Summary
It is well accepted that aging is an important contributing factor to the development of osteoarthritis (OA). The mechanisms responsible appear to be multifactorial and may include an age-related pro-inflammatory state that has been termed “inflamm-aging.” Age-related inflammation can be both systemic and local. Systemic inflammation can be promoted by aging changes in adipose tissue that result in increased production of cytokines such as interleukin (IL)-6 and TNFα. Numerous studies have shown an age-related increase in blood levels of IL-6 that has been associated with decreased physical function and frailty. Importantly, higher levels of IL-6 have been associated with an increased risk of knee OA progression. However, knockout of IL-6 in male mice resulted in worse age-related OA rather than less OA. Joint tissue cells, including chondrocytes and meniscal cells, as well as the neighboring infrapatellar fat in the knee joint, can be a local source of inflammatory mediators that increase with age and contribute to OA. An increased production of pro-inflammatory mediators that include cytokines and chemokines, as well as matrix degrading enzymes important in joint tissue destruction, can be the result of cell senescence and the development of the senescence-associated secretory phenotype. Further studies are needed to better understand the basis for inflamm-aging and its role in OA with the hope that this work will lead to new interventions targeting inflammation to reduce not only joint tissue destruction but also pain and disability in older adults with OA.
Keywords: osteoarthritis, chondrocyte, cartilage, senescence, cytokines, growth factors
Introduction
Aging is one of the most prominent risk factors for osteoarthritis (OA). Numerous studies have documented an increase in radiographic and symptomatic hand, hip, spine, and knee OA with increasing age (reviewed in1). Due to its prevalence, OA is a major cause of pain and disability in older adults2. Given the recent and dramatic increase in the older adult population in developed countries, the discovery of interventions that target the aging aspects of OA would have significant public health impact. This will require a better mechanistic understanding of how aging contributes to the development of OA, including the role of age-related inflammation.
Lopez-Otin et al3 have recently proposed nine candidate hallmarks of aging that include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Several of these processes contribute to the aging phenotype by promoting a low-grade pro-inflammatory state that will be discussed further below. The emergence of evidence to support a role for low-grade chronic inflammation in aging and age-related conditions suggests that inflammation could be an important link between aging and OA.
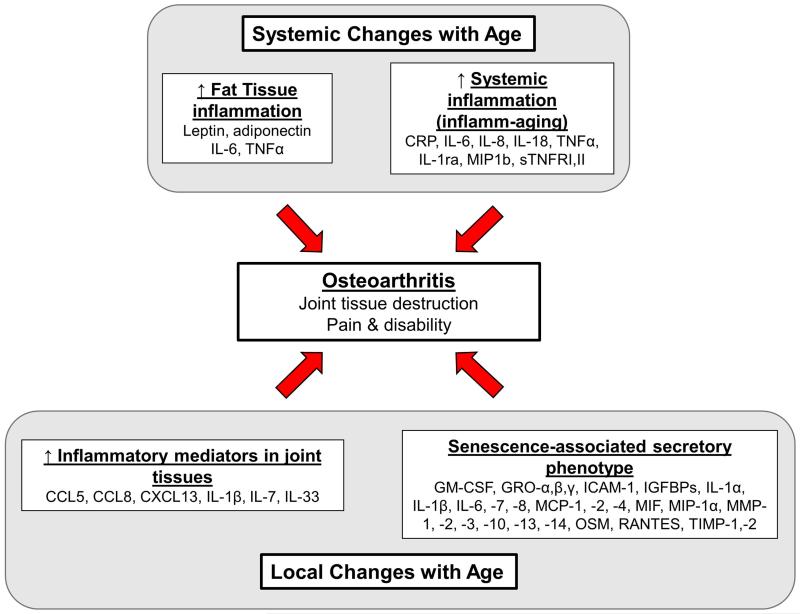
In this review, we will discuss various mechanisms by which an age-related pro-inflammatory state may contribute to the development of OA. OA is a clearly not just aging of the joints but rather is a multifactorial condition with a number of risk factors including joint injury, obesity, abnormal mechanics, genetics, gender, and so on. We would postulate that factors related to aging that promote both a systemic and a local pro-inflammatory state do not directly cause OA but rather serve as contributing factors to the development and progression of the disease as well as to increased pain and reduced physical function (Fig.1).
