Abstract
Duchenne muscular dystrophy (DMD) is caused by mutations in the DMD gene. It is the most common, severe childhood form of muscular dystrophy. We investigated an alternative to dystrophin replacement by overexpressing ITGA7 using adeno-associated virus (AAV) delivery. ITGA7 is a laminin receptor in skeletal muscle that, like the dystrophin–glycoprotein complex, links the extracellular matrix to the internal actin cytoskeleton. ITGA7 is expressed in DMD patients and overexpression does not elicit an immune response to the transgene. We delivered rAAVrh.74.MCK.ITGA7 systemically at 5–7 days of age to the mdx/utrn−/− mouse deficient for dystrophin and utrophin, a severe mouse model of DMD. At 8 weeks postinjection, widespread expression of ITGA7 was observed at the sarcolemma of multiple muscle groups following gene transfer. The increased expression of ITGA7 significantly extended longevity and reduced common features of the mdx/utrn−/− mouse, including kyphosis. Overexpression of α7 expression protected against loss of force following contraction-induced damage and increased specific force in the diaphragm and EDL muscles 8 weeks after gene transfer. Taken together, these results further support the use of α7 integrin as a potential therapy for DMD.
Introduction
Duchenne muscular dystrophy (DMD) is the most common, severe childhood form of muscular dystrophy affecting approximately 1:5000 males born.1 DMD is caused by mutations in the DMD gene, resulting in sarcolemmal fragility related to the absence of dystrophin and concomitant loss of anchors to binding partners in the dystrophin-associated glycoprotein complex. Absence of dystrophin also affects downstream signaling pathways.2 The outcome is a progressive muscle wasting disorder with replacement by fat and fibrosis. The quality of life is compromised by loss of ambulation in early teens and premature death in early 20s from complications related to respiratory muscle weakness and cardiac deficiency of dystrophin.3 Glucocorticoid treatment of the disease results in mild improvement by extending ambulation, but has some cumbersome side effects.4 Direct gene replacement using mini- or microdystrophins poses potential barriers related to transgene immunogenicity, and shortfalls in correction of functional deficits imploring investigation of alternative strategies.5 α7 is a laminin receptor in cardiac and skeletal muscle that, like the dystrophin–glycoprotein complex, links the extracellular matrix to the internal actin cytoskeleton through its formation of a heterodimer with β1 integrin. Although normally concentrated at the myotendinous junction in postnatal muscle, α7 integrin is upregulated throughout the sarcolemma in DMD and the mdx mouse.6 Our lab and others have investigated the potential of α7 as a therapy for DMD. The path for this approach was first demonstrated in the transgenic mouse overexpressing the rat isoform of α7 in the dystrophin/utrophin double knockout (mdx/utrn−/−, DKO) that maintained muscle fiber integrity, promoted satellite cell proliferation and activation, and reduced kyphosis.7 Recently, we published the results of adeno-associated virus (AAV)-mediated overexpression of the human α7 protein in the mdx mouse model following isolated limb perfusion with AAV.MCK.ITGA7.8 Membrane injury was reduced as illustrated by fewer fibers, demonstrating cellular entry of Evan's blue dye and protection against eccentric contraction. In addition, histological improvement was shown by an increase in muscle fiber size.
It is important to point out that the mdx mouse is clinically normal despite lacking dystrophin compared with the human disease.9 In contrast, mice deficient for dystrophin and utrophin, mdx/utrn−/− die between 6and 20 weeks of age because of severe muscle weakness, pronounced growth retardation, and kyphosis.10 Thus, the DKO model offers phenotypic features upon which to judge the efficacy of a therapeutic product. This study had two primary objectives directly related to clinical translation. The first was to test whether overexpression of α7 provides continued protection against dystrophin deficiency at extended time points. The second goal was to test whether early systemic treatment could reverse the phenotype of the more severe mdx/utrn−/− DKO mouse model. The results showed that overexpression of α7 significantly improved specific force and showed protection against eccentric contraction induced injury following three months of treatment. Moreover, gene transfer of α7 reduced kyphosis and significantly improved the lifespan of the mdx/utrn−/− mice by more than 10 weeks. These results provide further support for α7 overexpression as a potential treatment for DMD.
Materials and Methods
Mice
All procedures were approved by The Research Institute at Nationwide Children's Hospital Institutional Animal Care and Use Committee. C57BL/10 and C57BL10/ScSnDMDmdx/J were purchased from Jackson Laboratory. Mdx/Utrn−/− mice were generated by breeding mdx/utrn+/− mice, a gift from Jill Rafael-Fortney (The Ohio State University). All animals were housed in standard mouse cages with food and water ad libitum.
