Short abstract
Analysis of secreted proteins indicate that they may be undergoing accelerated evolution, either because of relaxed functional constraints, or in response to stronger selective pressure from host immunity.
Abstract
Background
Parasitism is a highly successful mode of life and one that requires suites of gene adaptations to permit survival within a potentially hostile host. Among such adaptations is the secretion of proteins capable of modifying or manipulating the host environment. Nippostrongylus brasiliensis is a well-studied model nematode parasite of rodents, which secretes products known to modulate host immunity.
Results
Taking a genomic approach to characterize potential secreted products, we analyzed expressed sequence tag (EST) sequences for putative amino-terminal secretory signals. We sequenced ESTs from a cDNA library constructed by oligo-capping to select full-length cDNAs, as well as from conventional cDNA libraries. SignalP analysis was applied to predicted open reading frames, to identify potential signal peptides and anchors. Among 1,234 ESTs, 197 (~16%) contain predicted 5' signal sequences, with 176 classified as conventional signal peptides and 21 as signal anchors. ESTs cluster into 742 distinct genes, of which 135 (18%) bear predicted signal-sequence coding regions. Comparisons of clusters with homologs from Caenorhabditis elegans and more distantly related organisms reveal that the majority (65% at P < e-10) of signal peptide-bearing sequences from N. brasiliensis show no similarity to previously reported genes, and less than 10% align to conserved genes recorded outside the phylum Nematoda. Of all novel sequences identified, 32% contained predicted signal peptides, whereas this was the case for only 3.4% of conserved genes with sequence homologies beyond the Nematoda.
Conclusions
These results indicate that secreted proteins may be undergoing accelerated evolution, either because of relaxed functional constraints, or in response to stronger selective pressure from host immunity.
Background
A central tenet of parasitology is that parasites must secrete biologically active mediators that modify or customize their niche within the host in order to survive immune attack. Such secretions have long been the focus of biochemical and immunological analyses [1-4]. With larger-scale genomic approaches now possible, a screen can be designed in which the characteristic signal sequences, necessary for proteins to exit the eukaryotic cell via the secretory pathway, can be identified by bioinformatic methods [5-9]. We describe here an analysis of this nature, applied to a widely used model system, Nippostrongylus brasiliensis, the gastrointestinal nematode of rats [10-12].
N. brasiliensis biology encapsulates many key aspects of parasite infection and immunology. It is a multicellular metazoan belonging to the phylum Nematoda, which together with the platyhelminth groups (Cestoda and Trematoda) are collectively known as helminths. Helminth infections are typically accompanied by a polarized type-2 (Th2) immune response, characterized by IgE antibody production, eosinophilia and mastocytosis [13-15]. N. brasilensis drives extremely strong Th2 responses [16], and this bias can be reproduced with secreted proteins collected from parasites in vitro [17]. More than 100 secreted proteins have been found by two-dimensional SDS-PAGE analysis (Y.H. and R.M.M., unpublished work), and among those experimentally verified are acetylcholinesterases [18-20], cysteine proteases [21,22], and a hydrolase that degrades an important host inflammatory mediator, platelet activating factor [23,24].
The molecular biological analysis of N. brasiliensis genes and gene products is at a very early stage. Secreted and intracellular globins have been characterized [25], and genes for both secretory [26,27] and neuronal [28] acetylcholinesterases cloned. A recombinant cystatin (cysteine protease inhibitor) has been shown functionally to inhibit host antigen-processing pathways [29]. Structural genes for both tubulin [30] and a keratin-like protein [31] have been described, and an α-crystallin-like small heat-shock protein (Hsp20) has been reported [32]. However, these studies on individual genes have yet to be complemented by higher-throughput molecular analyses. The potential of N. brasiliensis as an experimental system for functional genomics has been greatly enhanced by the demonstration of successful RNAi knockdown in this species [33].
The genomes of parasitic nematode species are between 60 and 250 megabases (Mb) in size [34], and there are more than 20 species of medical, veterinary and scientific importance [35]. Over the past decade, the most tractable way of applying genomics to this group of organisms has been by expressed sequence tag (EST) projects [36]. Large-scale EST sequencing of the human filarial parasite Brugia malayi [37,38] has been followed by similar studies in the sheep intestinal worm Haemonchus contortus [39], human hookworms [40], the river-blindness parasite Onchocerca volvulus [41], and important plant-parasitic species such as Meloidogyne incognita [42]. Smaller projects have added Litomosoides sigmodontis [43], Toxocara canis [44] and many other related species to the available database of parasitic nematode sequences [36]. In designing a study on N. brasiliensis, we wished to focus on the potential for secreted proteins that may interact with the host immune system. We therefore conducted an EST project that included a cDNA library specifically enriched for full-length inserts [45], allowing analysis of amino-terminal signal peptides to be carried out.
The evolutionary history of secreted immunomodulators is likely to be that of recent adaptation from ancestral genes which fulfilled other functions in free-living ancestors. Comparative studies on nematodes can take advantage of full-genome information available for the free-living species Caenorhabditis elegans [46] and C. briggsae [47], which are quite closely related to N. brasiliensis [48]. If rapid evolution of secreted gene products was required for efficient parasitism, this may be evident in greater diversity among signal peptide-bearing sequences than among genes coding for non-secreted proteins. We report here our results that support this hypothesis.
Results and discussion
A high proportion of N. brasiliensis ESTs encode proteins with predicted signal sequences
A total of 1,234 ESTs were collected from adult N. brasiliensis cDNA libraries constructed either by conventional means or by an oligo-capping method to select full-length cDNAs [45]. A full analysis of these has been posted on our website [49]. ESTs were then analyzed by SignalP, which predicted that 16.0% of total ESTs (197/1,234) contained either 5' signal peptide sequences (176/1,234) or signal anchors (21/1,234, Table 1). The oligo-capped cDNA library yielded a notably higher proportion of sequences with predicted signal peptides (20.4%) than did conventional cDNA libraries (10.1%).
Table 1.
Analysis of transcripts represented in conventional and oligo-capped cDNA libraries
| Conventional cDNA libraries | Oligo-capped cDNA library | |
| Total sequences providing peptide predictions | 734 | 500 |
| In-frame ATG followed by ≥ 99-nucleotide open reading frame (ORF) | 567 (77.2%) | 430 (86.0%) |
| Predicted ORF length (average) | 114.6 | 101.5 |
| % Signal peptide or signal anchor | SP: 74 (10.1%) | SP: 102 (20.4%) |
| SA: 16 (2.2%) | SA: 5 (1.0%) | |
| % Spliced leader | 0 | 37 (7.4%) |
The dataset was then clustered to account for multiple ESTs from highly expressed genes, and ESTs were assigned to 742 clusters, including 567 singletons. The proportion of clusters bearing potential signal sequences remained high (135/742; 18.2%), confirming that the dataset is not skewed by over-representation of a few abundant transcripts. The overall proportion of cDNAs encoding predicted signal peptides is within the 15-25% range estimated by analyis of whole-genome sequence data [50]. Of all predicted signal-sequence-bearing clones or clusters from N. brasiliensis, around 90% were classified as conventional signal peptides associated with export and secretion into the extracellular environment. The remaining approximately 10% were identified as potential signal anchors, in which the hydrophobic amino-terminal segment is retained, without cleavage, as a transmembrane domain for type II plasma membrane proteins [7].
Presence of trans-spliced leaders in N. brasiliensis
All nematodes undergo trans-splicing at the 5' end of a proportion of their mRNA transcripts; a short leader sequence is added upstream of the initiation codon. The leader is normally a 22-nucleotide sequence termed SL1 [51]. The precise SL1 sequence is highly conserved throughout the phylum, although the degree to which transcripts are trans-spliced varies between different nematode species [52]. To evaluate the prominence of SL1-trans-splicing in N. brasiliensis, we searched the 1,234 ESTs with the 3' 14 nucleotides of SL1, to allow for any minor truncation of cDNAs. Only 37 matches were found, all from the oligo-capped cDNA library (from 500 ESTs, giving a frequency of 7.4%); a few clones from the conventional libraries had 10 or fewer nucleotides identical to the SL1 sequence at their 5' termini. Although the overall frequency of trans-splicing in N.brasiliensis is not yet known, this level is well below those of other species, such as C. elegans. Moreover, transcripts bearing the spliced leader (and its unique tri-methylguanosine cap) are, in certain species, under-represented by the method we used to selectively amplify full-length mRNAs [45]. Hence the true extent of trans-splicing may be higher than the proportion evident in the current dataset.
N. brasiliensis sequences show closest similarity to those of other trichostrongyles
N. brasiliensis is a stronglylid nematode, closely related to veterinary parasites such as Haemonchus contortus and Teladorsagia (previously Ostertagia) circumcincta in the Superfamily Trichostrongyloidea, and within the Order Strongylida which includes human hookworm pathogens Ancylostoma duodenale and Necator americanus [53]. The closest free-living taxa to the Strongylida are members of the Rhabditina, including C. elegans, and both are grouped in Clade V of the Nematoda, on the basis of small subunit rRNA sequence analysis [48].
A more objective technique for visualizing the evolutionary relationships between species for which large datasets are available is to use SimiTri, which plots in two-dimensional space the relative similarities of gene sequences between one species (N. brasiliensis) and three comparators [54]. As shown in Figure 1a, N. brasiliensis sequences group slightly closer to Haemonchus than to Ancylostoma, consistent with the relationship described above. Likewise, in Figure 1b, N. brasiliensis sequences group more towards Teladorsargia than Necator.
