Abstract
8-OxodGuo and Fapy•dG induced 10–22% mutations, predominantly G→T transversions, in HEK 293T cells in four TG*N sequence contexts, where N = C, G, A, or T. siRNA knockdown of pol λ resulted in a 34% and 55% increase in mutations in the progeny from the 8-oxodGuo construct in the TG*T and TG*G sequence, respectively, suggesting that pol λ is involved in error-free bypass of 8-oxodGuo. For Fapy•dG, in contrast, G→T mutations were reduced by 27% and 46%, respectively, in the TG*T and TG*G sequence, suggesting that pol λ is responsible for a significant fraction of Fapy•dG-induced G→T mutations.
Oxidative stress produces many different lesions in DNA, of which 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) received much attention.1, 2 Approximately 20% of the hydroxyl radicals that react with dG add to C8 to produce a radical at N7.3 The N7-radical generates 8-oxodGuo via one-electron oxidation, whereas fragmentation followed by reduction gives rise to the ring-opened formamidopyrimidine derivative, Fapy•dG (Scheme 1).4 The relative amounts of these two lesions depend on the oxidation conditions, but frequently they are formed in comparable levels.4 8-OxodGuo and Fapy•dG are mutagenic in bacteria and mammalian cells.5–8 Many studies in prokaryotes and eukaryotes have demonstrated that 8-oxodGuo induces G→T transversion as the predominant mutation.5, 6
Scheme 1.
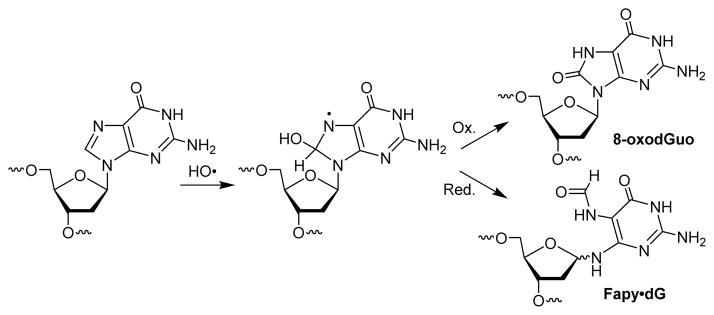
Formation of 8-oxodGuo and Fapy•dG by hydroxyl radical through a common intermediate.
Structural studies suggest that 8-oxodGuo leads to misincorporation of adenine in its syn conformation leading to G:C→T:A mutations.9 Fapy•dG also induces G→T mutations.7, 8 However, a recent study using a carbocyclic analog of Fapy•dG indicates that the Fapy lesions remain in the anti conformation during replication, but that base-shifting and tautomerization result in mispairing with dA, ultimately leading to G→T transversions.10 There are only a limited number of studies in which replication of 8-oxodGuo and Fapy•dG have been explored in the same sequence context using the same approach. In one comparative study in Escherichia coli, the bypass efficiency of Fapy•dG was found to be lower than 8-oxodGuo in four sequence contexts.7 Induction of the SOS functions resulted in increased bypass efficiency of 8-oxodGuo, whereas it has no effect on Fapy•dG, suggesting that the SOS enzymes are not involved in the translesion synthesis (TLS) of Fapy•dG. In addition, Fapy•dG is less mutagenic than 8-oxodGuo in E. coli in all four sequences.7 In contrast to this result, in two sequence contexts examined in the simian kidney (COS-7) cells, Fapy•dG was found to be ~25% more mutagenic than 8-oxodGuo. Fapy•dG-induced G→T mutation frequency (MF) was as high as 30% in TG*T sequence, which was nearly 4-fold relative to that in the TG*A sequence.8
For the last two decades, the role of oxidative stress and 8-oxodGuo in human diseases has been a very active area of research.11, 12 As such, the mechanism of TLS of 8-oxodGuo was studied extensively in vitro using purified human DNA polymerases (pols).13–16 It was also investigated in human cells.17–19 8-OxodGuo does not severely block the human replicative pols, but pol α, pol δ, and pol ε extend an 8-oxodGuo:dA mispair much more efficiently than the correct 8-oxodGuo:dC pair.2, 13, 19 Despite this, TLS of 8-oxodGuo in human cells is largely error-free.19, 20 In comparison to the B-family enzymes pol α, pol δ, and pol ε, the X-family enzyme pol λ bypasses 8-oxodGuo more faithfully.21 It incorporates the correct nucleotide (dC) opposite 8-oxodGuo 1,200-fold more efficiently than dA in the presence of RP-A and PCNA.22 A key role of MUTYH and pol λ in the repair of 8-oxodGuo:dA mispair was recognized.23 A subsequent study showed the existence of a pathway in which error-free TLS of 8-oxodGuo is accomplished by a switch of pol δ with pol λ.24 The importance of this switch is enhanced in this work in which it was established that pol β and pol η are not involved in the error-free pathway.
