Abstract
Aging and age-related diseases are one of the most important health issues that the world will confront during the 21st century. Only by understanding the proximal causes will we be able to find treatments to reduce or delay the onset of degenerative diseases associated with aging. Currently, the prevalent paradigm in the field is the accumulation of damage. However, a new theory that proposes an alternative explanation is gaining momentum. The hyperfunction theory proposes that aging is not a consequence of a wear and tear process, but a result of the continuation of developmental programs during adulthood. Here we use Drosophila melanogaster, where evidence supporting both paradigms has been reported, to identify which parameters that have been previously related with lifespan best predict the rate of aging in wild type flies cultured at different temperatures. We find that mitochondrial function and mitochondrial reactive oxygen species (mtROS) generation correlates with metabolic rate, but not with the rate of aging. Importantly, we find that activation of nutrient sensing pathways (i.e. insulin-PI3K/Target of rapamycin (Tor) pathway) correlates with lifespan, but not with metabolic rate. Our results, dissociate metabolic rate and lifespan in wild type flies and instead link nutrient sensing signaling with longevity as predicted by the hyperfunction theory.
Keywords: aging, damage theories, hyperfunction, mitochondria, Target of rapamycin
Introduction
Aging is a complex process that causes a progressive deterioration of the organism and reduces its capacity to manage stress. Because of its debilitating nature, aging is one of the main problems society faces during the 21st century. The only way to delay or reverse the onset of age-related diseases is to understand its proximal causes. Hundreds of theories have been proposed to explain aging,1 but few of them have stood the test of time. Nowadays, the dominant paradigm is that a progressive accumulation of damage causes aging. The most prominent example of this paradigm is the Mitochondrial Free Radical Theory of Aging (MFRTA) that provides a logical mechanistic explanation and has shown remarkable resilience in adapting to criticism and conflicting results. MFRTA posits that aging is a consequence of the accumulation of oxidative damage caused by mitochondrial reactive oxygen species (ROS).2 Mainly support for MFRTA comes from descriptive data,3,4 with less support coming from experimental evidence.5 For example, long-lived animals, in general are characterized by low levels of ROS and membranes resistant to oxidation.3 Unsaturated membranes are more sensitive to oxidation 6,7 and a reduction in unsaturation has been shown to extend the lifespan of C. elegans 8 in accordance with the free radical theory. However, depletion of all the superoxide dismutases (SODs) increases oxidative stress, but does not shorten lifespan in C. elegans.9 Similarly, heterozygous mutant mice for superoxide dismutase 2 (Sod2) “suffer” higher levels of oxidative damage, but have no reduction in lifespan.10
There are 3 different possibilities to explain these results: (i) oxidative damage is not properly assessed, (ii) the damage which causes aging is not oxidative or (iii) aging is not a consequence of damage accumulation.11 In line with this last possibility, a new hypothesis to explain aging independently of damage accumulation is gaining momentum. It proposes that aging is a later consequence of the growth program initiated during development.12 Therefore hyperfunction, a consequence of the activation of Target of rapamycin beyond the point where growth has concluded, would cause the degenerative processes which result in aging. Supporting this idea, aging is better explained by hyperfunction than damage accumulation in C. elegans13 and it has been demonstrated that pharmacological inhibition of Tor extends lifespan in yeast, worms, flies and mammals.14-17 Interestingly, rapamycin extends lifespan even when administered at old ages18 or in obese mice,19 indicating that inhibitors of Tor may be an option to extend health-span in humans.
Drosophila melanogaster is probably the model organism where most experimental support for MFRTA has been accumulated (reviewed in20). For instance, in Drosophila it has been shown that depletion of antioxidants shortens lifespan, and overexpression of some of these antioxidants extends lifespan.21,22 However, many other data do not support MFRTA. High levels of protein damage (including oxidative damage) do not shorten lifespan,23 low levels of ROS do not delay aging24 and reduced mitochondrial function increases ROS but prolongs lifespan.25 A recurrent explanation for these and other contradictory data is that these models are non-physiological, i.e., these models do not represent aging in wild type individuals.
