Abstract
Background:
The oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC), although initiated by tobacco carcinogens, their progression is due to inability of Langerhans cells (LCs) to detect these abnormal cells and promote lymphocytes to destroy these cells. We assessed and quantified the tumor associated LCs and inflammation in OED and OSCC to understand their role.
Materials and Methods:
Fifty-five microscopic sections were assessed (27 OED and 28 OSCC). The LCs were detected using S-100 immunohistochemical marker. The number of tumor associated LCs were counted. The presence of abnormal appearing large cells and its relation to histopathologic grade and inflammation was assessed.
Results:
Significant increase in the LC count was observed in OSCC when compared to dysplasia. Large, abnormal appearing cells were observed in dysplasia and carcinomas however, these were more pronounced in moderate dysplasia and poorly-differentiated carcinomas. The presence of these abnormal appearing cells was associated with decrease in lymphocytic infiltrate.
Conclusion:
The present study indicates more LC are recruited into the carcinoma. These accumulated nonfunctional LC in the tumor tissue are indicative of aggressive tumor with potential malignant transformation.
Keywords: Inflammation, Langerhans cell, oral epithelial dysplasia, oral squamous cell carcinoma, S-100
INTRODUCTION
In India, oral squamous cell carcinoma (OSCC) is the most common cancer among men and fourth most common cancer among women and accounts for one-third of the world cancer burden.[1,2] The major etiological factors are the use of the tobacco either in smoking and/or chewing forms and alcohol. The clinical changes (leukoplakia, erythroplakia or oral submucous fibrosis) are associated with the spectrum of histopathological changes starting from epithelial hyperplasia to different grades of dysplasia eventually progressing to cancer.[3,4]
Langerhans cells (LCs) are dendritic antigen presenting cells,[5,6] which originate from the bone marrow and migrate into the stratified squamous epithelium of the skin and mucosa of the upper aerodigestive tract.[7] The function of LCs is to recognize antigen, process it and present it to T cells. They intercept and bind new antigens detected in the squamous epithelium. They are responsible for initial stimulation of naïve T lymphocytes and secondary immune response by stimulating memory T cells.[8,9] In the oral mucosa other than LCs, melanocytes and Schwann cells can express positivity for S-100 antibody. However, these cells can be easily distinguished from other S-100 positive cells based on intraepithelial distribution, association with inflammatory cells and peculiar dendritic morphology.[10] We compared the distribution pattern and types of LCs in oral epithelial hyperplasia with leukoplakia, different grades of dysplasia and in carcinoma. We also analyzed the relationship of LC with the density lymphocytic infiltrations in the tumor-associated connective tissue.
MATERIALS AND METHODS
Buffered formalin fixed, paraffin wax embedded archival tissue blocks obtained from Department of Oral Pathology and Microbiology at our institution were used in this study. All the cases included in the category of dysplasia of hyperplasia were diagnosed clinically as leukoplakia. Histologically they were classified as epithelial hyperplasia, mild dysplasia, moderate dysplasia, and severe dysplasia. All the OSCCs were graded as well-, moderately- and poorly-differentiated carcinoma. Complete data on the type and duration of the habits was not available for all the patients, hence this criteria was not included in the study. Data collected included gender and age of the patients. The histological diagnosis was confirmed by review of the original Hematoxylin and Eosin slides by an oral pathologist. Ethics approval was obtained from the Institutional Human Ethics Committee.
