Abstract
Objective
The objective of the current study is to define whether intra-articular nerve growth factor (NGF), an inflammatory mediator that contributes to osteoarthritic pain, is necessary and sufficient for the development or maintenance of injury-induced facet joint pain and its concomitant spinal neuronal hyperexcitability.
Method
Male Holtzman rats underwent painful cervical facet joint distraction or sham procedures. Mechanical hyperalgesia was assessed in the forepaws, and NGF expression was quantified in the C6/C7 facet joint. An anti-NGF antibody was administered intra-articularly in additional rats immediately or 1 day following facet distraction or sham procedures to block intra-articular NGF and test its contribution to initiation and/or maintenance of facet joint pain and spinal neuronal hyperexcitability. NGF was injected into the bilateral C6/C7 facet joints in separate rats to determine if NGF alone is sufficient to induce these behavioral and neuronal responses.
Results
NGF expression increases in the cervical facet joint in association with behavioral sensitivity after that joint’s mechanical injury. Intra-articular application of anti-NGF immediately after a joint distraction prevents the development of both injury-induced pain and hyperexcitability of spinal neurons. Yet, intra-articular anti-NGF applied after pain has developed does not attenuate either behavioral or neuronal hyperexcitability. Intra-articular NGF administered to the facet in naïve rats also induces behavioral hypersensitivity and spinal neuronal hyperexcitability.
Conclusion
Findings demonstrate that NGF in the facet joint contributes to the development of injury-induced joint pain. Localized blocking of NGF signaling in the joint may provide potential treatment for joint pain.
Keywords: NGF, facet joint, pain, trauma, neuronal hyperexcitability
Introduction
Joint and neck/back pain are the most common types of chronic pain1. The fibers that innervate articular joints exhibit increased mechanosensitivity during joint inflammation and can be activated by joint loading2–4. Joint inflammation also sensitizes neurons in the spinal cord and expands receptive fields to include adjacent non-inflamed tissues4,5, supporting spinal neuronal sensitization in joint pain. However, the local joint mechanism(s) through which spinal neurons are sensitized and induce joint-mediated pain are not defined.
The spinal facet joint is the most common source of pain from neck injury6. Non-physiological loading of the facet activates nociceptors in its capsule, induces hyperexcitability of spinal neurons, and produces pain3,7–9. Intra-articular treatment with an NSAID alleviates pain after experimental facet trauma and reduces spinal astrocytic activation10, suggesting inflammation is involved in loading-induced facet pain. Neuro-inflammatory responses also contribute to osteoarthritis-induced joint pain; inflammatory cytokines and spinal neuronal hyperexcitability increase in arthritis models11,12. Because similar inflammatory and neuronal responses are associated with both arthritis-induced and injury-induced joint pain5,8–10,12,13, common mechanisms may contribute to both syndromes. Despite evidence suggesting that notion, the local molecular mechanisms that lead to facet pain are unclear.
Nerve growth factor (NGF) sensitizes adult sensory neurons and increases in inflamed tissues14. NGF injection into peripheral tissues induces sensitivity to mechanical stimuli in animal models and humans15,16. Anti-NGF antibody treatment alleviates pain from inflammation and nerve injury in rat models17,18, and systemic anti-NGF reduces osteoarthritic joint pain19, supporting NGF’s role in joint pain. NGF and its high-affinity receptor, trkA, have been identified in osteoarthritic joints and degenerative facets11,20–22. Although studies collectively suggest intra-articular NGF contributes to degenerative joint pain, its contribution to injury-induced facet pain is unknown.
We have developed a rat model of painful facet joint injury, in which inflammatory cytokines and prostaglandin E2 (PGE2) increase in association with pain, and in which intra-articular NSAID administration alleviates pain8,10,23,24. Since those findings suggest either local or widespread inflammation to be involved in injury-induced joint pain, and because NGF is upregulated in painful inflamed and arthritic joints clinically and experimentally11,20,25–27, intra-articular NGF is hypothesized to contribute to the development and/or maintenance of injury-induced facet joint pain. This study quantifies expression of NGF in the facet joint in order to determine whether NGF is a local mediator leading to joint pain. Based on those findings, complementary studies blocking intra-articular NGF signaling after facet injury and applying exogenous NGF intra-articularly to the facet joints of naïve rats were performed to determine if NGF is necessary and sufficient for the development and maintenance of facet-mediated pain and associated spinal neuronal hyperexcitability.
Methods
All procedures were approved by the University of Pennsylvania IACUC and performed under the guidelines of the Committee for Research and Ethical Issues of the IASP28. Complementary studies were performed to: (1) characterize NGF expression in the facet joint after its painful injury, (2) define the contribution of intra-articular NGF to injury-induced facet pain and spinal neuronal hyperexcitability, and (3) identify whether intra-articular NGF alone is sufficient to induce pain and spinal neuronal hyperexcitability. Rats were doubly-housed with 12-hour light/dark cycles. For all studies, rats were randomly assigned to groups before any surgical procedure or behavioral assessment. Multiple groups were evaluated simultaneously, and all quantitative analyses performed without group identification, to eliminate bias.
Facet Joint Distraction & Pain Assessment
Surgical procedures were performed using male Holtzman rats (weight 398±31g) under inhalation isoflurane anesthesia (4% induction; 2.5% maintenance). The painful facet joint distraction (FJD) has been described previously23,24. The C5-T1 laminae and facet joints were exposed and cleared of paraspinal musculature. The interspinous ligaments from C5-T1 were resected, and a customized loading device applied a symmetric distraction across the bilateral C6/C7 facet joints by displacing the C6 vertebra rostrally and holding C7 fixed. In separate control rats, sham procedures included device attachment with no joint distraction. Wounds were closed with polyester suture and surgical staples, and rats were recovered in room air with weight gain monitored regularly until each rat’s study endpoint.
Forepaw mechanical withdrawal thresholds were quantified using customary methods29,30 and performed between 8am and noon. An ascending series of von Frey filaments (Stoelting; Wood Dale, IL) was applied to the forepaw of each rat; the lower of two consecutive filaments eliciting an emphatic lifting was taken as the threshold for that paw. The bilateral responses were averaged to obtain the withdrawal threshold for each rat on each day. Thresholds were quantified prior to any surgical procedure to establish baseline responses, as well as until the time of tissue harvest or electrophysiological experiments.
