Abstract
Objective
Pro- and anti-inflammatory mediators, such as IL-1β and IL1Ra, are produced by joint tissues in osteoarthritis (OA), where they may contribute to pathogenesis. We examined whether inflammatory events occurring within joints are reflected in plasma of patients with symptomatic knee osteoarthritis (SKOA).
Design
111 SKOA subjects with medial disease completed a 24-month prospective study of clinical and radiographic progression, with clinical assessment and specimen collection at 6-month intervals. The plasma biochemical marker IL1Ra was assessed at baseline and 18 months; other plasma biochemical markers were assessed only at 18 months, including IL-1β, TNFα, VEGF, IL-6, IL-6Rα, IL-17A, IL-17A/F, IL-17F, CRP, sTNF-RII, and MMP-2.
Results
In cross-sectional studies, WOMAC (total, pain, function) and plasma IL1Ra were modestly associated with radiographic severity after adjustment for age, gender and BMI. In addition, elevation of plasma IL1Ra predicted joint space narrowing (JSN) at 24 months. BMI did associate with progression in some but not all analyses. Causal graph analysis indicated a positive association of IL1Ra with JSN; an interaction between IL1Ra and BMI suggested either that BMI influences IL1Ra or that a hidden confounder influences both BMI and IL1Ra. Other protein biomarkers examined in this study did not associate with radiographic progression or severity.
Conclusions
Plasma levels of IL1Ra were modestly associated with the severity and progression of symptomatic knee osteoarthritis in a causal fashion, independent of other risk factors. The findings may be useful in the search for prognostic biomarkers and development of disease-modifying OA drugs.
Keywords: osteoarthritis, causal analysis, radiographic progression, interleukin-1 receptor antagonist (IL1Ra)
INTRODUCTION
Osteoarthritis (OA), the most common adult joint disease, is increasing in frequency and severity, with an estimated U.S. prevalence of over 25 million affected adults1. Disease progression is associated with cartilage degradation, synovial proliferation and bony changes, including osteophytes, subchondral sclerosis and bone marrow lesions.
Identification of patients at risk for disease progression remains a challenge, particularly since routine radiography is an insensitive measure of molecular changes that presage cartilage and bone abnormalities. There is consensus that development of disease-modifying treatment of OA would be aided by validated biomarkers, which identify patients at risk for progressive disease development2. Indeed, the quest for improved imaging markers (including x-ray) is an active area of investigation. To date, most biochemical biomarker studies have focused on degradation or synthetic products of cartilage, bone or synovium and have yielded mixed results3–7. Conventional inflammatory markers such as C-reactive protein (CRP) have not consistently been associated with severity or progression of knee OA2,8.
While traditionally considered a non-inflammatory joint disease, it is now well-appreciated that inflammatory mediators are produced by articular tissues in OA and have been implicated in disease pathogenesis9–12. Indeed, cytokines and prostaglandins produced by cartilage promote cartilage degeneration, while synovitis has been associated with greater risk of cartilage loss in patients with knee OA13,14. Emerging literature indicates that local inflammation within OA joint tissues can be reflected in elevated plasma biomarkers8. Work by Robinson and associates11, for example, has revealed increased inflammatory proteins in sera of OA patients with joint effusions compared to controls, including interleukin-1 beta (IL-1β), IL-1 receptor antagonist (IL1Ra), IL-6, monocyte chemo-attractant protein-1 (MCP-1), monocyte interferon-gamma-inducible protein (MIG), vascular endothelial growth factor (VEGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF). The levels of these cytokines were higher in OA synovial fluid than sera, consistent with their origin from joint tissues9–12. We have recently reported that circulating blood leukocytes in patients with knee OA exhibit increased IL-1β gene expression, consistent with exposure to cytokines produced by OA joint tissues15. Increased leukocyte IL-1β expression was associated with increased knee pain and predicts risk for progression of symptomatic knee OA (SKOA)15.
In the current study, we examined demographic and anthropometric features as well as plasma levels of a panel of inflammatory markers in a two-year prospective analysis of radiographic progression in patients with SKOA. Our data indicate that among 12 candidate markers studied, plasma IL1Ra is a potential prognostic biomarker of radiographic progression in OA.
PATIENTS AND METHODS
Patients
As part of an NIH-funded study, 183 patients with SKOA were assessed at baseline and enrolled in a 24-month prospective study at the NYU Hospital for Joint Diseases (“NYUHJD SKOA progression cohort”). All patients complained of pain in the signal knee, met American College of Rheumatology (ACR) clinical symptomatic criteria, and had Kellgren-Lawrence (KL) grade ≥116,17. Patients were excluded if they had any other form of arthritis; body mass index (BMI) >33 kg/m2; any disorder requiring the use of systemic corticosteroids; history of bilateral knee replacements; major comorbidities including diabetes mellitus, noncutaneous cancer within 5 years of screening, chronic hepatic or renal disease, chronic infectious disease, or congestive heart failure; or had received a hyaluronan and/or corticosteroid injection to the affected knee within 3 months of screening. All patients were examined by one of two NYUHJD investigators (SK, JS) every 6 months; 146 patients had completed the 24-month observation period at the time of this analysis. Radiographic assessments at baseline and 24 months included bilateral (signal and non-signal knee) KL determination, quantitative measurement of joint space width (JSW), all performed by a musculoskeletal radiologist (LR) blinded to patient information. Baseline clinical information and radiographic images of joints other than the knee are not available for these patients.
Prior to study initiation, the protocol was approved by the IRB of NYU School of Medicine, and written informed consent was obtained from all study participants.
Knee radiographs
All NYUHJD SKOA patients underwent standardized weight-bearing fixed-flexion posteroanterior knee radiographs using the SynaFlexer™ X-ray positioning frame (Synarc). X-rays were scored for KL grade 0–4, and medial and lateral joint space width (JSW). Radiographic progression (JSN) was defined as narrowing of medial JSW in signal knee between baseline and 24-month follow-up. JSW was measured at the narrowest portion of the joint space via electronic calipers linked to computer monitor.
Sample collection
Non-fasting blood samples were collected at baseline when patients came for knee radiographs, and at 18-month clinical visit, in pyrogen-free heparinized tubes for isolation of plasma. After drawing, the tubes were inverted several times, immediately placed on ice for 30 min, and then spun at 3000 rpm for 15 min at 4°C to separate the plasma. Plasma samples were immediately aliquoted and stored at −70°C for future use.
