Abstract
Context:
Rivaroxaban is a direct factor Xa inhibitor approved for the prevention of thromboembolism. Drug induced liver injury has been increasingly reported with rivaroxaban recently, but actual liver failure has not been reported.
Case Report:
We present a case report on the probable occurrence of acute liver failure with rivaroxaban therapy. An 89 year old woman with history of atrial fibrillation was hospitalized for biventricular congestive heart failure with passive congestion of liver, which responded to furosemide. She was discharged home on rivaroxaban for prevention of thrombo-embolism. Liver function tests upon discharge returned to almost normal range. One week later, she presented with abdominal pain and was found to have highly elevated liver enzymes, elevated bilirubin, and an abnormal coagulation profile. A day later, she developed hepatic encephalopathy, suggesting liver failure.
Conclusion:
Liver enzymes declined rapidly with the discontinuation of all of her medications, however patient died because of multi-organ failure. The causality assessment in this patient was “probable” with rivaroxaban.
Keywords: Drug toxicity, liver failure, rivaroxaban
Introduction
Rivaroxaban is the first oral, direct factor Xa inhibitor approved (in 2008) for the prevention of venous thromboembolism (VTE) after elective hip and knee replacement surgery.[1] It has also been approved for the treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE), prevention of recurrent DVT and PE, and prevention of stroke in non-valvular atrial fibrillation. In some countries, it is approved for the prevention of atherothrombotic events in acute coronary syndrome (ACS) with elevated troponins.[2] Rivaroxaban is rapidly absorbed with maximum plasma concentration occurring 2-4 hours after oral intake. It is metabolized mainly by cytochrome P450 (CYP) 3A4 isoform and therefore its efficacy is affected by CYP 3A4 inhibitors and inducers. One third of the dose is eliminated unchanged in urine and two third undergoes hepatic degradation with metabolites being excreted in urine and bile. Rivaroxaban is administered in a fixed dose, once a day because of its predictable pharmacokinetics and pharmacodynamics. It does not require routine monitoring of the coagulation profile.[2,3]
Major and clinically relevant non-major bleeding is the most common side effect of rivaroxaban therapy.[4] Drug induced liver injury (DILI) has been reported with rivaroxaban. In pre marketing phase III RECORD (Regulation of Coagulation in Major Orthopedic Surgery Reducing the Risk of Deep Venous Thrombosis and Pulmonary Embolism) studies of rivaroxaban, 2.3 % of the patients showed elevation in alanine aminotransferase (ALT) more than 3 times the upper limit of normal (ULN). Nine of those patients had bilirubin >2 x ULN and after validation one of those was confirmed as an actual ‘Hy's law’ case.[5] Symptomatic liver dysfunction associated with rivaroxaban has recently been reported in two case series.[6,7] In our search of the literature, acute liver failure with rivaroxaban use has not been reported. We present a case in which a patient developed acute liver failure after initiation of rivaroxaban.
Case Presentation
An 89 year old woman with history of paroxysmal atrial fibrillation was admitted in the hospital for acute on chronic biventricular congestive heart failure. She was found to have left ventricular ejection fraction (LVEF) of 25% with mild passive congestion of the liver on admission (ALT: 94 U/L [ULN: 68]; aspartate aminotransferase [AST]: 104 U/L [ULN: 37]; alkaline phosphatase [ALP]: 160 U/L [ULN: 117]; bilirubin total: 0.7 mg/dL [ULN: 1.0]), which was resolving after she was treated with furosemide (ALT: 62 U/L; AST: 41 U/L; ALP: 124 U/L; bilirubin: 0.6 mg/dl). Her chronic amiodarone dose was increased from 200 mg to 400 mg daily because of paroxysmal atrial fibrillation, suspected secondary to frequent mode switching noted on pacemaker interrogation. She was stable and discharged home on rivaroxaban 20 mg daily for prevention of thromboembolism in atrial fibrillation.
