Abstract
Nymphaea nouchali Burm. f. (Family – Nymphaeaceae) is a well-known medicinal plant used in the Indian ayurvedic system of medicine for treating diabetes. The seeds especially have been prescribed for diabetes. The hydroalcoholic extract of N. nouchali seeds has been demonstrated to possess anti-hyperglycemic effects in diabetic rats, but the functional mechanism remains unknown. The nuclear receptor, peroxisome proliferator-activated receptor gamma (PPARγ) is noted to play an important role in glucose and lipid homeostasis. This study was hence focused in evaluating the effect of the extract on PPARγ activation, adipocyte differentiation, and glucose consumption in 3T3-L1 cells. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), followed by adipogenesis assay using Oil Red O technique. Glucose consumption of preadipocytes and adipocytes in the presence of the extract was also determined. Real-time polymerase chain reaction was performed to identify the expression of genes involved in glucose consumption in the adipocytes. MTT assay confirmed the extract to be nontoxic, and Oil Red O staining confirmed enhanced adipocyte differentiation of 3T3-L1 cells in a dose-dependent manner. The extract also increased the expression of PPARγ target gene, which in turn enhanced the expression of GLUT-4. The data, therefore, suggests that N. nouchali seed extract promotes adipocyte differentiation and glucose consumption by inducing PPARγ activation, which in turn increases mRNA GLUT-4 expression and subsequently enhances insulin-responsiveness in insulin target tissues.
Keywords: Adipogenesis, glucose uptake, GLUT-4, herbal drugs, insulin resistance, Nymphaea stellata, type 2 diabetes
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a metabolic disorder that has affected 2.8% of the world's population in 2000 and is expected to rise to 4.4% in 2030.[1] Insulin resistance is found to play a central role in the development of T2DM, which co-exists with central obesity, hypertension, dyslipidemia, and atherosclerosis.[2]
Among the various organs, adipose tissue plays a vital role in the onset and development of insulin resistance by ectopic lipid deposition or by the increased release of free fatty acids into the circulation. Peroxisome proliferator-activated receptor gamma (PPARγ) is a class of ligand-dependent nuclear receptor transcription factors, which are regulators of lipid metabolism and decreases the plasma concentration of fatty acids by their activation. PPARγ has the highest expression levels in adipose tissue compared with other metabolic organs, such as skeletal muscle, liver, and pancreas.[3] PPARγ plays an important role in converting preadipocytes into differentiated adipocytes. Expression and activation of PPARγ induces adipose conversion.[4] Also to its role in adipocyte differentiation and lipid metabolism, PPARγ is crucial for controlling the expression of genes, which are involved in glucose homeostasis.[5] PPARγ activation results in an increase in the expression of a number of downstream target genes that are critically involved in glucose and lipid metabolism[6] as well as an aid in insulin signal transduction.[7]
The glucose transporter, GLUT-4 is responsible for the uptake of glucose by an insulin-regulated mechanism in both muscle and adipose tissue.[8] GLUT-4 is directly involved in whole body glucose homeostasis and peripheral tissue glucose uptake by insulin receptor stimulation.[9] PPARγ ligand binding directly plays a key role in glucose uptake by increasing GLUT-4 expression and subsequently enhancing insulin responsiveness.[10] Increased PPARγ expression in adipose tissue results in increased GLUT-4. Glucose transport occurs after the expression of PPARγ.[4]
Recent advances have demonstrated that pharmacological agents of thiazolidinedione (TZD) class can reverse insulin resistance and profoundly improve many of the associated symptoms. TZDs and even few other non-TZDs mediate antidiabetic effects in the insulin-resistant states. The insulin-sensitizing effect of such chemicals mediates via the stimulation of PPARγ. Hence, it appears that PPARγ is a reasonable, viable drug target in treating insulin resistance and diabetes.[11] However, their adverse effects limit their, long-term usage. There is a need for identifying new antidiabetic compounds that can efficiently restore blood glucose levels without side-effects of the current treatments.[12] Hence, the demand for new antidiabetic compounds from plant source continues.[13] Therefore, it is desirable to identify plant-based molecules with limited or no adverse effects.