Plasma cytokine determination
At baseline, we determined only IL1Ra and no other biomarkers, because of limited plasma samples. More plasma was available at 18 months, so additional plasma protein biomarkers were assayed – IL-1β, tumor necrosis factor a (TNFα), IL1Ra, VEGF, IL-6, soluble IL-6 receptor α (sIL-6Rα), IL-17 isoforms (IL-17A, IL-17A/F, IL-17F), CRP, soluble TNF receptor II (sTNF-RII) and matrix metalloproteinase-2 (MMP-2) – using the highly sensitive Erenna Immunoassay system (Singulex, Inc.), based upon single molecule counting technology, as described18. The limits of detection (pg/mL; least amount of analyte per sample that could be detected but not necessarily quantitated as an exact value), limits of quantification (pg/mL; least amount of analyte per sample that could be quantitatively determined with suitable precision) and average coefficients of variation of the assays, respectively, were: IL-1β (0.03, 0.20, 7%); TNFα (0.02, 0.20, 7%); IL1Ra (1.64, 3.91, 7%); VEGF (0.06, 0.39, 6%); IL-6 (0.01, 0.10, 6%); IL-6Rα (0.01, 0.39, 8%); IL-17A (0.01, 0.10, 13%); IL-17A/F (0.10, 0.59, 6%); IL-17F (0.69, 3.13, 9%); CRP (0.12, 1.95, 7%), sTNF-RII (0.01, 0.04, 9%); MMP-2 (0.588, 3.91, 6%).
Statistical methods
We assessed associations and discrimination/predictive power of single biomarkers with outcomes of baseline and 24-month KL severity (KL1/2 vs. KL3/4) and radiographic JSN over 24 months using the following statistical tools:
Analysis of Fisher’s partial correlations (adjusted for age, gender, BMI) of biomarkers with continuous JSN outcome variables.
Assessing predictivity of biomarkers for dichotomized outcome variables (e.g., KL1/2 vs. KL3/4) with the area under receiver operating characteristic curve (AUC) and logistic regression (either unadjusted or adjusted for age, gender, BMI), as described19,20.
Comparing the means of a continuous outcome variable (e.g., JSN) for groups of patients defined based on biomarker values using Student’s two-tailed t-test.
We used 5% alpha level and corrected for multiple comparisons using the approach of Benjamini and Hochberg for assessing statistical significance21.
For the radiographic JSN outcome variable, we defined non-progressors as having JSN ≤0.0mm/24months, and three subgroups of progressors with JSN >0.0 or >0.2mm (“slow-progressors”), or >0.5 mm (“fast-progressors”) per 24 months. These thresholds were based on Emrani et al22 and recommendations of The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO)23.
For building predictive multivariable models of a dichotomized outcome variable [medial JSN and radiographic severity (KL1/2 vs KL3/4)] based on multiple biomarkers, we used support vector machines24. For measuring predictive performance, we used the AUC. For estimating predictivity of multivariate models, we used 10-fold stratified cross-validation repeated with 100 different splits of data into 10-folds25. We used strict multiple comparison control procedures to ensure no type I error inflation. All tests were corrected using the Bonferroni correction.
For causal graph analysis, we have used the Fast Causal Inference (FCI) algorithm26. This method discovers the causal graph consistent with the data and also identifies hidden confounding. The algorithm has been proven to be correct (i.e., to identify underlying causal structure) under very broad distributional assumptions. No data manipulation of any kind (e.g., transformation, imputation, thresholding) was applied for this analysis, so that it was not biased toward particular causal hypotheses. We used implementation of the FCI algorithm in the TETRAD software package (http://www.phil.cmu.edu/projects/tetrad/) with Fisher’s z-test and 0.05 significance level.
RESULTS
NYUHJD Symptomatic Knee OA (SKOA) Patient Cohort
Baseline demographic and clinical characteristics of SKOA are summarized in Table 1. Mean (±standard deviation) age of patients was 62.5 (±10.5) years; 64.5% were female; mean BMI was 26.7 (±3.5). Mean total WOMAC score (0–300 scale) was 114.6 (±67.8) and mean pain score on a 0–100 visual analog scale (VAS) was 43.4 (±28.6).
Table 1.
Comparison of demographic and clinical characteristics of patients with symptomatic knee osteoarthritis (SKOA) and of plasma biomarkers levels at baseline and 18 months.*
| Variable | Baseline (N=180) | 18 months (N=125) |
|---|---|---|
| Age, years (mean ± SD) | 62.6± 10.5 | 63.3 + 9.73 |
| Gender | F = 116; M=64 (Female 64.5%) |
F = 78; M=47 (Female 62.0%) |
| BMI | 26.7 ± 3.5 | 26.5 ± 3.6 |
| RACE | Caucasians (n=126), Blacks (n=41) Asians (n=13) |
Caucasians (n=84), Blacks (n=30) Asians (n=11) |
| Ethnicity | Non-Hispanic (n=155) Hispanic (n=25) |
Non-Hispanic (n=114) Hispanic (n=11) |
| WOMAC total | 114.6 ± 67.8 (0–294.6) |
93.03 ± 66.38 (0–267.9) |
| VAS | 43.4 ± 28.6 (0–100) |
32.7 ± 26.82 (0–100) |
| Percent KL distribution (n=146) | KL1/2 (30%) KL3/4 (60%) |
– |
| IL1Ra pg/ml (n=146) | 316. 01 ± 202.46 (36.70–1290.0) |
265.99 ± 112.05 (83.0–676.0) |
| MMP-2 ng/ml | – | 83.3 ± 63.5 (33.0–399.0) |
| IL-1β pg/ml | – | 0.44 ± 0.55 (0.08–3.5) |
| TNFα pg/ml | – | 2.41 ± 2.89 (0.4–20.50) |
| sTNF-RII pg/ml | – | 3455 ± 1470 (1451–10983) |
| IL-6 pg/ml | – | 1.99 ± 2.05 (0.1–12.1) |
| IL-6R alpha pg/ml | – | 25.49 ± 9.45 (8.0–59.0) |
| IL-17A pg/ml | – | 0.39 ± 1.08 (0.0–10.70) |
| IL-17F pg/ml | – | 41.24 ± 162.8 (4.4–1597) |
| IL-17A/F pg/ml | – | 2.92 ± 3.04 (0.2–23.60) |
| CRP ng/ml | – | 1513 ± 2864 (25–23997) |
Data are presented as mean ± SD unless otherwise indicated; data range is provided in parentheses below each value.
Abbreviations: BMI, body mass index; WOMAC, Western Ontario and McMaster Universities Arthritis Index; VAS, visual analog scale.