Figure 1.
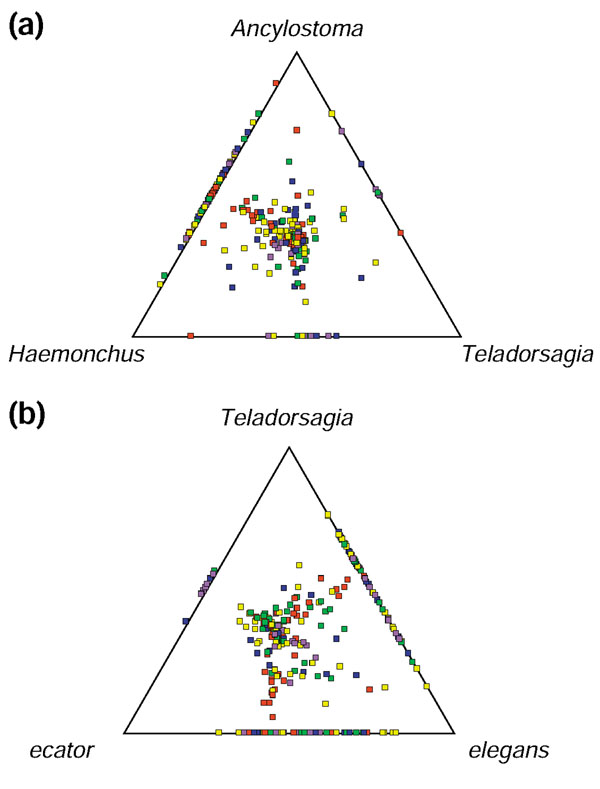
Similarity of N. brasiliensis ESTs to sequences from other nematodes. SimiTri [54] was used to plot 736 N. brasiliensis EST clusters against related species database entries. For each consensus sequence associated with the 736 Nippo clusters, a BLAST was performed against a series of different databases. Each tile in the graphic represents a unique consensus sequence and its relative position is computed from the raw BLAST scores derived above (with a cutoff of ≥ 50). Hence each tile's position shows its degree of sequence similarity to each of the three selected databases. Sequences showing similarity to only one database are not shown. Sequences showing sequence similarity to only two databases appear on the lines joining the two databases. Tiles are colored by their highest TBLASTX score to each of the databases: red ≥ 300; yellow ≥ 200; green ≥ 150, blue ≥ 100 and purple < 100. (a) SimiTri plot showing sequence similarity relationships between N. brasiliensis consensus sequences and database entries of Ancylostoma caninum/duodenale ESTs (20,177 entries, 386 hits), Haemonchus contortus ESTs (22,337 entries, 384 hits) and Teladorsagia circumcincta ESTs (5,300 entries, 264 hits). Database comparisons were performed using TBLASTX. (b) SimiTri plot showing sequence similarity relationships between N. brasiliensis consensus sequences and database entries of Necator americanus ESTs (4,821 entries, 244 hits), Teladorsagia circumcincta ESTs (5,300 entries, 264 hits), and C. elegans wormpep (21,600 entries, 466 hits). Database comparisons were performed using TBLASTX for N. americanus and T. circumcincta, while C. elegans wormpep comparions used BLASTX.
A compilation of the N. brasiliensis clusters, for which assigned homologs exist in protein databases, is presented in Table 2. Many sequences with high similarities to biosynthetic, structural, signaling and regulatory pathway proteins can readily be identified, corresponding to predicted nuclear or cytoplasmic proteins. Interestingly, multiple clusters encode categories of genes which are prominent in other nematode parasites, such as the five clusters encoding homologs of Ancylostoma secreted protein [2], five clusters of C-type and S-type lectins [55] and seven clusters for cysteine proteinases [56].
Table 2.
ESTs from adult cDNAs with known homologs, classified by function
| Cluster number | Conventional cDNAs | Oligo-capped cDNAs | P | Accession | Description |
| Proteases/proteosome/ubiquitin | |||||
| NBC00018 | 2 | 0 | 1e-33 | S66528 | 26S proteinase regulatory complex, non-ATPase chain (Drosophila melanogaster) |
| NBC00030 | 2 | 0 | 8e-56 | U41556 | Cysteine protease CPR-6 (Caenorhabditis elegans) |
| NBC00086 | 1 | 0 | 3e-29 | A48454 | Cathepsin B-like cysteine proteinase (Ostertagia ostertagi) |
| 5e-28 | D48435 | Cysteine proteinase AC-3 (Haemonchus contortus) | |||
| NBC00168 | 1 | 0 | 2e-42 | NM_065563 | Calpain thiol protease (Caenorhabditis elegans) |
| NBC00198 | 1 | 0 | 7e-60 | NM_073736 | Cysteine protease (legumain, asparaginyl endopeptidase) (Caenorhabditis elegans) |
| NBC00204 | 3 | 0 | 2e-32 | NM_072733 | Protease (aspartic) (Caenorhabditis elegans) |
| NBC00231 | 2 | 0 | 5e-90 | NM_064106 | Serine carboxypeptidase (Caenorhabditis elegans) |
| NBC00307 | 1 | 0 | 2e-32 | NM_015277 | Ubiquitin-protein ligase NEDD4-like; neural precursor (Homo sapiens) |
| NBC00311 | 1 | 0 | 5e-31 | NM_073736 | Cysteine protease (legumain, asparaginyl endopeptidase) (Caenorhabditis elegans) |
| NBC00352 | 2 | 0 | 6e-31 | NM_065253 | Ubiquitin (Caenorhabditis elegans) |
| NBC00348 | 1 | 0 | 2e-83 | A48145 | Ubiquitin-conjugating enzyme, UBC-2 (Caenorhabditis elegans) |
| NBC00362 | 1 | 0 | 1e-76 | S17521 | Multicatalytic endopeptidase complex (proteasome) zeta chain (Caenorhabditis elegans) |
| NBC00368 | 1 | 0 | 9e-13 | LCE_ORYLA | Low choriolytic enzyme precursor (zinc metalloprotease) (Oryzias latipes) |
| NBC00377 | 1 | 0 | 3e-75 | PSA4_CAEEL | Proteasome subunit, alpha type 4, PAS-3 (Caenorhabditis elegans) |
| NBC00459 | 2 | 1 | 2e-26 | NM_072733 | Protease (aspartic) (Caenorhabditis elegans) |
| NBC00469 | 1 | 0 | 7e-17 | NM_060215 | Zinc metalloprotease (Caenorhabditis elegans) |
| NBC00509 | 1 | 1 | 4e-71 | AL161503 | Polyubiquitin, UBQ10 (Arabidopsis thaliana) |
| NBC00664 | 0 | 1 | 5e-09 | NM_074798 | Cathepsin-like (cysteine) protease (Caenorhabditis elegans) |
| NBC00670 | 0 | 1 | 3e-18 | S17435 | Polyubiquitin 6 (Helianthus annuus) |
| NBC00772 | 0 | 1 | 4e-24 | NM_003352 | Sentrin, ubiquitin-like small protein (Gallus gallus) |
| NBC00783 | 0 | 1 | 2e-89 | U41556 | Cysteine protease CPR-6 (Caenorhabditis elegans) |
| NBC00828 | 0 | 1 | 9e-63 | NC_003424 | Pad1 protein; 26S proteasome subunit (Schizosaccharomyces pombe) |
| Enzymes (other than proteases) | |||||
| NBC00045 | 2 | 0 | 2e-92 | NM_065870 | Fructose-biphosphate aldolase (Caenorhabditis elegans) |
| NBC00049 | 1 | 0 | 9e-50 | NM_070783 | Lipase (Caenorhabditis elegans) |
| NBC00066 | 2 | 1 | 7e-76 | NM_074348 | Peptidyl-prolyl cis-trans isomerase (Caenorhabditis elegans) |
| NBC00079 | 1 | 0 | 2e-35 | NM_058712 | Helicase (Caenorhabditis elegans) |
| NBC00102 | 1 | 0 | 7e-37 | NM_074031 | Peroxidase-like (Caenorhabditis elegans) |
| NBC00139 | 1 | 0 | 8e-29 | NM_060074 | Hexokinase (Caenorhabditis elegans) |
| NBC00143 | 1 | 0 | 4e-66 | ADHX_MYXGL | Alcohol dehydrogenase class III (Caenorhabditis elegans) |
| NBC00147 | 1 | 0 | 6e-19 | XM_087230 | Similar to Uridine phosphorylase (UDRPase) (Homo sapiens) |
| NBC00157 | 1 | 0 | 3e-13 | XM_058660 | Similar to Protein tyrosine phosphatase 1E (Homo sapiens) |
| NBC00173 | 1 | 0 | 5e-72 | AJ440747 | Protein disulphide isomerase 1 (Ostertagia ostertagi) |
| NBC00183 | 1 | 0 | 3e-56 | T46280 | Isocitrate dehydrogenase, NADP+, cytosolic (Homo sapiens) |
| NBC00189 | 1 | 0 | 1e-21 | XM_129069 | Similar to Acetyltransferase (GNAT) family (Mus musculus) |
| NBC00212 | 1 | 0 | 6e-57 | NM_016100 | N-terminal acetyltransferase complex ard1 subunit (Homo sapiens) |
| NBC00283 | 1 | 0 | 4e-27 | NM_012088 | 6-phosphogluconolactonase (Homo sapiens) |
| NBC00285 | 1 | 0 | 2e-47 | LDHA_ANGRO | L-lactate dehydrogenase A chain (Anguilla rostrata) |
| NBC00290 | 1 | 0 | 3e-17 | I55976 | Dihydrolipoamide S-acetyltransferase (Rattus norvegicus) |
| NBC00292 | 1 | 0 | 1e-40 | NM_006223 | Peptidyl-prolyl cis/trans isomerase (Homo sapiens) |
| NBC00304 | 1 | 0 | 4e-12 | NM_073341 | Glucose-1-dehydrogenase (Caenorhabditis elegans) |
| NBC00309 | 1 | 0 | 1e-18 | NM_066225 | Hydroxymethylglutaryl-coA reductase (Caenorhabditis elegans) |
