Abstract
Introduction:
Stroke is one of the most important causes of disability in developed countries and, unfortunately, there is no effective treatment for this major problem of central nervous system (CNS); cell therapy may be helpful to recover this disease. In some conditions such as cardiac surgeries and neurosurgeries, there are some possibilities of happening brain stroke. Inflammation of CNS plays an important role in stroke pathogenesis, in addition, apoptosis and neural death could be the other reasons of poor neurological out come after stroke. In this study, we examined the preventive effects of the neural stem cells (NSCs) and mesenchymal stem cells (MSCs) intra-ventricular injected on stroke in rats.
Aim:
The aim of this study was to investigate the preventive effects of neural and MSCs for stroke in rats.
Materials and Methods:
The MSCs were isolated by flashing the femurs and tibias of the male rats with appropriate media. The NSCs were isolated from rat embryo ganglion eminence and they cultured NSCs media till the neurospheres formed. Both NSCs and MSCs were labeled with PKH26-GL. One day before stroke, the cells were injected into lateral ventricle stereotactically.
Results:
During following for 28 days, the neurological scores indicated that there are better recoveries in the groups received stem cells and they had less lesion volume in their brain measured by hematoxylin and eosin staining. Furthermore, the activities of caspase-3 were lower in the stem cell received groups than control group and the florescent microscopy images showed that the stem cells migrated to various zones of the brains.
Conclusion:
Both NSCs and MSCs are capable of protecting the CNS against ischemia and they may be good ways to prevent brain stroke consequences situations.
Keywords: Brain stroke, Mesenchymal stem cells, Neural stem cells, Preventive effects
Introduction
Brain stroke, growing incident, is the third major reason for mortality in the developed country.[1,2,3] Although there are some treatments for stroke; the patients’ neurological outcomes remain poor and the most of them are permanently paralyzed.[4] Nowadays, thrombolytic therapy is one of the best choices for treating acute ischemic stroke. However, it has some serious complications such as hemorrhage.[5,6] After some surgeries, especially cardiac surgeries, brain lesion including white matter and nonwhite matter occurs frequently (40-80%), this risk is higher in old ages and females.[7] Therefore, finding a suitable way such as prophylactic treatment for stroke could reduce the complications which caused by brain stroke. In stroke pathogenesis, inflammation plays an important role that caused by secreting some cytokines including tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and expression some adhesion molecules such as intercellular adhesion molecule, vascular cell adhesion molecule-1, and selectins.[8,9,10] Recently, cell therapy has opened a new attractive and hopeful window for treating brain stroke. Mesenchymal stem cells (MSCs), an available source of adult stem cell, have been used in different studies due to their anti-inflammatory effects by secreting transforming growth factor-β, and IL-10, etc.,[11,12,13] and neuroprotective effects by secreting vascular endothelial growth factor (VEGF), nerve growth factor, brain-derived neurotrophic factor (BDNF), cerebral dopamine neurotrophic factor, glial cell line-derived neurotrophic factor.[14] Neural stem cells (NSCs) are a type of stem cells that could be isolated from different areas in central nervous system (CNS) such as subventricular zone, hypothalamus, etc., and differentiated to three neural lineages containing neurons, astrocytes, and oligodendrocytes.[15] Furthermore, NSCs have been transplanted for treating ischemic stroke and the results show they have improved neurological function and reduced brain lesion via the capability of immunomodulatory, trophic, and regeneration.[16,17,18] According to mentioned above, we tested the preventive effects of NSCs and MSCs that injected stereotactically into the lateral ventricle of the middle cerebral artery occlusion (MCAO) model Sprague-Dawley male rats. There are some previous studies which used NSCs and MSCs but they did not find out these stem cells preventive effects on brain stroke and we have decided to distinguish their preventive effects in this study.
Materials and Methods
All procedures were approved by the Shiraz University of Medical Sciences Ethical committee.