Biomarkers associated with SKOA radiographic severity (KL1/2 vs. KL3/4)
Baseline IL1Ra and clinical markers were assessed for association with baseline radiographic severity in SKOA subjects. IL1Ra was measured at two time points, baseline and 18 months. Elevation of baseline IL1Ra was associated statistically significant with severity in bivariate analysis (p<0.04; Figure 1A, Table 2), but did not reach significance when adjusted for multiple comparisons and for age, gender and BMI (KL1/2 vs. KL3/4 with AUC=0.59; p=0.105). Repeat measurements of IL1Ra at 18 months confirmed these findings and were highly significant: in a logistic regression model, plasma IL1Ra was associated statistically significantly with radiographic severity at baseline after adjustment for age, gender and BMI (AUC=0.67; p=0.018).
Figure 1. Association of baseline IL1Ra with radiographic severity and progression in SKOA patients.
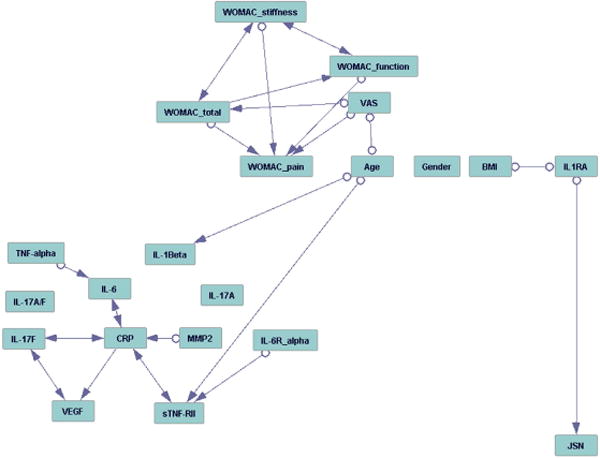
A) Distribution of log-transformed plasma baseline and 18 month IL1Ra between KL1/2 (n=43) vs. KL3/4 (n=89) radiographic severity groups. Data are presented as box plots; the lines within the boxes represent the median, and the lines outside the boxes represent the 25th and 75th percentiles. B) Correlation of log-transformed baseline IL1Ra and 18 month IL1Ra and with 18 month plasma IL-1β. C) Correlation of log-transformed baseline IL1Ra with medial JSN (n=109). D) Distribution of plasma baseline between radiographic non-progressors (JSN<0.0mm; n=39) and progressors [JSN >0.0 (n=72); 0.2 (n=64) and 0.5mm (n=44)] over 24 months. Data are presented as box plots; the lines within the boxes represent the median, and the lines outside the boxes represent the 25th and 75th percentiles. R and p values are indicated in the figures. Abbreviations: IL1Ra, interleukin 1 receptor antagonist; KL, Kellgren-Lawrence score; JSN, joint space narrowing.
Table 2.
Relationship of baseline and 18-month biomarkers to baseline radiographic severity (KL 1/2 vs. KL 3/4) in SKOA patients
| Markers | AUC | p-values | $P-values adjusted for multiple comparisons | GLM-based (Logistic Regression) | KL 1/2: Mean (± SD) | KL 3/4: Mean (± SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (no adjustment) | Age | Gender | BMI | Age, Gender, BMI | ||||||
| Baseline* | ||||||||||
| IL1RA | 0.59 | 0.042 | 0.105 | 0.048 | 0.083 | 0.037 | 0.105 | 0.121 | 262.25 (120.09) | 322.90 (183.25) |
| Age | 0.63 | 0.011 | 0.105 | 58.90 (11.82) | 63.73 (9.14) | |||||
| Gender | 0.60 | 0.018 | 0.105 | 0.26 (0.45) | 0.45 (0.5) | |||||
| BMI | 0.55 | 0.182 | 0.253 | 26.36 (3.82) | 27.25 (3.13) | |||||
| 18 months** | ||||||||||
| IL1RA | 0.67 | 0.001 | 0.018 | 0.001 | 0.006 | 0.0002 | 0.003 | 0.005 | 229.13 (79.13) | 290.14 (106.04) |
| VEGF | 0.60 | 0.042 | 0.105 | 0.127 | 0.229 | 0.077 | 0.203 | 0.238 | 94.63 (40.66) | 109.21 (60.06) |
| IL-17F | 0.41 | 0.053 | 0.107 | 0.563 | 0.686 | 0.476 | 0.682 | 0.719 | 29.45 (35.39) | 45.26 (190.24) |
| IL-17A/F | 0.46 | 0.255 | 0.306 | 0.217 | 0.052 | 0.197 | 0.186 | 0.043 | 2.62 (1.46) | 3.33 (3.81) |
| IL-17A | 0.54 | 0.269 | 0.306 | 0.724 | 0.671 | 0.600 | 0.828 | 0.679 | 0.34 (0.6) | 0.41 (1.23) |
| IL-1Beta | 0.45 | 0.163 | 0.239 | 0.832 | 0.656 | 0.897 | 0.872 | 0.624 | 0.40 (0.3) | 0.39 (0.45) |
| IL-6 | 0.56 | 0.152 | 0.237 | 0.227 | 0.388 | 0.217 | 0.295 | 0.426 | 1.83 (1.59) | 2.26 (2.05) |
| TNF-alpha | 0.46 | 0.267 | 0.306 | 0.941 | 0.720 | 0.851 | 0.978 | 0.752 | 2.39 (2.07) | 2.36 (2.90) |
| CRP | 0.61 | 0.024 | 0.105 | 0.241 | 0.447 | 0.192 | 0.379 | 0.550 | 1191.71 (2020.03) | 1775.25 (3193.1) |
| sTNF-RII | 0.59 | 0.039 | 0.105 | 0.233 | 0.515 | 0.238 | 0.284 | 0.570 | 3254.87 (1522.5) | 3572.68(1364.1) |
| IL-6R alpha | 0.48 | 0.345 | 0.345 | 0.936 | 0.715 | 0.774 | 0.755 | 0.778 | 25.90 (7.9) | 25.77 (8.96) |
| MMP2 | 0.59 | 0.056 | 0.107 | 0.103 | 0.197 | 0.114 | 0.132 | 0.268 | 73.39 (46.41) | 92.87 (73.21) |
Baseline: association of biomarkers measured at baseline with SKOA severity at baseline;
18 Months: association of biomarkers measured at 18 months with SKOA severity at baseline (radiographs not available at 18 months). For measuring biomarker predictivity for radiographic severity, we used the area under the receiver operating characteristic (ROC) curve. The mean and standard deviation of each biomarker is provided for each group. Generalized Linear Model (GLM) -based (logistic regression) p-values are provided without adjustment and after adjusting individually and in combination for three covariates: age, gender and body mass index (BMI).
Adjusted using Bonferroni correction.
p-values significant at 5% alpha level are shown in bold.
Abbreviations: KL, Kellgren-Lawrence; AUC, area under receiver-operator curve.