Here we study parameters that have been proposed to regulate aging using wild type flies cultured at different temperatures. Several factors make this model appropriate: firstly, temperature is the most important modulator of Drosophila lifespan, and changes in temperature modify longevity more than 4-fold;26 27 secondly, temperature variation is the most common stress flies confront in the wild forcing them to adapt their metabolic rate; and thirdly it has been proposed that the metabolic and aging rate are connected. The rate of living theory postulates that the faster the metabolism of one species the shorter it will live28 because more damage is generated. However, many important exceptions have been described, such as bats, birds, primates and the naked-mole rat.29 Moreover, when appropriate corrections for body size and phylogeny are introduced into the analysis, the correlation between the metabolic rate and longevity disappears.30 Similarly, metabolic rate fails to predict lifespan in worms,31 fruit flies 32 and mice.33 MFRTA explains these data by arguing that the rate of ROS production, independently of metabolic rate, determines the rate of aging.34 To test this hypothesis, we generated a fly model where aging rate and ROS production can be distinguished. We found that mtROS generation correlated both with mitochondrial respiration and metabolic rate, but not with lifespan. Interestingly, a different picture was observed when we studied the activation of nutrient sensing pathways. We found that activation of components of the Insulin/Insulin-like growth factor signaling (IIS) and Target of rapamycin (Tor) pathways correlated with lifespan, however activation of IIS or Tor did not correlate with metabolic rate. Altogether we show that generation of oxidative damage does not explain the differences in longevity found in wild type individuals, and provide evidence that Tor pathway activation is a better predictor of longevity in fruit flies.
Results
Lifespan does not correlate with metabolic rate in fruit flies cultured at different temperatures
In order to identify parameters that could explain or predict the lifespan of fruit flies, we cultured our wild type Dahomey (DAH) strain at 3 different temperatures: 10, 18 and 29°C and recorded the mean, median and maximum lifespan of the flies (Fig. 1A, Table 1). We observed that metabolic rate, indirectly measured by fly activity and food intake (Fig. 1B, C), positively correlated with temperature (R = 0.55/0.50 P < 0.001), but not with lifespan (R = 0 .05/0.11 P > 0.05). Interestingly, flies lived the longest at 18°C despite moving and eating the least at 10°C. On the other hand, fly weight was lowest at 18°C, the temperature at which flies lived the longest (Fig. 1D) and Tor signaling was attenuated (see below).
Figure 1.
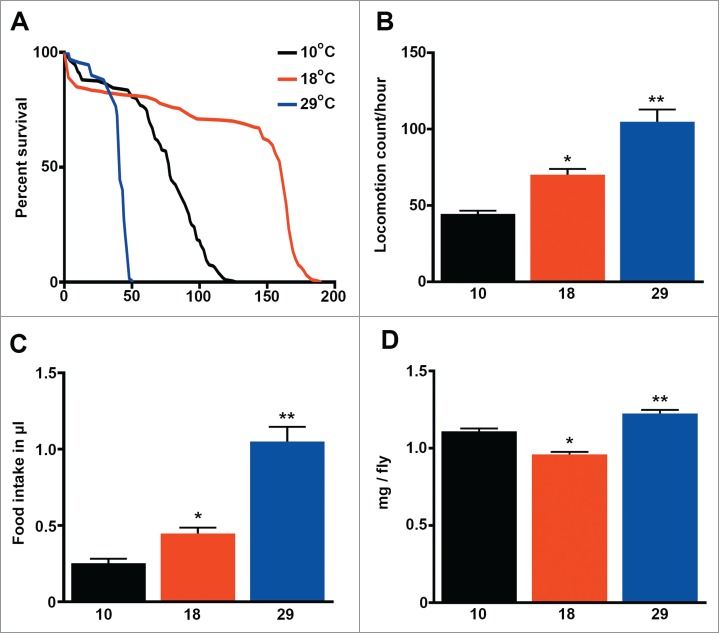
Lifespan does not correlate with the metabolic rate in Drosophila melanogaster. (a) Survival (days) of wild type flies cultured at 3 different temperatures. See details and statistics in Table 1 (n=200 ). (b) Fly activity expressed as the locomotion count per hour per fly (n = 40 ). (c) Food consumption expressed as μl of food ingested per fly (n = 20 ). (d) Weight expressed as mg per fly (n = 9 −10). The mean ±SEM is shown (n indicates independent replicates per group). Different numbers of * indicate statistically significant differences (ANOVA, p < 0.05) between temperatures.
Table 1.
Lifespan of wild type Drosophila melanogaster flies cultured at different temperatures
| Experiment 1 (d) |
Experiment 2 (d) |
Combined (d) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Med | Mean | Max | Med | Mean | Max | Med | Mean | Max | Statistics* |
| 10 | 84 | 78 | 119 | 70 | 67 | 107 | 78 | 73 | 113 | |
| 18 | 164 | 130 | 181 | 155 | 122 | 175 | 162 | 122 | 175 | p<0.001 |
| 29 | 41 | 40 | 46 | 44 | 39 | 49 | 41 | 39 | 49 | p<0.001 |
Analyzed using the Kaplan Meier log-rank test, differences with respect to flies cultured at 10°C.