Rat neural stem cells isolation and expansion
The 14-day rat embryo was provided and the ganglion eminence was dissected carefully, then the culture media (Dulbecco's modified Eagle's medium [DMEM]/F12, 2% B27, 1% pen/strep, basic fibroblast growth factor [bFGF] 10 ng/ml, and epidermal growth factor 20 ng/ml) was added to the plate. The tissue was digested mechanically. After that the cells were counted by Neubauer chamber and 250,000 cells were cultured in T-25 cm2 flask containing 5 ml culture media. After 1-week, NSCs marker, Nestin (Millipore MAB353A4, 1:100), was assessed for the cultured NSCs. After 5 days, some sphere formed in the T-25 cm2 called as neurospheres. The cells were incubated in 37°C, 5% CO2, and 95% humidity. For neurospheres passaging, first, the spheres were collected and centrifuged at 110 g for 5 min, and then 1 ml of trypsin 0.05% (Gibco) was added for three. After the duration trypsin inhibitor (Gibco) was added to neutralize the trypsin. For the next step, the cells were centrifuged at 110 g for 5 min and the enzymes were removed, the provided single cells were cultured in T-25 cm2 with density 50,000 cells/ml [Figure 1].[19]
Figure 1.
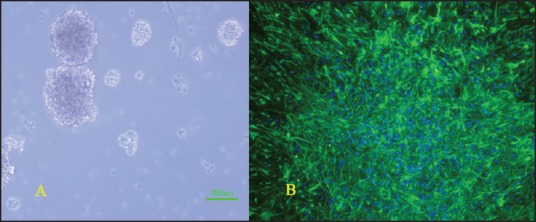
(a) Neurospheres formed by rat neural stem cells after 5 days (b) immunocytochemistry for nestin antibody (green) and 4’,6-diamidino-2-phenylindole
Differentiation of neural stem cells
The most important feature of NSCs is their ability to provide three neural lineages containing neurons, oligodendrocytes, and astrocytes. To differentiate the NSCs into these neural lineages, the single cells were cultured on poly-ornithine coated plates (50,000 cells/ml), then the differentiation media (DMEM/F12, 2%B27, 1% pen/strep, and 5% fetal bovine serum) was added to the plates and the cultured cells were incubated for 2 days in 37°C, 5% CO2, and 95% humidity [Figure 2].
Figure 2.
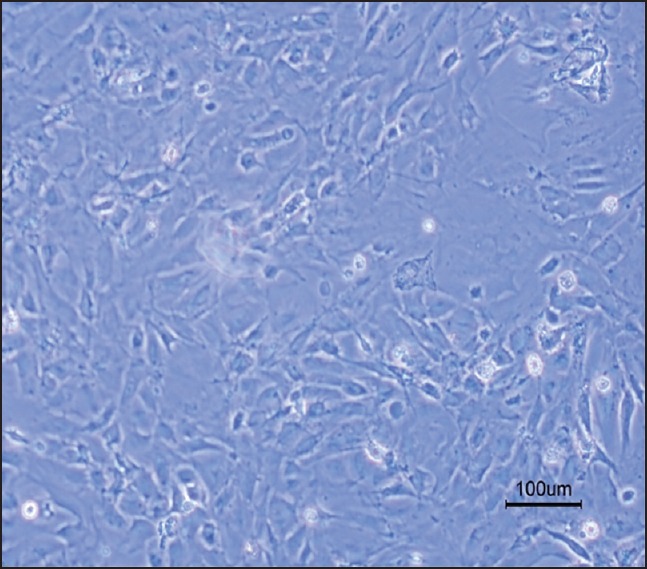
Neural stem cells differentiation to three neural lineages
Immunocytochemistry was performed for neurons with β-tubulin III antibody (PromegaG7121, 1:2000) and for astrocyte with glial fibrillary acidic protein antibody (DakoCytomation, Code No. Z0334 1:500) [Figure 3], briefly, the primary antibody was added to the fixed cells and kept for 1 hour at room temperature, after 1 hour the slides were washed 3 times with PBS. After washing the cells the secondary antibody was added and the slides were incubated for 1 hour at room temperature.
Figure 3.