Immunohistochemistry
Four μm sections of formalin-fixed, paraffin-embedded tissues were mounted on 3-aminopropyltriethoxysilane coated slides and incubated at 27°C for 24 h. Sections were then deparaffinized using xylene, followed by hydration using descending grades of alcohol. Antigen retrieval was performed in citrate buffer using pressure cooker. Endogenous peroxidize activity blocking was done using 3% hydrogen peroxide in distilled water for 10 min. After a tris buffer wash, nonspecific antigen blocking was done by incubation with power block solution. Sections were incubated in primary antibody at room temperature in 100% moisture for 60 min. The primary antibody was prediluted, purified bovine S-100 protein (Biogenix, AM058-5M) raised against mouse. Following the primary antibody further staining was performed using SuperSensitive™ polymer-HRP-immunohistochemistry (IHC) Detection System with DAB as a chromogen (BioGenex Laboratories, SanRamon, CA, USA) according to the manufacturer's protocol. Known control section (normal oral mucosal tissue) was included for each batch of staining to confirm the presence of appropriate immunostaining activity. Negative controls were included to assess the nonspecific staining. All slides were counter stained with hematoxylin and after dehydration were mounted with DPX.
Visualization and analysis
Langerhans cells were identified using the following criteria:
Brownish colored S-100 positive cells.
Elongated, pyramidal, round to ovoid brown stained cell with clearly visible cell body.
Presence of at least one dendritic process seen radiating from the cell surface.
All the slides were analyzed and counted independently by three senior oral pathologists using ×40 magnification of binocular light microscope. All the oral pathologists were blinded with respect to the clinical and histopathological details. During counting, the photographs of the counting field were obtained using photomicrograph. Any discrepancy of more than 5 LC cells slides were recounted and consensual agreement was obtained after recounting using the photographs. Mean value of all the three counts were entered in the data sheet. The number of LC were counted as total number of cells per high power field. The total LC associated with the tumor and in the peritumoral connective tissue were also counted. We classified the LC as:
Normal-When the all the cells showed normal appearance that is, pyramidal or small round to oval shaped cells with dendrites.
Few large cells-When at least 25% of the cells in 10 high power fields show large, abnormal appearing, irregular round to ovoid shape.
Predominantly large cells-When more than 25% of the cells in 10 high power fields show abnormally large cells.
The lymphocytic infiltration in the connective tissue was graded as mild, moderate and dense. Data were collated into a spreadsheet and analyzed using SPSS version 10 (IBM corporation, Somers, NY, USA).
RESULTS
A total of 55 cases were included in the study. Of these, 28 were OSCC and 27 were oral leukoplakia. The mean age of the OSCC group was 51 years (range: 31-70 years, standard deviation = 10.72). The mean age of oral epithelial dysplasia (OED) group was 52.85 years (range: 24-85 years, standard deviation = 16.46). All the patients were males, except for 2 patients in carcinoma and 3 in dysplasia being females.
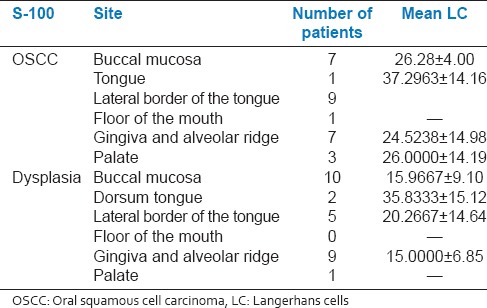
A total number of S-100 positive LC were compared at different sites. In both the dysplasia and SCC of the tongue, increase in LC was observed. There was definite increase in the total number of LC in OSCC compared to OED at all the sites [Table 1].
Table 1.
Mean LCs in OSCC and dysplasia at different sites
The total number of LC was counted in different grades of OED and OSCC [Table 2]. The total LC in different grades of dysplasia and OSCC was found to be statistically significant (P < 0.05, one-way ANOVA) [Figure 1].
Table 2.
Mean of total number of LCs in OSCC and different grades of dysplasia
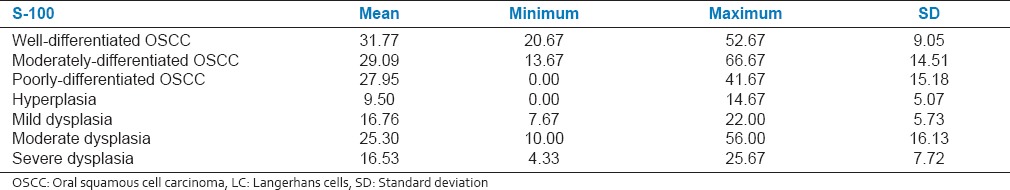
Figure 1.
