Abstract
Aim:
We evaluated the periodontal health status and oral health behavior among hospitalized patients with chronic obstructive pulmonary disease (COPD) to assess the association of COPD with dental health.
Materials and Methods:
A group of 100 hospitalized patients with COPD and a group of 100 age, sex, and race-matched control patients were included in this study. Detailed case histories along with standardized measures of oral health including gingival index, plaque index (PI), and simplified oral hygiene index (OHI) were estimated and compared. Probing depths and clinical attachment levels (CALs) were recorded at four sites per tooth. C-reactive protein (CRP) levels in saliva and serum were also measured.
Results:
The study subjects had similar demographics and distribution in either group. Patients with COPD had significantly lower brushing frequency, poor periodontal health (OHI and PI), greater gingival inflammation, and deeper pockets/CALs compared to controls. Further COPD patients had significantly higher serum and salivary CRP levels compared to control groups.
Conclusions:
Lower brushing frequency, poor oral health, and presence of destructive periodontal disease were observed among patients with COPD, which warrants promoting dental care and oral health knowledge as an integrated approach to treating COPD patients.
Keywords: Chronic obstructive pulmonary disease, C-reactive protein, periodontal infection, poor periodontal health, risk factor
INTRODUCTION
Systemic illness may often impact dental health. Indeed, several studies have associated periodontitis with other systemic diseases such as type 2 diabetes mellitus, cardiovascular and pulmonary diseases.[1,2,3] Hence, it can be hypothesized that oral health may be an important indicator of systemic status, including respiratory diseases such as chronic obstructive pulmonary disease (COPD).
COPD is a progressive chronic disease which is characterized by an inexorable decline in respiratory function, exercise capacity, and health status.[4] Over 3 million deaths globally every year are due to COPD, making it the fourth highest cause of death worldwide, which is progressively increasing.[5] Acute exacerbations of COPD are defined as the worsening of COPD symptoms, which are commonly observed as an increase in cough, sputum production, purulence, and dyspnea. In 50-70% of acute exacerbations of COPD, the pathophysiological basis is usually infectious.[6] Haemophilus influenzae is the most frequent bacterium isolated in most cases, followed by Streptococcus pneumoniae, Moraxella catarrhalis, and Pseudomonas aeruginosa.[6,7,8,9]
The relationship between periodontitis and COPD has recently received much attention. Periodontitis is a chronic inflammatory condition consequence to bacterial infections that results in the destruction of the tooth-supporting connective tissue and bone. Oral pathogens and inflammatory cytokines from periodontal lesions induce systemic inflammation, which may contribute to the pathogenesis of COPD.[10] Poor host defense may further exacerbate microbial growth and promote subsequent tissue destruction.[11,12,13] Indeed, dental plaque may serve as a reservoir for systemic pathogens, which may be evident as poor oral hygiene.[11]
Exacerbations of COPD are one of the most common causes for hospital admission globally and in India. Hence to evaluate the role of periodontal status contributing to exacerbations of COPD, we assessed the oral health among COPD inpatients.
MATERIALS AND METHODS
Study population
One-hundred hospitalized patients with COPD at Department of General Medicine, Civil Hospital, Ahmedabad, Gujarat, India were included in the study. Similarly, 100 age, sex, and race matched systemically healthy subjects from the outpatient clinic, Department of Periodontics, Government Dental College and Hospital, Ahmedabad, Gujarat, India were considered as potential controls. Inclusion exclusion criteria were set and systematic random sampling was used to select cases and controls.
Inclusion criteria
-
(1)
Patients who were hospitalized for >3 days and diagnosed with acute respiratory disease (i.e., pneumonia, acute bronchitis, or a lung abscess) or an exacerbation of chronic respiratory disease (i.e., COPD, which included chronic bronchitis and emphysema);
-
(2)
Patients with no past or present history of respiratory disease were considered potential controls; and
-
(3)
Patients were aged 25-75 years and having at least 20 remaining natural teeth were considered for the study.