| NBC00326 | 1 | 0 | 1e-65 | NM_065761 | Protein phosphatase 2A (Caenorhabditis elegans) |
| NBC00337 | 1 | 0 | 2e-60 | GMD1_CAEEL | Probable GDP-mannose 4,6 dehydratase 1 (Caenorhabditis elegans) |
| NBC00353 | 1 | 0 | 2e-56 | NM_065537 | ATP synthase B chain (Caenorhabditis elegans) |
| NBC00378 | 1 | 0 | 2e-43 | NM_073253 | Acetyltransferase (GNAT) family (Caenorhabditis elegans) |
| NBC00382 | 1 | 0 | 4e-49 | NM_063827 | Phospholipase A2 (Caenorhabditis elegans) |
| NBC00389 | 2 | 0 | 1e-48 | NM_058626 | Phosphotransferase (Caenorhabditis elegans) |
| NBC00404 | 1 | 0 | 2e-76 | NM_064078 | Glucosamine-fructose-6-phosphate aminotransferase (Caenorhabditis elegans) |
| NBC00413 | 1 | 0 | 6e-22 | NM_078324 | AMP-activated protein kinase (Caenorhabditis elegans) |
| NBC00427 | 1 | 0 | 2e-20 | NC_003423 | 3-oxoacyl-(acyl-carrier-protein)-synthase (Schizosaccharomyces pombe) |
| NBC00475 | 1 | 0 | 3e-42 | NM_065313 | Serine/threonine protein phosphatase (Caenorhabditis elegans) |
| NBC00483 | 1 | 0 | 4e-25 | NM_059984 | Phospholipase, similar to ADRAB-b (Caenorhabditis elegans) |
| NBC00504 | 1 | 0 | 7e-65 | AF292096 | Protein kinase AIRK2 (Xenopus laevis) |
| NBC00508 | 1 | 2 | 5e-64 | PPCK_HAECO | Phosphoenolpyruvate carboxykinase (Haemonchus contortus) |
| NBC00528 | 1 | 0 | 5e-66 | PPCK_HAECO | Phosphoenolpyruvate carboxykinase (Haemonchus contortus) |
| NBC00561 | 0 | 7 | 1e-54 | NDKB_RAT | Nucleoside diphosphate kinase B (Rattus norvegicus) |
| NBC00713 | 0 | 1 | 1e-08 | XM_140038 | Similar to tau-tubulin kinase (Mus musculus) |
| NBC00729 | 0 | 2 | 4e-21 | NM_079041 | Flap endonuclease 1 (Drosophila melanogaster) |
| NBC00743 | 0 | 1 | 3e-64 | G3P_BRUMA | Glyceraldehyde 3-phosphate dehydrogenase (Brugia malayi) |
| NBC00745 | 0 | 1 | 4e-13 | NM_068436 | Casein kinase (Caenorhabditis elegans) |
| NBC00689 | 0 | 3 | 2e-17 | CLYC_CAEEL | Serine hydroxymethyltransferase MEL-32 (Caenorhabditis elegans) |
| NBC00696 | 0 | 2 | 2e-15 | NM_000414 | Hydroxysteroid (17-beta) dehydrogenase 4 (Homo sapiens) |
| NBC00770 | 0 | 1 | 3e-45 | NM_066907 | Serine/threonine kinase, casein kinase-like (Caenorhabditis elegans) |
| NBC00777 | 0 | 1 | 8e-21 | OAZ_PRIPA | Ornithine decarboxylase antizyme (Pristionchus pacificus) |
| NBC00796 | 0 | 1 | 8e-52 | XM_125017) | Putative lysophosphatidic acid acyltransferase (Mus musculus) |
| NBC00802 | 0 | 1 | 4e-49 | NM_078623 | Enoyl Coenzyme A hydratase, short chain 1 (Rattus norvegicus) |
| Structural | |||||
| NBC00056 | 1 | 0 | 4e-58 | NM_071024 | Actin depolymerizing factor (Caenorhabditis elegans) |
| NBC00062 | 1 | 0 | 1e-11 | NM_006400 | Dynactin 2; dynactin complex 50 kD subunit; dynamitin (Homo sapiens) |
| NBC00078 | 2 | 0 | 0 | NM_059538 | Calponin (Caenorhabditis elegans) |
| NBC00097 | 1 | 0 | 1e-42 | MLR1_CAEEL | Myosin regulatory light chain 1 (Caenorhabditis elegans) |
| NBC00142 | 1 | 0 | 2e-76 | S53776 | Beta-tubulin isotype I (Haemonchus contortus) |
| NBC00172 | 2 | 0 | 0 | NM_073416 | Actin (Caenorhabditis elegans) |
| NBC00224 | 1 | 0 | 2e-40 | NM_063850 | Troponin C (Caenorhabditis elegans) |
| NBC00239 | 4 | 1 | 2e-39 | NM_077559 | Collagen (Caenorhabditis elegans) |
| NBC00241 | 2 | 0 | 2e-47 | NM_069715 | Collagen (Caenorhabditis elegans) |
| 6e-47 | NM_077291 | Cuticular collagen (Caenorhabditis elegans) | |||
| NBC00246 | 1 | 1 | 3e-19 | NM_077087 | Troponin I (Caenorhabditis elegans) |
| NBC00287 | 2 | 0 | 2e-61 | MLR1_CAEEL | Myosin regulatory light chain 1 (Caenorhabditis elegans) |
| NBC00360 | 1 | 1 | 3e-30 | NM_145671 | Actinfilin (Rattus norvegicus) |
| NBC00396 | 1 | 0 | 2e-67 | MYSP_CAEEL | Paramyosin (Caenorhabditis elegans) |
| NBC00403 | 1 | 0 | 3e-32 | NM_077291 | Cuticular collagen (Caenorhabditis elegans) |
| NBC00418 | 1 | 0 | 6e-27 | NM058881 | Calponin (Caenorhabditis elegans) |
| NBC00430 | 1 | 0 | 3e-11 | NM_011722 | Dynactin 6; p27 dynactin subunit (Mus musculus) |
| NBC00526 | 1 | 0 | 2e-44 | NM_060857 | Profilin (Caenorhabditis elegans) |
| NBC00552 | 0 | 1 | 9e-47 | MYSP_CAEEL | Paramyosin (Caenorhabditis elegans) |
| NBC00569 | 0 | 1 | 1e-23 | NM_060369 | Alpha crystallin B chain (Caenorhabditis elegans) |
| NBC00749 | 0 | 1 | 3e-43 | NM_060857 | Profilin (Caenorhabditis elegans) |
| Embryo/egg/mating etc | |||||
| NBC00068 | 3 | 0 | 1e-25 | VIT5_CAEEL | Vitellogenin 5 precursor (Caenorhabditis elegans) |
| NBC00161 | 1 | 0 | 2e-15 | VIT5_CAEEL | Vitellogenin 5 precursor (Caenorhabditis elegans) |
| NBC00397 | 1 | 9 | 7e-61 | MS10_CAEEL | Major Sperm Protein 10 (Caenorhabditis elegans) |
| NBC00523 | 1 | 0 | 4e-69 | XM_038960 | Similar to preimplantation protein 3 (Homo sapiens) |
| NBC00585 | 0 | 5 | 2e-30 | NM_076467 | Vitellogenin (Caenorhabditis elegans) |
| NBC00611 | 0 | 1 | 1e-25 | NM_060189 | Placental protein 11 (Caenorhabditis elegans) |
| Transporters/receptors/lectins and other binding proteins | |||||
| NBC00027 | 2 | 0 | 9e-17 | NM_062882 | Lectin, C-type (Caenorhabditis elegans) |
| 5e-15 | NM_076712 | Asialoglycoprotein receptor (C-type lectin) (Caenorhabditis elegans) | |||
| NBC00110 | 1 | 0 | 4e-17 | NC_001263 | Acyl-CoA-binding protein (Deinococcus radiodurans) |
| NBC00118 | 1 | 0 | 4e-41 | T31073 | Multidrug resistance P-glycoprotein (Haemonchus contortus) |
| NBC00128 | 3 | 0 | 1e-92 | NM_067381 | ADP/ATP carrier protein/translocase (Caenorhabditis elegans) |
| NBC00167 | 1 | 0 | 2e-12 | NM_130415 | Lysosomal amino acid transporter 1 (Rattus norvegicus) |
| NBC00175 | 1 | 0 | 7e-15 | A48925 | Mannose receptor (C-type lectin), macrophage (Mus musculus) |
| NBC00319 | 1 | 0 | 8e-15 | NXT2_HUMAN | NTF2-related export protein 2 (p15-2 protein) (Homo sapiens) |
| NBC00324 | 2 | 0 | 7e-15 | AJ243873 | Galectin (S-type lectin) (Haemonchus contortus) |
| NBC00340 | 1 | 0 | 2e-61 | NM_077246 | Galectin (S-type lectin) LEC-10 (Caenorhabditis elegans) |
| NBC00355 | 1 | 0 | 8e-21 | NM_059527 | Fatty acid-binding protein LBP-6 (Caenorhabditis elegans) |
| NBC00363 | 1 | 0 | 6e-48 | NM_016208 | Vacuolar protein sorting 28 homolog (Homo sapiens) |
| NBC00583 | 0 | 5 | 4e-35 | NM_065836 | Low density lipoprotein receptor (Caenorhabditis elegans) |
| NBC00593 | 0 | 2 | 2e-26 | NM_059525 | Fatty acid-binding protein LBP-6 (Caenorhabditis elegans) |
| NBC00752 | 0 | 1 | 3e-08 | NM_059071 | Acetylcholine receptor UNV-38 (Caenorhabditis elegans) |
| NBC00766 | 0 | 1 | 7e-44 | POR2_MELGA | Voltage-dependent anion-selective channel protein 2 (VDAC-2) (Meleagris gallopavo) |
| NBC00808 | 0 | 1 | 6e-53 | NM_072174 | Calreticulin precursor (Caenorhabditis elegans) |
| NBC00838 | 0 | 1 | 1e-78 | NM_063349 | T-complex protein, delta subunit (cytosolic chaperonin CCT-4) (Caenorhabditis elegans) |