Nymphaea nouchali Burm. f. (syn. Nymphaea stellata Willd.), commonly known as water lily, is an extensively-used medicinal plant by the Indian Siddha and Ayurvedic practitioners for the treatment of diabetes, inflammation, liver disorders, urinary disorders and as a bitter tonic.[14] It belongs to the family Nymphaeaceae. The seeds of this plant have been reported to be an excellent antioxidant[15] and antihepatotoxic agent.[16] Few phenolic compounds have been quantified[15] although, only a single compound, called nymphasterol has been so far isolated and characterized.[17] The antidiabetic activity of the seeds has been established very recently in vivo in streptozotocin-induced diabetic rats.[18] However, its potential mechanism of action has not been clearly elucidated at the molecular level. Since insulin-mediated glucose uptake in the peripheral tissues is one of the major therapeutic approaches in treating diabetes, this study was focused to evaluate the effect of the hydroalcoholic seed extract of N. nouchali on glucose consumption in 3T3-L1 adipocytes.
MATERIALS AND METHODS
Chemicals and reagents
Dulbecco's Modified Eagle's Medium (DMEM), fetal calf serum (FCS), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and bovine serum albumin (BSA) were obtained from HiMedia Laboratories, India. 3-isobutylmethyl xanthine (IBMX), dexamethasone (DEX) and gene specific primers were procured from Sigma-Aldrich Chemicals, India.
Plant material and extraction
N. nouchali seeds were collected from a pond in Kanyakumari, South India. The plant was identified and authenticated by a plant taxonomist, Plant Anatomy Research Centre, Chennai and a voucher specimen was deposited in the herbarium (PARC/2012/1248). The seeds were harvested after the flowering stage, air-dried under shadow and ground to obtain a coarse powder. About 500 g of the powder was extracted using a Soxhlet apparatus in 70% ethanol for 24 h. The liquid extract was filtered, and the filtrate was concentrated using a rotary evaporator at 40°C until the solvent was completely removed. The resultant powder, N. nouchali hydroalcoholic seed (NHS) extract was stored at 4°C until further use.
Cell culture and adipocyte differentiation
3T3-L1 murine preadipocytes were purchased from National Centre for Cell Science, Pune, India and cultured in DMEM-high glucose media supplemented with 10% FCS, 2 mM L-glutamine and antibiotics (50 kIU/l penicillin and 0.05 g/l streptomycin); at 37°C under a humidified 5% CO2 and 95% air atmosphere. For 3T3-L1 preadipocyte differentiation, cells at 2 days postconfluency were cultured in differentiation medium (MDI) comprising DMEM supplemented with 250 mM IBMX, 1 mM DEX, 670 nM insulin and 10% FBS. After 3 days, the differentiated adipocytes were maintained in DMEM containing 670 nM insulin and 10% FBS.[19]
Cell viability assay
The viability of the cells was assessed by performing MTT assay with minor modifications.[20] 3T3-L1 preadipocytes were seeded in 200 μl DMEM in a 96-well microplate at a density of 5000 cells/well. The plate was incubated at 37°C overnight to allow the cells to attach and multiply. After 48 h of growth, NHS extract at concentrations ranging from 1 ng to 10 μg was added to the cells. After 24 h of incubation, cells were washed with phosphate-buffered saline (PBS) and incubated with 50 μl MTT, 5 mg/ml dissolved in DMEM for 3 h at 37°C. The medium was then removed, and formazan crystals formed in live cells were solubilized with DMSO. The optical density was measured at 560 nm using an ELISA reader.
Adipogenesis assay
For adipogenesis study, 3T3-L1 preadipocytes cultured in DMEM supplemented with 10% FCS were preincubated with or without various concentrations of NHS extract (50 ng to 1 μg) for 24 h. Differentiation was induced by MDI media containing 250 mM IBMX, 1 mM DEX and 670 nM insulin with 10% FBS for 3 days. Differentiated adipocytes were maintained in DMEM containing 10% FBS and 670 nM insulin for 8–10 days. Fresh DMEM containing the same, with or without NHS extract was replenished every 3 days. The differentiated cells were fixed with 10% formalin in PBS and incubated for 1 h at room temperature. Further, it was washed thrice with PBS and stained with Oil Red O (0.5% in 60% isopropanol, soaked overnight and filtered) for 10 min. After washing thrice with distilled water, the cells were photographed under a microscope. Lipid and Oil Red O were eluted with isopropanol, and the absorbance was read at 540 nm using UV/Vis spectrophotometer.[21,22]