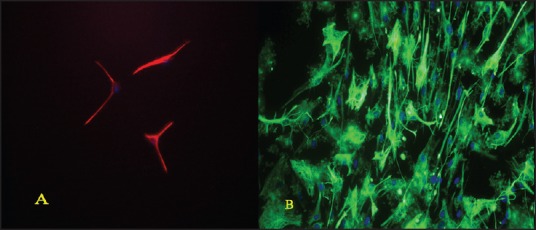
(a) Immunocytochemistry for β-tubulin (red) and 4’,6-diamidino-2-phenylindole (blue) (b) immunocytochemistry for glial fibrillary acidic protein (green) and 4’,6-diamidino-2- phenylindole (blue)
Bone marrow mesenchymal stem cell isolation and characterization
All procedures were supervised and confirmed by Shiraz University of Medical Sciences Ethical Committee. To isolate bone marrow MSCs, adult Sprague-Dawley rats weighing 250-300 g were used, they were killed and their tibia and femurs were excised under sterile condition and they were flashed with MSC culture media (DMEM, 10% fetal bovine serum, and 1% pen/strep), the suspension was transferred to T-25 cm2 flask. The cells were incubated in 37°C, 5% CO2, and 95% humidity. After 2 days, the media was changed with the fresh one and after 10 days the flasks confluence almost 80% with the MSCs.[20] To confirm the stemness feature of the isolated cells, they were differentiated into osteocyte and adipocyte by Miltenyi Biotech differentiation Medias. The osteogenic and adipogenic differentiation were confirmed by Alizarin Red and Oil Red staining [Figures 4 and 5].
Figure 4.
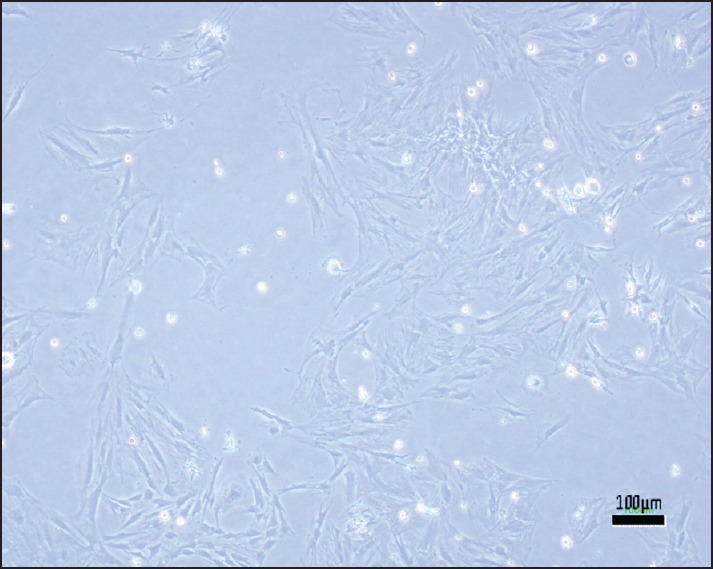
Rat mesenchymal stem cells after 1-week
Figure 5.
(a) Adipogenic differentiation of rat mesenchymal stem cells (b) osteogenic differentiation of rat mesenchymal stem cells
Animal preparation
All procedures were approved by ethical committee of Shiraz University of Medical Sciences. Sixty (n = 60) adult Sprague-Dawley rats were randomly selected and divided into four groups. The first one is the sham-operated group, the second one is the control group received 200 λL phosphate buffered saline stereotactic injection in their right side of lateral ventricles 1-day before MCAO, the third one received 2 × 105 NSCs stereotactical injection in their right side of lateral ventricles 1-day before MCAO, and the fourth one received 2 × 105 MSCs stereotactical injection in their right side of lateral ventricles 1-day before MCAO.
Middle cerebral artery occlusion
The rats were anesthetized by halothane (5% induction and 1.5% for maintenance) in the mixture of NO2 and O2 (1:1). MCAO induction was performed according to Koizumi's method. Briefly, a vertical incision was excised in the midline of the neck and the connective tissues, and muscles were dissected gently till the carotid sheath was observed, the sheath was removed, and carotid artery was separated from vagus nerve, then two loose sutures were prepared in common carotid artery and the external carotid artery was clamped, then a small incision was excised between the sutures and a silicon coated 4.0 nylon with round tip was passed through the common carotid artery till occlusion of middle cerebral artery occurred, after 45 min the 4.0 nylon was removed and the sutures were tighten up.[21]
Neurological function assessment
Neurological examinations were performed every 2 days for all rats during 28 days of experiment. The neurological examination was scored on six-score scale. The scores are described as below:[18,22]
Score of 0: No neurological deficit.