Intra-Articular NGF Characterization after Painful FJD
NGF expression was quantified in the facet joint soft tissues, including the capsular ligament and synovium, from a group of rats at 1 day after injury (FJD n=5; sham n=5) using Western blot (Table 1). Following behavioral assessment, rats were given an overdose of sodium pentobarbital (65mg/kg) and transcardially perfused with phosphate buffered saline (PBS). Tissue was harvested from the bilateral C6/C7 facet joints and protein extracted using the RIPA Lysis Buffer System (Santa Cruz Biotechnology; Santa Cruz, CA). Proteins were prepared, separated, and transferred to an Immobilon-FL transfer membrane (Millipore; Billerica, MA), as described previously31. Membranes were blocked for 1 hour with 5% nonfat dry milk in 0.1% Tween-20 Tris-buffered saline (TBS) and incubated overnight at 4°C with a rabbit anti-NGF antibody (1:200; Santa Cruz Biotechnology). Membranes were then washed three times with 0.1% Tween-20 TBS and incubated for 2 hours at room temperature with a goat anti-rabbit IRDye 800CW secondary antibody (1:15,000; LI-COR; Lincoln, NE). Membranes were imaged using the Odyssey Infrared Imaging System (LI-COR), then stripped and re-probed for β-tubulin using mouse anti-β-tubulin primary (1:2000; Covance; Princeton, NJ) and goat anti-mouse IRDye 680LT secondary (1:15,000 with 0.02% SDS; LI-COR) antibodies. Quantitative analysis of NGF (27kDa) and β-tubulin (50kDa) was performed using Image Studio Lite software (version 3.1; LI-COR). NGF expression was normalized to β-tubulin for each sample.
Table 1.
Number of rats for each experimental group and experimental outcomes.
| Study | Group | Study Endpoint (day) |
Rats/ Group |
Experimental Outcomes |
|---|---|---|---|---|
| NGF Characterization |
FJD | 1 | 8 | Forepaw withdrawal threshold NGF level (western blot; IHC) |
| sham | 8 | |||
| Intra-Articular Anti-NGF Injection |
sham+veh | 7 | 5 | Forepaw withdrawal threshold Evoked spinal neuronal firing |
| sham+anti-NGF | 5 | |||
| FJD+anti-NGF | 6 | |||
| FJD+veh | 5 | |||
| FJD+anti-NGFD1 | 8 | |||
| sham+veh | 1 | 5 | Forepaw withdrawal threshold Evoked spinal neuronal firing |
|
| FJD+anti-NGF | 5 | |||
| FJD+veh | 5 | |||
| Intra-Articular NGF Injection |
NGF | 7 | 6 | Forepaw withdrawal threshold |
| vehicle | 3 | |||
| NGF | 1 | 7 | Forepaw withdrawal threshold Evoked spinal neuronal firing |
|
| vehicle | 6 |
FJD: facet joint distraction; IHC: immunohistochemistry; NGF: nerve growth factor.
NGF expression was also assessed in the facet joints of additional rats at day 1 (FJD n=3; sham n=3) using immunohistochemistry (Table 1). The C4-T2 spines were harvested and post-fixed in 4% paraformaldehyde in PBS, transferred to 30% sucrose in PBS for 7 days, and decalcified in 10% Ethylenediaminetetraacetic Acid in PBS for 3 weeks. The C6/C7 spinal levels were embedded in Tissue-Tek OCT Compound (Sakura Finetek; Torrance, CA). The bilateral facet joints were sectioned (16µm) in the frontal plane, thaw-mounted onto slides, and labeled for NGF as previously described32. Endogenous peroxidase activity was quenched, and sections were incubated in DeCal Antigen Retrieval (BioGenex; Fremont, CA) solution for 30 minutes. Slides were washed, blocked with normal horse serum (Vector; Burlingame, CA), and incubated in rabbit anti-NGF (1:250; Santa Cruz Biotechnology) antibody overnight at 4°C. Sections were then incubated with a biotinylated horse anti-rabbit secondary antibody (1:1000; Vector) for 30 minutes and developed with 3,3-diaminobenzidine (Vector). Additional tissue sections that were not incubated with the primary antibody were included as negative controls.
Contribution of Intra-Articular NGF to Injury-Induced Pain & Neuronal Hyperexcitability
In order to determine if intra-articular NGF contributes to the development and/or maintenance of injury-induced joint pain, NGF signaling was blocked in the joint using an anti-NGF antibody either at the time of injury or 1 day after injury. Separate groups of rats underwent FJD or sham procedures as described above and received bilateral intra-articular injections of either a commercially available rabbit polyclonal anti-NGF antibody (IgG fraction) (Millipore #AB1526SP; Billerica, MA) or a control rabbit IgG (Millipore; Billerica, MA) in the C6/C7 facet joints, using customary methods30. Immediately following FJD, groups of rats received a bilateral 10µg intra-articular injection of either the anti-NGF antibody (FJD+anti-NGF) or control IgG (FJD+veh) in 5µL of PBS, a dose previously used in a rat model of inflammatory pain33. In a separate group of rats, anti-NGF was injected into the bilateral facet joints on day 1 after FJD (FJD+anti-NGFD1). Following behavioral testing on day 1, those rats were re-anesthetized, the bilateral C6/C7 facet joints were re-exposed, and 10µg of anti-NGF in 5µL of PBS was injected intra-articularly. Separate rats received bilateral intra-articular injections of the anti-NGF antibody (sham+anti-NGF) or control IgG (sham+veh) immediately following sham procedures. Previous work with this model demonstrates group sizes of 4–6 rats as sufficient to detect injury-induced behavioral hypersensitivity and increased evoked firing from spinal neurons7,10. As such, group sizes of 5 or 6 rats were utilized, with 8 rats used in the FJD+anti-NGFD1 group (Table 1).
Forepaw mechanical withdrawal thresholds were measured in all rats as described above in order to assess baseline and post-surgical responses on days 1, 3, 5, and 7, according to each group’s designated endpoint in the study. Rats were prepared for spinal electrophysiological recordings on day 1 (FJD+veh n=5; FJD+anti-NGF n=5; sham+veh n=5) or day 7 (FJD+veh n=5; FJD+anti-NGF n=6; sham+veh n=5; sham+anti-NGF n=5; FJD+anti-NGFD1 n=8) after behavioral testing (Table 1)7. Rats were anesthetized with sodium pentobarbital (45mg/kg, i.p.), and anesthesia was maintained with supplementary doses (5–10mg/kg, i.p.). The mid-cervical trachea was exposed, cannulated, and ventilated (CWE Inc.; Ardmore, PA); the rat was mounted onto a stereotaxic frame. The C6-C8 spinal cord was exposed and bathed in 37°C mineral oil. Core temperature was maintained at 35–37°C.