| Signaling | |||||
| NBC00207 | 1 | 0 | 0 | RAB2_LYMST | RAS-Related protein RAB-2 (Lymnea stagnalis) |
| NBC00252 | 1 | 0 | 8e-97 | NM_070558 | RAS-like GTP-binding protein RhoA (Caenorhabditis elegans) |
| NBC00297 | 1 | 0 | 4e-17 | NM_009106 | Rhotekin (Mus musculus) |
| NBC00312 | 1 | 0 | 4e-46 | A35350 | Protein kinase C inhibitor (Bos bovis) |
| NBC00269 | 1 | 0 | 1e-43 | NM_058274 | RAS-related protein RAB-11 (Caenorhabditis elegans) |
| NBC00282 | 1 | 0 | 9e-25 | NP_741191 | A kinase anchor protein 1 (Caenorhabditis elegans) |
| NBC00395 | 1 | 0 | 2e-29 | NM_07328 | RAS-like GTP-binding protein (cdc42-like) (Caenorhabditis elegans) |
| NBC00436 | 1 | 0 | 2e-44 | NM_070985 | Calmodulin (Caenorhabditis elegans) |
| NBC00462 | 1 | 0 | 2e-13 | SSRP_DROME | Single-strand recognition protein (SSRP) (Chorion-factor 5) (Drosophila melanogaster) |
| NBC00409 | 1 | 0 | 1e-16 | NM_019746 | Programmed cell death 5/TFAR19 protein (Mus musculus) |
| NBC00440 | 1 | 0 | 3e-72 | S43599 | SNF5 homolog R07E5.3 (Caenorhabditis elegans) |
| NBC00510 | 1 | 0 | 2e-28 | XM_129572 | Calcyclin (S100 family) binding protein (Mus musculus) |
| NBC00629 | 0 | 1 | 1e-20 | NM_026297 | RAB (RAS oncogene family-like 3) (Mus musculus) |
| NBC00648 | 0 | 1 | 3e-20 | NM_002624 | Prefoldin 5 isoform alpha; myc modulator-1; c-myc binding protein (Homo sapiens) |
| NBC00727 | 0 | 1 | 3e-17 | AB091687 | TGF-beta induced apotosis protein 3 (Mus musculus) |
| NBC00768 | 0 | 1 | 3e-18 | NM_078471 | TGF-beta-1 induced anti-apoptotic factor 1 isoform 1 (Homo sapiens) |
| NBC00829 | 0 | 1 | 1e-42 | A49146 | Developmental regulator WNT-4 (Xenopus laevis) |
| NBC00841 | 0 | 1 | 1e-31 | NM_012453 | Transducin (beta)-like 2, isoform 1 (Homo sapiens) |
| DNA-related/transcription/DNA binding/regulation | |||||
| NBC00024 | 1 | 0 | 1e-37 | NM_003752 | Eukaryotic translation initiation factor 3, subunit 8 (Homo sapiens) |
| NBC00048 | 1 | 0 | 1e-28 | NM_069150 | Glycine-rich RNA-binding protein (Caenorhabditis elegans) |
| 5e-21 | NM_007007 | Cleavage and polyadenylation specific factor 6 (Homo sapiens) | |||
| NBC00050 | 1 | 0 | 2e-12 | HEXP_LEIMA | DNA-binding protein HEXBP (Hexamer-binding protein) (Leishmania major) |
| NBC00055 | 1 | 1 | 2e-24 | NM_060622 | RNA recognition motif (RRM, RBD, or RNP domain) (Caenorhabditis elegans) |
| NBC00090 | 2 | 1 | 0 | NM_066119 | Elongation factor 1-alpha (Caenorhabditis elegans) |
| NBC00099 | 1 | 0 | 2e-30 | NM_067248 | Splicing factor (Caenorhabditis elegans) |
| NBC00170 | 1 | 0 | 2e-56 | NM_011304 | RuvB DNA helicase -like protein 2 (Mus musculus) |
| NBC00181 | 1 | 0 | 4e-13 | NM_001698 | AU RNA-binding protein/enoyl-Coenzyme A hydratase (Homo sapiens) |
| NBC00192 | 1 | 0 | 2e-26 | NM_060622 | RNA recognition motif (RRM, RBD, or RNP domain) (Caenorhabditis elegans) |
| NBC00210 | 1 | 0 | 3e-15 | NM_018403 | Transcription factor (SMIF gene) (Homo sapiens) |
| NBC00267 | 1 | 0 | 4e-20 | T2EB_XENLA | Transcription initiation factor IIE, beta subunit (Xenopus laevis) |
| NBC00321 | 1 | 0 | 1e-16 | NM_033224 | Purine-rich element binding protein B (Homo sapiens) |
| NBC00280 | 1 | 0 | 3e-58 | NM_006578 | Guanine nucleotide-binding protein, beta-5 subunit (Homo sapiens) |
| NBC00350 | 1 | 0 | 6e-40 | DPOD_DROME | DNA polymerase delta catalytic subunit (Drosophila melanogaster) |
| NBC00366 | 2 | 0 | 6e-79 | NM_066119 | Elongation factor 1-alpha (Caenorhabditis elegans) |
| NBC00370 | 1 | 0 | 1e-17 | NM_031992 | Eukaryotic translation initiation factor 4H, isoform 2 (Homo sapiens) |
| NBC00374 | 1 | 2 | 2e-53 | NM_070415 | Elongation factor 1-beta/delta chain (Caenorhabditis elegans) |
| NBC00480 | 1 | 0 | 3e-21 | NM_061014 | Regulator of chromosome condensation, RCC1 (Caenorhabditis elegans) |
| NBC00543 | 0 | 2 | 5e-23 | NM_065536 | Zinc finger, C3HC4 type (RING finger) (Caenorhabditis elegans) |
| NBC00577 | 0 | 7 | 2e-31 | NP_872244 | Translation elongation factor EFT-4 (Caenorhabditis elegans) |
| NBC00600 | 0 | 1 | 3e-74 | NM_063406 | Initiation factor 5A (Caenorhabditis elegans) |
| NBC00630 | 0 | 1 | 9e-39 | SFR4_MOUSE | Splicing factor, arginine/serine-rich 4 (Mus musculus) |
| NBC00764 | 0 | 1 | 4e-16 | XM_132357 | Similar to Translation Initiation factor EIF-2B alpha (Mus musculus) |
| NBC00776 | 0 | 1 | 6e-27 | SN2L_CAEEL | Potential global transcription activator SNF2L (Caenorhabditis elegans) |
| NBC00791 | 0 | 1 | 5e-38 | NM_001207 | Basic transcription factor 3 (Homo sapiens) |
| NBC00816 | 0 | 1 | 2e-24 | S3B2_HUMAN | Splicing factor 3B subunit 2 (Spliceosome associated protein 145) (Homo sapiens) |
| Other homologs of interest | |||||
| NBC00025 | 1 | 0 | 3e-16 | AF352714 | HC40 putative secretory protein precursor (ASP homolog) (Haemonchus contortus) |
| NBC00065 | 1 | 0 | 6e-20 | AA063577 | Secreted protein 5 precursor (ASP homolog) (Ancylostoma caninum) |
| NBC00095 | 1 | 0 | 8e-59 | GLB2_NIPBR | Myoglobin (body wall isoform globin) (Nippostrongylus brasiliensis) |
| NBC00103 | 1 | 0 | 9e-12 | DIM1_CAEEL | Protein dim-1 (2D-page protein spot 8) (Caenorhabditis elegans) |
| NBC00029 | 1 | 0 | 5e-17 | NM_001545 | Immature colon carcinoma transcript 1 (Homo sapiens) |
| NBC00141 | 1 | 0 | 2e-35 | NM_018984 | Slingshot 1 (Homo sapiens) |
| NBC00160 | 1 | 0 | 5e-12 | NM_053810 | Synaptosomal-associated protein, 29kD (Rattus norvegicus) |
| NBC00199 | 1 | 0 | 9e-39 | AF278538 | Nucleosome assembly protein 1 (Xenopus laevis) |
| NBC00256 | 2 | 0 | 2e-09 | NM_075227 | Transthyretin-like family (Caenorhabditis elegans) |
| NBC00293 | 1 | 0 | 7e-08 | NC_003424 | F-box protein (Schizosaccharomyces pombe) |
| NBC00399 | 1 | 0 | 2e-22 | NM_076443 | Calumenin, calcium-binding protein (Caenorhabditis elegans) |
| NBC00429 | 1 | 0 | 4e-14 | XM_122362 | Chromobox homolog 2 (Drosophila Pc class) (Mus musculus) |
| NBC00491 | 1 | 0 | 3e-21 | NM_076885 | Thrombospondin (Caenorhabditis elegans) |
| NBC00518 | 1 | 0 | 3e-73 | T37461 | Mago nashi-like protein (Caenorhabditis elegans) |
| NBC00544 | 0 | 1 | 2e-45 | NM_061213 | Alpha-2-macroglobulin family (Caenorhabditis elegans) |
| NBC00560 | 0 | 1 | 1e-35 | NM_021305 | SEC61, alpha subunit 2 (Saccharomyces cerevisiae) |
| NBC00705 | 0 | 1 | 3e-31 | DVA1_DICVI | DVA-1 nematode polyprotein allergen precursor (NPA) (Dictyocaulus viviparus) |
| 2e-12 | ABA1_ASCSU | ABA-1 nematode polyprotein allergen precursor (Body fluid allergen-1) (Ascaris suum) | |||
| NBC00753 | 0 | 1 | 4e-10 | AF089728 | Ancylostoma-secreted protein 2 precursor, ASP-2 (Ancylostoma caninum) |
| NBC00755 | 0 | 1 | 2e-40 | TCPB_CAEEL | T-complex protein 1, beta subunit (CCT-beta) (Caenorhabditis elegans) |