All COPD patients were clinically checked for barrel-shaped chest, hyper resonant note on percussion and bilateral bronchi. Criteria used for diagnosis were based on the Global Initiative for Chronic Obstructive Lung Disease spirometry guidelines.[32,36]
Exclusion criteria
Patients with:
-
(a)
History of systemic diseases other than respiratory diseases.
-
(b)
Under any medication known to influence periodontal status.
-
(c)
Having history of any periodontal treatment in past 6 months (day) hospitalized in intensive care units were excluded from the study.
Following institutional and ethical board approval, written consent of patients was sought. The hospital records pertaining to each patient during the study period were reviewed to match inclusion criteria. All the data of the survey were obtained on the basis of the detailed case history and included information regarding patient's age, sex, socioeconomic status (SES), and brushing frequency. SES variables included educational qualification and monthly income. All patients who had a history of smoking were heavy smokers (>20 cigarettes/day).
Clinical evaluation
The following standardized measures of oral health were performed: The gingival index (GI),[14] the plaque index (PI),[15] and the simplified oral hygiene index (OHIS).[16] Probing depth and clinical attachment level (CAL) were measured to the nearest mm. The CAL was obtained by subtracting the distance from the free gingival margin (FGM) to the cement-enamel junction as a reference point of each tooth from the distance from the FGM to the bottom of the sulcus in the absence of recession. In the presence of recession, CAL was calculated by adding distance from FGM to the cementoenamel junction to the pocket depth. An oral examination was performed under proper illumination with the case group patient sitting erect on a bed and with a mouth mirror and University of North Carolina-15 periodontal probe. A single qualified clinician performed the clinical evaluation of the parameters.
A whole unstimulated saliva sample was obtained from the patients and controls and stored as aliquots at −20°C for C-reactive protein (CRP) determination. A sample of 2 mL blood from patients and controls was collected into vacutainer tubes, centrifuged, and the serum was separated and stored at −20°C for the estimation of CRP level.
Statistical Analysis
The data (mean [standard deviation [SD]) were analyzed by SPSS statistical package (version 12.0, SPSS Inc., Chicago, IL, USA) using un-paired t-test to compare means between cases and controls for brushing frequency, PI score, GI score, OHIS score, PD, CAL, and CRP values in saliva and blood. P < 0.05 were considered to be statistically significant. Correlation between serum and saliva CRP was assessed by Pearson's correlation coefficient.
RESULTS
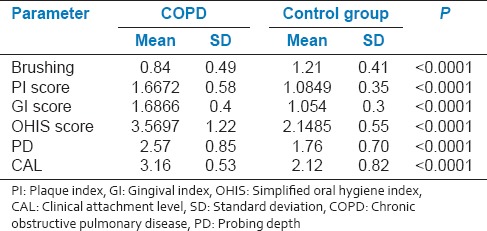
Demographic data of the study population are compared in Table 1. There was no significant difference in SES and educational qualification between COPD and control group. Mean values of the brushing frequency were significantly (P < 00001) lesser while GI, PI, OHIS, probing depth, and CAL were higher for patients with COPD compared to control group [Table 2].
Table 1.
Study population demographic
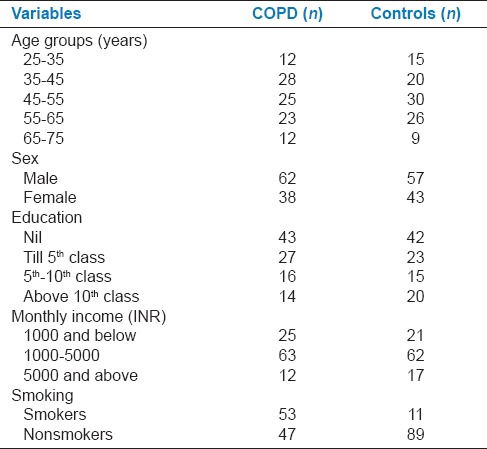
Table 2.
