Abstract
Context:
Glycyrrhizic acid (GA) is a triterpene glycoside representing the main active component of licorice root extract obtained from plants of the Glycyrrhiza glabra L. and widely used as a complex-forming agent for the synthesis of new transport forms of the well-known drugs.
Aims:
For the first time, the complexation of GA with chloramphenicol antibiotic (ChlA) was investigated by electrospray ionization mass spectrometry (ESI MS).
Subjects and Methods:
ESI MS was utilized in order to determine the composition and evaluate the stability of complexes of the GA and ChlA. The validation of the complex formation was confirmed by ultraviolet/visible and infrared (IR) spectroscopy.
Results:
MS data confirmed the noncovalent interactions between chloramphenicol and GA. Formation of the host: guest complexes of GA and chloramphenicol with the ratio 1:1 and 2:1 were registered in the negative ion mode. Binding of GA and ChlA was accompanied by changes in absorbance and IR spectrum of ChlA indicating the complex formation of these compounds.
Conclusions:
The research results confirmed the considerable potential of ESI MS as a technique for simple and fast detection of formation of the complexes of GA and the well-known drugs.
Keywords: Chloramphenicol, complexation, glycyrrhizic acid, mass spectrometry
INTRODUCTION
Chloramphenicol(ChlA) [Figure 1a], refers to antibiotics of a wide spectrum of biological activity. It is used for the treatment of typhoid, salmonellosis, brucellosis, meningitis, chlamydiosis, eye infections, septic wounds, and other illnesses.[1] It is utilized both individually and within complex formulations. However, the ChlA may cause toxic effects on the blood production system, initiate psychomotor disorders, confused mental states, hallucinations, allergic reactions, and depress senses of vision and audition; it also suppresses intestine microflora. The most serious adverse effect associated with chloramphenicol treatment is bone marrow toxicity, which may occur in two distinct forms: Bone marrow suppression, which is a direct toxic effect of the drug and is usually reversible, and aplastic anemia, which is idiosyncratic (rare, unpredictable, and unrelated to dose) and generally fatal. The therapeutic dosage of drugs may be reduced by means of their molecular encapsulation. As molecular carriers of medical agents, that is, pharmacons, plant glycosides are used.[2] The glycoside clathration to the most degree was studied for glycyrrhizic acid (GA) [Figure 1b], which represents a most abundant triterpene glycoside of various genera of licorice. Recently, novel complex compounds of GA with antimicrobial drugs have been prepared. For example, it was shown that the complex of GA with ChlA favors the resistance of mice to infection diseases and promotes immune protection. Nonetheless, the complex appeared to be more active than its individual components.[3] However, there is still no published data on the mass spectrometry (MS) investigation of molecular complexation of ChlA with GA so far. In this paper, the formation of glycoside molecular complexes with ChlA was studied by electrospray ionization (ESI) MS. Earlier we have successfully utilized that method in order to determine the composition and evaluate the stability of the α-hederin and hederasaponin C complexes of some drug ingredients, amino acids, and bases of nucleic acids.[4,5]
Figure 1.