To better understand the mutagenic mechanism of 8-oxodGuo and Fapy•dG in human cells, we replicated four sets of vectors containing 8-oxodGuo or Fapy•dG located in the TG*N sequence context (where N = C, G, A, or T and G* = 8-oxodGuo or Fapy•dG) in human embryonic kidney (HEK) 293T cells. In each case the progeny were analyzed for mutations using oligonucleotide hybridization, followed by DNA sequencing.25, 26
8-OxodGuo and Fapy•dG were significantly mutagenic in HEK293T cells (Figure 1). The total MF ranged from 10–22%, and in each sequence context they exhibited a distinct pattern of mutations. In the TG*T sequence the MF of Fapy•dG was ~75% higher than that of 8-oxodGuo, whereas in the TG*C and TG*G sequences the MF of 8-oxodGuo was 20–30% higher than that of Fapy•dG (Figure 1 & Table S1 in SI). In the TG*A sequence, the MFs of the lesions were comparable. Although the most prevalent mutations induced by both Fapy•dG and 8-oxodGuo were G→T transversions, in the TG8-oxoG and TGFapyC sequences, significant targeted G→A transitions also occurred (Figure 1 & Table S1 in SI). In contrast to the results in simian kidney (COS-7) cells,8 in which the MF in the TGFapyT sequence was 4-fold higher compared to that in the TGFapyA sequence, the MF in HEK293T cells was only ~75% higher in the TGFapyT sequence relative to that in the TGFapyA sequence. It should be noted, however, that the DNA sequence contexts beyond the immediate neighbors of the lesions in the work in COS-7 cells were different from the ones used in the current study.
Figure 1.
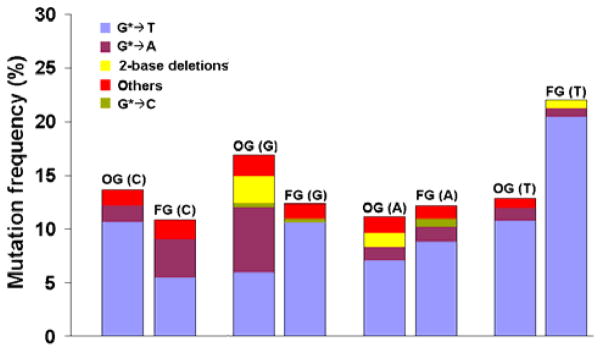
Comparative mutagenesis of 8-oxodGuo (OG) and Fapy•dG (FG) in HEK293T cells in TG*N sequence, where N represents C, G, A, or T shown in parenthesis. The data represent the average of two independent experiments (shown in Table S1 in the SI). Others include semitargeted mutations near the lesion as indicated in Table S1 in SI.
Given the recognized role of pol λ in error-free bypass of 8-oxodGuo,21, 24 we compared Fapy•dG and 8-oxodGuo mutagenesis in pol λ knockdown HEK293T cells. We chose the TG*T and TG*G sequences, because in the TG*T sequence Fapy•dG was more mutagenic than 8-oxodGuo, whereas in the TG*G sequence the reverse was true (Figure 1). The location of 8-oxodGuo in the TG*G sequence is also of interest owing to the diverse mutational spectrum observed in this sequence context, which included a high frequency of G→A mutations and moderate frequency of dinucleotide deletions (Figure 1). The control for this experiment was HEK293T cells treated with negative control (NC) siRNA (Figure 2), which had essentially no effect on mutagenesis and showed results very consistent with that induced by the lesions in untreated HEK293T cells (Figure 1).
Figure 2.