Glucose consumption assay
Preadipocytes and fully differentiated adipocytes in 24-well plates were incubated with serum-free DMEM containing 0.2% BSA for 24 h and then incubated with various concentrations of NHS extract (1 ng to 500 ng). The medium was removed, and its glucose concentration was determined by glucose oxidase – peroxidase method. The amount of glucose consumed was calculated by subtracting the glucose in the medium from the glucose in blank wells. Results were expressed as fold change in glucose consumption by comparing with the untreated control.[23]
RNA isolation and real-time polymerase chain reaction analysis
Total RNA was isolated from cells using the TRI reagent[24] and 1 μg of total RNA from each sample was reverse transcribed to cDNA employing random primers, reverse transcriptase and dNTPs suspended in buffer[25] according to the manufacturer's protocol (high cDNA, Applied Biosystems). After cDNA synthesis, target cDNA levels were determined by SYBR green-based real-time polymerase chain reaction (RT-PCR) performed in Applied Biosystems 7500 according to the manufacturer's instruction in 20 μl reaction buffer containing 1X SYBR green master mix, 300 nM of forward and reverse primers and 25 ng/μl cDNA. The PCR conditions were 1 cycle of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The primer sequences used for the PCR amplification were as follows:
β-actin, 5’-GGCCAACCGTGAAAAGATG-3’ and 5’-GGATCTTCATGAGGTAGTCTGTC-3’;
PPARα, 5’-GCCATCTTCACGATGCTGTCCTCC-3’ and 5’-GTAGATCTCTTGCAACAGTGGGTGC-3’;
PPARγ, 5’-GGATTCATGACCAGGGAGTTCCTC-3’ and 5’-GCGGTCTCCACTGAGAATAATGAC-3’;
GLUT-4, 5’-CTCATGGGCCTAGCCAATG-3’ and 5’-GGGCGATTTCTCCCACATAC-3’.
The change of fluorescence of SYBR green dye in every cycle was monitored and threshold (ct) above background for each reaction was calculated. The expression levels were normalized to the housekeeping gene, β-actin in the same reaction and the results were expressed as fold change.
Statistical analysis
Data are presented as mean ± standard error of mean and were analyzed using SPSS software 20 (Armonk, New York: IBM Corp.). Further, it was determined by Bonferroni's test using one-way analysis of variance. The statistical difference at P < 0.05 was considered significant.
RESULTS
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
Even though N. nouchali is a naturally occurring water plant that is being used traditionally for treating diabetes, its hydroalcoholic extract may exert toxic effect depending on their concentration and duration of exposure. Hence to assess the viability concentration range of NHS extract, the cell viability assay was performed in various concentrations by employing MTT assay in 3T3-L1 cell line. The cell viability assessed after 24 h of incubation with NHS extract was found to be nontoxic till a concentration of 2.5 μg, beyond, which it showed reduced cell viability when compared with the control [Figure 1]. Henceforth, further cell-based assays were performed within this concentration range.
Figure 1.
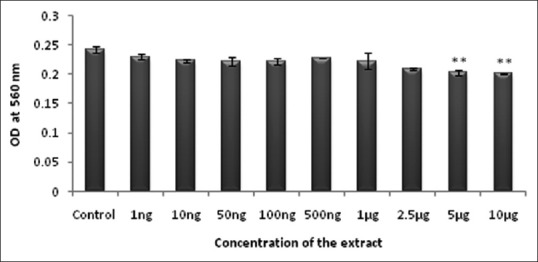
Effect of Nymphaea nouchali on cell viability. Values represent mean ± standard error of mean. **P < 0.01 versus the control group. Control: Untreated preadipocytes
Impact of Nymphaea nouchali hydroalcoholic seed extract on adipogenesis
Adipogenesis is a cellular process in which metabolically less active preadipocytes differentiate to metabolically active adipocytes. Once the cells get differentiated to adipocytes, it becomes a depot for lipid storage. Hence, studying the impact of the antidiabetic drug on this crucial process helps in the elucidation of the mechanism of action and also categorization of the drug. NHS extract at different concentrations tested on adipocyte differentiation showed a dose-dependent increase in the accumulation of lipid up to 1 μg concentration [Figure 2]. The cells treated with NHS extract showed distinct oil droplets when stained with Oil Red O [Figure 3]. Hence, the extract promotes adipogenesis which may be helpful in reducing circulating lipids in blood and thereby reducing the risk of cardiovascular diseases.
Figure 2.
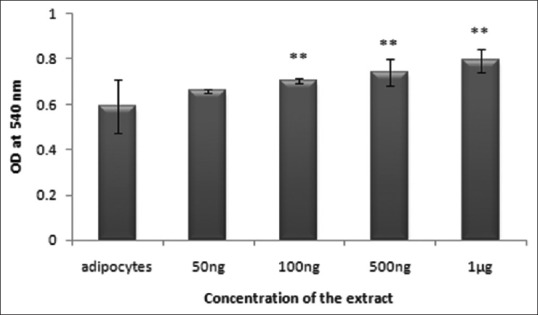
Effect of Nymphaea nouchali on lipid accumulation by Oil Red O staining. Values represent mean ± standard error of mean. **P < 0.01 versus the control group. Adipocytes: Untreated control
Figure 3.