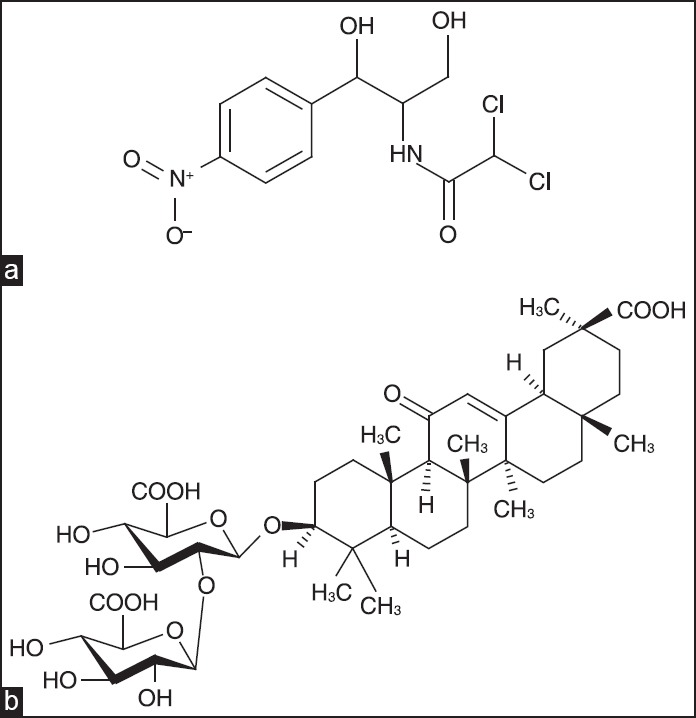
(a) Chloramphenicol, C11H12Cl12N2O5 (Mw = 323, 13); (b) glycyrrhizic acid, C42H62O16 (Mw = 822, 93)
MATERIALS AND METHODS
Complexes were prepared by mixing solutions contacting glycyrrhizic acid (>95%, Fluka) and chloramphenicol (local pharmacy company “Farmatsevt Plus”) (1 mmol each) in a 1:1 molar ratio (solvent, 50% aqueous EtOH, v/v). The resulting mixture was held at 40°C for 2.0 h with constant stirring. The organic solvents were vacuum distilled. The MS measurements were carried out on a Bruker Daltonics micrOTOF-Q mass spectrometer with ESI. The micrOTOFcontrol TM2.2 software manufactured by Bruker Daltonik GmbH was used. The calibration of the mass spectrometer was accomplished using electrospray calibrant solution by Fluka. The detection of positive and negative ions was performed in the range m/z from 50 to 3000. According to the instrument description, the precision of measurements by micrOTOF_Q was higher than 1-2 ppm, the accuracy of mass determination was 2 mDa. The voltage on the spray capillary was set at ±4200 V; the parameters of the dryer gas, nitrogen of extra high purity grade at 5 L/min, 200°C, were optimized for monitoring peaks of ion associates. For direct injection, we used substance solutions in acetonitrile, from Merck, qualified for high-performance liquid chromatography -MS, in concentration up to 0.2 mg/mL (10−7-10−6 M).
Absorption spectra and infrared (IR) spectra were recorded on spectrophotometer SPECS SSP 705 (190-1100 nm) and spectrometer Varian 3100 FT-IR-Excalibur Series, respectively.
RESULTS AND DISCUSSION
To evaluate the effectiveness of complex formation of GA with ChlA, the mass spectra of chloramphenicol and the mixture containing chloramphenicol and glycoside were studied [Table 1]. ChlA in the negative ion mode forms the deprotonated mono anion [MChlA−H]− more effectively with m/z 321.0, which is registered as the major peak in the mass spectrum. The mass spectrum also contains peaks of deprotonated dimer [2MChlA−H]− with m/z 645.0. The relative intensity of the peak is 14 times lesser for [2MChlA-H]−. In the positive ion mode, ChlA forms heteroclusters with Na+: [MChlA+Na]+ and [2MChlA+Na]+ more effectively. The mass spectrum contains a single type of K+ adduct. However, the relative intensity of their peaks is 90 times lesser for [MChlA+K]+ and 29 times lesser for [2MChlA+K]+ than that for heteroclusters with Na+: [MChlA+Na]+. The relative intensity of heteroclusters, [2MChlA+Na]+ and [2MChlA+K]+, differed by 1.4 times only. Mass spectra of mixture of ChlA and GA showed peaks belonging to both deprotonated ions of chloramphenicol and deprotonated monomer, dimer and trimer of GA in addition to various complexes consisting of monomers, dimers of GA with the one molecule of ChlA [Table 1]. In mass spectrum of a mixture of ChlA and GA, the base peak m/z 410.2 in negative ion mode relates to [MGA-2H]2−. Single-charged anion [MGA-H]− with m/z 821.3 is also present in the sample, its relative peak