Score of 1: Failure to extend left forepaw completely. It shows mild focal neurological deficit.
Score of 2: Circling to the left. It means a moderate focal neurological deficit
Score of 3: Falling to the left. It indicates a sever focal neurological deficit
Score of 4: Not walking spontaneously and decreasing level of consciousness.
Score of 5: Death due to brain ischemia.
Histology
After 28 days, the rats were anesthetized with halothane and were fixed with normal saline followed paraformaldehyde 4%; cryosections (10 μm) were mounted on silicon coated slides and stained with hematoxylin and eosin.
Stem cells labeling with PKH26-GL
The fluorescent membrane dye, PKH26-GL, was used to label the bone marrow derived MSCs and NSCs for tracking the cell in the rats’ brain. Briefly, the cells were trypsinized and resuspended in diluents C with concentration 2 × 107 cells/ml and then equal volume of PKH dye in diluents C was added to cell suspension to achieve 10 μM dye concentration, after 10 min incubation in 37°C the labeling process was stopped by adding equal volume of fetal bovine serum and washing with complete media three times.
Apoptosis evaluation with measurement of caspase-3 activity
Activation of IL-1β converting enzyme family proteases/caspases initiates apoptosis in mammalian cells. This evaluation is based on spectrophotometric detection of chromophore p-nitroaniline (p-NA) after cleavage from labeled substrate DEVD-p-NA. The p-NA light emission could be measured by using spectrophotometer at 400-405 nm. For this assay, caspase-3 assay kit was bought from Abcam Company (ab39401).
Stereotactic injection of stem cells
The animals were anesthetized with halothane (induction 5% and maintenance 1%) and fixed to the stereotactical frame, the labeled MSCs and NSCs were injected into right lateral ventricle at: Anterioposterior = –0.12 mm, mediolateral = 1.6 mm, dorsoventricular = 4.3 mm. Each rat received 200,000 MSCs and 200,000 NSCs in its right lateral ventricle.
Statistical analysis
Data were analyzed statistically using SPSS 16.00 (IBM Company) statistical software and expressed as the mean ± standard deviation. Differences between different groups were as compared by using one-way ANOVA test. P < 0.05 is considered statistically significant differences.
Results
Brain lesion evaluation by hematoxylin and eosin staining
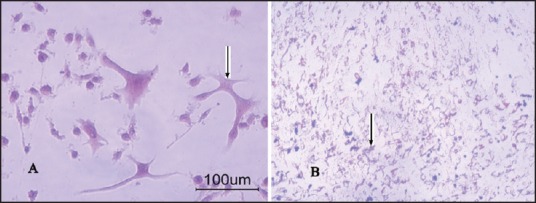
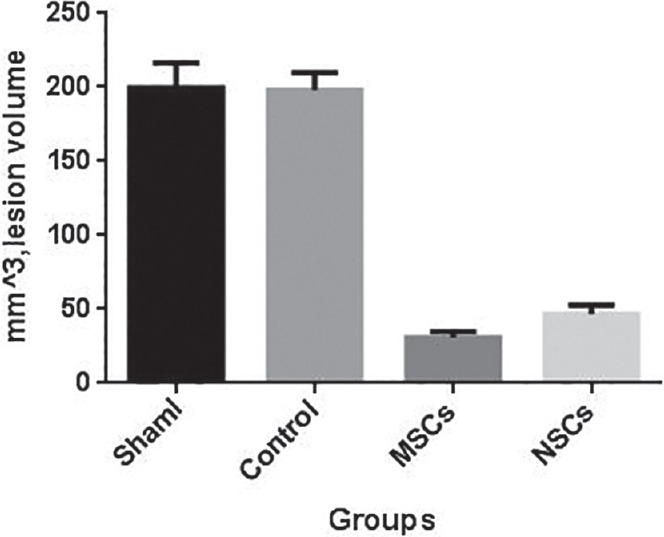
The coronal sections were selected with 1 mm interval and it began from 2 mm posterior to frontal tip, damaged area was determined by some ischemic signs such as eosinophilic cytoplasm and pyknotic nuclei. The lesion volume in control group was 197.88 ± 11.98 mm3, in MSCs injected group was 30.73 ± 3.90 mm3, and in NSCs injected group was 46.63 ± 6.14 mm3, there are significant differences between control group and two other groups (P < 0.05) [Figure 6].