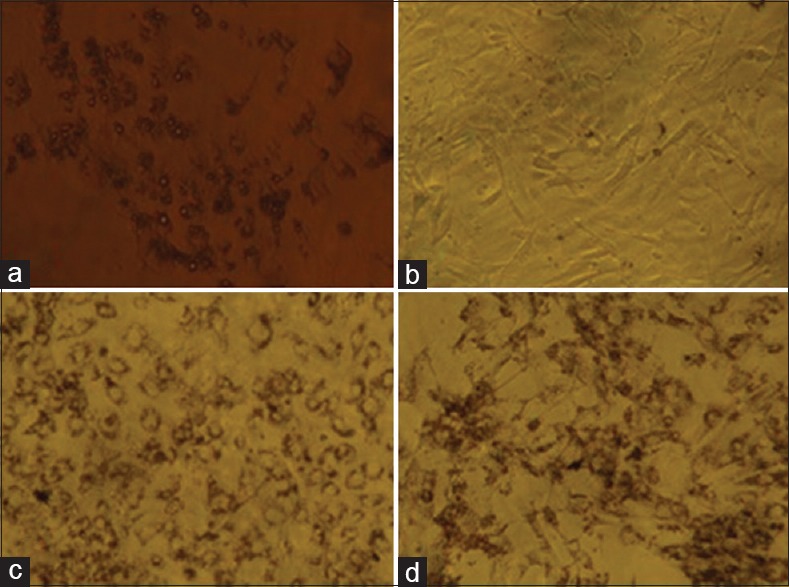
Oil Red O staining showing adipogenesis. (a) Unstained adipocytes showing lipid droplets around the nucleus due to adipocyte differentiation; (b) Preadipocyte stained with Oil Red O, showing no lipid accumulation in the cells; (c) Adipocyte stained with Oil Red O, showing red-colored lipid droplets in the cells; (d) Nymphaea nouchali extract-treated adipocyte stained with Oil Red O, showing red-colored lipid droplets in the cells
Improved glucose consumption by Nymphaea nouchali hydroalcoholic seed extract
Antidiabetic efficiency of NHS extract was evaluated both in preadipocytes and adipocytes. Drugs, which promote glucose transport into the cell will be useful in reducing hyperglycemia, as glucose uptake is the major event getting affected during diabetes. Treatment with NHS extract promoted glucose uptake in preadipocytes and adipocytes. A maximum of 3.3-fold increase in glucose consumption of preadipocytes was observed at 24th h incubation of 100 ng of NHS extract [Figure 4]. Likewise, a maximum of 3.2-fold increase in glucose consumption of adipocytes was observed at 6th h of incubation with 50 ng NHS extract [Figure 5]. The results of glucose consumption assay depict the antidiabetic property of NHS extract.
Figure 4.
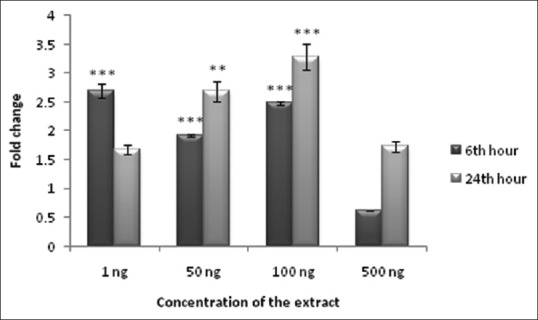
Glucose consumption in Nymphaea nouchali-treated 3T3-L1 preadipocytes. Values represent mean ± standard error of mean. **P < 0.01, ***P < 0.001 versus the control group. Preadipocytes without extract treatment were considered as control
Figure 5.