Fig. 1.
Potential age-related changes that increase systemic and local inflammation and promote the development of osteoarthritis. Systemic levels of multiple pro-inflammatory mediators, in particular IL-6 and TNF-α, increase with age due to production by adipose tissue which becomes more inflammatory with age and due to a chronic low grade pro-inflammatory state that has been termed “inflamm-aging”. Local changes within the joint have been associated with increased production of inflammatory mediators as has cell senescence through development of the senescence-associated secretory phenotype.
Aging and Systemic Inflammation
Studies demonstrating an increase with age in blood levels of C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-α (TNF-α) have been interpreted as evidence that age-related, chronic, systemic, low-grade inflammation contributes to the development of chronic diseases of aging, including dementia and cardiovascular disease4-7. The strongest association of these inflammatory markers with age-related disease, as well as with a decline in physical function, has been seen with IL-68. Epidemiologic studies have also shown a link between systemic markers of inflammation, including IL-6, and OA. Elevated levels of CRP and IL-6 were found in people with OA of the knee and levels of these pro-inflammatory markers were related to risk of progression9, 10. Likewise, a study of a cohort of 90-year-olds found an association between the absence of knee OA and lower production of IL-6 when whole-blood samples were stimulated with lipopolysaccharide11.
Levels of systemic markers of inflammation have been shown to correlate with pain and function in older adults with knee OA. Stannus et al12 noted an increase in serum TNF-α and high sensitivity(hs)-CRP corresponding to increased knee pain over a five year study, and Penninx et al13 reported that high levels of the soluble receptors for TNF-α correlated with decreased physical ability in older adults with knee OA. Although higher levels of TNF-α soluble receptors may seem to represent an increase in anti-inflammatory activity, previous studies have suggested they are a more stable marker of increased TNF-α activity than measures of TNF-α in serum which can be quite labile. Thus systemic inflammation in older adults with OA may not only be a factor associated with progression of OA but also with pain and physical function.
Franceschi et al14 coined the term “inflamm-aging” to describe the pro-inflammatory state that occurs with increasing age. Inflamm-aging was originally proposed to be the result of an accumulation over time of an increased “antigenic load” and the combined effects from various stressors on the immune system that resulted in immunosenescence. The macrophage was proposed to play a central role as a pro-inflammatory mediator in this process. In addition to immunosenescence, subsequent work has tied inflamm-aging to age-related endocrine, metabolic, and nutritional changes that also promote a pro-inflammatory state. These include an increase in fat mass that can be a source of pro-inflammatory mediators, increasing levels of reactive oxygen species that promote metabolic changes as well as increased activity of inflammatory mediators, and changes in levels of specific micro-RNAs that are involved in the regulation of the NFκB family of pro-inflammatory mediators15-17.
Inflamm-aging appears to be much more complex than just an age-related increase in production of pro-inflammatory mediators. Morrisette-Thomas et al18 examined levels of 19 inflammatory markers from an ongoing longitudinal study of older adults in Italy called the InCHIANTI study. They reported a significant age-related increase in pro-inflammatory markers, including hsCRP, IL-6, IL-15, IL-18 and macrophage inflammatory protein-1b (MIP-1b) while the anti-inflammatory cytokine IL-10 was found to decrease as age increased. However, they also noted an increase with age in several anti-inflammatory markers including IL-1 receptor antagonist (IL-1RA), soluble glycoprotein 130 (prevents IL-6 from binding to its membrane receptor), and soluble TNF receptors I and II (sTNFRI and sTNFRII).