Genotyping
The mdx/utrn−/− indentity was confirmed by genotyping. DNA from tail clippings was analyzed by using OneTaq DNA Polymerase (New England Biolabs) PCR. PCR analysis to determine utrophin-knockout status used a forward primer complementary to exon 7 of mouse utrophin (5′ GTG AAG GAT GTC ATG AAA G 3′) and reverse primers complementary to either intron 7 (5′ TGA AGT CCG AAA GAG ATA CC 3′) or to the PGK promoter located within the Neo-knockout cassette (5′ ACG AGA CTA GTG AGA CGT GC 3′). Reactions were carried out on genomic DNA for 30 cycles under the following conditions: 94°C, 30 s; 57°C, 30 s; 68°C, 20 s.
α7 integrin gene construction and rAAV production
The full-length human α7 cDNA (GenBank Accession No. AF072132) was codon optimized and synthesized by GenScript Inc. The cDNA was cloned into an AAV2 ITR containing plasmid that contained a consensus Kozak sequence, an SV40 intron, and a synthetic polyadenylation site.8 An MCK promoter/enhancer (GenBank Accession No. M21390)-derived sequence was used to drive muscle-specific gene expression. The promoter was synthesized by GenScript Inc. following derivation from previous work11,12 with some modifications. It is composed of the mouse MCK enhancer (206 bp) fused to the 351 bp MCK promoter (-351-0 MCK). After the promoter, the 53 bp endogenous mouse MCK exon1 (untranslated) was added for efficient transcription initiation. This inclusion has been shown to improve expression with other promoters, including CMV and troponin.13,14 Salva and colleagues have also shown that the addition of 50 bp from MCK exon1 improves expression.15 The MCK exon 1 was followed by the SV40 late 16S/19S splice signals (97 bp) and a small 5′UTR (61 bp). The intron and 5′ UTR are derived from plasmid pCMVb (Clontech).16
rAAV production
rAAV vectors were produced by a modified cross-packaging approach whereby the AAV type 2 ITRs can be packaged into multiple AAV capsid serotypes.17 Production was accomplished via a standard 3-plasmid DNA CaPO4 precipitation method using HEK293 cells.8
AAV vector delivery through isolated limb perfusion and intraperitoneal injection to transduce mouse muscle
mdx mice, 4–6 weeks old, were treated with 1×1012 vg of rAAVrh.74.MCK.ITGA7 by injection into the femoral artery as previously described.8,16 Animals were analyzed 12 weeks after gene transfer. In other experiments, mdx/utrn−/− mice (n=17) received an intraperitoneal (i.p.) injection of 1×1012 vg of AAVrh.74.MCK. ITGA7 into the body cavity (5–7 days old) using a 0.3 cc insulin syringe. Animals were analyzed at eight weeks postinjection and at an extended humane endpoint.
EDL force generation and protection from eccentric contractions
Mice were euthanized 8 and 12 weeks postinjection to allow for transgene expression. EDL muscles from both legs were dissected at the tendons and placed in Krebs–Henselet buffer. Muscles were subjected to physiological analysis using a protocol described by our lab8,16 with some adaptations. One tendon was tied to a force transducer and the other tendon was tied to a linear servomotor. Once the muscle was stabilized, the resting tension was set to a length (optimal length) where twitch contractions were maximal. After a rest period of 10 min without stimulation, a tetanic contraction was applied (500 ms tetanus at 150 Hz). Following 5 min of rest, an eccentric contraction protocol was used as previously described by Liu and colleagues18 with some modifications. The muscles were subjected to a series of 10 isometric 700 ms contractions, occurring at 2 min intervals, with a 5% stretch–re-lengthening procedure executed between 500 and 700 ms (5% stretch over 100 ms, followed by return to optimal length in 100 ms) for the 12-week mdx study and a series of 10 isometric 450 ms contractions, occurring at 2 min intervals, with a 3% stretch–re-lengthening procedure executed between 250 and 450 ms (3% stretch over 100 ms, followed by return to optimal length in 100 ms). Following the tetanus and eccentric contraction protocol, the muscle was removed, wet-weighed, mounted on chuck using gum tragacanth, and then frozen in isopentane-cooled in liquid nitrogen.