IL1Ra measured at 18 months was also associated statistically significant with 24- month radiographic severity (AUC=0.69; 95% CI 0.59–0.83; p=0.004) (Figure 1A, Table 2). Furthermore, there is a significant correlation between plasma IL1Ra levels at baseline and 18 months (r=0.37; p=0.001). In contrast, baseline plasma IL1Ra levels did not correlate with 18 month plasma IL-1β (r=0.11; p=0.31) (Figure 1B). In contrast to IL1Ra, no other plasma biomarkers tested showed significant association with baseline radiographic severity (Table 2). Several biomarkers (VEGF, CRP, sTNF-RII) were modestly associated with severity, but these associations were not independent of age, gender, and BMI.
We next tested multivariate predictive models with baseline plasma protein (IL1Ra) biomarkers alone and in combination with clinical markers (WOMAC and VAS pain), age, gender and BMI. The resulting predictivity of radiographic severity for IL1Ra alone was AUC=0.59 (95% CI 0.49–0.70; p=0.045) and for clinical biomarkers alone AUC=0.59 (95% CI 0.49–0.70; p=0.045). With combination of both clinical and baseline IL1Ra the predictivity of radiographic severity was AUC=0.61 (95% CI: 0.50–0.72) and p=0.02. Table 3 shows the parameters derived from ROC curves such as pre-specified sensitivity and specificity of baseline IL1Ra in discriminating OA patients with severe- KL3/4 from those with KL1/2.
Table 3.
Pre-computed sensitivity and specificity of pre-cut-off baseline plasma IL1Ra on predicting radiographic progressors.
| Sensitivity | Baseline IL1RA (pg/ml) cutoff | Specificity | |
|---|---|---|---|
| JSN ≤0 vs. JSN >0 mm | 0.5 | 298.9 | 0.72 |
| 0.6 | 245.0 | 0.64 | |
| 0.7 | 216.7 | 0.44 | |
| 0.8 | 168.9 | 0.15 | |
| 0.9 | 155.0 | 0.15 | |
| JSN ≤0 vs. JSN >0.2 mm | 0.5 | 298.9 | 0.72 |
| 0.6 | 248.3 | 0.64 | |
| 0.7 | 213.3 | 0.41 | |
| 0.8 | 168.9 | 0.15 | |
| 0.9 | 155.0 | 0.15 | |
| JSN ≤0 vs. JSN >0.5 mm | 0.5 | 336.7 | 0.79 |
| 0.6 | 285.0 | 0.69 | |
| 0.7 | 223.3 | 0.54 | |
| 0.8 | 188.3 | 0.26 | |
| 0.9 | 163.3 | 0.15 |
Baseline plasma IL1Ra predicts radiographic progression by medial JSN
From our cohort of patients who completed the 24-month study (n=146), 18 patients who had predominantly lateral compartment disease were excluded, since lateral and medial compartment OA differ with respect to risk factors for disease severity and progression27. In addition, 17 SKOA patients who had JSW=0mm at baseline were also excluded as they could not mechanically “progress.” We restricted our association analysis to patients (n=111) with medial compartment disease. While an association of biomarkers with severity may provide insight into disease mechanisms, a more essential function for a candidate biomarker in OA would be to predict disease progression. As shown in Figure 1C and Table 4, baseline IL1Ra had modest correlation (r=0.24; p=0.01) with medial JSN at 24 months and did not correlate with lateral JSN (r=0.012; p=0.89). After adjustment for age, gender and BMI, plasma IL1Ra at baseline retained positive correlation (r=0.193; p=0.047) with medial JSN and not lateral JSN at 24 months. In contrast, other biomarkers did not correlate with medial JSN (data not shown).
Table 4.
Medial and lateral joint space width (JSW) of patients with symptomatic knee osteoarthritis (SKOA) at baseline and 24 month; joint space narrowing (JSN) correlation with baseline plasma IL1Ra.
| n=111 | Mean | SD | Baseline IL1Ra | |
|---|---|---|---|---|
| Medial | baseline JSW | 3.65 | 1.34 | |
| 24 month JSW | 3.13 | 1.51 | ||
| JSN | 0.53 | 0.89 | r=0.24; p=0.01 | |
| Lateral | baseline JSW | 5.77 | 1.43 | |
| 24 month JSW | 5.53 | 1.45 | ||
| JSN | 0.24 | 1.17 | r=0.01; p=0.89 |
p values significant at 5% alpha level are shown in bold type.
Bivariate and multivariate predictors of JSN
In a bivariate model, we further assessed plasma IL1Ra as a predictor of radiographic progression. As shown in Table 5, SKOA patients with baseline IL1Ra levels above the median progressed more rapidly than patients with baseline IL1Ra at or below median level (medial JSW 0.36mm vs. 0.70mm/24 months, p=0.041). Box plot distributions of baseline IL1Ra between non-progressors (JSN<0.0mm) and progressors (JSN >0.0; >0.2 and 0.5mm) are shown in Figure 1D. We also examined increased BMI as a risk factor for development of OA and progression28,29. We did not observe significant difference between the two BMI groups (below and above median BMI) with respect to mean medial JSW at 24 months (Table 5).
Table 5.
Association of baseline plasma IL1Ra and BMI (dichotomized by median) with joint space narrowing (JSN) at 24 months in 111 patients with symptomatic knee osteoarthritis (SKOA) in the medial compartment.
| Biomarkers | Median biomarker level at baseline | Median JSN at 24 months in patients with baseline biomarker level: | P value (t-test) | |
|---|---|---|---|---|
| ≤ median | > median | |||
| IL1Ra (pg/ml) N=110 | 246.65 | 0.36 (0.75), N=55 | 0.7055 (0.99), N=55 | 0.041 |
| BMI N=111 | 26.8 | 0.4531 (0.27), N=57 | 0.6204 (1.02), N=54 | 0.27 |
IL1Ra and BMI were dichotomized based on median levels to define two groups of subjects (e.g., with biomarker above versus at or below the threshold); mean JSN values were compared in these two subject groups using a two-sample t-test. Among the 111 completers, BMI data were available for all and IL1Ra data were available for 110.
We next examined whether IL1Ra and BMI predicted SKOA progressors versus non-progressors, as assessed by changes in minimal medial JSW. Table 6 shows a statistically significant association of IL1Ra with JSN. Prediction accuracies for IL1Ra to distinguish non-progressors from “slow-progressors” (JSN >0.2mm/24 months) and “fast-progressors” (JSN>0.5mm/24 months) ranged from AUC 0.63 to 0.66 (Table 6). Additionally, in logistic regression models after adjusting for age, gender and BMI, baseline IL1Ra retained statistical significance (p=0.0069–0.01) in all three groups of progressors (Table 6). Our data indicate that elevated levels of IL1Ra at baseline are correlated statistically significantly with radiographic progression as measured by medial JSN and can help distinguish non-progressors (low plasma IL1Ra) from fast-progressors. Other protein biomarkers were measured at 18 months (baseline specimens not available); none were associated with radiographic severity (Table 2) or progression at 24 months (Supplemental Table 1).
