Abstract
The study is aimed to assess the incidence of drug-related problems (DRPs) and provide pharmacist interventions for identified DRPs. A prospective, observational study was conducted among 189 patients with cardiovascular disease who were aged 18 years or older and admitted to the general medicine in-patient ward. During the 6 months study period, the incidence of DRPs was identified using Pharmaceutical Care Network Europe Foundation classification system version 6.2. A total of 189 patients were screened for DRPs. Among them, 130 patients have at least one DRP. A total of 416 DRPs were identified (on average, 2.2 DRPs per each patient). Of the 416 DRPs, 125 (30.04%) interventions were accepted, 7 (1.68%) interventions were not accepted, while remaining (68.26%) accepted but no action taken. The results of the study indicate that incidence of DRPs is substantial and pharmacist-led interventions resulted in resolution of DRPs. This represents the need for the active role of the clinical pharmacist in the developing countries like India.
Keywords: Cardiovascular patients, classification, drug-related problems, Indian hospital, Pharmaceutical Care Network of Europe, pharmacist intervention, prospective study
INTRODUCTION
Drug-related problems (DRPs) lead to substantial morbidity and mortality and are statistically related to the clinical outcomes, healthcare costs, and quality of life of cardiovascular patients.[1] Several studies have demonstrated that cardiovascular patients are at the risk for medication-related problems. Also, cardiovascular drugs were most often associated with drug-related hospitalizations in adults and elderly patients.[2] Factors associated with medication-related problems in these patients include age (>65 years), poly-pharmacy, co-morbid medical conditions, concomitant medications, nonconformity to standard established guidelines, noncompliance by the patient, lack of proper laboratory and therapeutic drug monitoring, pharmacogenetic variations, medication errors, and patient-related factors.
Clinical pharmacists can play an important role in identifying and resolving DRPs through cooperation with patients and other health-care providers. Potential and actual DRPs can be identified through medication profile reviews, and these problems can be prevented by monitoring therapeutic plans.[3] A number of actual DRPs can be resolved with patient counseling and through appropriate clinical pharmacy interventions. Increased knowledge about the nature and frequency of DRPs with feedback to pharmacy personnel, physicians, drug manufacturers, and patients would enhance the rational use of drugs.[4] Our study was conducted to determine the incidence of DRPs in cardiovascular patients and to recommend pharmacist interventions to resolve the actual DRPs.
SUBJECTS AND METHODS
Ethical consideration
A prospective, observational study was carried out for a period of 6 months (from January 1 to June 30, 2014) in cardiovascular patients admitted to the general medicine ward of Dr. Pinnamaneni Siddhartha Institute of Medical Sciences and Research Foundation which is a 850-bedded tertiary-care teaching hospital at Chinaoutpalli, Gannavaram Mandal, Krishna district, Andhra Pradesh (India). A study protocol was designed and got approved by Institutional Ethics Committee of our institute (Protocol No.: KVSRSCOPS/IEC/2014/004).
Inclusion criteria
Patients who were willing to participate in the study, patients aged >18 years of either gender diagnosed with any cardiovascular illness, and admitted to in-patient ward of general medicine in the given study period.
Exclusion criteria
Outpatients, pregnant patients, pediatrics, and patients who were not willing to participate.
Study procedure and data analysis
A total of 189 patients (n = 189) who met the inclusion criteria were recruited in the study. Patient demographics, disease-specific information such as reason for admission, past medical history, and past medication history were collected in a specially designed data collection form. During the study period, patients were reviewed on a daily basis and any change either in the drug chart or in the laboratory details was collected. The collected data were analyzed and interpreted for the assessment of DRPs using standard databases like Micromedex, Lexicomp, etc. For identified DRPs, appropriate interventions were provided to prescribers with suitable strategies to resolve the DRPs. The acceptance level of a physician for each intervention was also recorded as either accepted and action taken, accepted but action not taken, or neither accepted nor action taken. The DRPs were categorized using Pharmaceutical Care Network Europe (PCNE) version 6.2 classification.[5,6] The number of DRPs per patient was calculated to estimate the incidence of DRPs. The medications that were most commonly implicated in DRPs were determined, and the drug risk ratio was calculated for each drug using the formula, the number of DRPs for the same drug by number of times the drug prescribed. Statistical analysis was carried out using GraphPad Prism version 5.0.
RESULTS
The demographic and health-related details of patient population were presented in Table 1. A total of 189 patients were screened for DRPs. Among them, 130 patients have at least one DRP. A total of 416 DRPs were identified (on average, 2.2 DRPs per each patient). A total of 416 DRPs were identified in our study. Their nature and frequency were evaluated. Frequency distribution of subtypes of DRPs was shown in Figure 1.