Next, we measured mitochondrial oxygen consumption in isolated mitochondria using a Clark electrode and found that mitochondrial respiration increased in parallel with environmental temperature (Fig. 2A), and as expected correlated with metabolic rate (R = 0 .93, P < 0.001), but not with lifespan (R = 0 .17, P > 0.05). Similarly we found that mitochondrial mass, measured using 2 different methods (citrate synthase and mitochondrial DNA copy number), increased in parallel with increases in temperature (Fig. 2B, C). Accordingly transcription of components of the electron transport chain was also elevated at high temperatures (Fig. 2D). In summary, our data shows that flies respire more per mitochondrion, and have more mitochondria at higher temperatures, but these changes do not correspond with the observed changes in lifespan.
Figure 2.
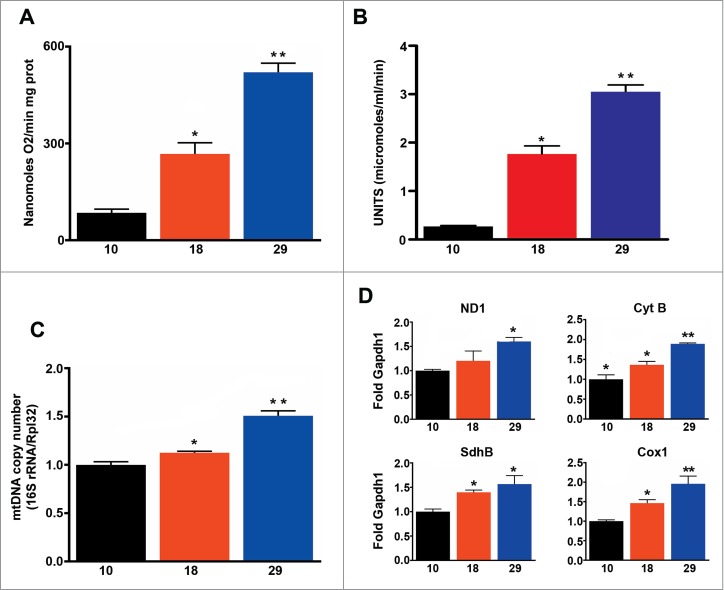
Mitochondrial function correlates with metabolic rate, but not with the rate of aging. (A) Mitochondrial oxygen consumption (n = 5 − 9). (B) Mitochondrial density (n = 6 − 7). (C) Mitochondrial copy number (n = 3 − 4). d) qPCR analysis of ND1, Cyt-b, SdhB and Cox1 (n = 4 − 5). The mean ±SEM is shown (n indicates independent replicates per group). Different numbers of * indicate statistically significant differences (ANOVA, p < 0.05) between temperatures.
Membrane unsaturation negatively correlates with metabolic rate, but not with lifespan
Homeoviscous adaptation of lipid membranes is a well-known mechanism used by poikilothermic animals to adapt to changes in temperature.35 Additionally, homeoviscous adaptation to longevity has also been proposed36 based on a negative correlation between the sensitivity to oxidation of lipid membranes and lifespan both in birds and mammals.37 As expected, we found that levels of membrane unsaturation were highest at the lowest temperature, and consequently membranes were more sensitive to lipid peroxidation (Fig. 3A, B and Table 2). Membrane unsaturation negatively correlated with metabolic rate (R = 0 .53, P < 0.01), but we found no association with lifespan (R = 0.14, P > 0.05). Finally, levels of ATP were similar at all temperatures (Fig. 3C), suggesting changes in respiration, mitochondrial density, and membrane unsaturation are effective for maintaining energy levels constant, independent of temperature.
Figure 3.
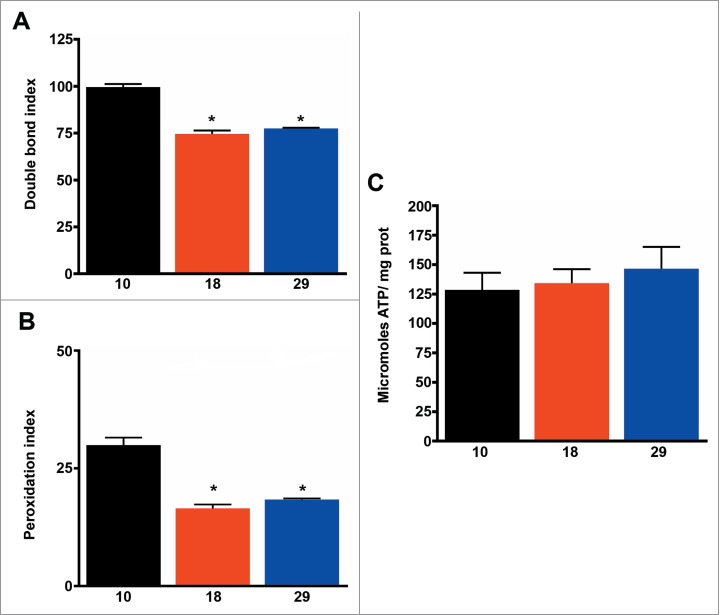
Membrane unsaturation negatively correlates with metabolic rate. (A) Double bond index of fatty acids (n = 4 −5). (B) Peroxidation index of fatty acid (n = 4 −5). (C) ATP content (n = 4 −5). The mean ±SEM is shown (n indicates independent replicates per group). Different numbers of * indicate statistically significant differences (ANOVA, p < 0.05) between temperatures.