In order to determine which of the inflammatory markers explained the most variance in the data and to examine the relationship of these groups of markers to age and chronic diseases they used principal components analysis (PCA). Two groups of inflammatory markers explained 19% and 10% of the total variance among all the markers. The first group (PCA1) included sTNF-R1, sTNF-RII, IL-6, TNF-α, hsCRP, IL-18, and IL1-RA and increased levels showed a strong correlation with age (r=0.56, p<0.0001). This group was also predictive of mortality and correlated with a number of chronic disease states. The second group (PCA2), which contained markers associated with innate immune system activation (MCP, IL-8 and IL-12), was weakly correlated with age (r=0.08, p=0.053) but was more strongly associated with chronic diseases that included arthritis. Unexpectedly, they found that higher levels of the markers in the second group were actually protective against chronic diseases, including arthritis which had an odds ratio of 0.74 (95%CI, 0.58-0.93) per unit change for the scores in PCA2. The authors suggested that an age-related increase in inflammation may stimulate production of anti-inflammatory factors and the balance of these factors in a given individual will determine their susceptibility to age-related diseases. Of potential relevance, male mice with a deletion of the IL-6 gene were found to have more severe age-related OA compared to age-matched wild-type controls rather than less severe OA as might be expected19. Clearly more work needs to be done in order to better understand the relationship between biomarkers of inflammation and age-related disease with the understanding that the balance of both pro-inflammatory and anti-inflammatory mediators is important to consider.
Aging and the Senescence-associated Secretory Phenotype
One of the mechanisms by which aging promotes chronic inflammation that could be important in OA is through cell senescence. Cell senescence was originally used to describe the state of growth arrest that occurred after extensive proliferation of fibroblasts induced by multiple passages in culture20. This form of replicative senescence has been thought to serve as a mechanism to prevent unlimited proliferation of cells that would lead to cancer. Various markers have been used to identify senescent cells, including senescence-associated β-galactosidase (SA-β-gal), increased expression of the cyclin-dependent kinase inhibitor p16INK4a, and formation of senescence-associated heterochromatin (reviewed in21). Cell senescence is not just a phenomenon observed with cultured cells but rather senescent cells have been shown to accumulate in various tissues with aging21.
There is surprisingly little in situ data to show that articular chondrocytes senesce in normal articular cartilage while there is evidence for senescence in OA cartilage. Price et al22 compared normal cartilage from older adults with hip fractures to OA cartilage removed during arthroplasty and found SA-β-gal staining only in the OA samples. In contrast, Martin and Buckwalter23 did find evidence of SA-β-gal and decreased telomere length with age in cultured chondrocytes from normal joints. Others have also shown evidence of cell senescence in OA cartilage including a reduction in telomere length and increased DNA damage24, 25 as well as an increase in p16INK4a26. Chondrocytes do not normally proliferate in the articular cartilage of adults27 making it unlikely that chondrocyte senescence would be the result of multiple cycles of cell proliferation. However, cell proliferation is not a requirement for cell senescence which can also occur after repetitive stress to tissues and cells28. This mechanism would be very relevant to the chronic repetitive loading experienced by joint tissues and the excessive loading associated with the development of OA which may explain why evidence of chondrocyte senescence has been detected in OA tissue.
In addition to growth arrest, senescent cells exhibit a number of other features that include secretion of pro-inflammatory cytokines, chemokines, growth factors, and matrix metalloproteinases (MMPs) which has been referred to as the senescence-associated secretory phenotype (SASP)29, 30. The SASP could promote age-related pathologies, such as osteoarthritis, by increasing local levels of pro-inflammatory mediators and matrix degrading enzymes. Freund et al31 composed a list of 83 SASP factors that had been reported in the literature in studies examining various inducers of cell senescence. The factors were divided by the level of increase from high (>4 fold) to intermediate (2-4 fold) to small (<2 fold). Interestingly, all of the SASP factors produced at high levels by senescent cells have been found in OA tissues and/or synovial fluid. These include GM-CSF, GROα,β,γ, IGFBP-7, IL-1α, IL-6, IL-7, IL-8, MCP-1, MCP-2, MIP1α, MMP-1, MMP-10, and MMP-332-38. Many of the SASP factors present at intermediate levels, such as ICAM-1, IL-1β, MCP-4, MIF, MMP-13, oncostatin M, RANTES, and TIMP-2 have also been recognized as potential mediators in OA32, 35, 39-41.