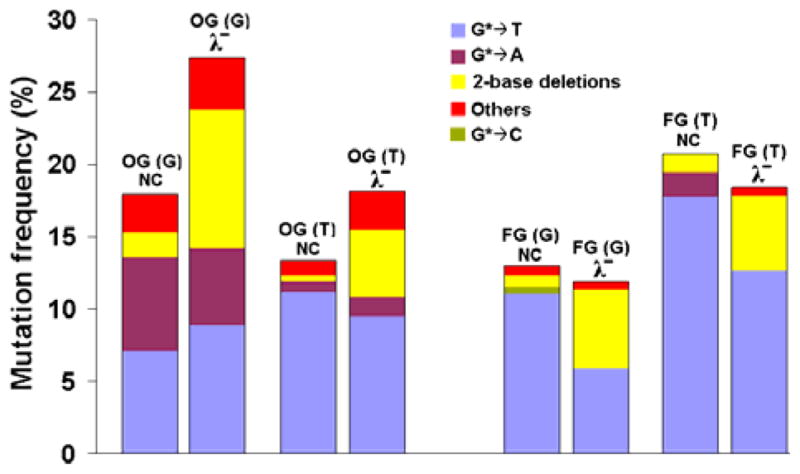
Mutational frequency of 8-oxodGuo (OG) and Fapy•dG (FG) in HEK293T cells also transfected with NC siRNA or siRNA for pol λ knockdown. HEK293T cells were plated in six-well plates at 25% confluence. After a 24-h incubation period, they were transfected with appropriate siRNA (NC or pol λ) and Lipofectamine. One day before the transfection of the plasmid, cells were seeded in 24-well plates at 50% confluence. Cells were then co-transfected with another aliquot of siRNA and either the control or the lesion-containing plasmid. After a 24-h incubation, progeny plasmids were isolated and analyzed as described.25 The data represent the average of two independent experiments (shown in Table S2 in the SI). Others include semitargeted mutations near the lesion as indicated in Table S2 in SI.
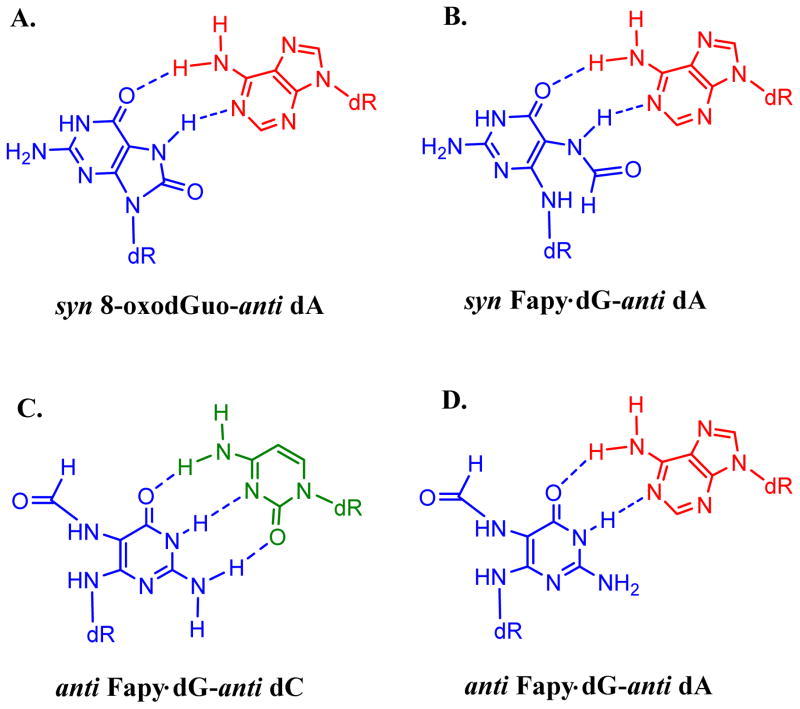
For 8-oxodGuo, the total MF increased in both sequence contexts upon knockdown of pol λ. In the TG8-oxoT sequence, the MF increased from 13% to 18%, and in the TG8-oxoG sequence it increased from 18% to 27% (Figure 2 & Table S2 in SI). This suggests that pol λ plays a role in the error-free bypass of 8-oxodGuo, as reported in murine and human cells.24 It is noteworthy that 8-oxodGuo-induced G→T transversions were not significantly affected upon knockdown of pol λ. Instead, a large increase in dinucleotide deletions occurred in pol λ-knockdown cells. It suggests, therefore, that pol λ prevents dinucleotide deletions induced by 8-oxodGuo. In contrast, MF of Fapy•dG in TGFapyT sequence decreased only marginally from 20.9±0.9% to 17.5±0.6% (Figure 2 & Table S2 in SI). In TGFapyG sequence, MF of Fapy•dG was nearly unaltered (i.e., changed from 12.8±1.3% to 11.4±1.6%) (Table S2 in SI). In striking divergence to the results from replication of the 8-oxodGuo construct, the G→T mutations induced by Fapy•dG dropped dramatically in the progeny from 11% to 6% in the TGFapyG construct and from 18% to 13% in the TGFapyT construct (Figure 2). This suggests a role of pol λ in part of Fapy•dG-induced G→T mutations. Even though structural studies are necessary to provide insight into the mechanism of these mutations, at least two different base-pairing modes of Fapy•dG with dA are possible (Figure 3). Based on the 8-oxodGuo mutagenic mechanism,9, 27 syn Fapy•dG can generate Hoogsteen pairing with anti dA (Figure 3B). Alternatively, anti Fapy•dG can pair with anti dA (Figure 3D) as reported at the active site of Bst Pol I.10 However, currently there is no experimental evidence to specify how pol λ might be involved in G→T mutations during TLS of Fapy•dG.