Table 2.
Fatty acid composition in wild-type flies cultured at 3 different temperatures
| DAHOMEY wild type |
|||
|---|---|---|---|
| 10°C | 18°C | 29°C | |
| 14:0 | 9.78±0.4 | 20.41±1.2* | 21.2±0.3* |
| 14:1 | 0.7±0.1 | 1.0±0.0* | 1.0±0.0* |
| 16:0 | 19.0±0.2 | 19.7±0.9 | 17.7±0.2 |
| 16:1 | 18.9±0.8 | 25.0±4* | 23.0±0.2** |
| 18:0 | 0.3±0.0 | 0.5±0.1 | 0.7±0.0 |
| 18:1 | 27.4±0.6 | 20.5±0.3* | 21.7±0.2* |
| 18:2n6 | 18.0±0.4 | 9.8±0.8* | 11.2±0.1* |
| 18:3n3 | 5.4±1.0 | 2.8±0.1* | 3±0.1* |
| 20:0 | 0.2±0.0 | 0.1±0.1 | 0.2±0.1 |
| 20:1n9 | 0.4±0.0 | 0.3±0.0 | 0.3±0.1 |
| ACL | 16.8±0.0 | 16.3±0.0* | 16.3±0.0* |
| SFA | 29.2±0.4 | 40.7±1.0* | 39.7±0.1* |
| UFA | 70.8±0.4 | 59.3±1.0* | 60.3±0.1* |
| MUFA | 47.4±0.7 | 46.7±0.3 | 46.1±0.0 |
| PUFA | 23.4±0.6 | 12.5±0.8* | 14.2±0.1* |
| PUFAn3 | 5.4±1.0 | 2.8±0.1* | 3.0±0.1* |
| PUFAn6 | 18.0±0.4 | 9.8±0.8* | 11.2±0.0* |
Values: mean ±SEM from n = 4 − 5 samples × group. *Statistical significant differences (ANOVA, p < 0.05) between flies cultured at 10°C and the rest of the groups. **Significant differences (ANOVA, p < 0.05) between flies cultured at 29°C and the rest of the groups.
mtROS levels correlate with mitochondrial respiration and metabolic rate, but not with lifespan
Higher rates of oxygen consumption increase ROS production (Fig. 4A), but higher levels of ROS were offset by increased expression of antioxidants that detoxify both superoxide and hydrogen peroxide (Fig. 4B). Accordingly, no significant increase in protein damage was observed at 29°C, where only one of 5 markers measured was increased (Fig. 4C). Interestingly, MDA-derived protein adducts (generated from lipoxidation reactions), were higher at 10°C than at 29°C, indicating that lipoxidative damage is mainly determined by the sensitivity of membranes to oxidation, and not by levels of mtROS (Figs. 3B and 4A). Consequently, mtROS levels correlated with metabolic rate (R = 0 .97, P < 0.001) but not with lifespan (R = 0 .11, P > 0.05) and lipoxidative damage did not correlate with either mtROS levels (data not shown) or lifespan (R = 0 .02, P > 0.05). It has been proposed that the rate of aging is regulated through nutrient signaling pathways such as IIS and Tor.11 In order to see if changes in the activation of these pathways explain differences observed in lifespan we measured the activation of IIS components and Tor at different temperatures. Firstly, we measured the expression levels of the 3 main Drosophila Insulin-like peptides (DILPs) that have previously been related with fly longevity: dilp2, 3 and 538. We observed that levels of dilp2 and 5 changed in parallel with changes in temperature but not with changes in lifespan (Fig. 5A). Furthermore, levels of phosphorylated Akt1 (pAKT), a central component of IIS signaling, negatively correlated with lifespan (R = 0 .58, P < 0.05). However, the ratio between pAKT and total AKT was unchanged, indicating that the same proportion of the total AKT is active at both 10 and 18°C (Fig. 5B, C). Surprisingly, phosphorylation of forkhead box sub-group O (pdFOXO), a downstream target of AKT, did not correlate with activation of AKT (data not shown). Moreover, pdFOXO was highest at 10°C, but total levels of dFOXO were also increased at this temperature. In fact, no correlation between the proportion of activated dFOXO and the metabolic (R = 0 .04, P > 0.05) or aging rate (R = 0 .31, P > 0.05) was found (Fig. 5B, C). A different picture emerged when activation of Tor was studied by measuring the phosphorylation levels of 2 downstream targets of Tor: (i) RPS6-p70-protein kinase (S6K1) and (ii) Thor (4E-BP) (Fig. 5D, E). Both total phosphorylation and the ratio between the phosphorylated and total amount of protein displayed a nice correlation with lifespan (Table S1), but not with metabolic rate (Table S1) indicating that activation of Tor is a better predictor of fly lifespan than other components of the insulin-PI3K/Tor pathway. The decreased weight detected in flies cultured at 18°C further confirmed the reduction of Tor signaling at this temperature (Fig. 1D).