Diaphragm physiology
After sacrificing the mouse, the thorax was opened and the diaphragm and ribcage were removed and placed in a modified Krebs–Henseleit solution (95% O2/5% CO2, 5 mM KCl, 137 mM NaCl, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 20 mM NaHCO3, 10 mM d-glucose, and 0.25 mM CaCl2) with the addition of 20 mM 2,3-butanedione monoxime (BDM) to prevent muscle damage.19 Muscles were subjected to physiological analysis using a protocol described previously by our lab20,21 with some adaptations. Two linear strips of muscle, approximately 2–3 mm in width, were carefully dissected from each diaphragm. On one end of the muscle, the rib tissue was left intact, allowing the diaphragm strip to be held in place by inserting the muscle through a stainless steel basket connecting to a force transducer (KG2; Scientific Instruments). The central tendon at the deep end of the diaphragm strip was pierced over the hook that was connected to a linear micromanipulator. The muscle strip was submerged in a bath that contained oxygenated Krebs–Henseleit solution as described above (without BDM and with 2.0 mM CaCl2) at 37°C. A single electrical stimulation pulse was delivered via two parallel platinum–iriduim electrodes on either side of the muscle. The muscle was then stretched to its optimal length, defined as the length at which maximum twitch force is measured. The muscles were then subjected to a protocol that consisted of a series of 6 tetanic contractions occurring at 2 min interval, each with a duration of 250 ms.
Immunofluorescence
Cryostat sections (12 μm) were incubated with a polyclonal human α7 primary antibody (Abcam) at a dilution of 1:100 in a block buffer (1× PBS, 10% goat serum, 0.1% Triton X-100) for 1 hr at room temperature in a wet chamber. Sections were then washed with PBS three times, each for 10 min, and reblocked for 30 min. AlexaFluor 488-conjugated goat antirabbit secondary was applied at a 1:250 dilution for 45 min. Sections were washed in PBS 3 times for 10 min and mounted with Vectashield mounting medium (Vector Laboratories).
Histology and cross-sectional area
Tibialis anterior (TA) and quad muscles from 8-week-old mice, treated with rAAVrh.74.MCK.ITGA7 versus control uninjected, were stained with hematoxylin and eosin, cut in cross section, and photographed using Zeiss Axiovision L4 software (4 random 20× images per section per animal). Fiber size diameters were compared between treated and controls. Approximately 450–600 fibers were counted per muscle per animal.
X-ray images
Whole-body x-rays were performed on anesthetized wild-type, treated, and untreated mice at 8 weeks of age using the Faxitron MX-20 digital x-ray system at 26 kV for 3 sec (Faxitron X-Ray Corp). The kyphotic index (KI) was then assessed by taking the measurement of the length from AB and dividing it by the length of CD to get a KI score.
Results
Isolated limb perfusion of ITGA7 by rAAVrh.74 in the mdx mouse
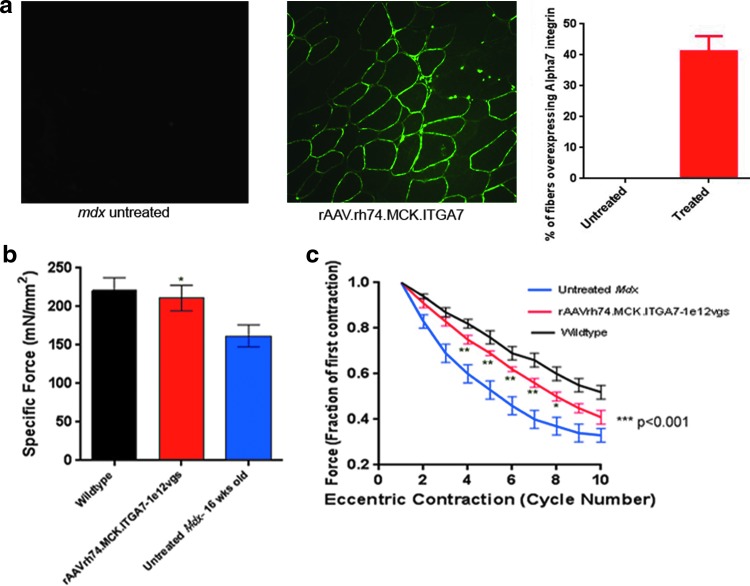
To assess the effects of extended treatment time, we perfused 1×1012 vg of rAAVrh.74.MCK.ITGA7 into the hind limb of 4-week-old mdx mice (n=6) using an established isolated limb perfusion methodology.8,16 The TA, extensor digitorum longus (EDL), and the gastrocnemius muscles were harvested 12 weeks postperfusion. Human α7 expression was quantified using immunofluorescent stained sections with a polyclonal antibody against the human protein. Fiber counts (n=6) revealed that 41.5%±5.18% of muscle fibers demonstrated sarcolemmal expression of human α7 (Fig. 1a). To test whether this increase of α7 continued to protect mdx muscle from contraction-induced injury and stabilized overall force, the functional properties of the EDL muscle were assessed. rAAVrh.74.MCK.ITGA7-treated muscles had a significantly higher specific force compared with the untreated, contralateral limb (treated 211.1±16.58 mN/mm2 vs. untreated 161.8±14.28 mN/mm2; p<0.05), which was not significantly different from age-matched wild-type mice (Fig. 1b). After assessment of specific force, the muscles were subjected to mechanical damage by repetitive contraction. α7 expression continued to protect against contraction-induced damage. By comparing the force ratio of each contraction versus the first contraction revealed that, after the 10th contraction, mdx-untreated muscle decayed to 0.32±0.04 versus treated 0.41±0.03 (p≤0.0001). The muscles receiving α7 were significantly more resistant compared with untreated mdx (*p<0.05 or **p<0.01, contractions 2–8); the treated group showed a slight decrease in the degree of protection compared with WT controls, which decayed to 0.52±0.03 (Fig. 1c). These data show that increasing expression of α7 integrin continues to lead to significant protection from contraction-induced injury, and preserves specific force in the treated muscle.