One week later, she presented to the emergency room with abdominal pain. On admission, vital signs were normal. Physical examination revealed scleral icterus and tenderness in epigastrium. She was alert and oriented, and did not have signs of encephalopathy. Laboratory values upon presentation revealed extremely elevated aminotransferases (ALT: 3011 U/L [44 x ULN]; AST: 5318 U/L [143 x ULN]), elevated bilirubin (2.5 mg/dL) and ALP levels (250 U/L [2x ULN]) along with elevated INR (8.54) and creatinine (1.6). Twenty four hours later, she developed hepatic encephalopathy and was subsequently diagnosed with acute liver failure. She did not have a prior history of chronic liver disease, alcohol abuse, illicit drug abuse, and was not taking herbal medications. Blood alcohol, acetaminophen levels, and viral hepatitis serology panel were negative. Screening for autoimmune liver disease was also negative. Hepatic ultrasound revealed post cholecystectomy changes, absence of biliary duct obstruction and normal hepato-petal blood flow. All of her medications were discontinued. She was managed medically as she was not the candidate for liver transplantation. Aminotransferase levels started to decline rapidly after discontinuation of medications [Figure 1]. Despite aggressive medical therapy and supportive care, patient developed multi-organ failure and died.
Figure 1.
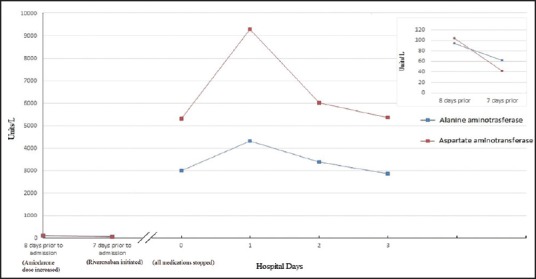
Trend in aminotransferase levels with rivaroxaban discontinuation
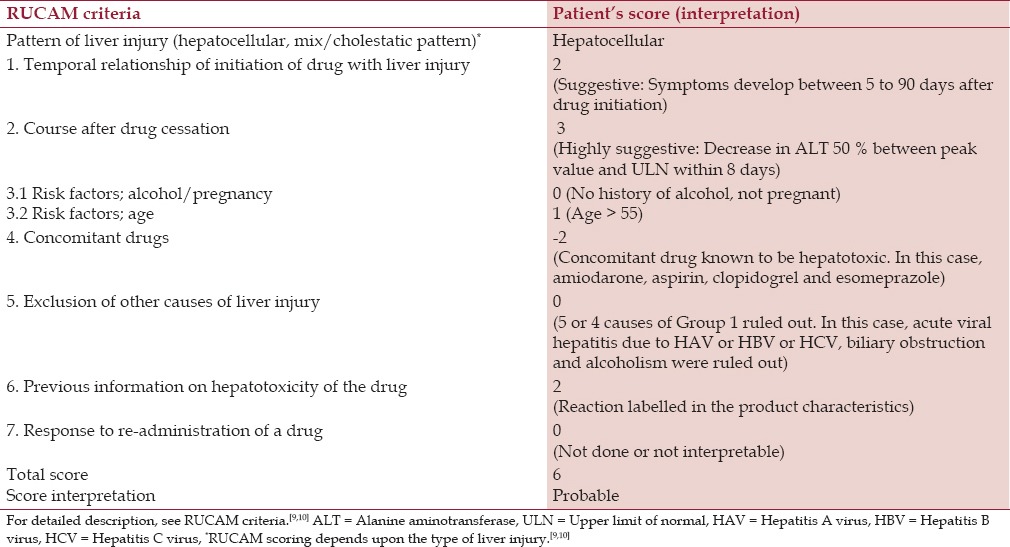
According to Roussel Uclaf Causality Assessment Method (RUCAM) for the causality risk assessment of drug-induced liver injury, it is “probable” (score 6) that rivaroxaban was the cause of liver injury and subsequent acute liver failure [Table 1].[8,9,10] However with the recent history of heart failure along with passive hepatic congestion, direct drug toxicity by rivaroxaban might have been aggravated by passive congestion of the liver, culminating in acute liver failure.