Figure 4.
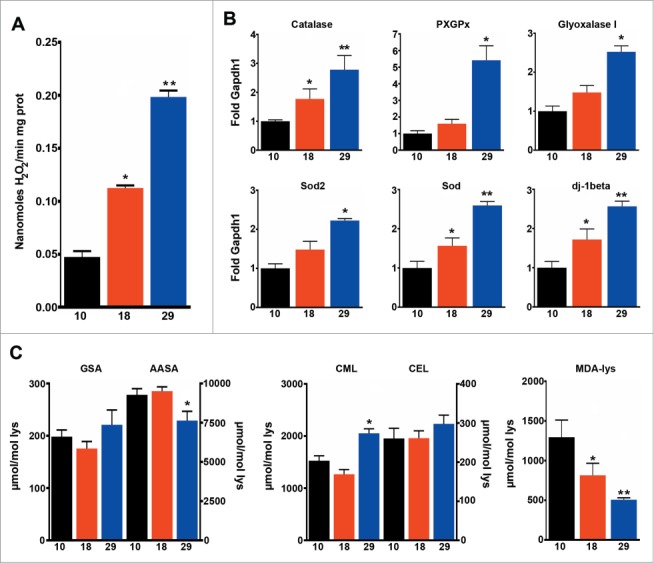
Mitochondrial ROS production correlates with metabolic rate, but not with lifespan. (A) Levels of mitochondrial H2O2 production (n = 4 − 7). (B) qPCR analysis of Catalase, PHGPx, Glyoxalase I, Sod2, Sod and dj-1beta (n = 3 − 4). (C) Markers of oxidative (GSA, AASA), glycoxidative (CML, CEL) and lipooxidative (MDA-lys) damage to proteins (n = 4 − 5). The mean ±SEM is shown (n indicates independent replicates per group). Different numbers of * indicate statistically significant differences (ANOVA, p < 0.05) between temperatures.
Figure 5.
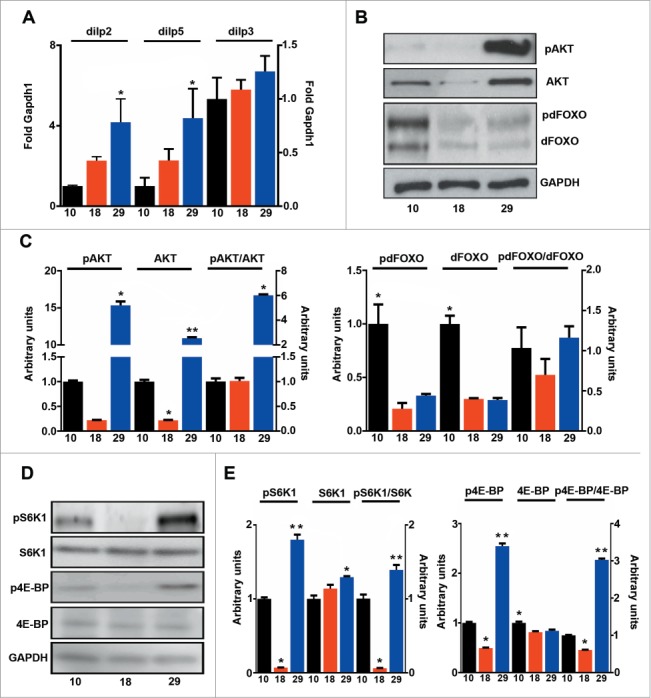
Tor activation correlates with lifespan. (A) qPCR analysis of dilp2, 3 and 5 (n = 3 − 4). (B) Western blot analysis of phosphorylated and total AKT and dFOXO. (C) Quantification of b (n = 3 ). (D) Western blot analysis of phosphorylated and total S6K1 and 4E-BP. (E) Quantification of d (n = 3 ). The mean ±SEM is shown (n indicates independent replicates per group). Different numbers of * indicate statistically significant differences (ANOVA, p <0.05) between temperatures.