Comparison of means and SD of different criteria of COPD and control group
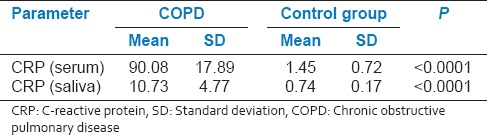
Mean CRP levels in serum and saliva of COPD patients (90.08 [SD 17.89] μg/mL and 10.73 [SD 4.77] μg/mL, respectively) were significantly (P < 0.0001) higher than those in the controls (1.45 [SD 0.72] μg/mL and 0.74 [SD 0.17] μg/mL, respectively) [Table 3]. A significant and positive correlation was observed between serum and saliva CRP levels in the COPD patients (r = 0.86) and control subjects (r = 0.62).
Table 3.
Comparison of means and SD of CRP level in serum and saliva of COPD and control group
DISCUSSION
Hospitalized patients with respiratory diseases consistently show poor periodontal status compared to controls. Cases with COPD had a significant difference in brushing frequency, PI, GI, OHIS scores, PD, and CAL compared to controls, which is consistent with previous reports.[17,18,19] Significantly greater mean OHI, mean PD, and mean CAL values were also reported to be associated with respiratory disease.[20,21,22] Although lack of association between gingival bleeding alone and respiratory disease is also reported.[20] Nevertheless, inappropriate tooth brushing method, lower regular supra-gingival scaling, and poor oral health knowledge remain significantly associated with COPD.[23]
Aspiration of pathogenic bacteria from the oropharynx into the lungs appears to play an important role in the pathogenesis of chronic pulmonary diseases.[11] Patients with periodontal disease have elevated levels of proteolytic bacteria (Porphyromonas gingivalis and Spirochetes) which produce proteases and together with elevated levels of various hydrolytic salivary enzymes destroy protective domains of host secretory components (e.g., mucins) and thus, diminish the nonspecific host defense against respiratory pathogens.[13] In light of complex and multifactorial nature of the respiratory disease, demonstration of a dose effect for such association between periodontitis and respiratory disease was not possible. However, high salivary concentrations of P. gingivalis were reported to enhance the risk of developing respiratory diseases.[24]
Oral pathogens and inflammatory cytokines from periodontal lesions induce systemic inflammation, which may contribute to the pathogenesis of COPD [Figure 1].[10] Bacteria can enhance pulmonary inflammation in COPD, which could potentially cause increased tissue damage due to leucocyte recruitment and proteinase secretion.[25,26,27] In periodontal disease, host response via immune defense system is considered important, specifically, the cellular response. Thus, the higher prevalence of periodontal diseases reported among COPD patients may possibly be also due to activation of the immune system, inhaled drugs or an interaction between them.[28]
Figure 1.
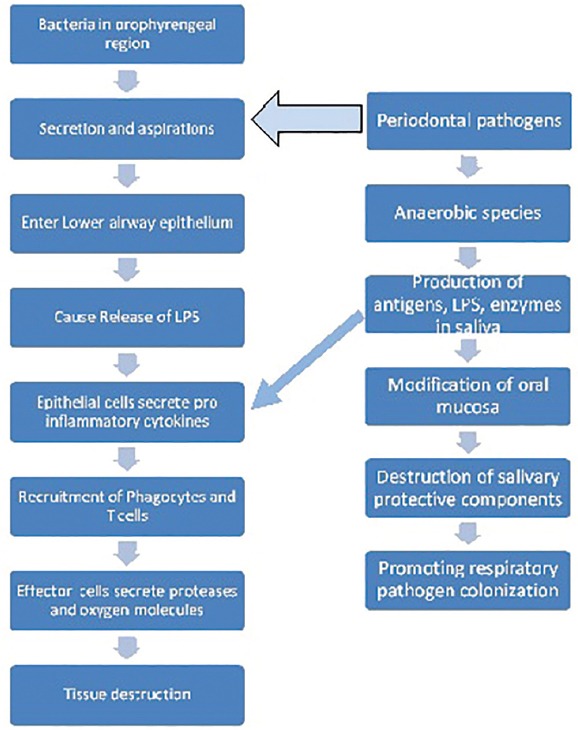
Mechanism for tissue destruction in COPD and role of periodontal pathogens
Quantitative changes of specific salivary biomarkers could have significance in the diagnosis and management of both oral and systemic diseases.[29] In our study, CRP levels in serum and saliva were significantly higher compared to patients in control group. Moreover, a significant and positive correlation was also observed between serum and salivary CRP levels of COPD and control patients. Indeed, significant differences in CRP levels between periodontitis patients with different grades of oral health are also reported.[30,31,32,33,34,35]