Figure 1.
Expression of human α7 in the mdx mouse hind limb 12 weeks following isolated limb perfusion of rAAVrh.74.MCK.ITGA7. Immunostaining with an antibody specific to ITGA7 reveals α7 integrin in rAAVrh.74.MCK.ITGA7 treated an mdx muscle that is not present in uninjected mice (a); ITGA7 antibody recognizes only human ITGA7 and does not cross-react with mouse α7. Quantification of the average percentage of myofibers overexpressing α7 integrin in rAAVrh.74.MCK.ITGA7-treated mdx muscles (n=6; error bar, SEM). Images taken at 20× magnification. Mdx muscles treated by isolated limb perfusion via the femoral artery with 1×1012 vg (red) of rAAVrh.74.MCK.ITGA7 (mouse) were compared with untreated contralateral mdx EDL muscles (blue) and WT (C57Bl/10) EDL muscles (black) 12 weeks after gene transfer. (b) Measurement of normalized specific force following tetanic contraction in rAAVrh.74.MCK.ITGA7-treated muscles was significantly increased, however (*p<0.05), compared with untreated contralateral mdx muscle. (c) Muscles were then assessed for loss of force following repetitive eccentric contractions. Treatment with rAAVrh.74.MCK.ITGA7 (red) significantly protected mdx muscle from loss of force compared with untreated (contralateral) muscles (blue). Two-way analysis of variance demonstrates significance in decay curves (***p<0.001). Moreover, Bonferroni post-hoc analysis revealed that force retention following contractions 3–9 (*p<0.05 and **p<0.01) was significantly higher than the untreated muscle. The treated muscle showed no significant difference from WT muscles (black). Error bars, SEM for n=6 (rAAVrh.74.MCK.ITGA7), 5 (WT, C57Bl/10), and 10 (mdx) muscles per condition.
Systemic delivery of rAAVrh.74.MCK.ITGA7 to mdx/utrn−/− muscle
To extend our studies in the mdx mouse to a model that better recapitulates DMD, we tested ITGA7 gene transfer in the mdx/utrn−/− mouse. To determine whether rAAVrh.74.MCK.ITGA7 could be delivered systemically to all muscle groups, 5–7-day-old mdx/utrn−/− pups received a one-dose i.p. injection of rAAVrh.74.MCK.ITGA7 at 1×1012 vg. One cohort of mice (n=5) was then analyzed at 8 weeks of age for expression. Immunofluorescence analysis revealed the presence of ITGA7 throughout the diaphragm, TA, gastrocnemius, quadriceps, and EDL muscles (Fig. 2a) in rAAVrh. 74.ITGA7-treated mice, whereas untreated mdx/utrn−/− controls were negative (inset in Fig. 2a). Quantification of muscle fibers expressing ITGA7 was performed in the TA, gastrocnemius, quadriceps, and diaphragm muscles. The muscles exhibited low-level, widespread expression (Fig. 2b).
Figure 2.
Systemic delivery of rAAVrh.74.MCK.ITGA7 results in expression of α7 integrin in multiple muscles in mdx/utrn−/− mice. Five- to seven-day-old mdx/utrn−/− mice (n=4) were intraperitoneally injected with 1×1012 vg of rAAVrh.74.MCK.ITGA7 and were taken out until 8 weeks of age. (a) Immunostaining shows expression of α7 integrin in multiple muscles such as the diaphragm, EDL, TA, gastroc, and quad muscles, 20× images. (b) Quantification of the average percentage of myofibers overexpressing α7 integrin in rAAVrh.74.MCK.ITGA7-treated muscles (n=4; error bar, SEM). Images taken at 20× magnification.