Extracellular potentials were recorded in the C6-C7 spinal cord using tungsten electrodes lowered into the deep laminae (III-VI) of the dorsal horn, where mostly wide dynamic range neurons are7,9,34, using a micropositioner. The signal was amplified, filtered, and sampled at 25kHz29. Sensory neurons with input from the forepaw were identified by brushing the plantar surface of the forepaw. A stimulus train, consisting of light brush (applied for 10 seconds), a series of non-noxious and noxious von Frey filaments (1.4g, 4g, 10g, 26g) each applied for 5 stimulations of 1-second followed by 1-second of recovery, and a noxious pinch (applied for 10 seconds), was applied to the paw. Stimulus trains were applied at 30 second intervals.
Recordings were spike-sorted using Spike2 (CED; Cambridge, UK). Evoked spikes were summed over the continuous 10-second stimulus period for both the brush and pinch stimuli. Evoked spikes during the pinch stimulus were used to classify neurons as either low threshold mechanoreceptive (LTM) or wide dynamic range (WDR)9. The number of spikes evoked from the initial application of a von Frey filament until 1-second after its 5th application were summed. For each filament, baseline firing was quantified by counting the number of spikes 1-second prior to its initial application and was subtracted from the total spike count.
Behavioral & Neuronal Response Assessments after Intra-Articular NGF
In order to determine if intra-articular NGF alone is sufficient to induce behavioral sensitivity and spinal neuronal hyperexcitability, rats received either 3µg of rat β-NGF (R&D Systems; Minneapolis, MN) in 5µL of sterile PBS (NGF) or the delivery vehicle alone (vehicle) injected intra-articularly into the bilateral C6/C7 facet joints. This dose was selected from an initial dose-response study administering intra-articular NGF at 1µg, 3µg, 6µg, or 10µg in separate groups. The 3µg dose was the lowest to elicit a behavioral response after injection and so was applied in the current study. Forepaw withdrawal thresholds to mechanical stimuli were measured to establish baseline responses, as well as at days 1, 3, 5, and 7 after injection (Table 1). Based on those behavioral studies, additional separate groups were followed for 1 day (NGF n=7; vehicle n=6) and prepared for spinal electrophysiological recordings after behavior testing on day 1 (Table 1). Evoked spikes from spinal neurons were quantified as described above.
Statistical Analyses
Statistical analyses were performed using JMP Pro v10.0.2 (SAS Institute Inc.; Cary, NC). A t-test compared NGF levels quantified via Western blot between the FJD and sham groups. Forepaw withdrawal thresholds were compared between groups using a two-way repeated measures ANOVA with group and time as factors and a post hoc Tukey’s HSD test, with a single animal as the experimental unit. For electrophysiological studies, the average number of evoked spikes for each stimulus was compared between groups using a two-way nested ANOVA with group and stimulus as factors, with neurons nested within rats and rats within groups, with post hoc Tukey’s HSD test. Differences in the ratio of WDR neurons were compared using a Pearson’s chi-square test. The experimental unit was a single neuron.
Results
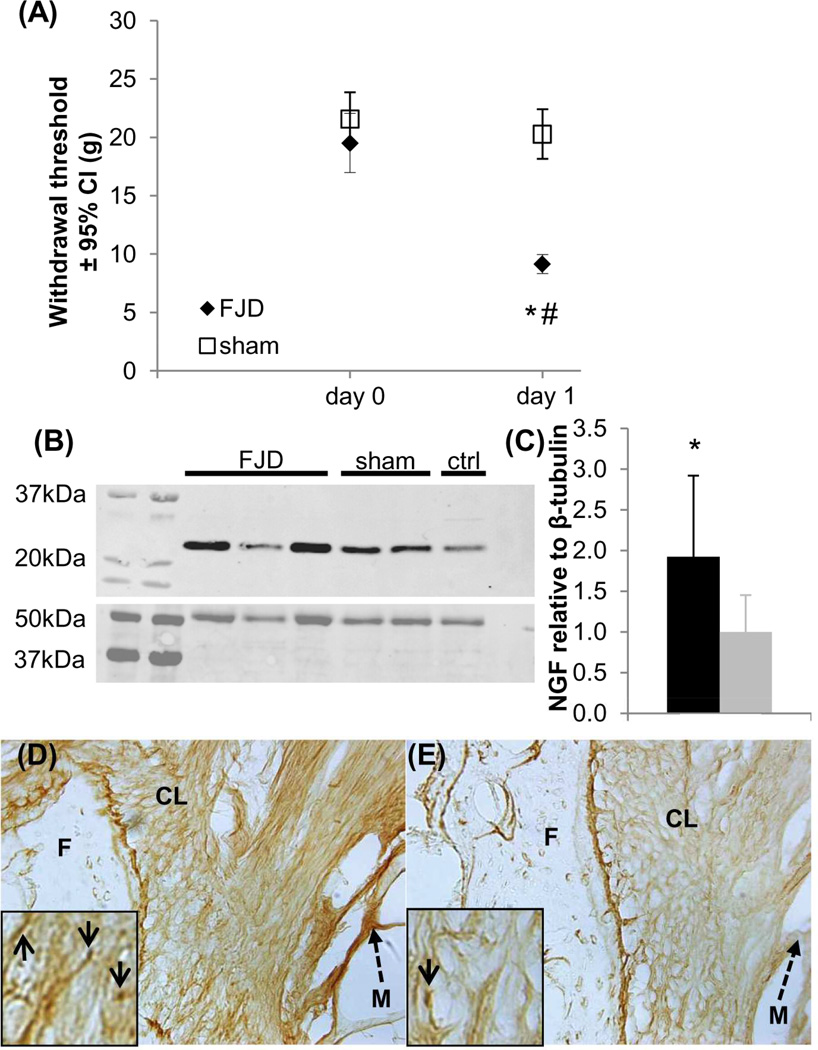
FJD induces significantly lower mechanical withdrawal thresholds on day 1 than at baseline (p<0.001), which are also lower than sham at day 1 (p<0.001) (Figure 1A). Thresholds in the sham group are unchanged from baseline (Figure 1A). NGF expression in the C6/C7 facet is significantly greater after FJD compared to sham (p=0.031) (Figures 1B & 1C). Immunohistochemical labeling of NGF in C6/C7 facet joints confirms that NGF expression is more prominent after FJD (Figure 1D) than after sham (Figure 1E).
Figure 1.