Several recent studies have used a proteomics approach to describe the secreted proteins or “secretome” produced by either chondrocytes or cartilage explants from human OA joints42-44 or from canine and equine cartilage explants stimulated with IL-1β45, 46. Some of the proteins identified in one or more of these studies are also on the list of SASP proteins including GROα, IGFBP-7, IL-8, MMP-1, MMP-2, MMP-3, MMP-14, IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5, IGFBP-6, TIMP-1 and TIMP-2. Although these 14 proteins only suggest a partial overlap of the chondrocyte secretome with the list of 83 SASP factors noted above, it should be noted that untargeted proteomics analysis tends to find the more abundant proteins in samples and would be less sensitive in detecting many of the SASP factors such as cytokines, chemokines and growth factors. Further studies are needed using more sensitive assays such as ELISAs or multiplex cytokine analysis to determine the degree of overlap between the factors produced by OA chondrocytes and those considered to be representative of the SASP.
The mechanisms responsible for cell senescence and in particular for the SASP are still being elucidated but appear to include DNA damage and activation of the p38 MAP kinase47. Another consistent finding has been expression of p16INK4a that activates the pRB tumor suppressor and promotes formation of senescence-associated heterochromatin foci which function to silence genes regulating cell proliferation28. Deletion of cells expressing p16INK4a in mice was found to prevent the development of age-related changes in tissues that included muscle, adipose tissue, and the eye48. As detailed above, chondrocytes in OA have been found to have evidence of DNA damage that could promote the development of a senescent phenotype24 and have been shown to have increased expression of p16INK4a26. Knockdown of p16INK4a in cultured OA chondrocytes restored matrix gene expression26. Studies of the role of p16INK4a in promoting the SASP in chondrocytes are warranted and would increase the understanding of the mechanisms by which chondrocyte senescence contributes to a pro-inflammatory state in cartilage.
Obesity, Adiposity, and Inflammaging
Obesity is a risk factor for OA due to the combined effects of abnormal joint loading and increased production of adipokines and other inflammatory mediators from fat depots throughout the body49, 50. As people age, there is a decrease in muscle mass and increase in fat mass51 which could contribute both to mechanical stress from abnormal joint loading and to an increase in inflammatory mediators produced by the increased fat mass. Age-related inflammation in adipose tissue has been attributed to senescence of pre-adipocytes resulting in adipocytes exhibiting the SASP, as well as from an influx of activated macrophages due to the release of cytokines and chemokines from stressed pre-adipocytes52.
The contribution of fat to systemic levels of pro-inflammatory mediators and adipokines was investigated by Messier et al 53 in the IDEA trial where overweight and obese older adults with symptomatic knee OA were randomly assigned to one of three intervention groups: intensive diet-induced weight loss and exercise, intensive diet-induced weight loss alone, or exercise alone. At the conclusion of the 18-month study, there were more significant decreases in circulating IL-6 levels and in total fat mass in participants assigned to the diet+exercise and diet groups than the exercise group. Regardless of intervention group, study participants who lost at least 10% of their body weight saw a greater reduction in knee compressive force, circulating IL-6, and pain than those who did not achieve the 10% weight loss goal. The connection between a decrease in fat mass and a corresponding decrease in circulating IL-6 support the role of obesity-related systemic inflammation in osteoarthritis.