| NBC00757 | 0 | 1 | 2e-68 | 1432_SCHMA | 14-3-3 Protein homolog 2 (14-3-3-2) (Schistosoma mansoni) |
| NBC00803 | 0 | 1 | 3e-09 | ASP_ANCCA | Ancylostoma secreted protein (ASP-1) precursor (Ancylostoma caninum) |
| 3e-09 | AF079521 | Ancylostoma-secreted protein 1 precursor (ASP-1 homolog) (Necator americanus) | |||
| NBC00827 | 0 | 1 | 3e-14 | NM_070108 | Testis-specific protein TPX-1 like (ASP homolog) (Caenorhabditis elegans) |
The table gives, for each numbered cluster, the highest homolog with a functional description where available; in a number of cases a C. elegans homolog exists with a higher similarity, but has no description. Similarities to entries described as 'hypothetical proteins' are excluded, as are heat-shock proteins, cytochromes, mitochondrial and ribosomal products. Where C. elegans protein description is ambiguous (for example, protease, lectin), further descriptors added manually are italicized. Different clusters may derive from a single gene if sequences are non-overlapping; for example, NBC00198 and NBC00311 align to different segments of the C. elegans protease gene NM_073736. This table does not include N. brasiliensis gene products discovered previously and/or reported by other laboratories. All entries for this species are aggregated on the NEMBASE website.
Proteins bearing signal sequences are less evolutionarily conserved
The set of 742 clusters was then divided into three categories according to their similarity to existing database sequences. 'Conserved' genes were defined as those with similarities to any non-nematode database entry above a given cutoff score; 'nematode-specific' genes were similar only to sequences from C. elegans or other nematode species, and 'novel' showed no similarity to any existing entry. BLASTX cutoff scores of 50 (P < e-6) and 80 (P < e-10) were both used to define these categories at different levels. Using the more stringent criterion, roughly one third (27-37%) of clusters fell into each category (Figure 2a), while the lower cutoff resulted in approximately half (48%) being classified as conserved, with the remainder evenly divided between nematode-specific (25%) and novel (27%).
Figure 2.
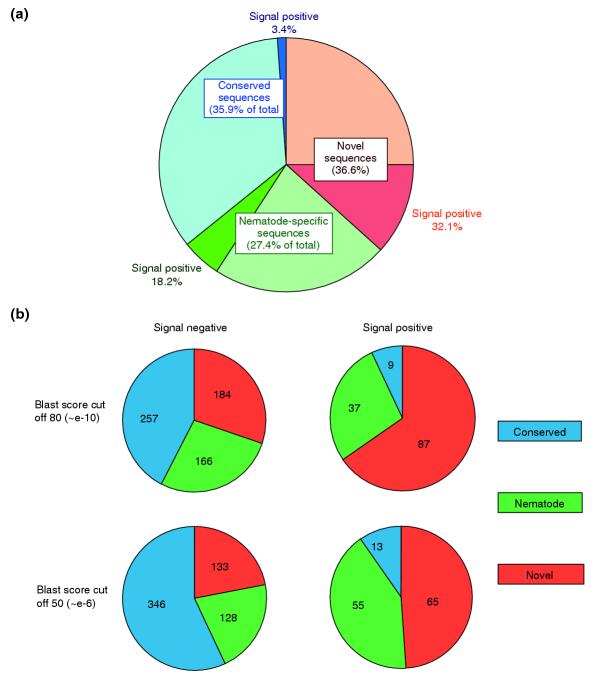
Proportion of ESTs predicted to encode signal sequences. (a) EST sequences were classified as conserved (similarities to non-nematode database entries), nematode-specific (similarities only to C. elegans or other nematode sequences), or novel (no similarities to existing entries), using a cutoff score of 80 in BLASTX (P < e-10). The number of ESTs bearing potential signal sequences was then calculated and the results are shown here. (b) Effects of relaxing cutoff scores on distribution of signal peptide-containing predicted gene products among conserved, nematode-specific and novel categories. Numbers of clusters in each category are given for cutoffs of 80 (P <e-10), as used in (a), and 50 (P <e-6).
The distribution of clusters containing signal sequences was, however, remarkably skewed towards the novel category. Because the primary classification of 92 novel genes was based on 5' EST sequences, all clusters initially designated as novel signal-sequence positive were further scrutinized. In 72 cases, clusters read through to a 3' poly(A) tail (either single reads from clones of 700 or fewer nucleotides or overlapping ESTs with at least one poly(A) tail present); in 20 cases, where no poly(A) tail was observed, 3' sequencing was carried out. Of these, three showed database homologies from 3' sequence and were reclassified as conserved, and two showed no poly(A) tail and were excluded from further analysis as presumed internal fragments. The remaining 15 clusters showed overlap between 3' and 5' cluster reads, without revealing any additional similarities. Thus, a total of 87 clusters were verified as novel signal-sequence positive.
Taking this more rigorously defined subset, some 65% (87/133) of sequences are predicted to encode either signal peptides or signal anchors when classified as novel at the higher cutoff (49% at the lower level), and only 4% were found in the conserved category (7% at the lower cutoff). Moreover, 32% of all novel sequences contained a signal peptide or anchor, compared to 18% of nematode-specific and only 3.4% of conserved.
Although the latter category will include many structural and housekeeping proteins for which secretion is unlikely to confer a selective advantage, the data suggest that nematode secreted proteins have diversified more rapidly than those that do not enter the secretory pathway.
This association between signal peptides and novel proteins may be falsely amplified where, for example, conserved domains are sufficiently distant from the amino terminus to have been omitted from EST sequences. Equally, some clones will have been sequenced from truncated transcripts, and a proportion of those erroneously classified as encoding non-signal sequence bearing proteins. However, neither of these considerations seems likely to account for the very large disparity in signal sequence frequency between the three categories we describe. A more general caveat with these analyses is that SignalP is a fallible prediction tool, with an accuracy of 70% or less when applied to non-mammalian sequences [6]. There is no reason, however, to expect that false-positive assignations would occur disproportionately in the novel group rather than the conserved, and the conclusion drawn here would remain valid over a wide range of prediction accuracies.
Has there been evolutionary acquisition of signal peptides?
The subset of signal-peptide-encoding N. brasiliensis clusters with similarity to predicted genes from C. elegans with either assigned function or of no known function was then identified. Examples of each category are given in Table 3. Some nine clusters were identified as bearing signal-peptide sequences, where in each case the C. elegans homologs appear not to possess a signal-pepide motif. Five of these clusters represent globins, which have previously been noted to possess signal peptides in N. brasiliensis even though the C. elegans paralogs do not [25,57]. One cluster (NBC00028) is almost identical to the recorded cuticular isoform precursor (P51536), but four additional clusters represent new members of this family in N. brasiliensis bearing signal peptides. In contrast, a distinct globin (NBC00095) closely related to the known body-wall isoform (P51535) lacks a predicted signal peptide. Hence, gene duplication may have predated the development in some globin forms, of a secretory function.
Table 3.