The association between periodontitis and COPD may have been confounded by shared risk factors especially smoking, which is the leading risk factor for periodontitis, emphysema, chronic bronchitis, and lung infections. As COPD is multifactorial and has a complex etiological profile, a demonstration of a dose effect for the association between periodontitis and COPD is challenging. Nevertheless, poor oral hygiene and poor periodontal status is consistently associated with the presence of respiratory disease specially aspiration pneumonia, hospital-acquired pneumonia, and COPD. It is, however, likely that periodontal pathogens may serve as the reservoir of microbiological etiology of COPD. Considering the pathogenesis of the periodontal and COPD, it seems possible that the inflammatory process underneath the periodontal disease may modify the respiratory epithelium and increase the risk of COPD. Hence, the CRP infection link cannot be ignored because CRP spikes during acute exacerbations of COPD and it will be very important to understand the effect of increased CRP levels in periodontitis on patients with COPD. The randomized clinical trials and clinicopathological study designs may be of long-term effectiveness when determining such association. As our study was cross-sectional and noninterventional with no microbiological evaluation, it limits determining a causal relationship between periodontitis and the COPD.
We conclude that presence of consistent poor oral hygiene, lower brushing frequency, and presence of destructive periodontal disease among COPD patients may be aggravating factors. Hence, oral health care maintenance must be given equal importance in the management of COPD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ryan ME, Carnu O, Kamer A. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134:34S–40. doi: 10.14219/jada.archive.2003.0370. [DOI] [PubMed] [Google Scholar]
- 2.Taylor GW. The effects of periodontal treatment on diabetes. J Am Dent Assoc. 2003;134:41S–8. doi: 10.14219/jada.archive.2003.0371. [DOI] [PubMed] [Google Scholar]
- 3.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 4.Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121:151S–5. doi: 10.1378/chest.121.5_suppl.151s. [DOI] [PubMed] [Google Scholar]
- 5.Salvi S. COPD: The neglected epidemic. In: Jindal SK, editor. Textbook of Pulmonary and Critical Care Medicine. 1/e ed. Vol. 2. New Delhi: Jaypee Publications; 2011. pp. 971–4. [Google Scholar]
- 6.Ball P. Epidemiology and treatment of chronic bronchitis and its exacerbations. Chest. 1995;108:43S–52. doi: 10.1378/chest.108.2_Supplement.43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monsó E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–20. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 8.Soler J, Sánchez L, Latorre M, Alamar J, Román P, Perpiñá M. The impact of COPD on hospital resources: The specific burden of COPD patients with high rates of hospitalization. Arch Bronconeumol. 2001;37:375–81. doi: 10.1016/s0300-2896(01)78818-7. [DOI] [PubMed] [Google Scholar]
- 9.Miravitlles M, Mayordomo C, Artés M, Sánchez-Agudo L, Nicolau F, Segú JL. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice. EOLO Group. Estudio Observacional de la Limitación Obstructiva al Flujo aEreo. Respir Med. 1999;93:173–9. doi: 10.1016/s0954-6111(99)90004-5. [DOI] [PubMed] [Google Scholar]
- 10.Terpenning MS. The relationship between infections and chronic respiratory diseases: An overview. Ann Periodontol. 2001;6:66–70. doi: 10.1902/annals.2001.6.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Scannapieco FA, Genco RJ. Relationships between periodontal disease and bacterial pneumonia. J Periodontol. 1996;67(Suppl 10):1114–22. doi: 10.1902/jop.1996.67.10s.1114. [DOI] [PubMed] [Google Scholar]
- 12.Fagon JY, Chastre J. Severe exacerbations of COPD patients: The role of pulmonary infections. Semin Respir Infect. 1996;11:109–18. [PubMed] [Google Scholar]
- 13.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 16.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 17.Raghavendran K, Mylotte JM, Scannapieco FA. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: The contribution of dental biofilms and periodontal inflammation. Periodontol 2000. 2007;44:164–77. doi: 10.1111/j.1600-0757.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma N, Shamsuddin H. Association between respiratory disease in hospitalized patients and periodontal disease: A cross-sectional study. J Periodontol. 2011;82:1155–60. doi: 10.1902/jop.2011.100582. [DOI] [PubMed] [Google Scholar]