Additional studies from the same group have shown that in overweight and obese older adults, the combination of diet-induced weight loss and physical activity reduced circulating leptin and IL-6 more than physical activity alone54, and that adiposity is the primary factor that explains poor physical performance in older adults with metabolic syndrome55. While the latter study did not focus on OA, the studies to date do provide supporting evidence for an effect of systemic inflammation caused by obesity in older adults.
In addition to a role in promoting systemic inflammation, fat tissue can also have local effects. The infrapatellar fat pad is a potential local source of adipokines and inflammatory mediators within the knee joint. Distel et al56 showed in a group of obese women with knee OA that the infrapatellar fat pad is a source of adipokines (adiponectin and leptin) as well as inflammatory mediators (IL-6 and sIL-6R). Relative to aging, Chuckpaiwong et al57 described a correlation between volume of the infrapatellar fat pad and the age of OA subjects where the size of the infrapatellar fat pad increased over the course of a 12 month study. Another group found that treatment of human articular chondrocytes with recombinant leptin or white adipose tissue (WAT) conditioned media containing leptin increased expression and activation of MMPs and stimulated collagen release from explant cultures58. The WAT samples were isolated from the infrapatellar fat pad of human OA knee joints suggesting that leptin and other factors produced by the infrapatellar fat pad may contribute to cartilage degradation in knee OA.
Age-related Changes in Inflammatory Mediators in Joint Tissues
In addition to aging fat, joint tissues including the cartilage and meniscus can be a source of pro-inflammatory mediators. In an animal study comparing gene expression in joint tissues from 12-week old mice and 12-month old mice undergoing either the destabilization of the medial meniscus (DMM) or sham surgery, Loeser et al59 described 493 genes that were differentially regulated between the young and old DMM groups, and 861 genes that were differentially expressed between young and old sham groups. Of note from the group of genes showing age-related differences, expression of IL-33 and three chemokines, CXCL13, CCL8, and CCL5, were significantly up-regulated in the old sham group compared to the young sham group.
Interleukin 7 (IL-7) is another cytokine that is up-regulated with age. Long et al34 found that human articular chondrocytes in monolayer culture from older donors release higher levels of IL-7 than young donors, and that OA chondrocytes release higher levels of IL-7 than age-matched normal donors. Chondrocytes respond to IL-7 treatment by increased levels of MMP-13 and increased proteoglycan release from cartilage explants. Rubenhagen et al60 also noted an age-related increase in IL-7 in synovial fluid samples from a cohort of 82 patients with varying degrees of OA. Likewise, there is evidence for an increase in production of IL-1β by cultured chondrocytes from older tissue donors61. In contrast, Peffers et al62 found a decrease in the RNA levels of IL-1β as well as IL-8 when comparing metacarpalphalangeal cartilage from young and old horses.
Recent studies have shown that the meniscus may be a source of inflammatory mediators in the joint. However, Brophy et al63 reported that the pro-inflammatory cytokine IL-1β and the matrix-degrading enzymes ADAMTS-5, MMP-1, MMP-9, and MMP-13 were more highly expressed in meniscal tissue isolated from young subjects (<40 years old) than from older subjects (>40). These changes may be due to an early inflammatory response promoting repair in young subjects while a low-grade chronic inflammatory response inhibits repair in older subjects. Whether similar soft-tissue structures such as ligaments in the knee also have increased production of inflammatory mediators with age would be of interest to investigate as would age-related changes in synovial tissue and the joint capsule.
Conclusions
There is epidemiologic as well as biologic evidence to suggest a link between age-related inflammation or “inflamm-aging” and the development of OA. The sources of age-related pro-inflammatory mediators that might contribute to OA include both peripheral sources, such as adipose tissue that increases with age, as well as local production within joint tissues. Although cell senescence and the development of the senescence-associated secretory phenotype serves as an attractive mechanism linking aging, inflammation, and OA, there is insufficient evidence that this occurs with normal aging in joint tissues. Excessive mechanical loading of the joint that is severe enough to lead to OA may result in stress-induced senescence and increased production of pro-inflammatory mediators but whether this phenotype occurs in normal aged joints that have not experienced excessive loading is less clear.