Table 1.
Demographic and health-related details of patient population
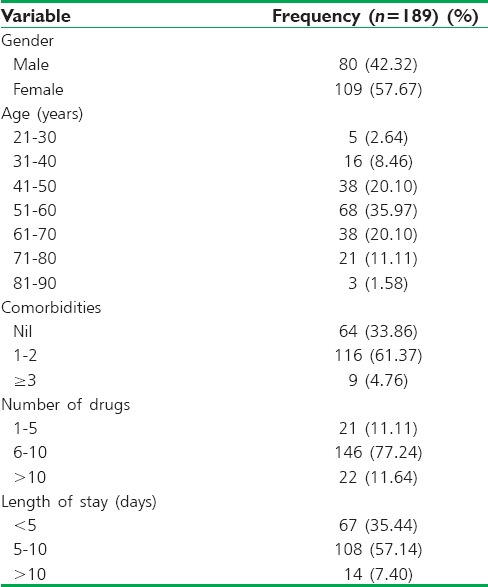
Figure 1.
Frequency distribution of subtypes of drug-related problems
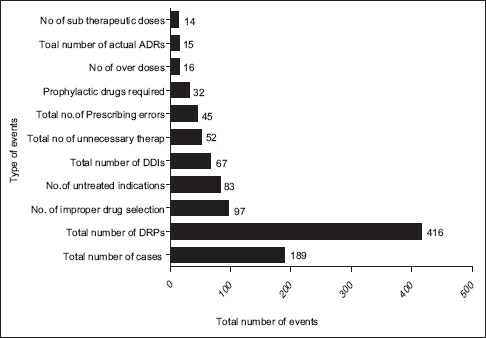
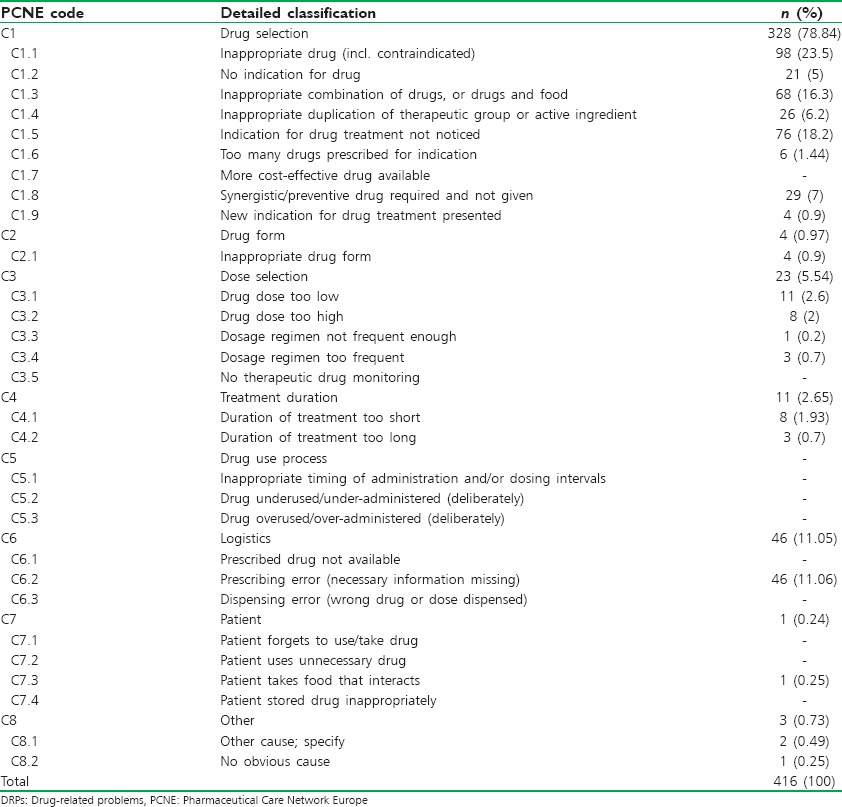
As per PCNE classification, the problems and the causes associated with the DRPs were categorized. The problem of wrong effect of drug treatment was found to be the highest accounted for 24.7% of DRPs followed by the effect of drug treatment not optimal with 20.4% and the remaining data were presented in Table 2. Among different causes of DRPs that were identified during the study, the problems caused due to inappropriate drug selection were found to be the highest with 22.17% which is followed by problems caused due to inappropriate drug combination with 16.39%. The problems caused due to food interaction were found to be least with 0.24%. The percentage of different causes of DRPs was mentioned in Table 3.