Facet joint distraction (FJD) induced pain associated with increased NGF expression in the joint. (A) FJD reduced the forepaw withdrawal threshold to mechanical stimulation at day 1 compared to baseline (#p<0.001) and sham procedures (*p<0.001). (B) Representative Western blots show NGF (top) and β-tubulin (bottom) expression in the joint tissue. (C) FJD significantly increased NGF in the joint tissue (*p=0.031) over levels in sham at day 1. Immunolabeling for NGF increased in the soft tissues surrounding the joint (solid arrows), including the capsular ligament, at day 1 after FJD (D) compared to labeling in shams (E). Scale bar in (D) is 50µm and applies to (D) and (E). The amplified inset boxes in (D) and (E) are 50µm wide. CL: capsular ligament; F: inferior facet of the superior vertebra; M: muscle (dashed arrow); NGF: nerve growth factor.
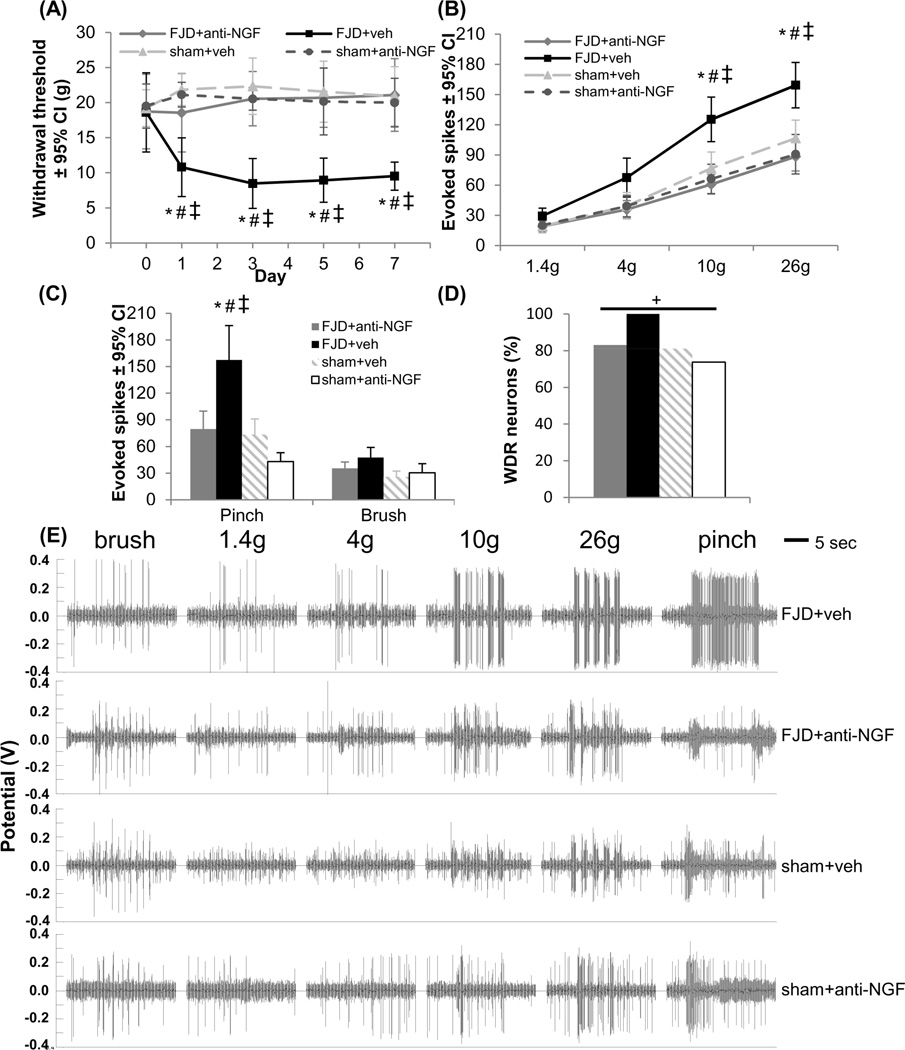
Consistent with behaviors after injury, the mechanical withdrawal threshold decreases from baseline 1 day after FJD with intra-articular injections of vehicle IgG (FJD+veh) and is maintained through day 7 (p<0.001) (Figure 2A). The withdrawal thresholds are unchanged from baseline after sham procedures (sham+veh or sham+anti-NGF) (Figure 2A). Intra-articular anti-NGF at injury (FJD+anti-NGF) inhibits the development of mechanical sensitivity, maintaining mechanical withdrawal thresholds at baseline levels comparable to sham+veh and sham+anti-NGF, and greater than FJD+veh (p<0.015) withdrawal thresholds (Figure 2A).
Figure 2.
Intra-articular anti-NGF immediately after FJD prevented the development of FJD-induced pain and spinal neuronal hyperexcitability. (A) The forepaw mechanical withdrawal threshold significantly decreased from baseline for FJD+veh at all times (p<0.001), with no change from baseline at any time in the FJD+anti-NGF, sham+veh, or sham+anti-NGF groups. The withdrawal threshold decreased for FJD+veh compared to FJD+anti-NGF (*p<0.015), sham+veh (#p<0.001), and sham+anti-NGF (‡p<0.001) at all days. (B) At day 7, the number of spikes evoked in spinal neurons by forepaw stimulation with 10g and 26g von Frey filaments significantly increased in the FJD+veh group compared to all other groups (*#‡p<0.012). There were no differences between FJD+anti-NGF, sham+veh, and sham+anti-NGF for any filament. (C) The pinch, but not brush, stimulus evoked significantly more spikes in the FJD+veh group than all others (*#‡p<0.001). (D) There was a significant effect of group on the proportion of WDR neurons (+p<0.005), with the largest number in the FJD+veh group. (E) Representative recordings show increased spikes evoked by the 10g, 26g, and pinch stimuli in the FJD+veh group compared to the other groups. FJD: facet joint distraction; NGF: nerve growth factor; WDR: wide dynamic range.
Recordings were made from 186 spinal neurons (depth 620±13µm for 9±2 applied forepaw stimulus trains and recorded neurons/rat) at day 7 (Table 2). Stimulation of the forepaw with either the 10g or 26g filament evokes significantly more spikes for the FJD+veh group than for any other group (FJD+anti-NGF p<0.001; sham+veh p<0.012; sham+anti-NGF p<0.001) (Figure 2B), with no differences detected between any other groups. Noxious pinch of the forepaw similarly elicits significantly more evoked spikes in the FJD+veh group (p<0.001) (Figure 2C); there are no differences in spikes between groups for light brushing of the paw (Figure 2C). There is a significant effect of injury group on the proportion of WDR neurons in the spinal cord (p<0.005), with the highest frequency of WDR neurons in FJD+veh (Figure 2D). Extracellular voltage recordings exhibit increased evoked firing in the FJD+veh group (Figure 2E). The behavioral and electrophysiological studies performed at day 1 after FJD in these same groups exhibit the same significant relationships as at day 7 (data not shown).