Figure 6.
H and E staining and brain lesion volume
Neurological scores
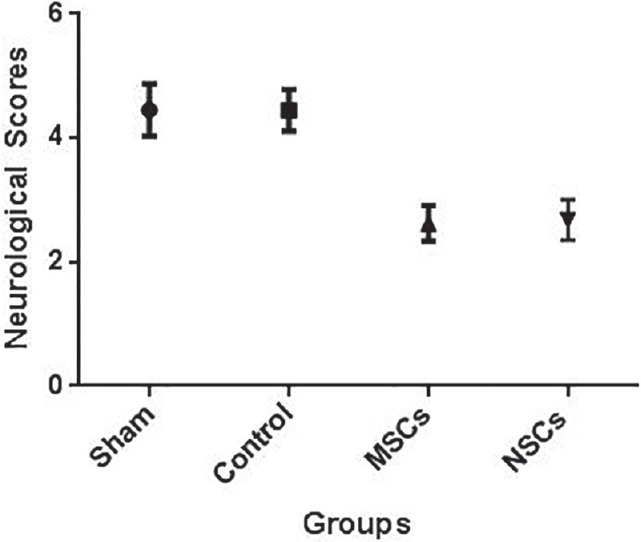
All rats were examined every 2 days till 28 days after MCAO, according to previously described scale. The neurological scores for control group was 4.48 ± 0.24, for MSCs injected group was 2.62 ± 0.29, and for NSCs injected group was 2.67 ± 0.32, and they were different significantly (P ≤ 0.05) [Figure 7].
Figure 7.
Neurological scores during 28 days
Stem cell tracking
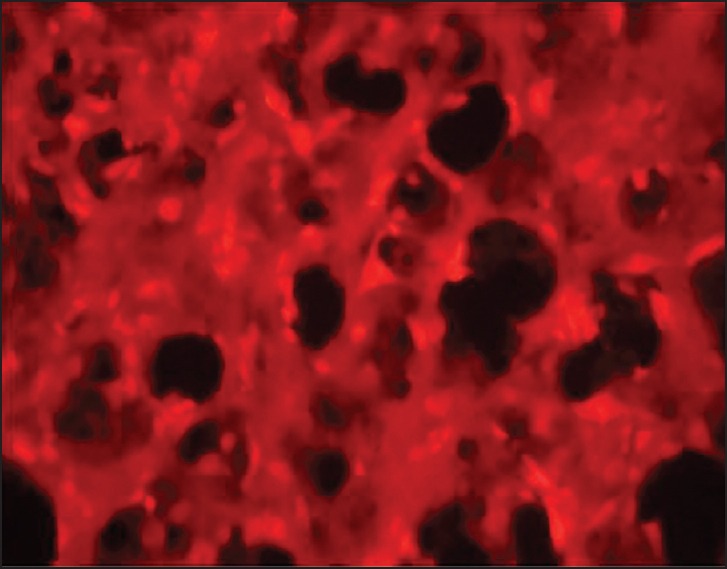
PKH26-GL labeling represents that some stem cells in both groups (MSCs and NSCs injected) migrate to upper zones of the brain such as cortex, striatum, and corpus callosum [Figure 8].
Figure 8.