ESTs from adult cDNAs with predicted amino-terminal signal peptides and with homologs in C. elegans
| Cluster | Score | P | Conventional cDNAs | Oligo-capped cDNAs | Wormpep ID | SignalP criteria | SignalP scores | Signal in C. elegans? | Description of C. elegans gene | |||
| C-p | Amino acids | SP-p | SP? | |||||||||
| (a) Signal peptides predicted in both N. brasiliensis and C. elegans | ||||||||||||
| NBC00012 | 86 | 6e-18 | 4 | 0 | CE20223 | YYYYS | 0.533 | 16 | 1.000 | Y | Y | Unknown (similar to NBC00237) |
| NBC00031 | 80 | 3e-16 | 2 | 2 | CE17924 | YYYYS | 0.932 | 18 | 0.999 | Y | Y | Unknown |
| NBC00237 | 84 | 5e-17 | 1 | 2 | CE20223 | YYYYS | 0.671 | 19 | 1.000 | Y | Y | Unknown (similar to NBC00012) |
| NBC00258 | 145 | 1e-35 | 1 | 0 | CE00133 | YYYYS | 0.524 | 19 | 0.999 | Y | Y | FAR-1 fatty acid/retinol-binding protein |
| NBC00266 | 129 | 6e-31 | 1 | 0 | CE19630 | YYYYS | 0.662 | 20 | 1.000 | Y | Y | Unknown |
| NBC00314 | 147 | 3e-36 | 1 | 1 | CE03639 | YYYYS | 0.708 | 19 | 0.987 | Y | Y | Transthyretin-like family |
| NBC00327 | 94 | 2e-20 | 1 | 0 | CE00906 | YYYYS | 0.542 | 25 | 0.998 | Y | Y | Unknown |
| NBC00336 | 138 | 2e-33 | 1 | 0 | CE23545 | YYYYS | 0.903 | 17 | 1.000 | Y | Y | Unknown |
| NBC00354 | 91 | 4e-21 | 4 | 0 | CE16530 | YYYYS | 0.511 | 17 | 0.943 | Y | Y | Unknown |
| NBC00472 | 215 | 8e-57 | 1 | 0 | CE04886 | YYYYS | 0.319 | 15 | 0.999 | Y | Y | Signal sequence receptor |
| NBC00487 | 55 | 7e-09 | 1 | 0 | CE05972 | YYYYS | 0.979 | 21 | 0.988 | Y | Y | Unknown |
| NBC00495 | 51 | 3e-07 | 1 | 1 | CE13171 | YYYYS | 0.566 | 19 | 0.999 | Y | Y | Transthyretin-like family |
| NBC00502 | 176 | 3e-45 | 1 | 0 | CE32298 | YYYYS | 0.634 | 20 | 1.000 | Y | Y | Ectonucleotide pyrophosphatase/phosphodiesterase |
| NBC00592 | 80 | 1e-15 | 0 | 3 | CE17924 | YYYYS | 0.920 | 16 | 1.000 | Y | Y | Unknown |
| NBC00606 | 81 | 4e-16 | 0 | 2 | CE02454 | YYYYS | 0.399 | 20 | 1.000 | Y | Y | Similar to O. volvulus hypodermal antigen Ov-17 |
| NBC00615 | 207 | 3e-54 | 0 | 1 | CE04533 | YYYYS | 0.995 | 18 | 1.000 | Y | Y | LBP-1 fatty acid-binding protein |
| NBC00616 | 61 | 3e-10 | 0 | 1 | CE20257 | YYYYS | 0.754 | 19 | 0.993 | Y | Y | Unknown |
| NBC00633 | 153 | 4e-38 | 0 | 1 | CE03639 | YYYYS | 0.450 | 17 | 1.000 | Y | Y | Transthyretin-like family |
| NBC00641 | 145 | 1e -35 | 0 | 1 | CE33289 | YYYYS | 0.219 | 19 | 0.930 | Y | Y | Unknown |
| NBC00643 | 102 | 2e-22 | 0 | 2 | CE27850 | YYYYS | 0.961 | 17 | 0.999 | Y | Y | Unknown |
| NBC00706 | 50 | 9e-07 | 0 | 1 | CE06014 | YYYYS | 0.466 | 20 | 1.000 | Y | Y | Unknown |
| NBC00720 | 12 | 3e-30 | 0 | 1 | CE16958 | YYYYS | 0.967 | 19 | 0.998 | Y | Y | NLP-13 neuropeptide |
| NBC00742 | 60 | 3e-10 | 0 | 1 | CE16731 | YYYYS | 0.880 | 21 | 0.993 | Y | Y | Unknown |
| NBC00748 | 50 | 4e-07 | 0 | 1 | CE02932 | YYYYS | 0.804 | 17 | 0.998 | Y | Y | Transthyretin-like family |
| NBC00767 | 79 | 7e-16 | 0 | 1 | CE31662 | YYYYS | 0.559 | 17 | 1.000 | Y | Y | Unknown |
| (b) Signal peptides predicted in N. brasiliensis but not C. elegans | ||||||||||||
| NBC00028 | 104 | 1e-23 | 1 | 1 | CE00431 | YYYYS | 0.731 | 18 | 0.999 | Y | N | Globin |
| NBC00124 | 128 | 8e-31 | 1 | 1 | CE00431 | YYYYS | 0.731 | 18 | 0.999 | Y | N | Globin |
| NBC00144 | 195 | 7e-51 | 1 | 0 | CE29663 | YYNYS | 0.866 | 19 | 0.963 | Y | N | Transport-secretion protein |
| NBC00197 | 143 | 8e-35 | 3 | 6 | CE00431 | YYYYS | 0.557 | 16 | 1.000 | Y | N | Globin |
| NBC00272 | 144 | 2e-35 | 1 | 0 | CE32475 | YYNYS | 0.262 | 22 | 0.513 | Y | N | Unknown |
| NBC00328 | 147 | 4e-36 | 3 | 4 | CE00431 | YYYYS | 0.523 | 17 | 0.999 | Y | N | Globin |
| NBC00581 | 122 | 7e-29 | 0 | 1 | CE00431 | YYYYS | 0.404 | 21 | 0.998 | Y | N | Globin |
| NBC00601 | 93 | 5e-20 | 0 | 1 | CE30218 | YYYYS | 0.535 | 34 | 0.944 | Y | N | Unknown |
| NBC00607 | 159 | 4e-40 | 0 | 1 | CE29597 | YYNYS | 0.529 | 18 | 0.786 | Y | N | Unknown |
Entries in table do not match numbers in Figure 2, which includes predicted signal anchors. SignalP criteria are C-score (raw cleavage site score); S-score (signal peptide score); Y-score (combined cleavage site score); mean S score; and assignation as signal peptide (S as in all entries above; otherwise A for signal anchor or N for neither). SignalP scores are as follows: C-p: probability of predicted cleavage site being correct; amino acids: length of predicted signal peptide in amino acids; SP-p: probability of existence of signal peptide; SP?: overall prediction for signal peptide. Note that NBC00028 is almost identical to the cuticular globin of N. brasiliensis (P51536), and NBC00197 and NBC00328 are closely related, whereas NBC0124 and NBC00581 are more similar to, but not identical to, the body-wall form of globin (P51535).
In these cases, and in the four additional examples given in Table 3, it is possible that pre-existing genes have been adapted for secretion or membrane expression in order to promote parasitism. Acquisition of secretory signals may not, in evolutionary terms, be demanding, in view of the report that approximately 20% of protein-coding fragments from Saccharomyces cerevisiae can function as a signal peptide [58]. In the case of the globins, conversion to the secretory pathway (as well as gene multiplication) may be interpreted as a physiological adaptation to the environment within the mammalian gastrointestinal tract [57]. Whether any of the four remaining genes in this category might have undergone a similar evolutionary process to counter immune attack is unknown at this stage.
Similar findings have previously been reported in individual genes from other nematode parasites. In B. malayi, the microfilarial secreted serpin gene (Bm-spn-2) is homologous to eight C. elegans genes, none of which encodes a signal peptide [59]. Likewise, the extracellular glutathione-S-transferase gene, Ov-gst-1, of Onchocerca volvulus has acquired a signal-peptide sequence [60], as has a gene for keratin-like protein (KLP) in N. brasiliensis itself [31]. Hence, conversion of key gene products to secretory function may be a common adaptive strategy for parasitic organisms.
Conclusions
Our study raises both methodological and evolutionary questions. First, it remains to be determined how valid is the assumption that signal sequences reflect secretion into the parasite environment. Clearly, this notion must be qualified in a metazoan parasite, because many such proteins will remain on the cell surface or be sorted to extracellular and extracytosolic compartments within the worm. However, the extent to which signal-peptide-bearing proteins are truly exported by these multicellular organisms will be clarified by current proteomic analyses on proteins secreted by the same adult-stage parasites as were used to construct the cDNA libraries. The same studies will answer a further methodological caveat: proteins can be secreted by non-signal-sequence-dependent pathways, and we have no information on the extent to which parasites may avail themselves of this possibility. One example already exists, of the macrophage migration inhibitory factor homolog of B. malayi which is exported despite lacking a signal peptide [61,62].
On a broader platform, we have addressed the question of whether secreted proteins of parasitic nematodes show accelerated evolution, and our results indicate that this is the case. The predominance of predicted secreted proteins in the novel class prevents us, at this stage, from discerning whether rapid evolution was consequent upon acquiring secretory status, or if the more divergent gene products were those most advantageous to co-opt into secretion. Parallel studies on other parasitic nematodes would now clarify these and additional issues. Have genes for parasite secreted proteins indeed acquired signal peptides, or have free-living lineages lost these motifs in the genes in question? Is more rapid diversification of secreted proteins a specific feature of parasitic nematodes, or can a similar phenomenon be observed in comparisons between divergent free-living organisms (such as C. elegans and C. briggsae)? These questions are now under study.