Table 2.
Number of neurons recorded for each experimental group.
| Group | Rats/ Group |
Study Endpoint (day) |
Total Neurons |
Neurons/ Group |
Max Neurons/Rat |
Min Neurons/Rat |
|---|---|---|---|---|---|---|
| FJD+anti-NGF | 6 | 7 | 186 | 65 | 15 | 5 |
| FJD+veh | 5 | 42 | 11 | 5 | ||
| sham+veh | 5 | 37 | 10 | 5 | ||
| sham+anti- NGF |
5 | 42 | 10 | 6 | ||
| FJD+anti- NGFD1 |
8 | 7 | 61 | 61 | 11 | 4 |
| NGF | 7 | 1 | 91 | 44 | 9 | 3 |
| vehicle | 6 | 47 | 10 | 6 |
FJD: facet joint distraction; NGF: nerve growth factor.
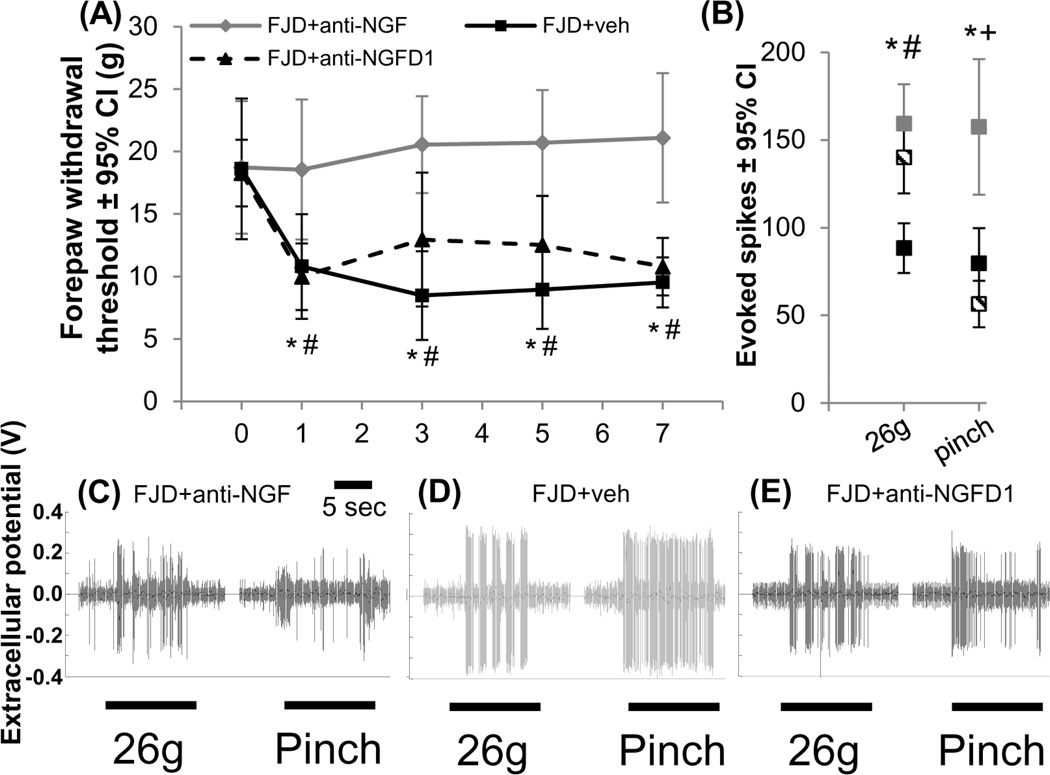
In contrast to anti-NGF injections immediately after injury, intra-articular anti-NGF injections given 1 day after FJD (FJD+anti-NGFD1) do not abolish injury-induced reductions in the withdrawal threshold (Figure 3A). On day 1 after FJD but before anti-NGF treatment, the withdrawal threshold decreases from baseline for the FJD+anti-NGFD1 group (p<0.001). The withdrawal threshold for FJD+anti-NGFD1 is not different from FJD+veh at day 1 but is lower than FJD+anti-NGF (p<0.012) (Figure 3A). After the intra-articular injection of the anti-NGF antibody given on day 1, the withdrawal threshold remains significantly lower than baseline (p<0.028) as well as FJD+anti-NGF (p<0.043) at all timepoints and is not different from FJD+veh on any day (Figure 3A).
Figure 3.
Inhibiting intra-articular NGF signaling at day 1 after FJD did not alter pain or spinal neuronal hyperexcitability. (A) The forepaw withdrawal threshold decreased at all days after FJD+veh compared to FJD+anti-NGF (*p<0.041). FJD+anti-NGFD1 also exhibited decreased thresholds at all days compared to FJD+anti-NGF (#p<0.043). (B) At day 7, there were fewer spikes evoked by noxious von Frey stimulation (26g) for FJD+anti-NGF relative to FJD+veh (*p<0.002) and FJD+anti-NGFD1 (#p<0.022), but both FJD+anti-NGF (*p<0.001) and FJD+anti-NGFD1 (+p<0.001) exhibited fewer evoked spikes than FJD+veh for a noxious pinch. (C–E) Representative spikes are shown for the 26g and pinch stimuli for each group. Datasets for the FJD+veh and FJD+anti-NGF groups are reproduced from Figure 2. FJD: facet joint distraction; NGF: nerve growth factor; d: day.
Quantification of evoked spikes from 61 spinal neurons (depth 724±25µm for 8±3 applied forepaw stimulus trains and recorded neurons/rat) at day 7 after FJD+anti-NGFD1 (Table 2) indicates more firing evoked by the 26g filament compared to FJD+anti-NGF (p<0.022); spike counts for that filament are not different between the FJD+anti-NGFD1 and FJD+veh groups (Figure 3B). However, the number of spikes evoked by the noxious pinch for the FJD+anti-NGFD1 group is significantly lower than FJD+veh (p<0.001) and is not different from FJD+anti-NGF. Extracellular voltage recordings show the differences between groups for the 26g and pinch stimuli (Figure 3C-3E).