PKH-labeled stem cells
Caspase-3 activity assay
MSCs and NSCs are capable to reduce apoptosis by down regulating some apoptotic genes such as caspase-3. Caspase-3 activity was evaluated by spectrophotometer, for control group it was 1.03 ± 0.08, for NSCs injected 0.75 ± 0.05, and for MSCs injected group it was 0.50 ± 0.06. The data indicates significant difference between each two groups (P ≤ 0.05), as it shows MSCs could reduce apoptosis more than two other groups [Figures 9 and 10].
Figure 9.
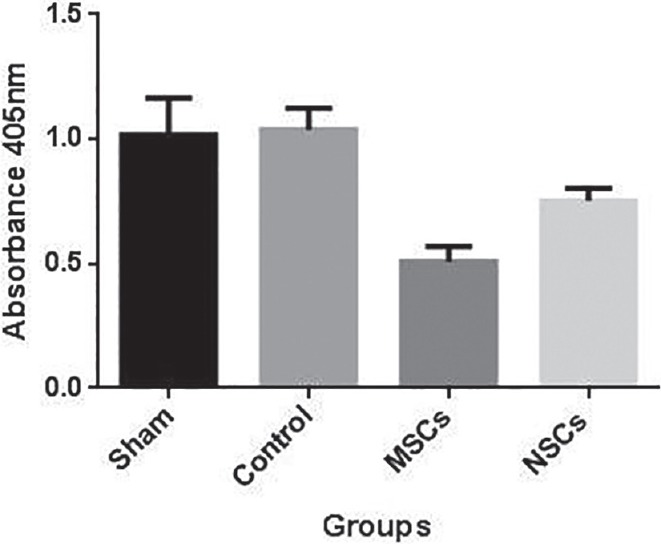
Caspase-3 activity
Figure 10.

The H and E staining for histology evaluation. (a) Noninfarction area (b) infarction area
Discussion
Our study represents that both NSCs and MSCs intra-ventricular injection before MCAO could make neurological disability less and provide more recovery after brain stroke; they can’t provide full recovery, although.
Gu et al. in 2014, showed that one of the most contributing factors involved in pathogenesis of brain stroke is the excessive inflammation and apoptosis and they could be diminished by MSCs injection through decreasing inflammatory cytokines including TNF-α, IL-1β, p-IκB-α, P-IKKβ, p53, and MSCs injection could increase anti-apoptotic genes expression such as B-cell lymphoma-2.[23] Furthermore, MSCs display their immune modulatory ability by up regulating TNF-α stimulated gene/protein 6 expression and nuclear factor-κB which could exert an anti-inflammatory effect by attenuating the inflammatory cascade in activated microglia.[24,25,26]
One more point in reducing inflammation ability of MSCs, is the diminishing T-cell invasion and white matter injury by inducing persistent peripheral T-cell tolerance and it could be one of the causes of neuroprotective effects of MSCs.[25] Brain edema made by astrocyte apoptosis and impaired blood brain barrier, plays a major role in brain lesion after ischemia. Tang et al., have designed a study which they found out that MSCs injection might protect blood brain barrier and astrocyte apoptosis by decreasing aquaporin-4 expression through the P38 pathway.[27]
Another effective MSCs’ feature which makes better neurological function after brain ischemia is their neurotrophic cytokines secretion which helps neural regeneration and axonal growth, MSCs are capable to secret some neurotrophic cytokines such as VEGF, vascular endothelial growth factor receptor-3, BDNF, insulin-like growth factor-2, tyrosine hydroxylase, and hepatocyte growth factor. So that the microenvironment becomes more enrich and suitable for regeneration.[28,29] Enhancing neural cells’ expression of FGF-2 that is induced by MSCs could help them to have more growth, too.[30]
NSCs are able to differentiate into neural lineages and have self-renewal ability.[31] The preischemic injection of them could have some effects on recovering brain ischemia by early increasing expressions of hypoxia-inducible factor 1alpha and VEGF which could make up a microenvironment for enhancing the neural plasticity of endogenous NSCs.[32] Expressing VEGF could induce angiogenesis in addition to neurogenesis and help neural regeneration.[33]
The NSCs seem to have some anti-inflammatory and anti-apoptotic effects mediated by cyclooxygenase-2 regulation.[34] The inflammation occurred after brain ischemia changes the NSCs committee and their gliogenicity would be decreased and they switched into neurogenesis and it could be a helpful change to regenerate CNS after brain ischemia.[35]
Regarding all above, NSCs and MSCs could help providing better neurological outcome and less brain lesion like our results show. So that, both these stem cells could be a useful method for preventing brain ischemia's complications in some high risk conditions such as neurosurgical and cardiac surgical procedures. Although, both NSCs and MSCs have protective effects against brain ischemia, NSCs transplantation has less anti-apoptotic effect comparing to MSCs.