Materials and methods
Parasite material
N. brasiliensis was maintained in Sprague-Dawley rats as previously described [10,63]. For cDNA synthesis, adult worms were recovered from gastrointestinal contents 5 or 6 days following subcutaneous injection of 3,000 infective L3 larvae. Adults were recovered by Baermannization in saline at 37°C, washed 6 × in saline and 6 × in RPMI1640 containing 100 μg/ml penicillin and 100 U/ml streptomycin. Worms were incubated with 10% gentamicin for 20 min and then washed a further 6 × in RPMI1640 with antibiotics before immersion in Trizol for mRNA preparation.
cDNA libraries
Conventional libraries were constructed in Uni-Zap (Stratagene) and propagated in pBluescript SK+ from mixed adult worm mRNA as previously described [27]. To construct an oligo-capped cDNA library, the technique of Fernández [45] was followed. mRNA was isolated from 1 ml of packed adult N. brasiliensis (approximately 10,000 worms) homogenized in 10 ml Trizol (Gibco Life Technologies). The homogenate was centrifuged (12,000g, 10 min), and the supernatant extracted with chloroform before isopropanol precipitation of RNA from the aqueous phase. mRNA was then purified with PolyA Purist oligo-dT cellulose (Ambion). Following dephosphorylation with calf intestinal phosphatase, mRNA was treated with tobacco acid pyrophosphatase to remove the 7-methylguanosine terminal cap on full-length mRNAs, leaving these with a reactive phosphate group. These were then adducted with the GeneRacer oligonucleotide (Invitrogen). Reverse transcription of mRNA was primed with a tagged oligo-dT (NotI primer-adapter). In this way, full-length transcripts contained specific extension sequences (5' Gene Racer and 3' oligo-dT tag) amenable to PCR amplification. Following PCR, products were ligated at both ends to SalI adapters, so that subsequent digestion with NotI provided inserts with cohesive ends to be directionally cloned into NotI/SalI-digested pSPORT1 vector.
EST sequencing
The library was used to transform DH10B Escherichia coli by electroporation, plated on ampicillin agar petri dishes, and colonies picked for sequencing. All colonies picked were grown overnight in 96-well plates, which were used to provide template samples for PCR before being directly archived. PCR reactions used M13 forward and reverse primers, and following shrimp alkaline phosphatase/exonuclease I treatment, products were directly sequenced with T7 primer on ABI automated sequencers. Archived clones are available on request from R.M.M. Where 3' sequencing was required, T3 primer was used.
Bioinformatics
Raw sequence trace data were processed to screen out vector and linking sequence, to remove low-quality sequence, and to trim poly(dA) tails using an in-house software solution. The resulting sequences were annotated with similarity information and library details and submitted to dbEST. To identify the nonredundant set of putative gene objects, sequences were clustered on the basis of sequence similarity using the CLOBB program [64]. Consensus sequences representing the putative gene objects were then generated from clusters containing more than one sequence using the assembly program phrap (Phil Green, University of Washington; available from [65]). Clusters containing only a single sequence ('singletons') and the consensuses generated from clusters containing more than one sequence ('clusters') were then subjected to the following BLAST analyses: BLASTN against a nonredundant DNA database (GenBank); BLASTX against a nonredundant protein database (SwissProt-trEMBL) and BLASTN against dbEST. Results from these analyses are available from our online database - NEMBASE [49]. Peptide predictions were performed on individual sequences using the program DEcoder [66]. Where DEcoder was unable to predict a peptide, ESTscan [67] was used. SignalP V2.0 [6] was used to predict the presence of secretory signal peptides and signal anchors for each of the predicted proteins. Peptides were defined as bearing a signal peptide if both the hidden Markov model (HMM) predicted the presence of a secretory leader and three of the four parameters defined by the neural network model (C-score, Y-score, S-score and S-mean, as described in legend to Table 3) were fulfilled. Signal anchors were predicted if both the HMM predicted a signal anchor and two of the four criteria specified by the neural network model were fulfilled. Selected clones were subject to comparative analysis with database entries from C. elegans and other species. Alignments were made using Clustal X within MacVector 7.0 (Oxford Molecular) and the SignalP V2.0 web server [68] was used to chart hydrophobicity and potential cleavage sites in predicted protein sequences.
Cross-taxon similarity analysis
The relative similarity between N. brasiliensis EST sequences and those from the related parasitic nematodes Ancylostoma caninum/duodenale, Haemonchus contortus and Teladorsagia circumcincta were plotted with the SimiTri program [54], downloadable from [69].
Acknowledgments
Acknowledgements
We thank Michelle Lizotte-Waniewski for constructing one of the original cDNA libraries in Edinburgh. The work was supported by through the Wellcome Trust, in programme grants to R.M.M. and M.E.S., a project grant to M.L.B. and an International Travelling Fellowship to C.F.
References
- Lightowlers MW, Rickard MD. Excretory-secretory products of helminth parasites: effects on host immune responses. Parasitology. 1988;96:S123–S166. doi: 10.1017/s0031182000086017. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Gomez-Escobar N, Gregory WF, Murray J, Zang X. Immune evasion genes from filarial nematodes. Int J Parasitol. 2001;31:889–898. doi: 10.1016/S0020-7519(01)00213-2. [DOI] [PubMed] [Google Scholar]
- Yatsuda AP, Krijgsveld J, Cornelissen AWCA, Heck AJ, De Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem. 2003;278:16941–16951. doi: 10.1074/jbc.M212453200. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Brunak S, von Heijne G. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng. 1999;12:3–9. doi: 10.1093/protein/12.1.3. [DOI] [PubMed] [Google Scholar]
- Menne KM, Hermjakob H, Apweiler R. A comparison of signal sequence prediction methods using a test set of signal peptides. Bioinformatics. 2000;16:741–742. doi: 10.1093/bioinformatics/16.8.741. [DOI] [PubMed] [Google Scholar]
- Chou KC. Prediction of protein signal sequences. Curr Protein Pept Sci. 2002;3:615–622. doi: 10.2174/1389203023380468. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Meghji M, Ogilvie BM. Restricted sets of parasite antigens from the surface of different stages and sexes of the nematode Nippostrongylus brasiliensis. Immunology. 1983;48:107–121. [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Holland MJ. Parasite immunity: pathways for expelling intestinal parasites. Curr Biol. 1998;8:R711–R714. doi: 10.1016/s0960-9822(98)70455-5. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Bundy DAP, Selkirk ME, Smith DF, Anderson RM. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- MacDonald AS, Araujo MI, Pearce EJ. Immunology of parasitic helminth infections. Infect Immun. 2002;70:427–433. doi: 10.1128/IAI.70.2.427-433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, Yazdanbakhsh M. Regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–743. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr, Madden KB, Svetic A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–1987. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ogilvie BM, Rothwell TLW, Bremner KC, Schnitzerling HJ, Nolan J, Keith RK. Acetylcholinesterase secretion by parasitic nematodes. I. Evidence for secretion by a number of species. Int J Parasitol. 1973;3:589–597. doi: 10.1016/0020-7519(73)90083-0. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Selkirk ME. Characterisation of the secretory acetylcholinesterases from adult Nippostrongylus brasiliensis. Mol Biochem Parasitol. 1992;53:79–88. doi: 10.1016/0166-6851(92)90009-9. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Tang L, Hussein AS, Selkirk ME. Purification and properties of monomeric (G1) forms of acetylcholinesterase secreted by Nippostrongylus brasiliensis. Mol Biochem Parasitol. 1997;90:513–524. doi: 10.1016/S0166-6851(97)00202-8. [DOI] [PubMed] [Google Scholar]
- Healer J, Ashall F, Maizels RM. Characterization of proteolytic enzymes from larval and adult Nippostrongylus brasiliensis. Parasitology. 1991;103:305–314. doi: 10.1017/s0031182000059588. [DOI] [PubMed] [Google Scholar]
- Kamata I, Yamada M, Uchikawa R, Matsuda S, Arizono N. Cysteine protease of the nematode Nippostrongylus brasiliensis preferentially evokes an IgE/IgG1 antibody response in rats. Clin Exp Immunol. 1995;102:71–77. doi: 10.1111/j.1365-2249.1995.tb06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn CC, Selkirk ME. Inactivation of platelet activating factor by a putative acetylhydrolase from the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunology. 1992;75:41–46. [PMC free article] [PubMed] [Google Scholar]
- Grigg ME, Gounaris K, Selkirk ME. Characterization of a platelet-activating factor acetylhydrolase secreted by the nematode parasite Nippostrongylus brasiliensis. Biochem J. 1996;317:541–547. doi: 10.1042/bj3170541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter ML, Ingram L, Tweedie S. Sequence, expression and evolution of the globins of the parasitic nematode Nippostrongylus brasiliensis. Mol Biochem Parasitol. 1994;68:1–14. doi: 10.1016/0166-6851(94)00127-8. [DOI] [PubMed] [Google Scholar]