rAAVrh.74.MCK.ITGA7 increases myofiber diameter in mdx/utrn−/− mice
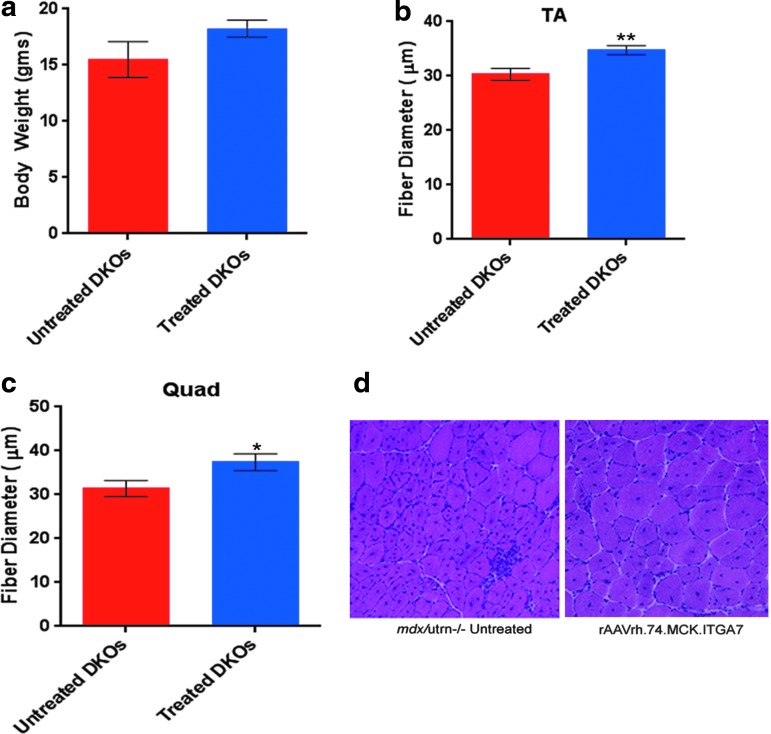
The mdx/utrn−/− mice tend to have a smaller body weight10 and treated mice showed a trend toward a higher body weight than their untreated mdx/utrn−/− littermates (untreated 15.49±1.59 g vs. treated 18.25±0.8 g; p=0.12) (Fig. 3a). Next, we analyzed the average fiber diameter in rAAVrh. 74.MCK.ITGA7-treated mdx/utrn−/− muscles versus control untreated mdx/utrn−/− mice. In rAAVrh.74.MCK.ITGA7-treated mdx/utrn−/− TA muscles (n=13), the mean fiber diameter of all muscle fibers was significantly larger compared with untreated mdx/utrn−/− TA muscles (n=11) (untreated 30.33±1.10 μm vs. treated 34.75±0.85 μm; **p<0.01) (Fig. 3b). The treated quadriceps muscle was also significantly larger compared with untreated mdx/utrn−/− quad muscle (untreated 31.39±1.84 μm vs. treated 37.40±1.95 μm; *p<0.05) (Fig. 3c). Taken together, these data show that α7 leads to an increase in myofiber diameter.
Figure 3.
rAAVrh.74.MCK.ITGA7 treatment increases myofiber diameter in mdx/utrn−/− mice. (a) Body weight between treated mdx/utrn−/− mice is not significantly different from untreated mdx/utrn−/− mice. (b) rAAVrh.74.MCK.ITGA7 treatment in mdx/utrn−/− mice results in an increase in myofiber diameter in the TA muscle compared with untreated mdx/utrn−/− controls (**p<0.01). (c) Quantification of the average fiber diameter of rAAVrh.74.MCK.ITGA7 treated vs. untreated mdx/utrn−/− quad muscle shows a significant increase in fiber diameter following hematoxylin and eosin staining (H&E) Error bars, SEM for (n=6). (d) H&E images of treated rAAVrh.74.MCK.ITGA7 mdx/utrn−/− muscle (right) illustrate an increase in fiber diameter compared with untreated mdx/utrn−/− muscle. DKO, double knockout. Color images available online at www.liebertpub.com/hum
rAAVrh.74.MCK.ITGA7 treatment reduces kyphosis in mdx/utrn−/− mice
By 8 weeks of age, untreated mdx/utrn−/− mice exhibit severe kyphosis (curvature of the thoracic spine) because of a weakness in the muscles that support the spinal column.10 Kyphosis results in the diaphragm being pushed forward, compromising lung capacity and diaphragm function. Eight-week-old untreated (n=4) and treated (n=7) mdx/utrn−/− underwent full-body x-rays to analyze kyphosis (Fig. 4a). The KI was then assessed by taking the measurement of the length from AB and dividing it by the length of CD to get a KI score.22 The treated mice had a significantly increased KI score compared with their untreated littermates (treated 4.38±0.22 vs. untreated 3.27±0.11; *p<0.05) (Fig. 4b). The treated mice still had a lower score than wild-type mice (6.4±0.17). Taken together, these data show that increased α7 integrin leads to a reduction in kyphosis.