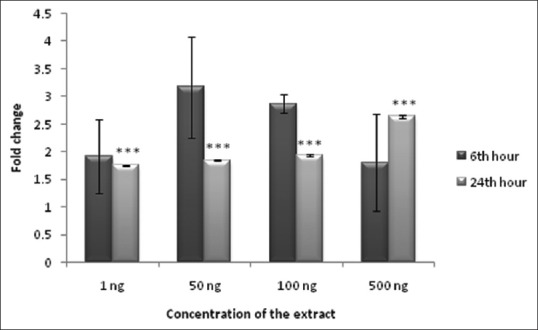
Glucose consumption in Nymphaea nouchali-treated 3T3-L1 adipocytes. Values represent mean ± standard error of mean. ***P < 0.001 versus the control group. Adipocytes without extract treatment were considered as control
Real-time polymerase chain reaction
Real-time PCR quantification of gene transcripts is more reliable and time-saving. Quantification of total RNA isolated for RT-PCR by using a Nanodrop exhibited a good yield. Expressions of PPARα, PPARγ and GLUT-4 were studied using RT-PCR. In the present study, expression of PPARα was seen increasing in a dose-dependent manner by NHS treatment. Treatment with 100 ng of the extract induced 2.75-fold, whereas treatment with 1 μg of the extract induced 3.31-fold activity. Fold change in the expression of PPARγ on treatment with NHS increased with the increase in concentration up to 100 ng with 2.89-fold increase after which there was a drop in the level of expression. GLUT-4 was found to be much elevated in NHS-treated cells and was found to be maximum at 100 ng showing 5.76-fold increase [Figure 6]. The expression of GLUT-4 was consistent with the expression of PPARγ.
Figure 6.
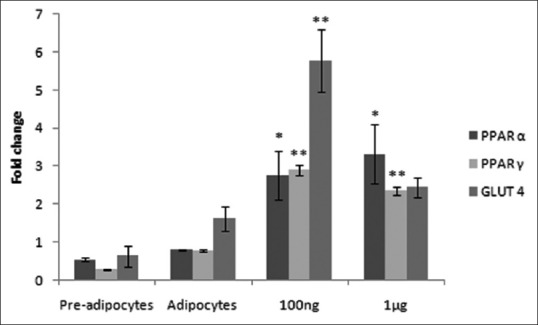
Expression of peroxisome proliferator-activated receptor α, peroxisome proliferator-activated receptor gamma and GLUT-4 genes. Values represent mean ± standard error of mean. *P < 0.05, **P < 0.01, versus the control group adipocytes
DISCUSSION
This study demonstrates that the hydroalcoholic extract of N. nouchali seeds stimulate adipocyte differentiation and glucose uptake in murine 3T3-L1 adipocytes and propose for the first time the mechanism of action for the antidiabetic property of the plant. 3T3-L1 preadipocyte cells derived from mice are an ideal cell line for screening antidiabetic drugs.[26]
Although the plant is known for its folklore medicinal value, it is necessary to find whether NHS exhibit toxicity in the cells. MTT assay was conducted to address this issue. Since MTT reduction occurs in active cells, the level of activity is a measure of the viability of the cells. Herbal products can either be toxic[27] or nontoxic to the cells.[28] However, NHS at lower concentration did not appreciably affect the cell viability. Subsequently the concentration of these cells was adjusted for analyzing differentiation and adipogenic factor expression at mRNA levels.
Impaired adipocyte differentiation contributes to the pathogenesis of obesity-related conditions such as insulin resistance and T2DM.[29] The adipocytes of type 2 diabetes patients are incapable of accumulating lipid due to insulin resistance.[23] NHS had the potential to stimulate adipogenesis, and this was evidenced by an increase in lipid accumulation after NHS treatment. Oil Red O staining showed that the intracellular lipid accumulation was greater in extract-treated cells. The adipocyte differentiation was accelerated on treatment, and the increase was dependent on NHS concentration. Accumulating large amounts of lipids in adipocytes is nontoxic to the cell or whole organism which, in a way, prevents extra-adipose lipid accumulation leading to lipotoxicity and insulin resistance.[30]
PPARγ has emerged as the prime regulator of adipocyte differentiation. It is an essential transcriptional regulator of adipogenesis and is required for the maintenance of adipocyte function.[31] Our present data showed that the mRNA expression of PPARγ increased in NHS-treated cells. A previous work has reported that enhanced PPARγ expression stimulated strong adipogenesis in 3T3-L1 cells.[32] PPARγ activation has also proved to promote adipocyte differentiation by increasing the number of small adipocytes and decreasing the number of hypertrophied adipocytes by apoptosis.[33]
PPARγ is a key transcriptional factor to regulate adipogenic gene expression during adipocyte proliferation and differentiation[34] and is also a key regulator of glucose homeostasis. It has been reported that PPARγ activation plays an important role in maintaining glucose homeostasis. Adipocyte differentiation leads to enhanced expression of adipocyte-specific genes, which are important components of the insulin receptor signal transduction pathway.[3] Hence, the effect of NHS on glucose consumption was investigated. NHS significantly enhanced insulin-stimulated glucose consumption in a dose-dependent manner when compared to control in both adipocytes and preadipocytes. NHS extract in adipose tissue acts as a key switch that favors lipid storage in adipocytes over fatty acid release into the circulation.