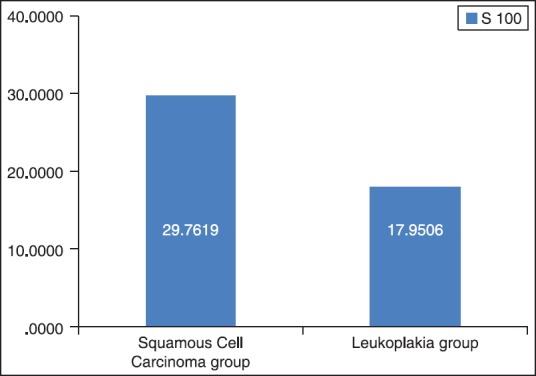
The mean total number of S-100 positive Langerhans cells in squamous cell carcinoma and dysplasia
A number of cases with different types of LC were compared in well-, moderate- and poorly-differentiated OSCC as well as different grades of dysplasia. Although, hyperplasia was into associated with any abnormal LC, abnormal cells (category b and c) were observed in both dysplasia and OSCC [Figures 2–7]. Interestingly moderate dysplasia cases and poorly-differentiated carcinomas showed more abnormal LC [Figure 8]. A number of S-100 positive LC were compared in the presence of mild, moderate and dense chronic inflammatory cell infiltrate in both dysplasia and SCC [Table 3]. There was increase in the number of LC in OSCC associated with mild inflammation when compared to moderate inflammation. In OED, LC were higher in mild and dense inflammation when compared to moderate inflammation. Types of LC were associated with different grades of inflammation in OED and OSCC [Table 4]. In OSCC, the mild inflammation was associated with the larger LC. This may indicate the failure of LC to perform their function and hence accumulate in the tumor.
Figure 2.
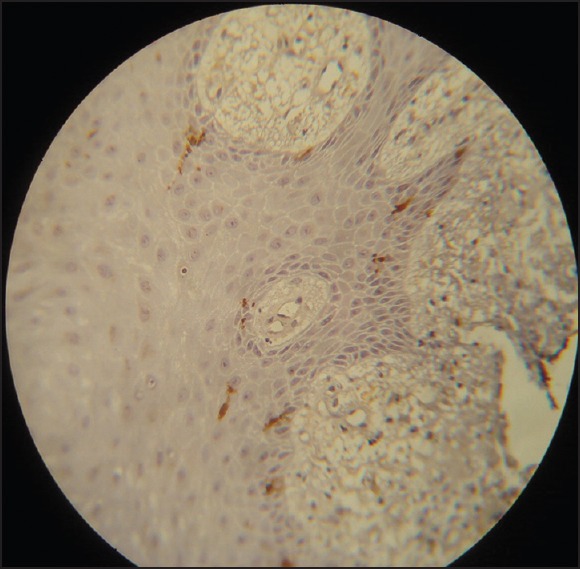
Normal appearing Langerhans cells in hyperplasia (Immunohistochemistry, ×40)
Figure 7.
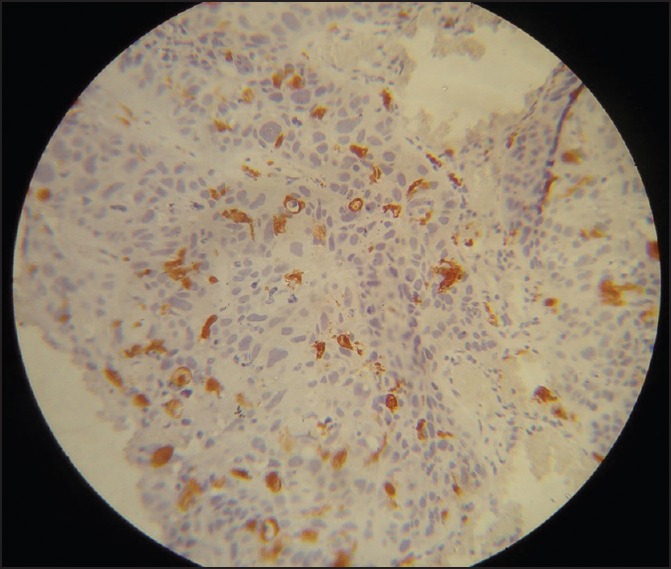
Poorly-differentiated squamous cell carcinoma with abnormal Langerhans cell and minimum inflammation (Immunohistochemistry, ×40)
Figure 8.