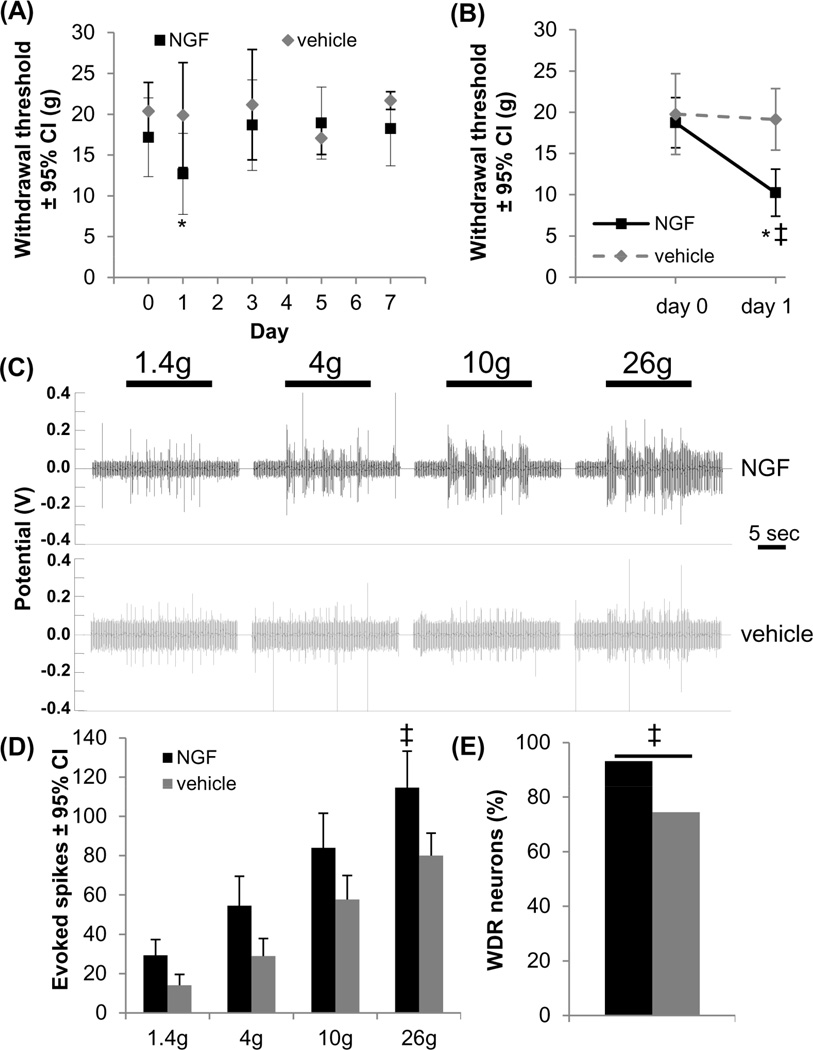
Intra-articular NGF is sufficient to induce behavioral and spinal neuronal hypersensitivity (Figure 4). The withdrawal threshold significantly decreases at day 1 (p<0.010) but returns to baseline for all later times (Figure 4A). Intra-articular PBS does not alter the withdrawal threshold (Figure 4A). For rats followed for only 1 day after intra-articular NGF injections, the withdrawal threshold is decreased at day 1 compared to baseline and to the vehicle group (p<0.001) (Figure 4B). Because NGF induces behavioral sensitivity that is evident only on day 1 (Figure 4), spinal neuronal excitability was only assessed at 1 day after injection. 91 mechanosensitive spinal neurons with input from the forepaw were recorded (depth 681±22µm; for 8±2 forepaw stimulus trains and recorded neurons/rat) (Table 2). Stimulation with all von Freys evoke increased firing in the NGF group compared to vehicle (Figure 4C). A trend towards increased firing at each filament strength is evident in the NGF group but is only significant for the noxious 26g filament (p<0.04) (Figure 4D). Significantly more neurons are classified as WDR on day 1 after NGF (93.2%) than after vehicle (74.5%) (p=0.016) (Figure 4E).
Figure 4.
Intra-articular NGF induced transient behavioral sensitivity that was associated with spinal neuronal hyperexcitability. (A) Intra-articular NGF significantly reduced the withdrawal threshold from baseline at day 1 (*p<0.010), but it returned to baseline by day 3. Injection of PBS vehicle did not alter the withdrawal threshold from baseline at any day. (B) At 1 day after intra-articular NGF the withdrawal threshold significantly decreased relative to baseline (*p<0.001) and to vehicle injection (‡p<0.001). (C) Representative extracellular recordings in the spinal cord at day 1 demonstrated increased evoked neuronal firing after NGF. (D) The number of evoked spikes significantly increased (‡p<0.040) for the noxious 26g filament after NGF injection. (E) Intra-articular NGF increased the number of spinal neurons classified as WDR neurons compared to the number identified after intra-articular vehicle administration (‡p=0.016). NGF: nerve growth factor; WDR: wide dynamic range.
Discussion
This study establishes a role for intra-articular NGF in the development of facet joint-mediated pain. NGF increases in the injured joint early after its painful injury, and local administration of anti-NGF immediately after injury prevents the development of both behavioral and spinal neuronal hypersensitivity. However, delayed administration of intra-articular anti-NGF, even at 1 day after injury, does not alter the behavioral or associated von Frey-evoked spinal neuronal sensitivity. Because NGF increases in the joint after injury in parallel with onset of pain and spinal neuronal hyperexcitability, we determined if intra-articular NGF alone is sufficient to induce those changes. Exogenous intra-articular NGF induces pain that lasts for only 1 day, further implicating intra-articular NGF in the initiation of joint pain.
Intra-articular NGF increases in the facet after a painful injury, similar to findings in experimental arthritis11,25. In inflamed tissues, there is increased NGF release from immune cells14, and elevated NGF has been reported in the soft tissues of experimental knee arthritis11. These modifications have also been reported in the synovial fluid of painful inflamed and arthritic joints in humans20,26,27. Yet, chondrocytes subject to mechanical stress are also potential sources of NGF35. Although the cellular source(s) of NGF in the facet joint must be defined, upregulation of the inflammatory mediator PGE2 has been reported in this model24 when intra-articular NGF is increased, suggesting that increase may result from joint inflammation. Because intra-articular NGF induces pain, albeit transiently, elevated intra-articular NGF may be a source of clinical joint pain, especially since intradermal and intramuscular NGF induce pain in humans15,36. The intra-articular injection itself may stimulate joint afferents by capsule distension37. Yet, neither joint afferent activation nor stimulation of surrounding tissues is likely to contribute to the current findings since no changes in any outcomes were evident in volume-matched vehicle control injected rats. The elevated intra-articular NGF that is evident across several types of painful conditions, together with the fact that local anti-NGF prevents pain, suggests that regardless of the etiology, NGF is involved in a broad range of painful joint conditions, including other joint injuries and arthritis.