Table 2.
Problems associated with DRPs as per PCNE classification system version 6.2
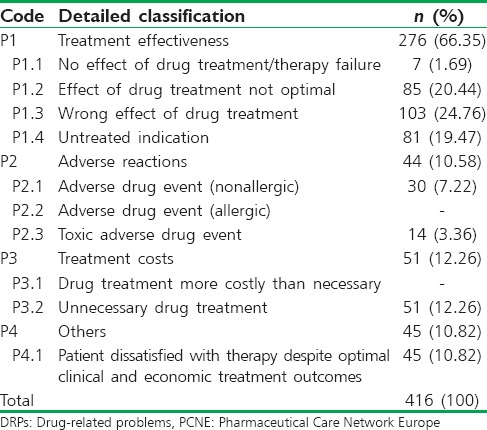
Table 3.
Causes associated with DRPs as per PCNE classification system version 6.2
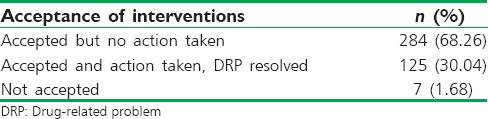
The pharmacist recommendations were shown in Table 4. Among the different types of recommendations in pharmacist interventions, the majority of them were regarding addition of the drug which is about 29.32% of total recommendations made, which is followed by cessation of the drug (24%). The recommendations regarding the change in the dosage form were observed to be the least with 1.44%. Results of pharmacist intervention include: 30.04% of pharmacist interventions were accepted by physicians and DRPs were resolved, while 68.26% interventions were accepted but no action was taken on them, and 1.68% interventions were not accepted at all [Table 5].
Table 4.
Frequency of pharmacist recommendations to the physician

Table 5.
Percentage frequency of pharmacist interventions in the therapy with the follow-up of action taken and DRP resolved
There was no significant association between number of drugs used and number of patients with or without DRPs [Table 6]. The patients who were hospitalized for 5–10 days were found to have the highest number of DRPs compared to the patients with length of stay <4 days. However, no association was found between the length of stay and incidence of DRPs with the Chi-square test [Table 7].
Table 6.
Cross table represents number of drugs used against number of patients with and without DRPs

Table 7.
Cross table represents the length of stay against number of patients with and without DRPs

DISCUSSION
A total of 416 DRPs were detected in 130 patients, with an average incidence rate of 2.2 DRPs per patient. This finding is in agreement with a recent study with an almost equivalent sample size (193), which also used the PCNE classification system, which reported 2.2 ± 1.6 per patient.[7] The incidence of DRPs was high (35.97%) among the patients aged between 51 and 60 years. In terms of the number of drugs, patients receiving 6–10 drugs were found to have more DRPs (77.24%). This observation was supported by a national survey of pharmacy practice in hospital settings.[8] From the graph illustrating the cumulative frequency of DRPs, it is evident that about 68.78% of the patients had at least one DRP. This shows the high incidence of DRPs among the cardiovascular inpatients in this prospective study.
The number of causes identified in our study was lower than the causes identified in other studies such as Chan et al. (2014).[7] This is because most of the problems identified were matched with the one most relevant cause rather than several causes, which might be seen in other studies. The DRPs caused due to improper drug selection were found to be the highest accounting for 22.17% of all DRPs and this finding was comparable to other studies. Most of the drug choice problems were related to the use of amlodipine in hypertensive patients with diabetes or renal failure. According to Arauz-Pacheco et al. and Joint National Committee-8 classification, angiotensin-converting enzyme inhibitors are preferred over calcium channel blockers in hypertensive patients with diabetes or chronic kidney disease or both.[9,10] The inappropriate drug combination was found to be the second most common cause of DRPs accounting for 16.39%.
The proportion of DRPs observed in this study was not in line with other similar studies.[11,12,13] Among the various types of recommendations in pharmacist interventions, the majority of them were “addition of the drug” which is about 29.32% of total recommendations provided followed by “cessation of drug” which is 24%. These findings correlate with the observation made in other studies where drug discontinuation was the most frequent recommendation.[11,12] However, our study differs from some other studies where drug dose change was reported as the most common suggestion made.[13]
The acceptance rate of clinical pharmacist recommendations and change in drug therapy was found to be low 30.04% [Table 5] when compared with other published Indian and International studies where high acceptance rate was recorded.[11,12,13,14,15] However, 68.26% of total recommendations were accepted but therapy was not changed, may be because the suggestion provided was thought to be insignificant with respect to patient's current major clinical condition by the physicians or due to a need for close monitoring before changing the therapy. Some of the recommendations (1.68%) were neither accepted nor was the therapy changed, which might be due to the lack of pharmacist understanding about the sophisticated physician prescribing behavior.