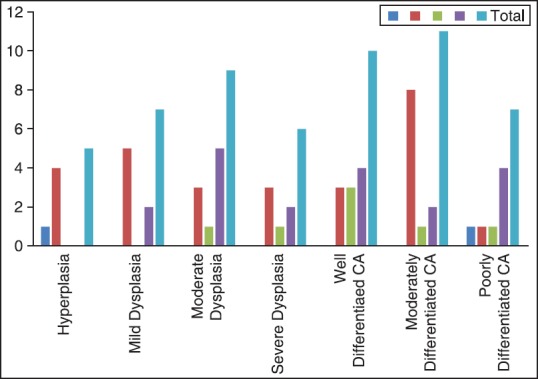
Number of cases with different types of S-100 positive Langerhans cells in dysplasia and oral squamous cell carcinoma
Table 3.
Total number of S-100 positive LCs in OSCC and OED associated with different grades lymphocytic infiltrate
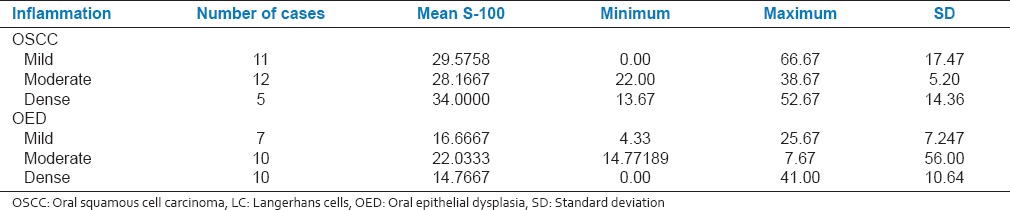
Table 4.
Number of cases showing different types of LCs were compared with different grades of inflammation in OED and OSCC
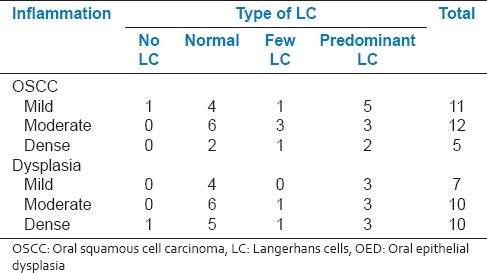
Figure 3.
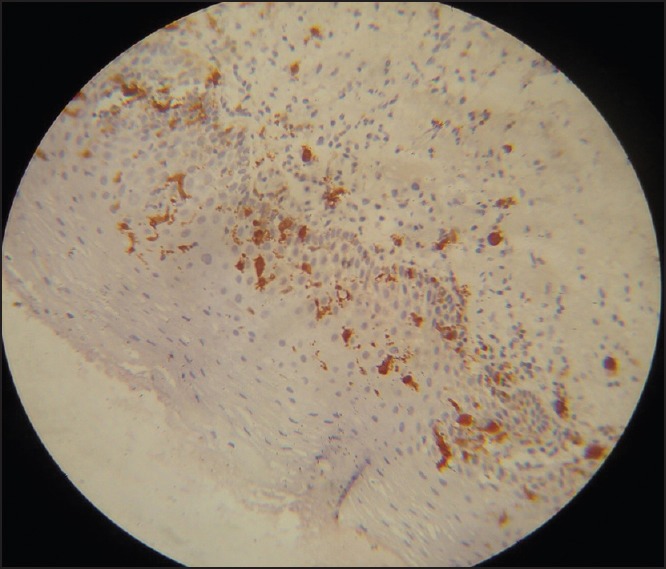
Numerous large abnormal appearing Langerhans cells (LCs) interspersed with normal appearing LCs in moderate dysplasia, connective tissue shows minimum inflammation (Immunohistochemistry, ×40)
Figure 4.