Table 6.
Relationship of plasma IL1Ra and BMI to progression of JSN at 24 months in patients with symptomatic knee osteoarthritis (SKOA).
| JSN ≤0 vs. JSN >0 (39 vs. 72) |
AUC | Lower CI | Upper CI | p-value | $Adjusted p-value | JSN ≤0 mean (SD) | JSN >0 mean (SD) | (no adjust.) | Adjusted for Age, Gender, BMI |
|---|---|---|---|---|---|---|---|---|---|
| IL1RA | 0.63 | 0.53 | 0.74 | 0.007 | 0.042 | 250.84 (121.06) | 354.09 (231.27) | 0.004 | 0.012 |
| BMI | 0.59 | 0.47 | 0.69 | 0.066 | 0.39 | ||||
|
JSN ≤0 vs. JSN >0.2 (39 vs. 64) |
AUC | Lower CI | Upper CI | p-value | JSN ≤0 mean (SD) | JSN >0.2 mean (SD) | (no adjust.) | Age, Gender, BMI | |
| IL1RA | 0.63 | 0.52 | 0.73 | 0.012 | 0.072 | 250.84 (121.06) | 360.15 (241.19) | 0.004 | 0.019 |
| BMI | 0.62 | 0.51 | 0.73 | 0.015 | 0.09 | ||||
|
JSN ≤0 vs. JSN >0.5 (39 vs. 44) |
AUC | Lower CI | Upper CI | p-value | JSN ≤0 mean (SD) | JSN >0.5 mean (SD) | (no adjust.) | Age, Gender, BMI | |
| IL1RA | 0.66 | 0.54 | 0.78 | 0.004 | 0.024 | 250.84 (121.06) | 375.51 (236.38) | 0.002 | 0.007 |
| BMI | 0.60 | 0.48 | 0.73 | 0.048 | 0.29 |
For measuring biomarker predictivity for radiographic progression in medial knee OA, we used the area under the receiver operating characteristic (ROC) curve. The mean and standard deviation of each biomarker is provided for each group. Generalized Linear Model (GLM)-based (logistic regression) p-values are provided after adjusting individually and in combination for three covariates: age, gender and body mass index (BMI).
Adjusted using Bonferroni correction.
p-values significant at 5% alpha level are shown in bold. JSN, joint space narrowing; AUC, area under ROC curve.
BMI at baseline was also assessed in analyses of JSN at 24 months. As reported above, when assessed using binary model (by ROC curve), Fisher partial correlation (Table 4), or dichotomized according to median BMI (Table 5), BMI did not associate with medial JSN progression. However, Table 6 shows that when dichotomized according to JSN, BMI did associate with medial JSN progression at 24 months. It is of interest therefore that the association of a given biomarker with progression may vary with the methods by which “progression” is assessed, and hence explain some of the disparate findings in the literature.
Causal graph analysis of medial JSN
In order to further explore interactions of clinical features and plasma biomarkers, we performed causal graph analysis to determine the interdependence of plasma protein biomarkers, covariates (BMI, age, gender), clinical markers [total WOMAC, WOMAC sub-scores (pain, stiffness and function), VAS pain] and continuous JSN. Only data for 111 patients with medial JSW >0.0mm were used for modeling.
The resulting graph of application of FCI is shown in Figure 2. Baseline IL1Ra either plays a causal role or positively influences JSN, or there is a hidden confounder that influences both IL1Ra and JSN. Interestingly, the analysis also indicates a causal relation between BMI and IL1Ra, which is ambiguous: either BMI is influencing IL1Ra, or IL1Ra is influencing BMI, or there is a hidden confounder that influences both BMI and IL1Ra.
Figure 2. Causal analysis of baseline biomarkers along with age, gender and BMI on medial JSN.
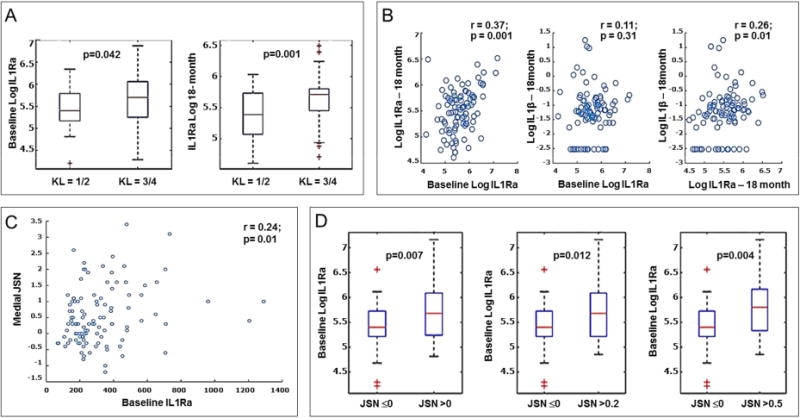
To determine the interdependence of plasma protein biomarkers covariates (BMI, age, gender), and clinical markers (WOMAC, VAS pain) on continuous radiographic joint space narrowing (JSN) over 24 months. Fast Causal Inference (FCI) algorithm26 was used for causal graph analysis of all variables. Edges with a single arrow denote causality, edges with double arrows denote hidden confounders, and marks (circles) on the edges denote uncertainty of causal orientation.
DISCUSSION
These studies demonstrate that elevation of plasma protein IL1Ra is modestly associated with radiographic severity (KL1/2 vs. KL3/4) as well as increased risk for progression of SKOA at 24 months. In addition, the data indicate that WOMAC (total, pain, function) and VAS pain are correlated with the severity of SKOA, as has been reported30,31. However, in these studies neither baseline WOMAC nor VAS pain predicted radiographic progression at 24 months.
These findings suggest that plasma IL1Ra reports increased production of cytokines and endogenous cytokine inhibitors by joint tissues involved in the pathophysiologic process of OA at both its early and end stages12. It is known that IL1Ra is produced by chondrocytes, macrophages and synovial cells following exposure to cytokines and chemokines, such as IL-1β, TNFα and IL-6. Therefore, we postulate that elevations of IL1Ra sufficient to be detected in plasma reflect joint tissues’ exposure to inflammatory cytokines. Histologic analyses of synovial biopsies, and both contrast and non-contrast MRI studies of knee joints, have demonstrated mild-to-moderate synovial inflammation in early and end stages of disease and association with increased risk of severe OA10,13,32,33. Noteworthy studies from Robinson and co-workers have demonstrated the importance of inflammation in both animal models and human OA11,34. OA is a heterogeneous disease, and multiple subgroups or phenotypes of OA have been identified based on etiological and risk factor such as metabolic, traumatic, inflammatory, and subchondral bone-driven progression of OA35,36. Our data indicate that, in a subset of patients, inflammation plays a role in OA pathology and disease progression15,37–39.
