK, Patil KD. Nicotine and smokeless tobacco effects on gingival and peripheral blood mononuclear cells. J Clin Periodontol. 1998;25:246–52. doi: 10.1111/j.1600-051x.1998.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 38.Payne JB, Johnson GK, Reinhardt RA, Schmid M. Histological alterations following short-term smokeless tobacco exposure in humans. J Periodontal Res. 1998;33:274–9. doi: 10.1111/j.1600-0765.1998.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 39.Johnson GK, Poore TK, Squier CA, Wertz PW, Reinhardt RA, Vincent SD. Prostaglandin E2 and interleukin-1 levels in smokeless tobacco-induced oral mucosal lesions. J Periodontal Res. 1994;29:430–8. doi: 10.1111/j.1600-0765.1994.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 40.Bascones A, Noronha S, Gómez M, Mota P, Gónzalez Moles MA, Villarroel Dorrego M. Tissue destruction in periodontitis: Bacteria or cytokines fault? Quintessence Int. 2005;36:299–306. [PubMed] [Google Scholar]
- 41.Arimilli S, Damratoski BE, Prasad GL. Combustible and non-combustible tobacco product preparations differentially regulate human peripheral blood mononuclear cell functions. Toxicol In Vitro. 2013;27:1992–2004. doi: 10.1016/j.tiv.2013.06.015. [DOI] [PubMed] [Google Scholar]