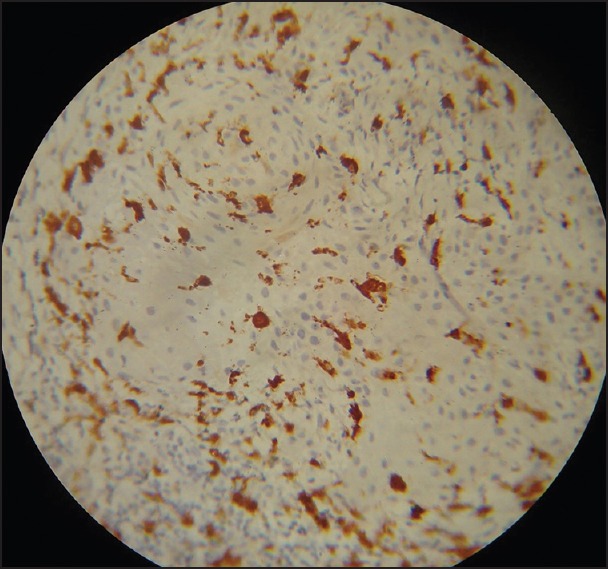
Well-differentiated squamous carcinoma with abnormal appearing Langerhans cell (Immunohistochemistry ×40)
Figure 5.
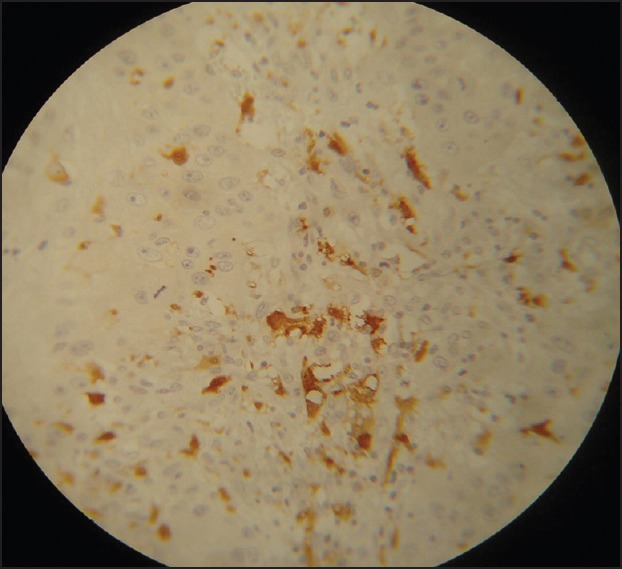
Moderately-differentiated squamous cell carcinoma showing large abnormal appearing Langerhans cell (Immunohistochemistry, ×40)
Figure 6.