Figure 3.
Similar to mispairing of syn 8-oxodGuo with anti dA (as shown in A),9, 27 the base-pairing of syn Fapy•dG may occur with anti dA (B). The postulated base-pairing modes of anti Fapy•dG with anti dC (C) for error-free and with anti dA (D) for mutagenic bypass are also shown. The anti Fapy•dG base-paring with anti dA (as shown in D) was observed at the active site of Bst Pol I.10
A common observation upon replication of vectors containing 8-oxodGuo and Fapy•dG in pol λ knockdown cells was the large increase in dinucleotide deletions, which involved the lesion-containing G and its 3′ or 5′ neighboring base. For 8-oxodGuo, the dinucleotide deletions increased from 0.4% to 4.3% in the TG8-oxoT sequence and 1.9% to 8.9% in the TG8-oxoG sequence in pol λ knockdown cells (Figure 2 & Table S2 in SI). For Fapy•dG, likewise, the dinucleotide deletions increased from 1.3% to 5.8% in the TGFapyT sequence and 0.9% to 5.2% in the TGFapyG sequence in pol λ knockdown cells (Figure 2 & Table S2 in SI). The polymerase responsible for the dinucleotide deletions was not identified. However, a four-fold increase in small deletions by 8-oxodGuo was reported in HeLa cells in which pol λ was knocked down.24
Though a similar study with Fapy•dG in human cells has not been performed, replication of the related MeFapy•dG (Figure 4) which contains a methyl group at the 5 position, in simian kidney cells resulted in a significant frequency of dinucleotide deletions in the TXT sequence, even in the presence of all functional pols.28
Figure 4.
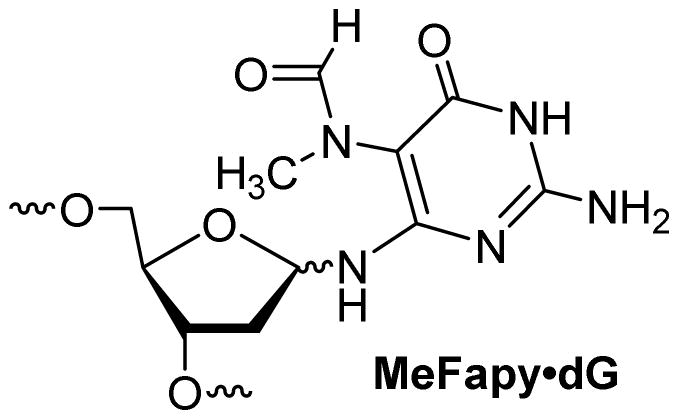
Structure of MeFapy·dG
In conclusion, the results of this study established a fundamental difference between the mechanism of TLS of 8-oxodGuo and that of Fapy•dG in human cells in that pol λ is responsible for a fraction of G→T mutations induced by Fapy•dG, whereas it conducts predominantly error-free bypass of 8-oxodGuo.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the NIH grants ES09127 and ES021762 to AKB and CA-74954 to MMG.
ABBREVIATIONS
- 8-oxodGuo
8-oxo-7,8-dihydro-2′-deoxyguanosine
- Fapy•dG
N-(2-deoxy-D-pentofuranosyl)-N-(2,6-diamino-4-hydroxy-5-formamidopyrimidine)
- G8-oxo and GFapy
respectively, represent the bases of 8-oxodGuo and Fapy•dG
- pol
DNA polymerase
- TLS
translesion synthesis
- HEK
human embryonic kidney
- MF
mutation frequency
- NC
negative control
Footnotes
Notes
The authors declare no competing financial interests.