Most of the recommendations that were implemented had been found to improve the clinical outcomes in the patients studied. The overall findings suggest that clinical pharmacists could effectively identify and prevent various DRPs by pharmaceutical care activities through their involvement in a healthcare team.
CONCLUSION
The results of our study indicate that incidence of DRPs is substantial, and pharmacist-led interventions resulted in resolution of DRPs. This represents the need for the active role of the clinical pharmacist in developing countries such as India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank our Principal, Prof. G. Devala Rao and management of KVSR Siddhartha College of Pharmaceutical Sciences for providing continuous support during this work. We also thank Director General, Dr. C. Nageswara Rao, Hospital Superintendent and HOD, Department of General Medicine, Dr. N. V. Krishna Rao and Principal, Dr. P. Satyanarayana Murthy, of Dr. Prinnamaneni Siddhartha Institute of Medical Sciences and Research Foundation for granting permission to do Pharm D Project work and providing the facilities for completion of this work.
REFERENCES
- 1.Niquille A, Bugnon O. Relationship between drug-related problems and health outcomes: A cross-sectional study among cardiovascular patients. Pharm World Sci. 2010;32:512–9. doi: 10.1007/s11096-010-9401-1. [DOI] [PubMed] [Google Scholar]
- 2.Urbina O, Ferrández O, Luque S, Grau S, Mojal S, Pellicer R, et al. Patient risk factors for developing a drug-related problem in a cardiology ward. Ther Clin Risk Manag. 2014;11:9–15. doi: 10.2147/TCRM.S71749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton ML, Lurvey PL. Impact of medication profile review on prescribing in a general medicine clinic. Am J Hosp Pharm. 1991;48:265–70. [PubMed] [Google Scholar]
- 4.Westerlund T, Almarsdottir AB, Melander A. Drug related problems and pharmacy interventions in community practice. Int J Pharm Pract. 1999;7:40–50. [Google Scholar]
- 5.Pharmaceutical Care Network Europe. DRP-Classification V6.2. 2010. Jan 14, Available from: http://www.pcne.org/upload/files/11_PCNE_classification_V6.2.pdf .
- 6.van Mil JW, Westerlund LO, Hersberger KE, Schaefer MA. Drug-related problem classification systems. Ann Pharmacother. 2004;38:859–67. doi: 10.1345/aph.1D182. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, Chen JH, Wen CJ, Chiu LS, Wu SC. Effectiveness of the medication safety review clinics for older adults prescribed multiple medications. J Formos Med Assoc. 2014;113:106–13. doi: 10.1016/j.jfma.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: Prescribing and transcribing-2013. Am J Health Syst Pharm. 2014;71:924–42. doi: 10.2146/ajhp140032. [DOI] [PubMed] [Google Scholar]
- 9.Arauz-Pacheco C, Parrott MA, Raskin P. American Diabetes Association. Hypertension management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):S65–7. doi: 10.2337/diacare.27.2007.s65. [DOI] [PubMed] [Google Scholar]
- 10.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 11.Algiriswami B, Ramesh M, Parthasarathi G, Basavanagowdappa H. A study of clinical pharmacist initiated changes in drug therapy in a teaching hospital. Indian J Pharm Pract. 2009;1:36–45. [Google Scholar]
- 12.Ganachari MS, Mahendra Kumar BJ, Shashikala CW, Fibin M. Assessment of drug therapy intervention by clinical pharmacist in tertiary care hospital. Indian J Pharm Pract. 2010;3:22–8. [Google Scholar]
- 13.Parthasarathi G, Ramesh M, Kumar JK, Madaki S. Assessment of drug related problems and clinical pharmacists’ interventions in an Indian teaching hospital. J Pharm Pract Res. 2003;33:272–4. [Google Scholar]
- 14.Langebrake C, Hilgarth H. Clinical pharmacists’ interventions in a German university hospital. Pharm World Sci. 2010;32:194–9. doi: 10.1007/s11096-010-9367-z. [DOI] [PubMed] [Google Scholar]
- 15.Tigabu BM, Daba D, Habte B. Drug-related problems among medical ward patients in Jimma university specialized hospital, Southwest Ethiopia. J Res Pharm Pract. 2014;3:1–5. doi: 10.4103/2279-042X.132702. [DOI] [PMC free article] [PubMed] [Google Scholar]