intensity does not exceed 25%. In this case, doubly-charged ion [MGA-2H]2− is 4 times more stable than single-charged one. Triple-charged anions of dimer and trimer of GA were identified as [2MGA+2H2O−2H]3− and [3MGA+3H2O−2H]3− with m/z 559.9 and 565.3, respectively. It was found that the anion [2MGA+2H2O−2H]3− is 6 times more stable than [3MGA+3H2O−2H]3−. The mass spectrum also contains single and double-charged anions of GA ammonium salt [MGA+NH3−H]− and [MGA+NH3−2H]2−. In this case, doubly-charged ion is 6 times more stable than single-charged one. In the mixture of GA - ChlA, a peak of the anions with m/z 1143.3 is registered and attributed to the complex [MGA+MChlA−H]−. The relative intensity of this peak was 5.3%. The complex of GA with ChlA occurred most stable among the recently investigated complexes of this glycoside with the paracetamol and streptocid.[6,7] Complex of GA dimer - ChlA is presented in mass spectra as double-charged anions with m/z 993.4 and 1001.3. The relative peak intensities of the ions [2MGA+MChlA+2H2O−2H]2− and [2MGA+MChlA+H2O−2H]2− differed by 3.5 times. Complex of GA ammonium salt-chloramphenicol is presented in mass spectra with peak m/z 579.2, but its intensity was small (~0.02%). In the positive ions mode, the complex GA and ChlA have not registrated. This may be due to the weak ionization of the complex to form a positively charged ion. In our previous studies, it was found that GA is ionized to form positive ions on the order of magnitude weaker than the negative ions. In this regard, MS analysis of the structure of the complexes with GA should be performed in negative ion mode to obtain more complete information.
Table 1.
Most characteristic ions in the mass spectra of chloramphenicol (ChlA), and its mixtures with GA
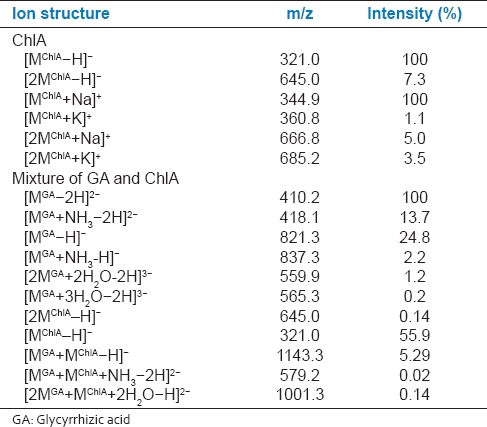
To the validation of the complex formation was used other experimental techniques (ultraviolet/visible [UV/Vis] and IR spectroscopy). Binding of GA and ChlA after mixing of their solutions was accompanied by changes in absorbance and IR spectrum of ChlA indicating the complex formation of these compounds. The UV-spectra of the mixture of GA and ChlA has shown that the increase in GA concentration is accompanied by the change in the shape of ChlA spectrum due to complex formation (its maximum becomes lower and shifts to a longer wavelength region) [Figure 2]. At low concentrations of GA (up to 0.1 mM) slight changes in the absorbance spectrum of ChlA were observed. However, the subsequent increase in GA concentration resulted in a significant change of the spectrum. Subsequent band shift of the complex absorbance to the longer wavelength regions observed at higher GA concentrations [Figure 2] indicates that the increase in GA concentrations up to 0.7 mM causes enlargement of this complex. These results are consistent with MS data, where peak of complex GA and ChlA (m/z 1143.3) are registrated and its intensity was enlarged (up to 30%) under increase in GA concentrations up to 0.4 mM.
Figure 2.