Given that OA is a chronic disease that becomes more prevalent with age, it is difficult to separate effects of age from disease, particularly in studies of human tissues. It seems unlikely that any single pro-inflammatory mediator plays a key role in linking aging and OA. At least in male mice, deletion of IL-6, the cytokine that has been perhaps most closely related to aging, resulted in more severe rather than less severe OA19. Future studies will need to examine the balance of multiple pro-inflammatory and anti-inflammatory factors in order to better understand mechanisms by which this balance is disrupted in aging. Targeting these mechanisms and restoring a proper balance may help to slow or stop the progression of age-related chronic conditions including OA.
Acknowledgements
We would like to acknowledge the contributions of the many lab members and collaborators who have contributed to improving our understanding of the contributions of aging to the development of OA.
Funding
National Institute on Aging [RO1 AG044034 and R21 AG044185 (RFL), F31 AG046990 (MAG)], National Institute of Arthritis, Musculoskeletal and Skin Disease [R37 AR049003 (RFL)] and the Herman and Louise Smith Professorship. The study sponsors had no role in the study design; in the acquisition, analysis, or interpretation of data; in drafting the manuscript; or in the decision to submit the manuscript to OAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Both MAG and RFL reviewed the literature and drafted the manuscript. RFL made final revisions and both authors approved the final manuscript.
Competing Interest Statement
The authors declare no conflicts of interest.
References
- 1.Anderson SA, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation --- United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1261–1265. [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 5.Strandberg TE, Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–1060. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- 6.Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002;13:389–391. [PubMed] [Google Scholar]
- 7.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 8.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 10.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009;60:2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goekoop RJ, Kloppenburg M, Kroon HM, Frolich M, Huizinga TW, Westendorp RG, et al. Low innate production of interleukin-1beta and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthritis Cartilage. 2010;18:942–947. doi: 10.1016/j.joca.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72:535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 13.Penninx BW, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP, et al. Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol. 2004;31:2027–2031. [PubMed] [Google Scholar]
- 14.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 15.Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9:228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- 16.Olivieri F, Rippo MR, Monsurro V, Salvioli S, Capri M, Procopio AD, et al. MicroRNAs linking inflamm-aging, cellular senescence and cancer. Ageing Res Rev. 2013;12:1056–1068. doi: 10.1016/j.arr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Calcada D, Vianello D, Giampieri E, Sala C, Castellani G, de Graaf A, et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech Ageing Dev. 2014;136-137:138–147. doi: 10.1016/j.mad.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Morrisette-Thomas V, Cohen AA, Fulop T, Riesco E, Legault V, Li Q, et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139:49–57. doi: 10.1016/j.mad.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Hooge AS, van de Loo FA, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage. 2005;13:66–73. doi: 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 21.Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 23.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 24.Rose J, Soder S, Skhirtladze C, Schmitz N, Gebhard PM, Sesselmann S, et al. DNA damage, discoordinated gene expression and cellular senescence in osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2012;20:1020–1028. doi: 10.1016/j.joca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Harbo M, Bendix L, Bay-Jensen AC, Graakjaer J, Soe K, Andersen TL, et al. The distribution pattern of critically short telomeres in human osteoarthritic knees. Arthritis Res Ther. 2012;14:R12. doi: 10.1186/ar3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou HW, Lou SQ, Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology (Oxford) 2004;43:555–568. doi: 10.1093/rheumatology/keh127. [DOI] [PubMed] [Google Scholar]