Adipocytes also regulate the whole-body insulin sensitivity. GLUT-4 abundantly expressed in adipocytes plays a vital role in the control of blood sugar.[35] Though many studies have focused on the mechanism underlying insulin-regulated GLUT-4 trafficking,[36] an established pathway is upregulation of GLUT-4 expression on activation of PPARγ. Pharmacological drugs which increase glucose transporter expression have a positive impact on whole body glucose homeostasis.[37] In this study exposure to NHS increased mRNA expression of GLUT-4, which confirms the potential of NHS to improve cellular glucose uptake. We hypothesize that this effect may be related to the ability of NHS to act as a ligand, binding to the PPARγ binding domain and up-regulate GLUT-4 expression. Adipose-specific PPARγ knock-out mice in a previous study displayed grossly elevated levels of free fatty acids and triglycerides and the anti-lipolytic effect of insulin was blunted in these animals which indicate a strong correlation between PPARγ expression and insulin sensitivity.[38]
Recently, several studies have shown that PPARα is expressed in adipose tissue, indicating that adipose tissue might also be a favorable target of PPARα activators. Although a weak target for promoting adipogenesis, PPARα is reported to stimulate peripheral glucose uptake by its expression in the brain.[39] In our study, the mRNA expression of PPARα was increased to 2.75-fold with 100 ng NHS. It is believed that PPARα activation results in hypolipidemic effects, insulin-stimulated glucose uptake and increase in the expression of adipogenic genes but, however, the role of PPARα in adipose tissue is not fully understood.[40] When there was an increase in PPARα to 3.31-fold in 1 μg NHS-treated cells, the expression of PPARγ and GLUT-4 were repressed explaining the effect of NHS in a dose-dependent manner.
Extracts from medicinal and food plants have been identified as potentially interesting PPAR agonists and are found to increase insulin-stimulated glucose uptake by activating PPARγ and PPARα.[41] Many natural compounds exhibit PPARγ agonist activity.[42] The phenolic compounds in NHS might have efficiently facilitated GLUT-4 expression and activation and simultaneous glucose uptake activity.[43] As dual activators of PPARγ and PPARα, NHS could ameliorate insulin resistance and dyslipidemia, however, the effects of NHS on PPARα expression is yet to be elaborated through further studies. The results suggest that NHS may modulate the insulin signaling pathway, thereby positively regulating glucose consumption in adipocytes which are attributed partly to the expression of PPARγ. However, further studies are required to clarify whether there are alternative pathways responsible for the NHS-induced effects to attenuate insulin resistance.
CONCLUSION
This is the first in vitro mechanistic study on N. nouchali seed extract confirming its traditional use in diabetes. The extract is shown to promote adipogenesis. It also enhances glucose uptake as evidenced by GLUT-4 expression mediated by PPARγ activation and thereby improves insulin sensitivity. Hence, this study provides evidence that the extract has an antidiabetic effect by activating the PPARγ gene in the adipocytes. Besides, it may also ameliorate insulin resistance in other insulin-responsive organs by reducing the free circulating lipids. Its pharmacological activity is probably due to the synergistic action of phytocompounds in the extract. Therefore, this plant can be further studied to identify the active pharmaceutical ingredients. Moreover, efficient methodologies have to be adopted to enhance its availability in order to develop N. nouchali as a natural anti-diabetic agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The first author thanks the MANF Scheme, University Grants Commission, New Delhi, India, for providing fellowship during the course of this study. The authors would like to acknowledge the help and support provided by Natural Products Research Laboratory, Department of Biotechnology, Pondicherry University, for carrying out the study.