Conclusion
Since we are facing the growing incidence of brain ischemia and some of them are the complication of some surgeries, MSCs and NSCs might be useful to protect the CNS against it. However, it needs more investigations for confirming this finding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank the research deputy and Prof. Imanie, the president of Shiraz University of Medical Sciences for supporting the Cell and Molecular Student Research Group's Laboratory.
References
- 1.Hankey GJ. Stroke: How large a public health problem, and how can the neurologist help? Arch Neurol. 1999;56:748–54. doi: 10.1001/archneur.56.6.748. [DOI] [PubMed] [Google Scholar]
- 2.Geneva: World Health Organization; 1999. World Health Organization. The World Health Report 1999 — Making A Difference; p. 65. [Google Scholar]
- 3.Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–7. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Andres RH, Choi R, Steinberg GK, Guzman R. Potential of adult neural stem cells in stroke therapy. Regen Med. 2008;3:893–905. doi: 10.2217/17460751.3.6.893. [DOI] [PubMed] [Google Scholar]
- 5.Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke. 2004;35:1490–7. doi: 10.1161/01.STR.0000126871.98801.6E. [DOI] [PubMed] [Google Scholar]
- 6.Thomalla G, Sobesky J, Köhrmann M, Fiebach JB, Fiehler J, Zaro Weber O, et al. Two tales: Hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38:313–8. doi: 10.1161/01.STR.0000254565.51807.22. [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Acker MA, Bilello M, Melhem ER, Stambrook E, Ratcliffe SJ, et al. Sex, aging, and preexisting cerebral ischemic disease in patients with aortic stenosis. Ann Thorac Surg. 2010;90:1230–5. doi: 10.1016/j.athoracsur.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JC, Cho GS, Kim HJ, Lim JH, Oh YK, Nam W, et al. Accelerated cerebral ischemic injury by activated macrophages/microglia after lipopolysaccharide microinjection into rat corpus callosum. Glia. 2005;50:168–81. doi: 10.1002/glia.20164. [DOI] [PubMed] [Google Scholar]
- 9.Bémeur C, Ste-Marie L, Desjardins P, Vachon L, Butterworth RF, Hazell AS, et al. Dehydroascorbic acid normalizes several markers of oxidative stress and inflammation in acute hyperglycemic focal cerebral ischemia in the rat. Neurochem Int. 2005;46:399–407. doi: 10.1016/j.neuint.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23:1367–79. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- 11.Barone FC, Schmidt DB, Hillegass LM, Price WJ, White RF, Feuerstein GZ, et al. Reperfusion increases neutrophils and leukotriene B4 receptor binding in rat focal ischemia. Stroke. 1992;23:1337–47. doi: 10.1161/01.str.23.9.1337. [DOI] [PubMed] [Google Scholar]
- 12.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–8. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 13.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: The dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paradisi M, Alviano F, Pirondi S, Lanzoni G, Fernandez M, Lizzo G, et al. Human mesenchymal stem cells produce bioactive neurotrophic factors: Source, individual variability and differentiation issues. Int J Immunopathol Pharmacol. 2014;27:391–402. doi: 10.1177/039463201402700309. [DOI] [PubMed] [Google Scholar]
- 15.Dai N, Sottile V. Neural stem cell approaches to CNS repair. Electron J Biol. 2008;4:79–87. [Google Scholar]