Table 1.
RUCAM (Roussel Uclaf causality assessment method) risk assessment of a patient
Mildly elevated aminotransferases trending downward after furosemide therapy on prior hospitalization (8 days prior to admission). Rivaroxaban was started (7 days prior). On subsequent hospitalization (day 0), patient had highly elevated aminotransferase levels, which declined after discontinuation of rivaroxaban.
Discussion
Rivaroxaban is approved for the prevention and treatment of VTE and for the prevention of stroke in non-valvular atrial fibrillation.[1,2] Its side effects include major and minor bleeding, and more rarely, drug induced liver injury (DILI).[4,5] DILI is the most common cause of acute liver failure in the United States.[11,12] The diagnosis of DILI is challenging since the histologic findings on liver biopsy are non-specific, and sensitive/specific biomarkers for drug induced hepatotoxicity are not available. Therefore, two major diagnostic processes are used for the assessment of suspected DILI cases; exclusion of other causes of liver injury and the recognition of a disease pattern that is temporally related to initiation and discontinuation of the suspected medication.[12]
Several clinical scoring systems are used to determine the causality risk assessment on suspected DILI cases. The RUCAM scoring system is widely used, although it has limitations.[13,14,15] In the present case, the patient was exposed to rivaroxaban in the setting of congestive heart failure and subsequently found to have symptomatic hepatocellular pattern of liver injury, six days after the initiation of rivaroxaban.[9] It progressed to acute liver failure 24 hours after admission when she developed hepatic encephalopathy along with coagulopathy and liver cell dysfunction.[11] RUCAM score in this case is “6” indicating that it is “probable” that rivaroxaban was the cause of DILI which led to acute liver failure.
Viral hepatitis, the second most common cause of acute liver failure in United States was excluded by normal viral hepatitis serology. Other less common causes of acute liver failure which include alcoholic liver disease, biliary tract obstruction, autoimmune hepatitis, sepsis, Wilson's disease, veno-occlusive liver disease and primary biliary cirrhosis, were excluded by history, absence of infection, normal imaging studies of liver (CT scan/ultrasound) and normal auto-immune serology.
Ischemic hepatitis which is the third most common cause of acute liver failure in United States after drugs and viruses, might be a contributing factor in causing acute liver failure, because of the recent episode of congestive heart failure and concomitantly elevated creatinine levels on subsequent admission, indicating hypo-perfusion.[11] However, the absence of significant hemodynamic instability on prior hospitalization and on subsequent admission, along with normal hepato-petal blood flow on ultrasound makes ischemic hepatitis doubtful as a primary cause of acute liver failure. It is likely that elevated creatinine on readmission was secondary to hepato-renal phenomenon from hepatic dysfunction.
Amiodarone, aspirin, clopidogrel and esomeprazole are other potential hepatotoxic medications the patient was taking at the time of presentation, however, it is unlikely that acute liver failure was caused from any of these, since the patient had been taking them for many years and did not have liver injury despite multiple episodes of congestive heart failure with passive congestion of liver in the past. Intravenous (IV) preparation of amiodarone has been reported to be associated with acute liver failure while the clear mechanism is not known.[16] Our patient was not exposed to an IV preparation of amiodarone recently or in the past. However, with the concomitant increase in the oral dose of amiodarone and initiation of rivaroxaban therapy simultaneously, there might be a possibility of drug-drug interaction between the two medications, which could have contributed to acute liver failure.