Preventing pain by immediate, but not delayed (day 1), intra-articular anti-NGF supports traumatic joint pain being mediated by early NGF signaling cascades. Yet, NGF-induced pain is only transient, so NGF is not the sole mediator. Because painful FJD is also associated with increases in PGE2 at day 124, which itself regulates inflammation and pain38, NGF may facilitate pain by increasing the duration of PGE2-mediated behavioral hypersensitivity39,40. Such priming may explain why intra-articular NGF induces only short-lasting pain and intra-articular anti-NGF given at, but not after, injury prevents long-lasting facet pain. Early block of NGF may prevent nociceptor priming after injury. An intra-articular NSAID, which disrupts prostaglandin synthesis, reverses established facet joint pain, but only when given one day after injury10. The different effects of the anti-NGF and NSAID may be due to early NGF facilitating later PGE2-mediated nociception; although NGF is sufficient to initiate pain, additional mediators may contribute to its maintenance.
It is unknown if painful FJD also induces joint degeneration. Joint laxity contributes to post-traumatic osteoarthritis41, so altered facet kinematics from joint laxity after injury may promote later degeneration. Indeed, joint laxity increases after this painful facet injury42. Further, joint inflammation is evident in both post-traumatic and chemically-induced osteoarthritis43–45. It is, therefore, possible that our findings, along with evidence of early inflammatory responses following FJD10,24, may reflect initial stages of a degenerative process. Transient pain is evident in a rat model of facet osteoarthritis in association with inflammation, but that pain also returns weeks later when joint degeneration is severe44. Since our latest endpoint is day 7, it is likely that joint degeneration may not contribute to the responses observed here. Increased intra-articular NGF is reported as late as four weeks after knee osteoarthritis induction in the rat11, suggesting intra-articular NGF may contribute to pain maintenance during joint degeneration. We did not probe later time points, and NGF expression was only quantified at day 1. Because behavioral hypersensitivity lasts for at least six weeks in this model46, studies are needed to define relationships between mechanical facet joint injury, intra-articular NGF, joint degeneration, and persistent pain.
Intra-articular NGF also induces dorsal horn neuron hyperexcitability. Not surprisingly, that hyperexcitability is only evident for a 26g stimulation, which is expected given the paw withdrawal threshold of slightly greater than 10g. Many of the neurons in the deep dorsal horn are WDR neurons, responding to both non-noxious and noxious signals38,47. WDRs contribute to central sensitization and many forms of persistent pain5,38. Intra-articular NGF increases the number of neurons responding to mechanical stimulation as WDRs. Increases in spinal neuronal excitability and WDRs suggest that intra-articular NGF may mediate central modifications underlying joint pain. One common consequence of central sensitization is expansion of sensory neuron receptive fields38, which has been reported for monoarthritis in the rat knee5. Whiplash patients exhibit hypersensitivity to mechanical stimuli in the neck, as well as in the shoulder, arm, and hand48,49. Intra-articular NGF in the facet inducing behavioral hypersensitivity in the forepaw further supports central sensitization in traumatic facet-mediated pain.
Painful facet joint injury increases dorsal horn neuronal excitability and shifts neurons from LTMs to WDRs7,9. Because NGF increases in the facet after its injury and is sufficient to induce pain and spinal neuronal hyperexcitability, early activity of intra-articular NGF likely mediates injury-induced facet pain. Administration of intra-articular anti-NGF immediately after joint injury prevents behavioral hypersensitivity and spinal neuronal hyperexcitability. Although current systemic anti-NGF therapies alleviate osteoarthritic joint pain and chronic low back pain, they are associated with many adverse events, including joint degeneration19,50. Our findings suggest local anti-NGF treatment to be effective for preventing traumatic joint pain. All rats receiving local anti-NGF in our study exhibited normal weight gain and grooming behavior and were indistinguishable from controls, with no obvious ill-effects.
In contrast to the effects of immediate intra-articular anti-NGF application, delayed intra-articular anti-NGF even 1 day after injury does not mitigate the pain or spinal neuronal firing evoked by filament stimuli. Despite not affecting neuronal firing evoked by von Frey stimulation, intra-articular anti-NGF on day 1 reduces pinch-evoked firing. The mechanism by which this occurs is currently unknown, but this differential effect warrants further study to identify that mechanism. Interestingly, a fast-acting anesthetic prevents the development of both of these correlates only when given within 8 hours of injury29. As such, studies varying the timing of anti-NGF treatment are needed to fully evaluate whether this more-specific, local treatment is effective in alleviating facet joint pain and is associated with fewer adverse events than systemic anti-NGF treatment for joint pain. Nevertheless, the prevention of injury-induced pain and neuronal hyperexcitability achieved by anti-NGF demonstrates that intra-articular NGF is necessary for the development of joint pain after facet injury.
Summarizing, these data demonstrate a role for intra-articular NGF in the development of pain and spinal neuronal hyperexcitability following facet injury. Despite reports of increased NGF in degenerative and arthritic joints11,20,25,26, this is the first study to establish that intra-articular NGF induces pain and spinal neuronal sensitization. Intra-articular anti-NGF given immediately after joint injury prevents pain development; yet, with a 1 day delay, that same dose is ineffective. Because only a single dose of anti-NGF was used, different anti-NGF treatment regimens may identify potential treatments for established pain and the intra-cellular signaling mechanisms through which NGF contributes to hyperexcitability of spinal neurons and the maintenance of joint pain. Regardless, this study provides the first evidence that intra-articular NGF is both necessary and sufficient for the development of joint-mediated pain and spinal neuronal hyperexcitability, identifying it as an intra-articular initiator of joint injury-induced pain and supporting early localized treatment targeting NGF as potential effective therapy.
Acknowledgements
This work was funded by a grant from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288).
Role of the Funding Source: The study sponsor had no involvement in the study design, collection, analysis, and interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication. This work was funded by a grant from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
(1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data: Kras JV, Kartha S, Winkelstein BA
(2) Drafting the article or revising it critically for important intellectual content: Kras JV, Winkelstein BA
(3) Final approval of the version to be submitted: Winkelstein BA
Competing Interest Statement: The authors declare no conflicts of interest.