- 27.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Ceuninck F, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323:960–969. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- 33.Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43:1734–1741. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Long D, Blake S, Song XY, Lark M, Loeser RF. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthritis Res Ther. 2008;10:R23. doi: 10.1186/ar2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Liu C, Zhang Y, Hao Y, Zhang X, Zhang YM. Protein interaction and microRNA network analysis in osteoarthritis meniscal cells. Genet Mol Res. 2013;12:738–746. doi: 10.4238/2013.March.13.2. [DOI] [PubMed] [Google Scholar]
- 37.Clutterbuck AL, Smith JR, Allaway D, Harris P, Liddell S, Mobasheri A. High throughput proteomic analysis of the secretome in an explant model of articular cartilage inflammation. J Proteomics. 2011;74:704–715. doi: 10.1016/j.jprot.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker N, Sharpe P, Culley K, Otero M, Bevan D, Newham P, et al. Dual regulation of metalloproteinase expression in chondrocytes by Wnt-1-inducible signaling pathway protein 3/CCN6. Arthritis Rheum. 2012;64:2289–2299. doi: 10.1002/art.34411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alaaeddine N, Olee T, Hashimoto S, Creighton-Achermann L, Lotz M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001;44:1633–1643. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Li WQ, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol. 2001;166:3491–3498. doi: 10.4049/jimmunol.166.5.3491. [DOI] [PubMed] [Google Scholar]
- 41.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peffers MJ, Beynon RJ, Clegg PD. Absolute quantification of selected proteins in the human osteoarthritic secretome. Int J Mol Sci. 2013;14:20658–20681. doi: 10.3390/ijms141020658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenberg J, Ruetschi U, Skioldebrand E, Karrholm J, Lindahl A. Quantitative proteomics reveals regulatory differences in the chondrocyte secretome from human medial and lateral femoral condyles in osteoarthritic patients. Proteome Sci. 2013;11:43. doi: 10.1186/1477-5956-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lourido L, Calamia V, Mateos J, Fernandez-Puente P, Fernandez-Tajes J, Blanco FJ, et al. Quantitative proteomic profiling of human articular cartilage degradation in osteoarthritis. J Proteome Res. 2014;13:6096–6106. doi: 10.1021/pr501024p. [DOI] [PubMed] [Google Scholar]
- 45.Swan AL, Hillier KL, Smith JR, Allaway D, Liddell S, Bacardit J, et al. Analysis of mass spectrometry data from the secretome of an explant model of articular cartilage exposed to pro-inflammatory and anti-inflammatory stimuli using machine learning. BMC Musculoskelet Disord. 2013;14:349. doi: 10.1186/1471-2474-14-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams A, Smith JR, Allaway D, Harris P, Liddell S, Mobasheri A. Carprofen inhibits the release of matrix metalloproteinases 1, 3, and 13 in the secretome of an explant model of articular cartilage stimulated with interleukin 1beta. Arthritis Res Ther. 2013;15:R223. doi: 10.1186/ar4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011 doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine. 2013;80:568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beavers KM, Ambrosius WT, Nicklas BJ, Rejeski WJ. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J Am Geriatr Soc. 2013;61:1089–1094. doi: 10.1111/jgs.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beavers KM, Hsu FC, Houston DK, Beavers DP, Harris TB, Hue TF, et al. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013;68:617–623. doi: 10.1093/gerona/gls213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009;60:3374–3377. doi: 10.1002/art.24881. [DOI] [PubMed] [Google Scholar]
- 57.Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J Orthop Res. 2010;28:1149–1154. doi: 10.1002/jor.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–462. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 59.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64:705–717. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubenhagen R, Schuttrumpf JP, Sturmer KM, Frosch KH. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 2012;83:59–64. doi: 10.3109/17453674.2011.645195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forsyth CB, Cole A, Murphy G, Bienias JL, Im HJ, Loeser RF., Jr. Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci. 2005;60:1118–1124. doi: 10.1093/gerona/60.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peffers M, Liu X, Clegg P. Transcriptomic signatures in cartilage ageing. Arthritis Res Ther. 2013;15:R98. doi: 10.1186/ar4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am. 2012;94:385–393. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]