Forty-two cases of suspected drug induced liver injury associated with rivaroxaban have been reported so far to the Pharmacovigilance Unit of the Swiss Agency of Therapeutic Products (Swissmedic). Recently, two case series, one included 14 patients and the other included 2 patients, reported drug induced liver injury in association with rivaroxaban. In both studies, patients had similar clinical presentation with symptomatic liver dysfunction and full recovery after the discontinuation of rivaroxaban.[6,7] We believe that our patient had drug induced liver injury from rivaroxaban, superimposed on passive congestion of liver which progressed to acute liver failure. Although, the close temporal relationship of rivaroxaban administration with the development of acute liver failure, along with the rapidly improving liver enzymes after its discontinuation and the reported hepatotoxicity with rivaroxaban suggests hepatotoxicity from rivaroxaban, the possibility of rivaroxaban-amiodarone interaction as a cause of acute liver failure cannot completely be ruled out.
Conclusion
Drug induced liver injury with rivaroxaban use has been increasingly reported recently, probably because of the increase in its prescription in recent years. This case suggests a causal relationship of acute liver failure with rivaroxaban. Physicians should be vigilant when starting a patient on rivaroxaban by monitoring liver function tests closely, especially in elderly patients who have acute congestive heart failure. Further studies are warranted to evaluate the causal relationship of rivaroxaban with acute liver failure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgement
We appreciate the assistance of Zainab Dandia in graph plotting and valuable suggestions for the manuscript.
References
- 1.Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov. 2011;10:61–75. doi: 10.1038/nrd3185. [DOI] [PubMed] [Google Scholar]
- 2.Harder S, Graff J. Novel oral anticoagulants: Clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol. 2013;69:1617–33. doi: 10.1007/s00228-013-1510-z. [DOI] [PubMed] [Google Scholar]
- 3.Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29(Suppl):S24–33. doi: 10.1016/j.cjca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharma Sci. 2013;16:74–88. doi: 10.18433/j33p49. [DOI] [PubMed] [Google Scholar]
- 5.Watkins PB, Desai M, Berkowitz SD, Peters G, Horsmans Y, Larrey D, et al. Evaluation of drug-induced serious hepatotoxicity (eDISH): Application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf. 2011;34:243–52. doi: 10.2165/11586600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Liakoni E, Rätz Bravo A, Terracciano L, Heim M, Krähenbühl S. Symptomatic hepatocellular liver injury with hyperbilirubinemia in two patients treated with rivaroxaban. JAMA Intern Med. 2014;174:1683–6. doi: 10.1001/jamainternmed.2014.3912. [DOI] [PubMed] [Google Scholar]
- 7.Russmann S, Niedrig D, Budmiger M, Schmidt C, Stieger B, Hürlimann S, et al. Rivaroxaban postmarketing risk of liver injury. J Hepatol. 2014;61:293–300. doi: 10.1016/j.jhep.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 9.Danan G, Benichou C. Causality assessment of adverse reactions to drugs — I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 10.Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6:17–32. doi: 10.4254/wjh.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: Unified list based on international collaborative work. Drug Saf. 2010;33:503–22. doi: 10.2165/11535340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs — II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–6. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 14.Andrade RJ, Camargo R, Lucena MI, González-Grande R. Causality assessment in drug-induced hepatotoxicity. Expert Opin Drug Saf. 2004;3:329–44. doi: 10.1517/14740338.3.4.329. [DOI] [PubMed] [Google Scholar]
- 15.Lucena MI, García-Cortés M, Cueto R, Lopez-Duran J, Andrade RJ. Assessment of drug-induced liver injury in clinical practice. Fundam Clin Pharmacol. 2008;22:141–58. doi: 10.1111/j.1472-8206.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 16.Nasser M, Larsen TR, Waanbah B, Sidiqi I, McCullough PA. Hyperacute drug-induced hepatits with intravenous amiodarone: Case report and review of the literature. Drug Healthc Patient Saf. 2015;5:191–8. doi: 10.2147/DHPS.S48640. [DOI] [PMC free article] [PubMed] [Google Scholar]