Discussion
Based on a comparative approach, the rate of living theory proposes that lifespan is directly proportional to metabolic rate.28 MFRTA provides a mechanistic explanation39 for the inverse relationship between metabolic rate and lifespan, and an explanation for those exceptions such as birds,34 although not for others such as naked-mole rat.29 Some of the strongest support for the rate of living theory comes from fly studies demonstrating a negative correlation between lifespan and metabolic rate.26,27,40 Unfortunately, most of these studies do not use temperatures below 18°C in spite of the fact that flies live at these temperatures in the wild and are able to eat, move and mate. Rearing flies at 3 different temperatures, including one lower than 18°C, we were able to show that it is possible to separate the metabolic and aging rate in wild type flies. We found that lifespan did not correlate with mitochondrial respiration or mitochondrial ROS production indicating that lower levels of ROS do not always correlate with a longer lifespan, as we have previously shown in genetically modified populations.24
ATP and protein damage levels remained constant at all temperatures, indicating that homeostatic mechanisms work efficiently at the 3 different temperatures studied. In order to maintain ATP levels, flies increased mitochondrial respiration and mitochondrial mass at 29°C, at 10°C the flies increased levels of unsaturation in lipid membranes. Interestingly, this meant that lipoxidative damage was highest at lower temperatures, whereas other types of damage were kept constant by adjusting the expression levels of antioxidants against production of ROS. This increase in unsaturation of lipid membranes is a well-known mechanism of temperature adaptation used by poikilothermic animals.41,42 In homeothermic vertebrates, changes in unsaturation have not only been related to the regulation of metabolic rate, but have also been associated with changes in lifespan observed between different animal species.6 Highly unsaturated membranes are more prone to oxidation 6,7 and although this has not been confirmed in other animal models,15,43-45 a reduction of membrane unsaturation has been shown to extend the lifespan of C. elegans.8 However, changes in lipoxidation have been related to changes in mtROS 46 and/or unsaturation.47 Our results indicate that in fruit flies, lipoxidative damage is determined by the level of membrane unsaturation and not by steady-state levels of ROS, and negatively correlates with metabolic rate but not with the rate of aging.
Our results do not support the idea that differences in mtROS generation or lipoxidation levels cause the divergent aging rates observed in flies cultured at different temperatures. However, we cannot discard other types of damage. We find that activation of nutrient signaling pathways better predicts the lifespan of wild type flies. Activation of Tor correlated with lifespan, however other components of the insulin-PI3K/Tor signaling pathway were not so clearly connected. For instance, we found that phosphorylation of AKT changed in parallel with lifespan, but this correlation disappeared after normalization with total levels, indicating that AKT concentration, more than its activation, may be important in determining fly lifespan. Interestingly, phosphorylation of dFOXO, a downstream target of AKT, was highest at 10°C but no correlation with AKT activation status or lifespan was observed. This was surprising since dFOXO or its ortholog gene DAF-16 are required for lifespan extension in models of reduced or attenuated IIS.11 Rapamycin is able to extend lifespan independently of dietary restriction and without altering IIS or AMP-activated protein kinase (AMPK) activity.16 Similarly, attenuated Tor signaling extends lifespan of DAF-16 null mutants. However, it does not further extend the lifespan of DAF-2 or chico mutants.16,48 These results indicate that IIS and Tor work in pathways that partially overlap. Accordingly, we found a consistent reduction in Tor activation independently of the activation status of other components of the insulin-PKI3-Tor pathway. Two different mechanisms have been proposed to explain the effects of Tor on lifespan: (i) a boost in autophagy and (ii) repression of protein translation. Accordingly, inhibition of autophagy or induction of protein translation prevents lifespan extension via rapamycin.49 In the future, testing these 2 mechanisms may prove instrumental in determining whether the hyperfunction or damage theory is correct.
Our results fit better with the hyperfunction theory than with MFRTA or similar theories. Recent findings indicate that pharmacological18 or genetic50 inhibition of Tor or its downstream targets17 extends lifespan in rodent models, whereas modifications in ROS levels mainly failed to modify longevity.20 Interestingly, females generally live longer than males, and are smaller being this last characteristic associated to attenuated Tor signaling.51 Similarly wild type flies live longer and are lighter at 18°C where Tor signaling is reduced. However, the extension in lifespan as a result of IIS/Tor inhibition is modest in comparison with the observed differences in lifespan between flies cultured at different temperatures.16,52,53 This indicates that other factors must be important in the determination of lifespan of wild type flies. The hyperfunction theory predicts that oxidative damage (as well as other forms of damage) is a consequence, and not a cause, of aging. However, the hyperfunction theory acknowledges that damage can play a role in the development of age-related diseases that affect individual survival reducing lifespan.12 Therefore, it is possible that low levels of mtROS and membranes more resistant to oxidation could protect individuals from age-related diseases contributing to a longer lifespan. In this model, aging would be driven by a de-regulation of essential signaling pathways. Beyond a certain threshold de-regulation would cause uncontrolled damage generation that would be responsible for age-related diseases resulting in death. Cellular systems generating lower levels of damage or those more resilient to damage would be protected against age-related diseases contributing to an extended lifespan. This agrees with models showing that increased levels of ROS cause age-related degenerative diseases but do not accelerate aging.54 Also from our own results we observed intermediate levels of ROS and lipoxidative damage at 18°C where flies lived the longest. The hyperfunction theory like any other theory challenging the established damage accumulation paradigm must be thoroughly tested. Our data suggest that these tests will be worth the time and effort.