- 19.Russell SL, Boylan RJ, Kaslick RS, Scannapieco FA, Katz RV. Respiratory pathogen colonization of the dental plaque of institutionalized elders. Spec Care Dentist. 1999;19:128–34. doi: 10.1111/j.1754-4505.1999.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 20.Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: Analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72:50–6. doi: 10.1902/jop.2001.72.1.50. [DOI] [PubMed] [Google Scholar]
- 21.Garcia RI, Nunn ME, Vokonas PS. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6:71–7. doi: 10.1902/annals.2001.6.1.71. [DOI] [PubMed] [Google Scholar]
- 22.Hayes C, Sparrow D, Cohen M, Vokonas PS, Garcia RI. The association between alveolar bone loss and pulmonary function: The VA dental longitudinal study. Ann Periodontol. 1998;3:257–61. doi: 10.1902/annals.1998.3.1.257. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhou X, Zhang J, Zhang L, Song Y, Hu FB, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol. 2009;36:750–5. doi: 10.1111/j.1600-051X.2009.01448.x. [DOI] [PubMed] [Google Scholar]
- 24.Page RC. Periodontitis and respiratory diseases: Discussion, conclusions, and recommendations. Ann Periodontol. 2001;6:87–90. doi: 10.1902/annals.2001.6.1.87. [DOI] [PubMed] [Google Scholar]
- 25.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14:1015–22. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 26.Khair OA, Devalia JL, Abdelaziz MM, Sapsford RJ, Tarraf H, Davies RJ. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-alpha and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur Respir J. 1994;7:2109–16. doi: 10.1183/09031936.94.07122109. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman D, Ben-Yaakov M, Lazarovich Z, Ohana B, Boldur I. Chlamydia pneumoniae infection in acute exacerbations of chronic obstructive pulmonary disease: Analysis of 250 hospitalizations. Eur J Clin Microbiol Infect Dis. 2001;20:698–704. doi: 10.1007/s100960100596. [DOI] [PubMed] [Google Scholar]
- 28.Karl L. Hakamima Asthma and oral health: A clinical and epidemiological study. Acta Electronica Universitatis Tamperensis. 2002. p. 193. Available from: http://acta.uta.f .
- 29.Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20:740–5. doi: 10.1097/00003246-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Christodoulides N, Floriano PN, Miller CS, Ebersole JL, Mohanty S, Dharshan P, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–28. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 31.Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA. C-reactive protein levels in patients with aggressive periodontitis. J Periodontol. 2006;77:933–9. doi: 10.1902/jop.2006.050165. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Bunjhoo H, Xiong W, Xu Y, Yang D. Concentration and chronic obstructive pulmonary disease: A systematic review and meta-analysis. J Int Med Res. 2012;40:1629–35. doi: 10.1177/030006051204000501. [DOI] [PubMed] [Google Scholar]
- 33.Biljak VR, Pancirov D, Cepelak I, Popovic-Grle S, Stjepanovic G, Grubišic TŽ. Platelet count, mean platelet volume and smoking status in stable chronic obstructive pulmonary disease. Platelets. 2011;22:466–70. doi: 10.3109/09537104.2011.573887. [DOI] [PubMed] [Google Scholar]
- 34.Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–24. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Torres JP, Cordoba-Lanus E, López-Aguilar C, Muros de Fuentes M, Montejo de Garcini A, Aguirre-Jaime A, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J. 2006;27:902–7. doi: 10.1183/09031936.06.00109605. [DOI] [PubMed] [Google Scholar]
- 36.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]