Consistent with the IL1Ra findings reported in the present work, multiplex cytokine analysis revealed increased inflammatory proteins in sera of OA patients compared to controls, including IL-1β, IL1Ra, IL-6, MCP-1, MIG, VEGF, and GM-CSF11. The novel finding in the current study is that IL1Ra predicts radiographic JSN progression in a causal fashion. This is particularly intriguing since polymorphisms in the IL1RN gene have been shown to predict radiographic severity and progression in OA40,41. We do not suggest that IL1Ra per se promotes joint damage, but rather that its production as an endogenous anti-inflammatory protein is a response to the degree of inflammation within the joint. IL1Ra levels are therefore an indirect measure of IL-1 and other cytokine activity within joint tissues. IL1Ra is mainly produced by synovial macrophages, where it is induced by cytokines such as IL-1 and IL-442, chemokines, and interferon gamma (IFN-γ)43. Synovitis has been associated with increased risk for progression of human OA; in animals, Blom et al. have shown that depletion of synovial macrophages greatly reduces induction of experimental OA in mice44. Our data are consistent with the hypothesis that plasma IL1Ra, produced by synovial macrophages, reports synovial inflammation, and therefore may be useful as a prognostic biomarker reflecting causal events that promote radiographic progression of SKOA.
Among the more intriguing findings of this study was the revelation by causal graph analysis of a causal relation between BMI, IL1Ra and SKOA progression, which remains unclear (Figure 2). BMI alone was associated with radiographic progression, when progression was dichotomized according to JSN (Table 6); however, unlike IL1Ra, BMI was not associated with JSN when JSN was assessed as a continuous variable (Table 4) or BMI dichotomized according to median levels (Table 5). Interestingly, IL1RN TGC haplotype (rs419598/rs9005/rs315943) was associated with radiographic progression of knee OA in the Johnston County Osteoarthritis Project cohort41. Consistent with the NYUHJD cohort, the rate of progression in this population was not significantly higher in subjects with BMI greater than the median (28.5 kg/m2) compared to those below the median. However, BMI association with progression was only evident in subjects who carried the IL1RN TGC haplotype. Taken together, these data may suggest that the relationship between IL1Ra and JSN depends upon BMI, but the relationship is governed by genetic variations. It is also of interest that IL1Ra has been reported to be positively correlated with BMI and insulin resistance45, and to play a role in cholesterol excretion46. Additionally, IL1Ra and leptin are markers of obesity47. Leptin is shown to be elevated in obese OA patients and promotes catabolic activities in cartilage48. In our current study we did not determine leptin levels in plasma samples.
Thus, the data from our studies and in the literature indicate an as-yet-undetermined interaction between IL1Ra and BMI: either BMI is influencing IL1Ra, or IL1Ra is influencing BMI, or there is a hidden confounder that influences both BMI and IL1Ra.
Inflammatory cytokines, notably IL-1β and TNFα, produced by joint tissues in the pathogenesis of OA have attracted increased attention. IL-1β exerts catabolic effects on chondrocyte metabolism, decreasing proteoglycan collagen synthesis and increasing aggrecan release via the induction of degradative proteases. IL-1β also induces IL1Ra expression in chondrocytes and synovial fibroblasts9. Richette et al have reported OA patients with lower median levels of IL-1β and IL1Ra in synovial fluids than RA patients, and also concluded that the ratio of IL1Ra/IL-1β was not associated with pain or disability in OA patients49. In addition to IL1Ra, other markers of inflammation have recently been reported in OA, including the observation that serum levels of TNFα and IL-6 inflammatory markers associate with knee pain in older adults11,50,51. Additionally, Kraus and associates have shown that synovial fluid IL-1β and IL-18 are associated with OA severity and progression52. Another acute phase protein, serum amyloid protein, is also elevated in OA53. Proteomics profiling of synovial fluid identified three dominant pathways that are dysregulated in OA relative to healthy controls: the acute-phase response signaling pathway, complement pathway, and coagulation pathway54. This further emphasizes that acute-phase response may be activated to resolve injury (such as cartilage damage) as response to an ongoing inflammatory process. In our studies, the other inflammatory protein biomarkers studied did not predict severity or progression in SKOA. However, we note that sufficient plasma for these studies was not available for assay until the 18-month time point. Therefore, we cannot determine whether elevations of one or more of these biomarkers might have predicted progression were a full 24 months of follow-up permitted. Such studies are ongoing.
We have previously reported that peripheral blood leukocytes (PBL) in selected patients with OA are activated to express inflammatory genes, including IL-1β, TNFα and COX-215. This observation has also been made in animal models of OA55,56. We speculate that one explanation for the inflammatory plasma signature in a subset of SKOA patients is that PBLs are activated as a result of repeated exposure to inflammatory stimuli as they circulate through the diseased synovium and subchondral bone of affected joints. Enhanced expression of mRNA for IL-6 and TNFα by CD34+ hematopoietic precursor cells is shown in the bone marrow of both OA and RA patients compared to normal marrow57. This raises the intriguing possibility that activated PBLs observed in our studies originate from a pool of activated precursor cells in the bone marrow of SKOA joints.
ESCEO has recently suggested that radiographic JSN >0.5mm over 2 or 3 years (fast-progressors) might be a reliable surrogate measure for total joint replacement23. Of note, Reginster et al have shown in a recent large clinical trial that >30% of OA patients had JSN >0.5mm over 24 months58, consistent with our data. Inflammatory biomarkers may be used to identify the subgroups among those with clinical knee OA in whom disease progresses at different rates. We would like to point out that, as with any other biomarker, there is significant overlap or distribution of IL1Ra between severity (KL1/2 vs. Kl3/4) or progressors (JSN <0.0mm to JSN>0.2 or 0.5mm) groups; nonetheless, it reaches significance in our studies. There are a number of limitations to this study, such as the modest size of the cohort, and current observations will need to be validated in other, larger cohorts. In addition, most of the plasma cytokine markers other than IL1Ra were assessed at 18 months only and not also at baseline, due to the availability of adequate specimens at that time point. In the current study, plasma IL-1Ra identified SKOA patients with severe disease or radiographic progressors at a group (but not at individual) level. Future studies will include all biomarkers at baseline to allow their evaluation at two or more years of follow-up.