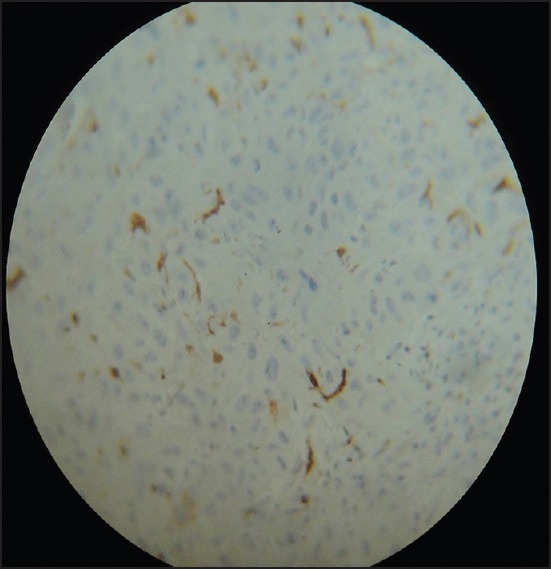
Moderately-differentiated squamous cell carcinoma showing normal appearing Langerhans cell (Immunohistochemistry, ×40)
DISCUSSION
The LC are intraepithelial dendritic cells (DCs) which process and present antigens to the immune cells, playing important role in tumor immunity.[9] The pyramidal shaped cells with long dendritic process located in suprabasal layer are Type I LC. The spherical cells with shorter DCs located in the basal layer are Type II LC.[11] Analyzing LC associated with tumor component is useful in evaluating the immunologic status of the patient.[10] Studies done on LC counts in OSCC and dysplasia have shown significant decrease of these DCs in OSCC compared to dysplasia.[12,13] The reduction was conspicuous in higher-grade tumors. Contrary to these previous reports we observed statistically significant increase in the number of LC in OSCC when compared to OED. Although, increase in LC in the peritumoral connective tissue is previously reported,[14] it is suggested that the greater influx of LC in the invasive tumors reflects increased tumor bulk resulting in increased production of the putative chemotactic factors. Chemokine MIP-3/CCL20 produced by tumor cells are selectively chemotactic to LC.[15] Interleukin-10, transforming growth factor-alpha, vascular endothelial growth factor could also regulate the recruitment and migration in the tumor microenvironment.[16]
Pronounced decrease of LC in poorly-differentiated OSCC is previously reported.[17,18] The opposite was observed in moderately- and well-differentiated tumors, which was associated with possible immune suppression induced by anaplastic tumor cells. Our observations are consistent with this report. However, lack of correlation between grading and ploidy status or LC count is also reported.[19] The number of LC infiltrating the tumor is a significant prognostic factor in OSCC patients. The increase in number of LC is regarded as a better prognostic factor.[20]
Increase in the LC density was seen at the lateral border of tongue and lip in subjects with history of smoking.[21] The tobacco smoke may cause increase in the local LC population helping to maintain normal protective function of oral mucosa. This is possibly due to increased rate of cell division of resident LC or influx of precursors from the circulation. Indeed smokeless tobacco usage reduced the number of LC due to inhibitory effect from the absorption of the tobacco contents.[22] In our cases, all the patients had the habit of tobacco chewing and some of them had smoking and chewing habits. However, the total duration and type of tobacco usage could not be obtained from the case records.
After the antigen capture, LC migrates to regional lymph nodes. During this migration, they undergo morphologic and ultrastructural modifications[23,24] as a result of the maturation process. Numerous peculiar S-100 positive, round-shaped cells scattered in the connective tissue as a result of phenotypic changes, during their migration toward regional lymph nodes is reported[13,25,26] wherein these cells may participate in antitumor immunity.[27] In contrast presence of immature CD207/Langerin+ in the primary OSCC and mature DCs were rare.[28] In our study, we found higher number of larger LC in moderate dysplasia and poorly-differentiated SCC. These altered LC may indicate accumulated immature cells. Due to reduced tumor immunity such lesions may behave aggressively.[28]
Antitumor response is initiated when immature LC come in direct contact with tumor cells. Defective host antitumor immune response causes immune evasion of the tumor cells often with invasion of immature immune cells.[29,30,31,32] Nevertheless an interesting question, which needs to be answered is, the role of LC in tumor immunity. The progression of tumor is unchecked even after increase in the number of infiltrating LC.[33] Low immunogenicity of the tumor cells may be the reason for lack of antigen recognition resulting in tumor cell immune evasion. Further, the functional capability of LC may be compromised within the tumor stroma[33] and the local environment of the dysplastic epithelium may further modulate the LC distribution.[34,35,36,37]