Figure 4.
Systemic delivery of rAAVrh.74.MCK.ITGA7 results in reduced kyphosis and joint contractures. (a) X-ray images show a reduction in kyphosis in ITGA7- injected mdx/utrn−/− mice compared with untreated mdx/utrn−/− mice and wild-type mice. (b) A kyphotic index score (KI) was given to each mouse and the KI was quantified. Color images available online at www.liebertpub.com/hum
Additional α7 integrin increases force and protects mdx/utrn−/− muscle from contraction-induced damage
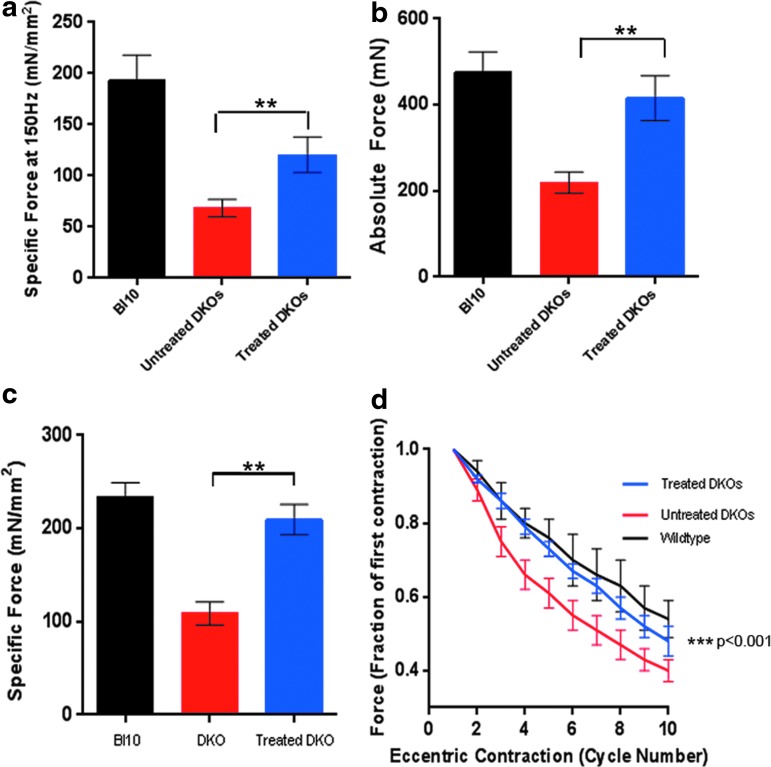
To test whether increasing expression of α7 could protect mdx/utrn−/− muscle from contraction-induced injury and increase overall force, the functional properties of the diaphragm and EDL muscle from mdx/utrn−/− mice treated with rAAVrh.74.MCK.ITGA7 were assessed. Eight weeks postinjection, animals were euthanized and the diaphragm and EDL were removed for in vitro force measurements. rAAVrh.74.MCK.ITGA7-treated diaphragm muscles showed a significant improvement in normalized specific force when compared with untreated diaphragm (untreated 68.29±8.7 mN/mm2 vs. treated 120.6±17.4 mN/mm2, **p<0.01), but did not reach wild-type levels (Fig. 5a). When we compared force production between rAAVrh.74.MCK.ITGA7-treated EDL muscles and untreated EDL muscles, the ITGA7-treated mice had significant improvement in absolute tetanic force and normalized specific force compared with untreated EDL muscles (absolute tetanic force: untreated 219±24.4 mN vs. treated 415.7±52.2 mN; p<0.005, and normalized specific force: untreated 108.9±12.33 mN/mm2 vs. treated 209.5±16.25 mN/mm2; p<0.01). The treated mice showed no significant difference from wild-type muscle (234.4±14.7 mN/mm2) (Fig. 5b and c). After assessment of specific force, the EDL muscle was subjected to mechanical damage by repetitive eccentric contractions. α7 overexpression significantly protected against contraction-induced injury (mdx/utrn−/− vs. treatment p<0.001; ANOVA) (Fig. 5d).
Figure 5.