Experimental procedures
Fly stocks and lifespan assessments
Wild-type Dahomey (DAH) female flies, used in this study, were maintained on standard media (1% agar, 1.5% sucrose, 3% glucose, 3.5% dried yeast, 1.5% maize, 1% wheat, 1% soya, 3% treacle, 0.5% propionic acid, 0.1% Nipagin). Flies were collected under CO2 anesthesia within 24 hours of eclosion and then maintained at a density of 20 flies per vial at 10°C, 18°C, or 29°C under a controlled 12 hour light:dark cycle. Flies were transferred to new vials every 2-3 d Lifespan assessments were performed with a minimum of 100 flies per temperature and repeated twice. The number of dead flies was recorded every 2-3 d. The median, mean and maximum lifespan (the last 10% of surviving flies) were calculated for each experiment. Ten days old flies were used in all experiments.
Mitochondrial O2 consumption and measurement of mitochondrial H2O2
Mitochondria were isolated as described elsewhere.55 Mitochondrial respiration rates were measured via polarography using a Clark-type oxygen electrode as previously described.24 Mitochondria were incubated in assay buffer (120 mM KCl, 5 mM KH2PO4, 3 mM HEPES, 1 mM EGTA, 1 mM MgCl2, and 0.2% bovine serum albumin, pH 7.2 at 25°C), supplemented with a combination of complex I (5 mM pyruvate+ 5 mM proline) linked substrates. State 3 respiration was initiated by adding 500 µM ADP to the sample. Mitochondrial ROS production was assayed according to the method described in24 using pyruvate+proline (5 mM each) to feed the electron transport chain.
Western blot analysis
Protein extraction, SDS-PAGE and Western blotting were performed as described in.55 The primary antibodies, employed together with the appropriate secondary antibodies, were as follows: anti-phospho-Drosophila-AKT (Ser505) (Cell Signaling, Massachusets, USA), 1:1,000; anti-Drosophila-AKT (Cell Signaling, Massachusets, USA), 1:1,000; anti-phospho-Fox01 (Ser256) (Cell Signaling, Massachusets, USA), 1:1,000; anti-FOXO (Cell Signaling, Massachusets, USA), 1:1,000; anti-p-p70 S6 Kinase (Thr389) (Cell Signaling, Massachusets, USA), 1:1,000; p70 S6 kinase α (Santa Cruz, Texas, USA), 1:250; anti-phospho-4E-BP1 (Thr37/46) (Cell Signaling, Massachusets, USA), 1:250; anti-4E-BP1(Cell Signaling, Massachusets, USA), 1:250; and anti-GAPDH (Everest Biotech, Oxfordshire, United Kingdom), 1:40,000. The secondary antibodies were as follows: HRP-conjugated horse anti-mouse IgG [H+L] (Vector Laboratories, Burlingame, USA), used at 1:10,000; HRP-conjugated horse anti-rabbit IgG [H+L] (Vector Laboratories, Burlingame, USA), 1:10,000; and HRP-conjugated horse anti-goat IgG [H+L] (Vector Laboratories, Burlingame, USA), 1:5,000. The intensity of the bands were quantified with ImageJ software.
ATP measurements
The total ATP content was measured using a luciferin-luciferase-based ATP determination kit (Molecular Probes, Eugene, USA). One to 5 flies were homogenized in 100 µl of 6 M guanidinium chloride, and the homogenates were centrifuged at 16,000 × g for 5 min. The supernatants were dissolved 1:750 in TE buffer. Ten microliters of the diluted sample was mixed with a reaction solution containing luciferin-luciferase, and triplicate samples were measured via luminometry. A standard curve of different ATP dilutions was run in parallel, and the results were normalized based on the protein content (mg), after interpolation with the standard curve.