REFERENCES
- 1.Sangeetha KN, Sujatha S, Muthusamy VS, Anand S, Nithya N, Velmurugan D, et al. 3beta-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim Biophys Acta. 2010;1800:359–66. doi: 10.1016/j.bbagen.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal R, Sethiya NK, Mishra SH. Antidiabetic activity of alkaloids of Aerva lanata roots on streptozotocin-nicotinamide induced type-II diabetes in rats. Pharm Biol. 2013;51:635–42. doi: 10.3109/13880209.2012.761244. [DOI] [PubMed] [Google Scholar]
- 3.Mansor F, Gu HF, Ostenson CG, Mannerås-Holm L, Stener-Victorin E, Wan Mohamud WN. Labisia pumila upregulates peroxisome proliferator-activated receptor gamma expression in rat adipose tissues and 3T3-L1 adipocytes. Adv Pharmacol Sci. 2013;2013:808914. doi: 10.1155/2013/808914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernyhough ME, Okine E, Hausman G, Vierck JL, Dodson MV. PPARgamma and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007;33:367–78. doi: 10.1016/j.domaniend.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARγ signaling and metabolism: The good, the bad and the future. Nat Med. 2013;19:557–66. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drira R, Sakamoto K. Modulation of adipogenesis, lipolysis and glucose consumption in 3T3-L1 adipocytes and C2C12 myotubes by hydroxytyrosol acetate: A comparative study. Biochem Biophys Res Commun. 2013;440:576–81. doi: 10.1016/j.bbrc.2013.09.106. [DOI] [PubMed] [Google Scholar]
- 7.Olefsky JM, Saltiel AR. PPAR gamma and the treatment of insulin resistance. Trends Endocrinol Metab. 2000;11:362–8. doi: 10.1016/s1043-2760(00)00306-4. [DOI] [PubMed] [Google Scholar]
- 8.Berenguer M, Zhang J, Bruce MC, Martinez L, Gonzalez T, Gurtovenko AA, et al. Dimethyl sulfoxide enhances GLUT4 translocation through a reduction in GLUT4 endocytosis in insulin-stimulated 3T3-L1 adipocytes. Biochimie. 2011;93:697–709. doi: 10.1016/j.biochi.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–93. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- 10.Karnieli E, Armoni M. Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: From physiology to pathology. Am J Physiol Endocrinol Metab. 2008;295:E38–45. doi: 10.1152/ajpendo.90306.2008. [DOI] [PubMed] [Google Scholar]
- 11.Guo L, Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacol Ther. 2006;111:145–73. doi: 10.1016/j.pharmthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Zapata-Bustos R, Alonso-Castro AJ, Gómez-Sánchez M, Salazar-Olivo LA. Ibervillea sonorae (Cucurbitaceae) induces the glucose uptake in human adipocytes by activating a PI3K-independent pathway. J Ethnopharmacol. 2014;152:546–52. doi: 10.1016/j.jep.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Kalekar SA, Munshi RP, Bhalerao SS, Thatte UM. Insulin sensitizing effect of 3 Indian medicinal plants: An in vitro study. Indian J Pharmacol. 2013;45:30–3. doi: 10.4103/0253-7613.106431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja MK, Sethiya NK, Mishra SH. A comprehensive review on Nymphaea stellata: A traditionally used bitter. J Adv Pharm Technol Res. 2010;1:311–9. doi: 10.4103/0110-5558.72424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parimala M, Shoba FG. Phytochemical analysis and in vitro antioxidant activity of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. Asian Pac J Trop Biomed. 2013;3:887–95. [Google Scholar]
- 16.Verma A, Ahmed B. Anti-hepatotoxicactivity of Nymphaea stellata seeds in carbon tetrachloride induced toxicity. Indian J Nat Prod. 2009;5:1–4. [Google Scholar]
- 17.Verma A, Ahmed B, Upadhyay R, Soni N. Nymphasterol, a new steroid from Nymphaea stellata. Med Chem Res. 2012;21:783–7. [Google Scholar]
- 18.Parimala M, Shoba FG. Evaluation of antidiabetic potential of Nymphaea nouchali Burm. f seeds in STZ-induced diabetic rats. Int J Pharm Pharm Sci. 2014;6:536–41. [Google Scholar]
- 19.Choi SS, Cha BY, Lee YS, Yonezawa T, Teruya T, Nagai K, et al. Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Life Sci. 2009;84:908–14. doi: 10.1016/j.lfs.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Castro AJ, Zapata-Bustos R, Domínguez F, García-Carrancá A, Salazar-Olivo LA. Magnolia dealbata Zucc and its active principles honokiol and magnolol stimulate glucose uptake in murine and human adipocytes using the insulin-signaling pathway. Phytomedicine. 2011;18:926–33. doi: 10.1016/j.phymed.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Hemati N, Ross SE, Erickson RL, Groblewski GE, MacDougald OA. Signaling pathways through which insulin regulates CCAAT/enhancer binding protein alpha (C/EBPalpha) phosphorylation and gene expression in 3T3-L1 adipocytes. Correlation with GLUT4 gene expression. J Biol Chem. 1997;272:25913–9. doi: 10.1074/jbc.272.41.25913. [DOI] [PubMed] [Google Scholar]