- Hussein A, Harel M, Selkirk M. A distinct family of acetylcholinesterases is secreted by Nippostrongylus brasiliensis. Mol Biochem Parasitol. 2002;123:125–134. [PubMed] [Google Scholar]
- Hussein AS, Chacón MR, Smith AM, Tosado-Acevedo R, Selkirk ME. Cloning, expression, and properties of a nonneuronal secreted acetylcholinesterase from the parasitic nematode Nippostrongylus brasiliensis. J Biol Chem. 1999;274:9312–9319. doi: 10.1074/jbc.274.14.9312. [DOI] [PubMed] [Google Scholar]
- Hussein AS, Grigg ME, Selkirk ME. Nippostrongylus brasiliensis: characterisation of a somatic amphiphilic acetylcholinesterase with properties distinct from the secreted enzymes. Exp Parasitol. 1999;91:144–150. doi: 10.1006/expr.1998.4360. [DOI] [PubMed] [Google Scholar]
- Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, Takashima M, Himeno K. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect Immun. 2001;69:7380–7386. doi: 10.1128/IAI.69.12.7380-7386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Prichard RK. Comparison of the properties of tubulin from Nippostrongylus brasiliensis with mammalian brain tubulin. Mol Biochem Parasitol. 1988;29:133–140. doi: 10.1016/0166-6851(88)90068-0. [DOI] [PubMed] [Google Scholar]
- Shibui A, Takamoto M, Shi Y, Komiyama A, Sugane K. Cloning and characterization of a novel gene encoding keratin-like protein from nematode Nippostrongylus brasiliensis. Biochim Biophys Acta. 2001;1522:59–61. doi: 10.1016/S0167-4781(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Tweedie S, Grigg ME, Ingram L, Selkirk ME. The expression of a small heat shock protein homologue is developmentally regulated in Nippostrongylus brasiliensis. Mol Biochem Parasitol. 1993;61:149–154. doi: 10.1016/0166-6851(93)90168-W. [DOI] [PubMed] [Google Scholar]
- Hussein AS, Kichenin K, Selkirk ME. Suppression of secreted acetylcholinesterase expression in Nippostrongylus brasiliensis by RNA interference. Mol Biochem Parasitol. 2002;122:91–94. doi: 10.1016/S0166-6851(02)00068-3. [DOI] [PubMed] [Google Scholar]
- Hammond MP, Bianco AE. Genes and genomes of parasitic nematodes. Parasitol Today. 1992;8:299–305. doi: 10.1016/0169-4758(92)90100-G. [DOI] [PubMed] [Google Scholar]
- Muller R. Worms and Human Disease. Wallingford, UK: CABI Publishing; 2002. [Google Scholar]
- Parkinson J, Mitreva M, Hall N, Blaxter M, McCarter JP. 400,000 nematode ESTs on the Net. Trends Parasitol. 2003;19:283–286. doi: 10.1016/S1471-4922(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Williams SA, Lizotte-Waniewski MR, Foster J, Guiliano D, Daub J, Scott AL, Slatko B, Blaxter ML. The filarial genome project: analysis of the nuclear, mitochondrial and endosymbiont genomes of Brugia malayi. Int J Parasitol. 2000;30:411–419. doi: 10.1016/S0020-7519(00)00014-X. [DOI] [PubMed] [Google Scholar]
- Blaxter M, Daub J, Guiliano D, Parkinson J, Whitton C, Filarial Genome Project The Brugia malayi genome project: expressed sequence tags and gene discovery. Trans R Soc Trop Med Hyg. 2002;96:7–17. doi: 10.1016/S0035-9203(02)90224-5. [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Visser A, Otsen M, Tibben J, Lenstra JA, Roos MH. EST sequencing of the parasitic nematode Haemonchus contortus suggests a shift in gene expression during transition to the parasitic stages. Mol Biochem Parasitol. 2000;110:53–68. doi: 10.1016/S0166-6851(00)00255-3. [DOI] [PubMed] [Google Scholar]
- Daub J, Loukas A, Pritchard D, Blaxter ML. A survey of genes expressed in adults of the human hookworm Necator americanus. Parasitology. 2000;120:171–184. doi: 10.1017/S0031182099005375. [DOI] [PubMed] [Google Scholar]
- Lizotte-Waniewski M, Tawe W, Guiliano DB, Lu W, Liu J, Williams SA, Lustigman S. Identification of potential vaccine and drug target candidates by expressed sequence tag analysis and immunoscreening of Onchocerca volvulus larval cDNA libraries. Infect Immun. 2000;68:3491–3501. doi: 10.1128/IAI.68.6.3491-3501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter JP, Mitreva MD, Martin J, Dante M, Wylie T, Rao U, Pape D, Bowers Y, Theising B, Murphy CV, et al. Analysis and functional classification of transcripts from the nematode Meloidogyne incognita. Genome Biol. 2003;4:R26. doi: 10.1186/gb-2003-4-4-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JE, Daub J, Guilliano D, McDonnell A, Lizotte-Waniewski M, Taylor D, Blaxter M. Analysis of genes expressed at the infective larval stage validate the utility of Litomosoides sigmodontis as a murine model for filarial vaccine development. Infect Immun. 2000;68:5454–5458. doi: 10.1128/IAI.68.9.5454-5458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh KKA, Loukas A, Tripp C, Maizels RM. Identification of abundantly-expressed novel and conserved genes from infective stage larvae of Toxocara canis by an expressed sequence tag strategy. Infect Immun. 1999;67:4771–4779. doi: 10.1128/iai.67.9.4771-4779.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández C, Gregory WF, Loke P, Maizels RM. Full-length-enriched cDNA libraries from Echinococcus granulosus contain separate populations of oligo-capped and trans-spliced transcripts and a high level of predicted signal peptide sequences. Mol Biochem Parasitol. 2002;122:171–180. doi: 10.1016/S0166-6851(02)00098-1. [DOI] [PubMed] [Google Scholar]
- The C. elegans Genome Consortium Genome sequence of Caenorhabditis elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent MR, Chen N, Chinwalla A, Clarke L, Clee C, Coghlan A, et al. The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 2003;1:E45. doi: 10.1371/journal.pbio.0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Blaxter lab nematode genomics http://www.nematodes.org
- Liu J, Rost B. Comparing function and structure between entire proteomes. Protein Sci. 2001;10:1970–1979. doi: 10.1110/ps.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen TW. Trans-splicing of nematode premessenger RNA. Annu Rev Microbiol. 1993;47:413–440. doi: 10.1146/annurev.mi.47.100193.002213. [DOI] [PubMed] [Google Scholar]
- Blaxter M, Liu L. Nematode spliced leaders - ubiquity, evolution and utility. Int J Parasitol. 1996;26:1025–1033. doi: 10.1016/S0020-7519(96)00060-4. [DOI] [PubMed] [Google Scholar]
- Anderson RC. Nematode Parasites of Vertebrates: Their Development and Transmission. Wallingford, UK: CAB International; 1992. [Google Scholar]
- Parkinson J, Blaxter M. SimiTri - visualizing similarity relationships for groups of sequences. Bioinformatics. 2003;19:390–395. doi: 10.1093/bioinformatics/btf870. [DOI] [PubMed] [Google Scholar]
- Loukas A, Maizels RM. Helminth C-type lectins and host-parasite interactions. Parasitol Today. 2000;16:333–339. doi: 10.1016/S0169-4758(00)01704-X. [DOI] [PubMed] [Google Scholar]
- Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Adv Parasitol. 1999;43:161–266. doi: 10.1016/s0065-308x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- Blaxter ML. Nemoglobins: divergent nematode globins. Parasitol Today. 1993;9:353–360. doi: 10.1016/0169-4758(93)90082-Q. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Preuss D, Grisafi P, Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987;235:312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Zang X, Maizels RM. Serine proteinase inhibitors from nematodes and the arms race between host and pathogen. Trends Biochem Sci. 2001;26:191–197. doi: 10.1016/S0968-0004(00)01761-8. [DOI] [PubMed] [Google Scholar]
- Sommer A, Nimtz M, Conradt HS, Brattig N, Boettcher K, Fischer P, Walter R, Liebau E. Structural analysis and antibody response to the extracellular glutathione S-transferases from Onchocerca volvulus. Infect Immun. 2001;69:7718–7728. doi: 10.1128/IAI.69.12.7718-7728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana DV, Raghavan N, FitzGerald P, Eisinger SW, Metz C, Bucala R, Schleimer RP, Bickel C, Scott AL. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang XX, Taylor P, Meyer D, Wang JM, Scott AL, Walkinshaw MD, Maizels RM. Homologues of human macrophage migration inhibitory factor from a parasitic nematode: gene cloning, protein activity and crystal structure. J Biol Chem. 2002;277:44261–44267. doi: 10.1074/jbc.M204655200. [DOI] [PubMed] [Google Scholar]
- Camberis M, Le Gros G, Urban J., Jr . Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. In: Coico R, editor. In Current Protocols in Immunology. New York: John Wiley and Sons; 2003. pp. 19.12.11–19.12.27. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Guiliano DB, Blaxter M. Making sense of EST sequences by CLOBBing them. BMC Bioinformatics. 2002;3:31. doi: 10.1186/1471-2105-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Phred/Phrap/Consed system home page http://www.phrap.org
- Fukunishi Y, Hayashizaki Y. Amino acid translation program for full-length cDNA sequences with frameshift errors. Physiol Genomics. 2001;5:81–87. doi: 10.1152/physiolgenomics.2001.5.2.81. [DOI] [PubMed] [Google Scholar]
- Iseli C, Jongeneel CV, Bucher P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Int Conf Intell Syst Mol Biol. 1999:138–148. [PubMed] [Google Scholar]
- SignalP server http://www.cbs.dtu.dk/services/SignalP-2.0
- Index of /SimiTri http://www.nematodes.org/SimiTri