Furthermore, there is a need for improved methods to assess the progression of OA, in order to understand differences among studies, since multiple joint compartments are involved and there are limitations to existing imaging methodologies. Radiographic progression of knee OA has been assessed using semi-quantitative measures of osteophytes, composite scores such as Kellgren-Lawrence grade, and quantitative or semi-quantitative measures of joint space width. Therefore, association of biomarkers with progression is dependent on multiple factors: the source (tissue), location (blood, synovial fluid) and stage and phenotype of the OA disease.
In summary, emerging multi-modal biomarkers, including wet (chemical, genomic or measurement of gene expression) and dry (imaging) biomarkers, contribute to a multi level, “systems” view of OA and hold promise for early diagnosis and as prognostic tests for managing osteoarthritis. Integrative analyses, which combine traditional statistical and multivariate predictive and other forms of advanced analytics, are helpful in synthesizing all available information as demonstrated in the present work. IL1Ra can potentially be used to identify and predict inflammatory knee OA phenotypes and monitor radiographic disease progression. As elegantly observed by Bruyere et al39, “Decisions on the selection of appropriate therapy can be made considering clinical presentation, underlying pathophysiology and stage of OA disease. Furthermore, identification of these patient profiles and phenotype of OA may lead to more personalized healthcare and more targeted treatment for osteoarthritis.” We investigated here the application of cutting-edge causal graph techniques that have recently been shown to have significant de novo mechanism and pathway discovery capabilities59–61; this approach has provided evidence that IL1Ra – in an as-yet-undefined interaction with BMI – predicts radiographic medial JSN progression, and our ongoing research in a larger OAI cohort may provide deeper understanding of the role of plasma IL1Ra in pathophysiology of OA.
Supplementary Material
Acknowledgments
This work was funded in part by research grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR052873 to SBA), National Center for Research Resources (1UL1 RR029893 to CFA), and National Library of Medicine (R01 LM011179-01A1 to AS) of the U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: MA and SBA have filed a provisional patent for the use of inflammatory biomarkers in OA diagnosis and prognosis. JT and QAL are employees of Singulex, Inc.
Author Contributions:
MA, AS, CFA, and SBA: conception and design, data acquisition, collection and assembly of data, analysis and interpretation of data, drafting of article, critically revising manuscript, final approval of manuscript, obtaining of funding.
JS, SK, JG, ZL and HZ: data acquisition, collection and assembly of data, drafting of article, critically revising manuscript, final approval of manuscript.
LR, JT and QAL: data acquisition, critically revising manuscript, and final approval of manuscript.
VBK and JMJ: analysis and interpretation of data, critically revising manuscript, and final approval of manuscript.
Contributor Information
M. Attur, Email: Mukundan.Attur@nyumc.org.
A. Statnikov, Email: Alexander.Statnikov@nyumc.org.
J. Samuels, Email: Jonathan.Samuels@nyumc.org.
Z. Li, Email: Zhiguo.Li@nyumc.org.
A.V. Alekseyenko, Email: Alexander.Alekseyenko@nyumc.org.
J.D. Greenberg, Email: JGreenberg@corrona.org.
S. Krasnokutsky, Email: krasns01@nyumc.org.
L. Rybak, Email: Leon.Rybak@nyumc.org.
Q.A. Lu, Email: A.Lu@singulex.com.
J. Todd, Email: J.Todd@singulex.com.
H. Zhou, Email: Hua.Zhou@nyumc.org.
J.M. Jordan, Email: joanne_jordan@med.unc.edu.
V.B. Kraus, Email: vbk@duke.edu.
C.F. Aliferis, Email: Constantin.Aliferis@nyumc.org.
S.B. Abramson, Email: StevenB.Abramson@nyumc.org.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25(1):136–44. doi: 10.1097/BOR.0b013e32835a9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50(8):2471–8. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 4.Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio-Le Graverand MP, et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage. 2007;15(4):379–85. doi: 10.1016/j.joca.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyère O, Chapurlat R, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72(11):1756–63. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos YF, Metrustry S, Arden N, Bay-Jensen AC, Beekman M, de Craen AJ, et al. Meta-analysis identifies loci affecting levels of the potential osteoarthritis biomarkers sCOMP and uCTX-II with genome wide significance. J Med Genet. 2014;51(9):596–604. doi: 10.1136/jmedgenet-2014-102478. [DOI] [PubMed] [Google Scholar]
- 7.Van Spil WE, Nair SC, Kinds MB, Emans PJ, Hilberdink WK, Welsing PM, Lafeber FP. Systemic biochemical markers of joint metabolism and inflammation in relation to radiographic parameters and pain of the knee: data from CHECK, a cohort of early-osteoarthritis subjects. Osteoarthritis Cartilage. 2015;23(1):48–56. doi: 10.1016/j.joca.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10(8):595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Abramson SB, Attur M, Yazici Y. Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol. 2006;2(6):304–12. doi: 10.1038/ncprheum0193. [DOI] [PubMed] [Google Scholar]
- 11.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Krasnokutsky S, Belitskaya-Levy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63(10):2983–91. doi: 10.1002/art.30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crema MD, Felson DT, Roemer FW, Niu J, Marra MD, Zhang Y, et al. Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced MRI and association with pain. The MOST study. Osteoarthritis Cartilage. 2013;21(3):413–8. doi: 10.1016/j.joca.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attur M, Belitskaya-Levy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, et al. Increased interleukin-1beta gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63(7):1908–17. doi: 10.1002/art.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 2007;53(11):1990–5. doi: 10.1373/clinchem.2007.091181. [DOI] [PubMed] [Google Scholar]
- 19.Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;27(8):861–74. [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J Royal Stat Society Series B. 1995;57(1):289–300. [Google Scholar]
- 22.Emrani PS, Katz JN, Kessler CL, Reichmann WM, Wright EA, McAlindon TE, et al. Joint space narrowing and Kellgren-Lawrence progression in knee osteoarthritis: an analytic literature synthesis. Osteoarthritis Cartilage. 2008;16(8):873–82. doi: 10.1016/j.joca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper C, Adachi JD, Bardin T, Berenbaum F, Flamion B, Jonsson H, et al. How to define responders in osteoarthritis. Curr Med Res Opin. 2013;29(6):719–29. doi: 10.1185/03007995.2013.792793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vapnik VN. Statistical learning theory. New York: Wiley; 1998. [Google Scholar]
- 25.Braga-Neto UM, Dougherty ER. Is cross-validation valid for small-sample microarray classification? Bioinformatics. 2004;20(3):374–80. doi: 10.1093/bioinformatics/btg419. [DOI] [PubMed] [Google Scholar]