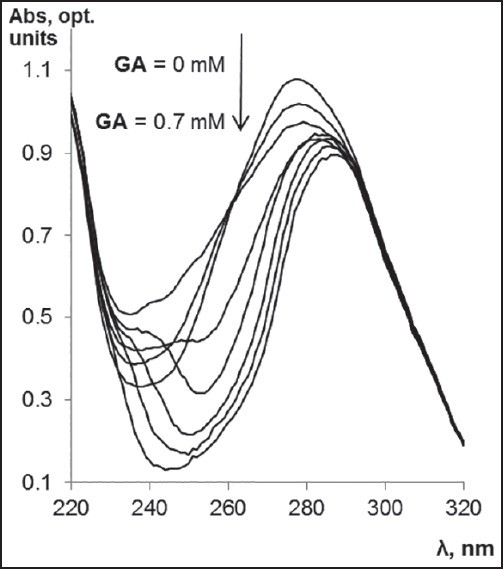
The dependence of the change in absorbance spectrum of chloramphenicol (ChlA) on glycyrrhizic acid (GA) concentration. ChlA concentration was 0.1 mM (in 20% methanol). The lower spectrum line corresponds to absorbance spectrum in the presence of 0.7 mM GA, the upper spectrum line corresponds to absorbance spectrum in the absence of GA
The IR spectra of complexes of GA - ChlA exhibit a shift of the absorption peaks 1655 cm−1 (C = O) by 5 cm−1, 1710 (−СООН) by 11 cm−1, 1450 (−СН2) by 7 cm−1 toward shorter frequencies and 1046 cm−1 (C-O-C) by 6 cm−1, 2968–2869 (δ С-Н) by 3-11 cm−1 toward lower frequencies as compared to their positions in the spectra of initial glycosides.
Research results confirm the formation of intermolecular bonds between drug and GA molecules.
CONCLUSIONS
The 1st time the formation of complex between GA and chloramphenicol (ChlA) was investigated by ESI MS. Mass spectra in the negative ion mode showed peaks belonging to various noncovalent complexes consisting of monomers, dimers of GA, and one molecule of ChlA. Formation of the host: guest complexes of GA and chloramphenicol with the ratio 1:1 and 2:1 were registered.
The validation of the complex formation was confirmed by UV/Vis and IR spectroscopy. Binding of GA and ChlA was accompanied by changes in absorbance and IR spectra indicating the formation of complex.
The research results open up the prospects for widespread use of ESI MS in the development of technology to create new low-dose drugs based on GA and ChlA.
ACKNOWLEDGMENTS
This research was supported by the internal grant of the Southern Federal University (project No 213.01-2014/005ВГ).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Brook I. Antibiotic Resistance of Anaerobic Bacteria. In: Mayers D. L, editor. Antimicrobial Drug Resistance. Clinical and Epidemiological Aspects. Humana Press; 2009. pp. 873–99. [Google Scholar]
- 2.Tolstikova TG, Tolstikov AG, Tolstikov GA. On the way to low-dose drugs. Vestn Ross Akad Nauk. 2007;77:867–4. [Google Scholar]
- 3.Kondratenko RM, Baltina LA, Mustafina SR, Ismagilova AF, Zarudii FS, Davydova VA, et al. Complex compounds of glycyrrhizic acid with antimicrobial drug. Pharm Chem J. 2003;37:32–5. [Google Scholar]
- 4.Lekar AV, Vetrova EV, Borisenko NI, Yakovishin LA, Grishkovets VI. Mass spectrometry of triterpene glycosides molecular complexation with purine bases of nucleic acids. Russian J Bioorg Chem. 2011;37:609–13. doi: 10.1134/s1068162011050116. [DOI] [PubMed] [Google Scholar]
- 5.Lekar AV, Yakovishin LA, Borisenko SN, Vetrova EV, Borisenko NI. Complexation of antibiotic levomycetin (Chloramphenicol) with α-hederin and hederasaponin C under the conditions of electrospray ionization. J Anal Chem. 2011;66:1437–40. [Google Scholar]
- 6.Lekar AV, Vetrova EV, Borisenko NI, Yakovishin LA, Grishkovets VI. Electrospray-ionization mass spectrometry of mixtures of triterpene glycosides with paracetamol. J Appl Spec. 2010;77:615–8. [Google Scholar]
- 7.Lekar AV, Vetrova EV, Borisenko NI, Yakovishin LA, Grishkovets VI. Mass spectrometric study on plant glycosides molecular complexation with streptocid (sulfanylamide) Russian J Bioorg Chem. 2012;38:749–52. [Google Scholar]