Increase in abnormal LCs in OED and OSCC associated with the mild inflammation in our study may be due to immune modulation by the locally secreted cytokines or due to immune escape. The reduction in the tumor immune response is reflected as lymphocytic infiltrate. Thus, the local immune modulation and absence of fully functional LC result in reduced immune response resulting in aggressive tumor. We conclude that an important observation of abnormal LC associated with the OEDs and OSCC and their association with reduced lymphocytic infiltrate reflects poor tumor immunity and aggressive tumor. Further studies are necessary to investigate the possibility of simple S-100 IHC as a surrogate marker for the surgeons to decide the aggressive treatment options in dysplasia and OSCC.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Subramanian S, Sankaranarayanan R, Bapat B, Somanathan T, Thomas G, Mathew B, et al. Cost-effectiveness of oral cancer screening: Results from a cluster randomized controlled trial in India. Bull World Health Organ. 2009;87:200–6. doi: 10.2471/BLT.08.053231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misra C, Majumder M, Bajaj S, Ghosh S, Roy B, Roychoudhury S. Polymorphisms at p53, p73, and MDM2 loci modulate the risk of tobacco associated leukoplakia and oral cancer. Mol Carcinog. 2009;48:790–800. doi: 10.1002/mc.20523. [DOI] [PubMed] [Google Scholar]
- 3.Lippman SM, Hong WK. Molecular markers of the risk of oral cancer. N Engl J Med. 2001;344:1323–6. doi: 10.1056/NEJM200104263441710. [DOI] [PubMed] [Google Scholar]
- 4.Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: An overview of the literature. J Oral Pathol Med. 2008;37:1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 5.Langerhans P. On the nerves of the human skin. Arch Pathol Anat. 1868;44:325–37. [Google Scholar]
- 6.Silberberg I. Apposition of mononuclear cells to Langerhans cells in contact allergic reactions. An ultrastructural study. Acta Derm Venereol. 1973;53:1–12. [PubMed] [Google Scholar]
- 7.Cutler CW, Jotwani R. Dendritic cells at the oral mucosal interface. J Dent Res. 2006;85:678–89. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett AW, Cruchley AT, Williams DM. Oral mucosal Langerhans’ cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi T, Hauser C, Budtz-Jörgensen E. Langerhans cells: Structure, function and role in oral pathological conditions. J Oral Pathol Med. 1993;22:193–202. doi: 10.1111/j.1600-0714.1993.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- 11.Breathnach AS. Variation in ultrastructural appearance of Langerhans cells in normal human epidermis. Br J Dermatol. 1977;97:14. [Google Scholar]
- 12.Girod SC, Kühnast T, Ulrich S, Krueger GR. Langerhans cells in epithelial tumors and benign lesions of the oropharynx. In vivo. 1994;8:543–7. [PubMed] [Google Scholar]
- 13.Albuquerque RL, Jr, Miguel MC, Costa AL, Souza LB. Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinoma. Int J Exp Pathol. 2003;84:259–65. doi: 10.1111/j.0959-9673.2004.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upadhyay J, Rao NN, Upadhyay RB. A comparative analysis of Langerhans cell in oral epithelial dysplasia and oral squamous cell carcinoma using antibody CD-1a. J Cancer Res Ther. 2012;8:591–7. doi: 10.4103/0973-1482.106565. [DOI] [PubMed] [Google Scholar]
- 15.Halliday GM, Lucas AD, Barnetson RS. Control of Langerhans’ cell density by a skin tumour-derived cytokine. Immunology. 1992;77:13–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: Friends or enemies? Cancer Metastasis Rev. 2006;25:333–56. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 17.Arany I, Adler-Storthz K, Chen Z, Tyring SK, Brysk MM. Tumor differentiation-dependent local immunity in human head and neck cancers. Cancer Lett. 1998;123:173–6. doi: 10.1016/s0304-3835(97)00432-1. [DOI] [PubMed] [Google Scholar]