- 26.Spirtes P, Glymour CN, Scheines R. Causation, prediction, and search. Cambridge, Massachusetts: MIT Press; 2000. [Google Scholar]
- 27.Hunter DJ, Niu J, Felson DT, Harvey WF, Gross KD, McCree P, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007;56(4):1212–8. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 28.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61(3):329–35. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou ZY, Liu YK, Chen HL, Liu F. Body mass index and knee osteoarthritis risk: A dose-response meta-analysis. Obesity (Silver Spring) 2014;22(10):2180–5. doi: 10.1002/oby.20835. [DOI] [PubMed] [Google Scholar]
- 30.Weiss E. Knee osteoarthritis, body mass index and pain: data from the Osteoarthritis Initiative. Rheumatology (Oxford) 2014;53(11):2095–9. doi: 10.1093/rheumatology/keu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cubukcu D, Sarsan A, Alkan H. Relationships between pain, function and radiographic findings in osteoarthritis of the knee: a cross-sectional study. Arthritis. 2012;2012:984060. doi: 10.1155/2012/984060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pessler F, Dai L, Diaz-Torne C, Gomez-Vaquero C, Paessler ME, Zheng DH, et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis. 2008;67(8):1184–7. doi: 10.1136/ard.2008.087775. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17(12):1674–9. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Esch M, Knoop J, van der Leeden M, Roorda LD, Lems WF, Knol DL, Dekker J. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthritis Cartilage. 2015;23(4):544–9. doi: 10.1016/j.joca.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Karsdal MA, Bihlet A, Byrjalsen I, Alexandersen P, Ladel C, Michaels M, et al. OA phenotypes, rather than disease stage, drive structural progression - identification of structural progressors from 2 phase III randomized clinical studies with symptomatic knee OA. Osteoarthritis Cartilage. 2015;23(4):550–8. doi: 10.1016/j.joca.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(11):375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67(4):956–65. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruyère O, Cooper C, Arden N, Branco J, Brandi ML, Herrero-Beaumont G, et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging. 2015;32(3):179–87. doi: 10.1007/s40266-015-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attur M, Wang HY, Kraus VB, Bukowski JF, Aziz N, Krasnokutsky S, et al. Radiographic severity of knee osteoarthritis is conditional on interleukin 1 receptor antagonist gene variations. Ann Rheum Dis. 2010;69(5):856–61. doi: 10.1136/ard.2009.113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Kondragunta V, Kornman KS, Wang HY, Duff GW, Renner JB, et al. IL-1 receptor antagonist gene as a predictive biomarker of progression of knee osteoarthritis in a population cohort. Osteoarthritis Cartilage. 2013;21(7):930–8. doi: 10.1016/j.joca.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1992;89(9):4076–80. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan L, Lin CW, Lin YJ, Sheu JJ, Chen BH, Liao CC, et al. Type I IFN induced IL1- Ra expression in hepatocytes is mediated by activating STAT6 through the formation of STAT2: STAT6 heterodimer. J Cell Mol Med. 2008;12(3):876–88. doi: 10.1111/j.1582-4934.2008.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blom AB, van Lent PL, Libregts S, Holthuysen AE, van der Kraan PM, van Rooijen N, et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56(1):147–57. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 45.Feve B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(6):305–11. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 46.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19(4):547–66. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Sandell LJ. Obesity and osteoarthritis: is leptin the link? Arthritis Rheum. 2009;60(10):2858–60. doi: 10.1002/art.24862. [DOI] [PubMed] [Google Scholar]
- 49.Richette P, François M, Vicaut E, Fitting C, Bardin T, Corvol M, et al. A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis. J Rheumatol. 2008;35(8):1650–4. [PubMed] [Google Scholar]
- 50.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–40. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 51.Honsawek S, Yuktanandana P, Tanavalee A, Chirathaworn C, Anomasiri W, Udomsinprasert W, et al. Plasma and synovial fluid connective tissue growth factor levels are correlated with disease severity in patients with knee osteoarthritis. Biomarkers. 2012;17(4):303–8. doi: 10.3109/1354750X.2012.666676. [DOI] [PubMed] [Google Scholar]
- 52.Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A. 2011;108(5):2088–93. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Seny D, Cobraiville G, Charlier E, Neuville S, Esser N, Malaise D, et al. Acute-phase serum amyloid a in osteoarthritis: regulatory mechanism and proinflammatory properties. PLoS One. 2013;8(6):e66769. doi: 10.1371/journal.pone.0066769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritter SY, Subbaiah R, Bebek G, Crish J, Scanzello CR, Krastins B, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65(4):981–92. doi: 10.1002/art.37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kyostio-Moore S, Nambiar B, Hutto E, Ewing PJ, Piraino S, Berthelette P, et al. STR/ort mice, a model for spontaneous osteoarthritis, exhibit elevated levels of both local and systemic inflammatory markers. Comp Med. 2011;61(4):346–55. [PMC free article] [PubMed] [Google Scholar]
- 56.Colitti M, Gaspardo B, Della PA, Scaini C, Stefanon B. Transcriptome modification of white blood cells after dietary administration of curcumin and non-steroidal anti-inflammatory drug in osteoarthritic affected dogs. Vet Immunol Immunopathol. 2012;147(3–4):136–46. doi: 10.1016/j.vetimm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Hirohata S, Yanagida T, Tomita T, Yoshikawa H. Enhanced expression of mRNA for interleukin 6 and tumor necrosis factor-alpha in CD34+ cells of the bone marrow in osteoarthritis [abstract #1771] Arthritis Rheum. 2011;63(10 Suppl):S694. [Google Scholar]
- 58.Reginster JY, Beaudart C, Neuprez A, Bruyere O. Strontium ranelate in the treatment of knee osteoarthritis: new insights and emerging clinical evidence. Ther Adv Musculoskelet Dis. 2013;5(5):268–76. doi: 10.1177/1759720X13500862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narendra V, Lytkin NI, Aliferis CF, Statnikov A. A comprehensive assessment of methods for de-novo reverse-engineering of genome-scale regulatory networks. Genomics. 2011;97(1):7–18. doi: 10.1016/j.ygeno.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alekseyenko AV, Lytkin NI, Ai J, Ding B, Padyukov L, Aliferis CF, et al. Causal graph-based analysis of genome-wide association data in rheumatoid arthritis. Biol Direct. 2011;6(1):25. doi: 10.1186/1745-6150-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Statnikov A, Henaff M, Lytkin NI, Aliferis CF. New methods for separating causes from effects in genomics data. BMC Genomics. 2012;13(Suppl 8):S22. doi: 10.1186/1471-2164-13-S8-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