- 16.Hermann DM, Peruzzotti-Jametti L, Schlechter J, Bernstock JD, Doeppner TR, Pluchino S. Neural precursor cells in the ischemic brain - Integration, cellular crosstalk, and consequences for stroke recovery. Front Cell Neurosci. 2014;8:291. doi: 10.3389/fncel.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132(Pt 8):2239–51. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 18.Doeppner TR, Ewert TA, Tönges L, Herz J, Zechariah A, ElAli A, et al. Transduction of neural precursor cells with TAT-heat shock protein 70 chaperone: Therapeutic potential against ischemic stroke after intrastriatal and systemic transplantation. Stem Cells. 2012;30:1297–310. doi: 10.1002/stem.1098. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Lu J, Wang X. Propofol and remifentanil at moderate and high concentrations affect proliferation and differentiation of neural stem/progenitor cells. Neural Regen Res. 2014;9:2002–7. doi: 10.4103/1673-5374.145384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S, et al. 2,5-hexanedione induced apoptosis in mesenchymal stem cells from rat bone marrow via mitochondria-dependent caspase-3 pathway. Ind Health. 2015;53:222–35. doi: 10.2486/indhealth.2014-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. 1: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 22.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Gu N, Rao C, Tian Y, Di Z, Liu Z, Chang M, et al. Anti-inflammatory and antiapoptotic effects of mesenchymal stem cells transplantation in rat brain with cerebral ischemia. J Stroke Cerebrovasc Dis. 2014;23:2598–606. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Zhang R, Yan K, Chen F, Huang W, Lv B, et al. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation. 2014;11:135. doi: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jellema RK, Wolfs TG, Lima Passos V, Zwanenburg A, Ophelders DR, Kuypers E, et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. 2013;8:e73031. doi: 10.1371/journal.pone.0073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang G, Liu Y, Zhang Z, Lu Y, Wang Y, Huang J, et al. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32:3150–62. doi: 10.1002/stem.1808. [DOI] [PubMed] [Google Scholar]
- 28.Liang CM, Weng SJ, Tsai TH, Li IH, Lu PH, Ma KH, et al. Neurotrophic and neuroprotective potential of human limbus-derived mesenchymal stromal cells. Cytotherapy. 2014:pii. doi: 10.1016/j.jcyt.2014.05.015. S1465-324900633-1. [DOI] [PubMed] [Google Scholar]
- 29.Berg J, Roch M, Altschüler J, Winter C, Schwerk A, Kurtz A, et al. Human adipose-derived mesenchymal stem cells improve motor functions and are neuroprotective in the 6-hydroxydopamine-rat model for Parkinson's disease when cultured in monolayer cultures but suppress hippocampal neurogenesis and hippocampal memory function when cultured in spheroids. Stem Cell Rev. 2015;11:133–49. doi: 10.1007/s12015-014-9551-y. [DOI] [PubMed] [Google Scholar]
- 30.Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, et al. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9:e110722. doi: 10.1371/journal.pone.0110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leker RR. Fate and manipulations of endogenous neural stem cells following brain ischemia. Expert Opin Biol Ther. 2009;9:1117–25. doi: 10.1517/14712590903130558. [DOI] [PubMed] [Google Scholar]
- 32.Song S, Park JT, Na JY, Park MS, Lee JK, Lee MC, et al. Early expressions of hypoxia-inducible factor 1alpha and vascular endothelial growth factor increase the neuronal plasticity of activated endogenous neural stem cells after focal cerebral ischemia. Neural Regen Res. 2014;9:912–8. doi: 10.4103/1673-5374.133136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, et al. Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. J Cereb Blood Flow Metab. 2014;34:1138–47. doi: 10.1038/jcbfm.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Sun W, Han DW, Moon HJ, Lee J. iNSC suppress macrophage-induced inflammation by repressing COX-2. In Vitro Cell Dev Biol Anim. 2015;51:157–64. doi: 10.1007/s11626-014-9816-4. [DOI] [PubMed] [Google Scholar]
- 35.Covacu R, Perez Estrada C, Arvidsson L, Svensson M, Brundin L. Change of fate commitment in adult neural progenitor cells subjected to chronic inflammation. J Neurosci. 2014;34:11571–82. doi: 10.1523/JNEUROSCI.0231-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