Systemic delivery of rAAVrh.74.MCK.ITGA7 leads to functional improvement in mdx/utrn−/− mice. (a) Measurement of normalized specific force following tetanic contraction in rAAVrh.74.MCK.ITGA7-treated diaphragm muscles was increased compared with untreated mdx/utrn−/− muscle. There is a significant improvement in specific force in treated diaphragms. Measurement of absolute (b) and normalized (c) specific force following tetanic contraction in rAAVrh.74.MCK.ITGA7-treated EDL muscles was significantly increased compared with untreated mdx/utrn−/− mdx/utrn−/− muscle (**p<0.01). (d) Muscles were then assessed for loss of force following repetitive eccentric contractions. rAAVrh.74.MCK.ITGA7 (red) shows a protection of mdx/utrn−/− muscle from loss of force compared with untreated mdx/utrn−/− muscles (blue). Two-way analysis of variance demonstrates significance in decay curves (***p<0.001). Error bars, SEM for n=4 (rAAVrh.74.MCK.ITGA7), n=4 (mdx/utrn−/− mice), and n=5 (C57Bl10, WT) for diaphragm; n=6 (rAAVrh.74.MCK.ITGA7), n=12 (mdx/utrn−/− mice), and n=11 (C57Bl10, WT) for EDL. Color images available online at www.liebertpub.com/hum
rAAVrh.74.MCK.ITGA7 gene transfer increases longevity of mdx/utrn−/− mice
To determine whether systemic delivery of ITGA7 increased longevity, a separate cohort of mdx/utrn−/− mice (n=8) was intraperitoneally injected with rAAVrh.74.MCK.ITGA7 (1×1012 vg). Longevity was significantly extended in the treated mice. Kaplan–Meier survival analysis of 10 treated mice and 21 untreated mice demonstrated statistically significant differences in survival. Log rank and Wilcoxon tests also showed differences in survival (**p<0.005). The median age of death of the untreated mdx/utrn−/− mice was 9 weeks, whereas the median age of the treated mdx/utrn−/− was 21 weeks of age, with the oldest mouse at 34 weeks (Fig. 6). Expression was checked by immunofluorescence in the treated mice and we saw ITGA7 expression in all muscles compared with the mice treated to 8 weeks of age, except in the diaphragm, which no longer had detectable ITGA7 expression (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum).
Figure 6.
rAAVrh.74.MCK.ITGA7 improves longevity in mdx/utrn−/− mice. Kaplan–Meier survival curves of rAAV.ITGA7-treated mdx/utrn−/− mice (n=10) and untreated mdx/utrn−/− mice (n=21). Wilcoxon and log-rank tests show that there is a significant difference in survival curves. The treated mice had a median life expectancy of 21 weeks, whereas untreated mice had a median life expectancy of 9 weeks. Color images available online at www.liebertpub.com/hum
Despite gene transfer, at 18 weeks kyphosis did develop and the natural history of respiratory complications and wasting led to the death of the DKOs. Increased doses or adjunctive therapy may be required for a sustained effect in severe disease.
Discussion
Our results demonstrate the benefit of increased overexpression of ITGA7 as a potential therapy for DMD. We first tested the efficacy of gene transfer following vascular delivery by isolated limb perfusion in the mdx mouse. Immunostaining showed an increased number of α7 integrin-positive fibers accompanied by functional improvement in specific force that reached wild-type levels, and significant improvement in protection against eccentric contraction. Gene replacement therapy using rAAVrh.74.MCK.ITGA7 was also remarkably beneficial in the more severely affected mdx/utrn−/− mouse. The severity of the dystrophic process in the DKO mouse and premature death more closely resembles DMD compared with the mdx mouse. The mdx/utrn−/− mice were treated shortly after birth and analyzed at 8 weeks of age. Widespread gene expression was seen that included the diaphragm, TA, gastrocnemius, quadriceps, and EDL. This was accompanied by stabilized body weight, increase in myofiber diameter, and reduction in contraction-induced injury. A remarkable finding was the reduction of kyphosis and improved diaphragm function. The net effect was prolonged survival in this severely affected dystrophic mouse model treated in the first few days after birth. Early treatment efficacy in the days immediately following birth supports clinical efforts to bring newborn screening forward as a potential tool for early recognition of DMD providing potential treatment intervention before catastrophic muscle loss. This will benefit multiple forms of molecular-based treatment that is evolving.23
A fundamental translational outcome from this study is the observed increase in the median survival time of the mdx/utrn−/− mice treated with rAAVrh.74.MCK.ITGA7 compared with untreated mdx/utrn−/− mice. It is important to emphasize that this was not a cure, but improving lifespan by 12 weeks for a lethal disease is a significant accomplishment. Given that both dystrophin and utrophin are deficient, it is difficult to know if the findings in the DKO mouse underestimate a treatment effect that might be found in DMD. We attribute death in the mdx/utrn−/− treated mice to loss of vector in diaphragm and compromised respiratory capacity. Treated mice began to revert back to the typical mdx/utrn−/− phenotype. A caveat to treating early is that if the muscle is not transduced efficiently, the regeneration process will contribute to the loss of vector genome transgene copies over time. This is especially striking in muscles such as the diaphragm that tend to exhibit more severe histopathology.24 This phenomenon has been reported previously by Le Hir et al.25 Following AAV-U7 exon skipping treatment in mdx/utrn−/− mice, they showed a loss of viral genomes in partially rescued muscle. This was accompanied by a drastic reduction in dystrophin expression in skeletal muscle and no detectable dystrophin in the diaphragm near the endpoint of survival. It would be of use to evaluate ITGA7 gene therapy in a larger animal model such as canine dystrophy with exclusive deficiency in dystrophin and phenotypic features simulating DMD and ask what levels of α7 integrin correlate with reversal of disease. This might provide a clearer picture of what is required in clinical trials.