References
- 1.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Guilbaud G, Iggo A, Tegnér R. Sensory receptors in ankle joint capsules of normal and arthritic rats. Exp Brain Res. 1985;58:29–40. doi: 10.1007/BF00238950. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005;23:779–787. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, et al. Joint pain. Exp Brain Res. 2009;196:153–162. doi: 10.1007/s00221-009-1782-9. [DOI] [PubMed] [Google Scholar]
- 5.Martindale JC, Wilson AW, Reeve AJ, Chessell IP, Headley PM. Chronic secondary hypersensitivity of dorsal horn neurons following inflammation of the knee joint. Pain. 2007;133:79–86. doi: 10.1016/j.pain.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash: A placebo-controlled prevalence study. Spine. 1996;21:1737–1744. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Crosby ND, Weisshaar CL, Winkelstein BA. Spinal neuronal plasticity is evident within 1 day after a painful cervical facet joint injury. Neurosci Lett. 2013;542:102–106. doi: 10.1016/j.neulet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–1393. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 9.Quinn KP, Dong L, Golder FJ, Winkelstein BA. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–421. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong L, Smith JR, Winkelstein BA. Ketorolac reduces spinal astrocytic activation and PAR1 expression associated with attenuation of pain after facet joint injury. J Neurotrauma. 2013;30:818–825. doi: 10.1089/neu.2012.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, et al. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 2010;62:3666–3676. doi: 10.1002/art.27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu D, Liu F, Liu M, Zhao X, Wang X, Li Y, et al. The inhibition of subchondral bone lesions significantly reversed the weight-bearing deficit and the overexpression of CGRP in DRG neurons, GFAP and Iba-1 in the spinal dorsal horn in the monosodium iodoacetate induced model of osteoarthritis pain. PLoS One. 2013;8:e77824. doi: 10.1371/journal.pone.0077824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- 15.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, et al. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- 16.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 17.Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, et al. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther. 2007;322:282–287. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- 18.Woolf CJ, Safieh-Garabedian B, Ma QP, Crill P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 19.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13:790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Barthel C, Yeremenko N, Jacobs R, Schmidt RE, Bernateck M, Zeidler H, et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res Ther. 2009;11:R82. doi: 10.1186/ar2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Surace MF, Prestamburgo D, Campagnolo M, Fagetti A, Murena L. Presence of NGF and its receptor TrkA in degenerative lumbar facet joint specimens. Eur Spine J. 2009;18 Suppl 1:122–125. doi: 10.1007/s00586-009-0994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;27:163–174. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kras JV, Dong L, Winkelstein BA. Increased interleukin-1α and prostaglandin E2 expression in the spinal cord at 1 day after painful facet joint injury: evidence of early spinal inflammation. Spine. 2014;39:207–212. doi: 10.1097/BRS.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aloe L, Tuveri MA, Levi-Montalcini R. Studies on carrageenan-induced arthritis in adult rats: presence of nerve growth factor and role of sympathetic innervation. Rheumatol Int. 1992;12:213–216. doi: 10.1007/BF00302155. [DOI] [PubMed] [Google Scholar]
- 26.Raychaudhuri SP, Raychaudhuri SK, Atkuri KR, Herzenberg LA, Herzenberg LA. Nerve growth factor: a key local regulator in the pathogenesis of inflammatory arthritis. Arthritis Rheum. 2011;63:3243–3252. doi: 10.1002/art.30564. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Koshino T. Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin Orthop Relat Res. 2000;376:172–182. doi: 10.1097/00003086-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 29.Crosby ND, Gilliland TM, Winkelstein BA. Early afferent activity from the facet joint after painful trauma to its capsule potentiates neuronal excitability and glutamate signaling in the spinal cord. Pain. 2014;155:1878–1887. doi: 10.1016/j.pain.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kras JV, Tanaka K, Gilliland TM, Winkelstein BA. An anatomical and immunohistochemical characterization of afferents innervating the C6-C7 facet joint after painful joint loading in the rat. Spine. 2013;38:E325–E331. doi: 10.1097/BRS.0b013e318285b5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kras JV, Weisshaar CL, Quindlen J, Winkelstein BA. Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res. 2013;91:1312–1321. doi: 10.1002/jnr.23254. [DOI] [PubMed] [Google Scholar]
- 32.Kartha S, Zeeman ME, Baig HA, Guarino BB, Winkelstein BA. Upregulation of BDNF and NGF in cervical intervertebral discs exposed to painful whole-body vibration. Spine. 2014;39:1542–1548. doi: 10.1097/BRS.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, et al. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 34.Dostrovsky JO, Craig AD. Ascending projection systems. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Textbook of Pain. Philadelphia: Saunders; 2013. pp. 182–198. [Google Scholar]
- 35.Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon MC, et al. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther. 2014;16:R16. doi: 10.1186/ar4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 37.Ferrell WR. The effect of acute joint distension on mechanoreceptor discharge in the knee of the cat. Q J Exp Physiol. 1987;72:493–499. doi: 10.1113/expphysiol.1987.sp003091. [DOI] [PubMed] [Google Scholar]
- 38.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Dare D, Rodeo S. Mechanisms of post-traumatic osteoarthritis after ACL injury. Curr Rheumatol Rep. 2014;16:448. doi: 10.1007/s11926-014-0448-1. [DOI] [PubMed] [Google Scholar]
- 42.Quinn KP, Lee KE, Ahaghotu CC, Winkelstein BA. Structural changes in the cervical facet capsular ligament: potential contributions to pain following subfailure loading. Stapp Car Crash J. 2007;51:169–187. doi: 10.4271/2007-22-0008. [DOI] [PubMed] [Google Scholar]
- 43.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 44.Gong K, Shao W, Chen H, Wang Z, Luo ZJ. Rat model of lumbar facet joint osteoarthritis associated with facet-mediated mechanical hyperalgesia induced by intra-articular injection of monosodium iodoacetate. J Formos Med Assoc. 2011;110:145–152. doi: 10.1016/S0929-6646(11)60024-7. [DOI] [PubMed] [Google Scholar]
- 45.Huebner KD, Shrive NG, Frank CB. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J Orthop Res. 2014;32:566–572. doi: 10.1002/jor.22568. [DOI] [PubMed] [Google Scholar]
- 46.Rothman SM, Hubbard RD, Lee KE, Winkelstein BA. Detection, transmission, and perception of pain. In: Slipman CW, Simeone FA, Derby R, Mayer TG, editors. Interventional Spine: An Algorithmic Approach. Philadelphia: Saunders; 2007. pp. 29–37. [Google Scholar]
- 47.Pezet S, Onténiente B, Grannec G, Calvino B. Chronic pain is associated with increased TrkA immunoreactivity in spinoreticular neurons. J Neurosci. 1999;19:5482–5492. doi: 10.1523/JNEUROSCI.19-13-05482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández-Pérez AM, Villaverde-Gutiérrez C, Mora-Sánchez A, Alonso-Blanco C, Sterling M, Fernández-de-Las-Peñas C. Muscle trigger points, pressure pain threshold, and cervical range of motion in patients with high level of disability related to acute whiplash injury. J Orthop Sports Phys Ther. 2012;42:634–641. doi: 10.2519/jospt.2012.4117. [DOI] [PubMed] [Google Scholar]
- 49.Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but no chronic idiopathic neck pain. Clin J Pain. 2005;21:175–181. doi: 10.1097/00002508-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]