- 22.Kasturi R, Joshi VC. Hormonal regulation of stearoyl coenzyme A desaturase activity and lipogenesis during adipose conversion of 3T3-L1 cells. J Biol Chem. 1982;257:12224–30. [PubMed] [Google Scholar]
- 23.Choi SS, Cha BY, Iida K, Lee YS, Yonezawa T, Teruya T, et al. Artepillin C, as a PPARγ ligand, enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biochem Pharmacol. 2011;81:925–33. doi: 10.1016/j.bcp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–5. [PubMed] [Google Scholar]
- 25.Bonetta L. Prime time for real-time PCR. Nat Methods. 2005;2:305–12. [Google Scholar]
- 26.Sangeetha MK, ShriShri Mal N, Atmaja K, Sali VK, Vasanthi HR. PPAR's and Diosgenin a chemico biological insight in NIDDM. Chem Biol Interact. 2013;206:403–10. doi: 10.1016/j.cbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Kamalakkannan S, Rajendran R, Venkatesh RV, Clayton P, Akbarsha MA. Effect of Caralluma fimbriata extract on 3T3-L1 pre-adipocyte cell division. Food Nutr Sci. 2011;2:329–36. [Google Scholar]
- 28.Zhang CH, Xu GL, Liu YH, Rao Y, Yu RY, Zhang ZW, et al. Anti-diabetic activities of gegen qinlian decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine. 2013;20:221–9. doi: 10.1016/j.phymed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Guerre-Millo M. Adipose tissue and adipokines: For better or worse. Diabetes Metab. 2004;30:13–9. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 30.Christodoulides C, Vidal-Puig A. PPARs and adipocyte function. Mol Cell Endocrinol. 2010;318:61–8. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Imai T, Takakuwa R, Marchand S, Dentz E, Bornert JM, Messaddeq N, et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci U S A. 2004;101:4543–7. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Park KK, Lee SK, Lee SE, Hwang JK. Cornus kousa F. Buerger ex Miquel increases glucose uptake through activation of peroxisome proliferator-activated receptor γ and insulin sensitization. J Ethnopharmacol. 2011;133:803–9. doi: 10.1016/j.jep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276:41245–54. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 34.Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr. 2010;92:5–15. doi: 10.3945/ajcn.2009.28435. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya A, Kanno T, Nishizaki T. Diacylglycerol promotes GLUT4 translocation to the cell surface in a PKCe-dependent and PKCε/dependent and -ζ-independent manner. Life Sci. 2013;93:240–6. doi: 10.1016/j.lfs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Honma N, Kobayashi K, Jia LN, Hosono T, Shindo K, et al. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS One. 2014;9:e87894. doi: 10.1371/journal.pone.0087894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangeetha MK, Priya CD, Vasanthi HR. Anti-diabetic property of Tinospora cordifolia and its active compound is mediated through the expression of Glut-4 in L6 myotubes. Phytomedicine. 2013;20:246–8. doi: 10.1016/j.phymed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 38.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knauf C, Rieusset J, Foretz M, Cani PD, Uldry M, Hosokawa M, et al. Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: Role in liver and brain. Endocrinology. 2006;147:4067–78. doi: 10.1210/en.2005-1536. [DOI] [PubMed] [Google Scholar]
- 40.Goto T, Lee JY, Teraminami A, Kim YI, Hirai S, Uemura T, et al. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res. 2011;52:873–84. doi: 10.1194/jlr.M011320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen KB, Minet A, Svenstrup H, Grevsen K, Zhang H, Schrader E, et al. Identification of plant extracts with potential antidiabetic properties: Effect on human peroxisome proliferator-activated receptor (PPAR), adipocyte differentiation and insulin-stimulated glucose uptake. Phytother Res. 2009;23:1316–25. doi: 10.1002/ptr.2782. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi N, Goto T, Taimatsu A, Egawa K, Katoh S, Kusudo T, et al. Bixin regulates mRNA expression involved in adipogenesis and enhances insulin sensitivity in 3T3-L1 adipocytes through PPARgamma activation. Biochem Biophys Res Commun. 2009;390:1372–6. doi: 10.1016/j.bbrc.2009.10.162. [DOI] [PubMed] [Google Scholar]
- 43.Prasad CN, Anjana T, Banerji A, Gopalakrishnapillai A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010;584:531–6. doi: 10.1016/j.febslet.2009.11.092. [DOI] [PubMed] [Google Scholar]