Mutation data of 8-oxodGuo and Fapy•dG in TG*N sequences in HEK293T cells (Table S1) and in pol λ knockdown cells (Table S2), and RT-PCR efficiency of pol λ knockdown (Figure S1). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA Damage and Disease: Induction, Repair and Significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.van Loon B, Markkanen E, Hubscher U. Oxygen as a Friend and Enemy: How to Combat the Mutational potential of 8-Oxo-guanine. DNA Repair (Amst) 2010;9:604–616. doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Chatgilialoglu C, D‚Angelantonio M, Kciuk G, Bobrowski K. New Insights into the Reaction Paths of Hydroxyl Radicals with 2′-Deoxyguanosine. Chem Res Toxicol. 2011;24:2200–2206. doi: 10.1021/tx2003245. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg MM. The Formamidopyrimidines: Purine Lesions Formed in Competition with 8-Oxopurines from Oxidative Stress. Acc Chem Res. 2012;45:588–597. doi: 10.1021/ar2002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood ML, Esteve A, Morningstar ML, Kuziemko GM, Essigmann JM. Genetic Effects of Oxidative DNA Damage: Comparative Mutagenesis of 7,8-Dihydro-8-Oxoguanine and 7,8-Dihydro-8-Oxoadenine in Escherichia coli. Nucleic Acids Res. 1992;20:6023–6032. doi: 10.1093/nar/20.22.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriya M. Single-Stranded Shuttle Phagemid for Muta-genesis Studies in Mammalian Cells: 8-Oxoguanine in DNA Induces Targeted G.C-->T.A Transversions in Simian Kidney Cells. Proc Natl Acad Sci U S A. 1993;90:1122–1126. doi: 10.1073/pnas.90.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patro JN, Wiederholt CJ, Jiang YL, Delaney JC, Essigmann JM, Greenberg MM. Studies on the Replication of the Ring Opened Formamidopyrimidine, Fapy, dG in Escherichia coli. Biochemistry. 2007;46:10202–10212. doi: 10.1021/bi700628c. [DOI] [PubMed] [Google Scholar]
- 8.Kalam MA, Haraguchi K, Chandani S, Loechler EL, Moriya M, Greenberg MM, Basu AK. Genetic Effects of Oxidative DNA Damages: Comparative Mutagenesis of the Imidazole Ring-Opened Formamidopyrimidines (Fapy Lesions) and 8-Oxo-Purines in Simian Kidney Cells. Nucleic Acids Res. 2006;34:2305–2315. doi: 10.1093/nar/gkl099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAuley-Hecht KE, Leonard GA, Gibson NJ, Thomson JB, Watson WP, Hunter WN, Brown T. Crystal Structure of a DNA Duplex Containing 8-Hydroxydeoxyguanine-Adenine Base Pairs. Biochemistry. 1994;33:10266–10270. doi: 10.1021/bi00200a006. [DOI] [PubMed] [Google Scholar]
- 10.Gehrke TH, Lischke U, Gasteiger KL, Schneider S, Arnold S, Muller HC, Stephenson DS, Zipse H, Carell T. Unexpected Non-Hoogsteen-Based Mutagenicity Mechanism of FaPy-DNA Lesions. Nature chemical biology. 2013;9:455–461. doi: 10.1038/nchembio.1254. [DOI] [PubMed] [Google Scholar]
- 11.D’Errico M, Parlanti E, Dogliotti E. Mechanism of Oxidative DNA Damage Repair and Relevance to Human Pathology. Mutat Res. 2008;659:4–14. doi: 10.1016/j.mrrev.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Hoeijmakers JH. DNA Damage, Aging, and Cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 13.Einolf HJ, Guengerich FP. Fidelity of Nucleotide Insertion at 8-Oxo-7,8-Dihydroguanine by Mammalian DNA Polymerase Delta. Steady-State and Pre-Steady-State Kinetic Analysis. J Biol Chem. 2001;276:3764–3771. doi: 10.1074/jbc.M006696200. [DOI] [PubMed] [Google Scholar]
- 14.Maga G, Villani G, Crespan E, Wimmer U, Ferrari E, Bertocci B, Hubscher U. 8-Oxo-Guanine Bypass by Human DNA Polymerases in the Presence of Auxiliary Proteins. Nature. 2007;447:606–608. doi: 10.1038/nature05843. [DOI] [PubMed] [Google Scholar]