- 18.Gallo O, Libonati GA, Gallina E, Fini-Storchi O, Giannini A, Urso C, et al. Langerhans cells related to prognosis in patients with laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1991;117:1007–10. doi: 10.1001/archotol.1991.01870210079015. [DOI] [PubMed] [Google Scholar]
- 19.van Heerden WF, Raubenheimer EJ, van Rensburg EJ, le Roux R. Lack of correlation between DNA ploidy, Langerhans cell population and grading in oral squamous cell carcinoma. J Oral Pathol Med. 1995;24:61–5. doi: 10.1111/j.1600-0714.1995.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 20.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–45. [PubMed] [Google Scholar]
- 21.Cruchley AT, Williams DM, Farthing PM, Speight PM, Lesch CA, Squier CA. Langerhans cell density in normal human oral mucosa and skin: Relationship to age, smoking and alcohol consumption. J Oral Pathol Med. 1994:55–9. doi: 10.1111/j.1600-0714.1994.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 22.Daniels TE, Chou L, Greenspan JS, Grady DG, Hauck WW, Greene JC, et al. Reduction of Langerhans cells in smokeless tobacco-associated oral mucosal lesions. J Oral Pathol Med. 1992;21:100–4. doi: 10.1111/j.1600-0714.1992.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 23.Ardavín C, Martínez del Hoyo G, Martín P, Anjuère F, Arias CF, Marín AR, et al. Origin and differentiation of dendritic cells. Trends Immunol. 2001;22:691–700. doi: 10.1016/s1471-4906(01)02059-2. [DOI] [PubMed] [Google Scholar]
- 24.Keller R. Dendritic cells: Their significance in health and disease. Immunol Lett. 2001;78:113–22. doi: 10.1016/s0165-2478(01)00247-4. [DOI] [PubMed] [Google Scholar]
- 25.Zavala WD, De Simone DS, Sacerdote FL, Cavicchia JC. Variation in Langerhans cell number and morphology between the upper and lower regions of the human esophageal epithelium. Anat Rec. 2002;268:360–4. doi: 10.1002/ar.10147. [DOI] [PubMed] [Google Scholar]
- 26.Katlic MR, Wilkins EW, Jr, Grillo HC. Three decades of treatment of esophageal squamous carcinoma at the Massachusetts General Hospital. J Thorac Cardiovasc Surg. 1990;99:929–38. [PubMed] [Google Scholar]
- 27.Wright-Browne V, McClain KL, Talpaz M, Ordonez N, Estrov Z. Physiology and pathophysiology of dendritic cells. Hum Pathol. 1997;28:563–79. doi: 10.1016/s0046-8177(97)90079-4. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell RK, Mick R, Feldman M, Hino S, Wang Y, Brose MS, et al. Distribution of dendritic cell subtypes in primary oral squamous cell carcinoma is inconsistent with a functional response. Cancer Lett. 2007;255:145–52. doi: 10.1016/j.canlet.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 30.Lucas AD, Halliday GM. Progressor but not regressor skin tumours inhibit Langerhans' cell migration from epidermis to local lymph nodes. Immunology. 1999;97:130–7. doi: 10.1046/j.1365-2567.1999.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang RR, Wen DR, Guo J, Giuliano AE, Nguyen M, Offodile R, et al. Selective modulation of paracortical dendritic cells and T-Lymphocytes in breast cancer sentinel lymph nodes. Breast J. 2000;6:225–232. doi: 10.1046/j.1524-4741.2000.98114.x. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi K, Kusama K, Taguchi K, Ishikawa F, Okamoto M, Shimada J, et al. Dendritic cells in human squamous cell carcinoma of the oral cavity. Anticancer Res. 2002;22:545–57. [PubMed] [Google Scholar]
- 33.Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008;272:186–96. doi: 10.1016/j.canlet.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Gottfried E, Kreutz M, Mackensen A. Tumor-induced modulation of dendritic cell function. Cytokine Growth Factor Rev. 2008;19:65–77. doi: 10.1016/j.cytogfr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 36.Grabbe S, Bruvers S, Gallo RL, Knisely TL, Nazareno R, Granstein RD. Tumor antigen presentation by murine epidermal cells. J Immunol. 1991;146:3656–61. [PubMed] [Google Scholar]
- 37.Furumoto K, Soares L, Engleman EG, Merad M. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113:774–83. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]