Supplementary Material
Acknowledgments
We would like to thank the NCH Viral Vector Manufacturing Facility for supplying vector for this study. This work has been funded by the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (1U54HD066409) at Nationwide Children's Hospital, and a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Graduate Student Training Fellowship (to K.N.H).
Author Disclosure
All the authors declare that no competing financial interests exist.
References
- 1.Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012;71:304–313 [DOI] [PubMed] [Google Scholar]
- 2.O'Brien KF, Kunkel LM. Dystrophin and muscular dystrophy: past, present, and future. Mol Genet Metab 2001;74:75–88 [DOI] [PubMed] [Google Scholar]
- 3.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve 1983;6:91–103 [DOI] [PubMed] [Google Scholar]
- 4.Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med 1989;320:1592–1597 [DOI] [PubMed] [Google Scholar]
- 5.Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010;363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodges BL, Hayashi YK, Nonaka I, et al. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci 1997;110:2873–2881 [DOI] [PubMed] [Google Scholar]
- 7.Burkin DJ, Wallace GQ, Milner DJ, et al. Transgenic expression of {alpha}7{beta}1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol 2005;166:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller KN, Montgomery CL, Janssen PM, et al. AAV-mediated overexpression of human alpha7 integrin leads to histological and functional improvement in dystrophic mice. Mol Ther 2013;21:520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sicinski P, Geng Y, Ryder-Cook AS, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 1989;244:1578–1580 [DOI] [PubMed] [Google Scholar]
- 10.Deconinck AE, Rafael JA, Skinner JA, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 1997;90:717–727 [DOI] [PubMed] [Google Scholar]
- 11.Shield MA, Haugen HS, Clegg CH, Hauschka SD. E-box sites and a proximal regulatory region of the muscle creatine kinase gene differentially regulate expression in diverse skeletal muscles and cardiac muscle of transgenic mice. Mol Cell Biol 1996;16:5058–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther 2002;9:1576–1588 [DOI] [PubMed] [Google Scholar]
- 13.Nikovits W, Jr, Mar JH, Ordahl CP. Muscle-specific activity of the skeletal troponin I promoter requires interaction between upstream regulatory sequences and elements contained within the first transcribed exon. Mol Cell Biol 1990;10:3468–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simari RD, Yang ZY, Ling X, et al. Requirements for enhanced transgene expression by untranslated sequences from the human cytomegalovirus immediate-early gene. Mol Med 1998;4:700–706 [PMC free article] [PubMed] [Google Scholar]
- 15.Salva MZ, Himeda CL, Tai PW, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther 2007;15:320–329 [DOI] [PubMed] [Google Scholar]
- 16.Rodino-Klapac LR, Janssen PM, Montgomery CL, et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med 2007;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002;76:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Yue Y, Harper SQ, et al. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 2005;11:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulieri LA, Hasenfuss G, Ittleman F, et al. Protection of human left ventricular myocardium from cutting injury with 2,3-butanedione monoxime. Circ Res 1989;65:1441–1449 [DOI] [PubMed] [Google Scholar]
- 20.Rodino-Klapac LR, Montgomery CL, Bremer WG, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther 2010;18:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grose WE, Clark KR, Griffin D, et al. Homologous recombination mediates functional recovery of dysferlin deficiency following AAV5 gene transfer. PLoS One 2012;7:e39233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laws N, Hoey A. Progression of kyphosis in mdx mice. J Appl Physiol 2004;97:1970–1977 [DOI] [PubMed] [Google Scholar]
- 23.Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 2013;74:637–647 [DOI] [PubMed] [Google Scholar]
- 24.Stedman HH, Sweeney HL, Shrager JB, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 1991;352:536–539 [DOI] [PubMed] [Google Scholar]
- 25.Le Hir M, Goyenvalle A, Peccate C, et al. AAV genome loss from dystrophic mouse muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol Ther 2013;21:1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.