- 15.Haracska L, Yu SL, Johnson RE, Prakash L, Prakash S. Efficient and Accurate Replication in the Presence of 7,8-Dihydro-8-Oxoguanine by DNA Polymerase eta. Nature Genetics. 2000;25:458–461. doi: 10.1038/78169. [DOI] [PubMed] [Google Scholar]
- 16.Haracska L, Prakash S, Prakash L. Yeast DNA Polymerase ζ is an Efficient Extender of Primer Ends Opposite from 7,8-Dihydro-8-Oxoguanine and O-6-Methylguanine. Molecular and Cellular Biology. 2003;23:1453–1459. doi: 10.1128/MCB.23.4.1453-1459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Page F, Guy A, Cadet J, Sarasin A, Gentil A. Repair and Mutagenic Potency of 8-OxoG:A and 8-OxoG:C Base Pairs in Mammalian Cells. Nucleic Acids Res. 1998;26:1276–1281. doi: 10.1093/nar/26.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DH, Pfeifer GP. Translesion Synthesis of 7,8-Dihydro-8-Oxo-2′-Deoxyguanosine by DNA Polymerase eta in vivo. Mutat Res. 2008;641:19–26. doi: 10.1016/j.mrfmmm.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avkin S, Livneh Z. Efficiency, Specificity and DNA Polymerase-Dependence of Translesion Replication Across the Oxidative DNA Lesion 8-Oxoguanine in Human Cells. Mutat Res. 2002;510:81–90. doi: 10.1016/s0027-5107(02)00254-3. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya H, Yamaguchi A, Suzuki T, Harashima H. Roles of Specialized DNA Polymerases in Mutagenesis by 8-Hydroxyguanine in Human Cells. Mutat Res. 2010;686:90–95. doi: 10.1016/j.mrfmmm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Picher AJ, Blanco L. Human DNA Polymerase Lambda is a Proficient Extender of Primer Ends Paired to 7,8-Dihydro-8-Oxoguanine. DNA Repair (Amst) 2007;6:1749–1756. doi: 10.1016/j.dnarep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Maga G, Crespan E, Wimmer U, van Loon B, Amoroso A, Mondello C, Belgiovine C, Ferrari E, Locatelli G, Villani G, Hubscher U. Replication Protein A and Proliferating Cell Nuclear Antigen Coordinate DNA Polymerase Selection in 8-Oxo-Guanine Repair. Proc Natl Acad Sci U S A. 2008;105:20689–20694. doi: 10.1073/pnas.0811241106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Loon B, Hubscher U. An 8-Oxo-Guanine Repair Pathway Coordinated by MUTYH Glycosylase and DNA Polymerase Lambda. Proc Natl Acad Sci U S A. 2009;106:18201–18206. doi: 10.1073/pnas.0907280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markkanen E, Castrec B, Villani G, Hubscher U. A Switch Between DNA Polymerases Delta and Lambda Promotes Error-Free Bypass of 8-Oxo-G Lesions. Proc Natl Acad Sci U S A. 2012;109:20401–20406. doi: 10.1073/pnas.1211532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pande P, Malik CK, Bose A, Jasti VP, Basu AK. Mutational Analysis of the C8-Guanine Adduct of the Environmental Carcinogen 3-Nitrobenzanthrone in Human Cells: Critical Roles of DNA Polymerases eta and kappa and Rev1 in Error-Prone Translesion Synthesis. Biochemistry. 2014;53:5323–5331. doi: 10.1021/bi5007805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weerasooriya S, Jasti VP, Basu AK. Replicative Bypass of Abasic Site in Escherichia coli and Human Cells: Similarities and Differences. PloS one. 2014;9:e107915. doi: 10.1371/journal.pone.0107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beard WA, Batra VK, Wilson SH. DNA Polymerase Structure-Based Insight on the Mutagenic Properties of 8-Oxoguanine. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010;703:18–23. doi: 10.1016/j.mrgentox.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earley LF, Minko IG, Christov PP, Rizzo CJ, Lloyd RS. Mutagenic Spectra Arising from Replication Bypass of the 2,6-Diamino-4-Hydroxy-N(5)-Methyl Formamidopyrimidine Adduct in Primate Cells. Chem Res Toxicol. 2013;26:1108–1114. doi: 10.1021/tx4001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.