Fly activity
The locomotor activity of individual flies was measured in a Digitherm CircKinetics monitoring incubator (Tritech Research, Los Angeles, USA) at the appropriate temperature under a controlled 12:12-hour dark:light cycle. Individual flies were placed in capillary tubes with standard fly food, and their activity was monitored over 72 hours using TriKinetics Activity Monitors (TriKinetics Inc.., Waltham, USA). The flies were acclimated for the first 24 h, and their activity was monitored during the next 48 h. The number of times that flies crossed the center of the vial per hour was counted and integrated using TriKinetics software.
Mitochondrial density measurements via the citrate synthase assay
Approximately 40-60 flies were immobilised on ice and then transferred to a chilled mortar. The flies were homogenized in 500 µl of ice-cold mitochondria isolation medium (250 mM sucrose, 5 mM Tris-HCL, 2 mM EGTA), and the homogenate was filtered through cheesecloth. Then, an additional 500 µl of the mitochondria isolation medium containing 1 mM DTT was added, and the samples were frozen at −80°C overnight. Next, samples were defrosted and 50 µl of the sample was diluted 1:5 in mitochondria isolation medium containing 1 mM PMSF. The remainder of the sample was used to isolate mitochondria as described elsewhere.55 The mitochondria were subsequently diluted 1:4 in mitochondria isolation buffer containing 1 mM PMSF. Measurements were performed in a 96-well plate, in which 182 µl of fresh reaction buffer (100 mM Tris-HCL (pH 7.5) and 2.5 mM EDTA), 2 µl of 30 mM acetyl-CoA and 2 µl of 10 mM DTNB were added to each well. Finally, the samples (either the whole homogenate or isolated mitochondria) were added. The reaction was initiated by adding 10 µl of 10 mM oxaloacetate (OAA), and the linear increase in absorbance at 412 nm was followed for 3-4 minutes using a PerkinElmer EnVision 2104 plate reader. Blanks were made from the same samples without the addition of OAA and then measured. Mitochondrial density was calculated by dividing the specific citrate synthase activity measured in the whole-fly homogenates by the specific citrate synthase activity measured in isolated mitochondria.
CAFE assay
Tubes were punched with holes on the top and the sides to allow the introduction of capillary tubes (containing the food) and air circulation. Flies were anesthetised with CO2 and transferred to a 1.5-ml tube. Capillary tubes were filled with CAFE assay food (5% sucrose and 5% yeast extract). Quantification of food intake was performed by adding a known amount of food to capillaries and then measuring changes in this volume every 24 hours. Food evaporation was controlled for by carrying out measurements in capillary tubes in 1.5-ml tubes without flies. The analysis was performed for approximately 120 hours.
Weight measurements
Approximately 10 flies were anesthetised with CO2 and then collected into a tube. Their weight was calculated by subtracting the weight of the tube without flies from the weight of the tube with the flies.
RNA/DNA quantification
The methods for the isolation of mRNA and cDNA and q-RT-PCR synthesis have been described in detail in.55 To determine mtDNA copy number, total DNA was isolated according to 23 and analyzed via qPCR using the same conditions employed for mRNA analysis. The data were extracted and analyzed using Applied Biosystems StepOne software version 2.1. Primer sequences are available upon request.
Fatty acid analyses and global fatty acid unsaturation indexes
The fatty acids in lipids were analyzed as methyl ester derivatives via gas chromatography/mass spectrometry (GC/MS) as previously described.46 The following fatty acyl indices were also calculated: saturated fatty acids (SFA); unsaturated fatty acids (UFA); monounsaturated fatty acids (MUFA); polyunsaturated fatty acids (PUFA) of n-3 and n-6 series (PUFAn-3 and PUFAn-6); and the average chain length (ACL) = [(Σ%Total14 × 14) + (Σ% Total16 × 16 ) + (Σ%Total18 × 18 ) + (Σ%Total20 × 20 )]/100. The density of double bonds in the membrane was calculated according to the double bond index, DBI = [(1 × Σmol% monoenoic) + (2×Σmol% dienoic) + (3×Σmol% trienoic)]. The susceptibility of the membrane to peroxidation was calculated using the peroxidizability index, PI = [(0.025 × Σmol% monoenoic) + (1 × Σmol% dienoic) + (2 × Σmol% trienoic)].
Analysis of protein damage markers via mass spectrometry
The levels of the markers aminoadipic semialdehyde (AASA - oxidation), glutamic semialdehyde (GSA - oxidation), carboxymethyl-lysine (CML - glycoxidation), carboxyethyl-lysine (CEL - glycoxidation) and malondialdehydelysine (MDAL - lipoxidation) were determined via GC/MS according to.55
Statistical analysis
The data were analyzed using GraphPad Prism 6 software. One-way ANOVA followed by the Newman-Keuls multiple comparisons test. Lifespan data were analyzed using the Kaplan Meier log-rank test. Linear regression analysis (equation y = a +b*x) was used to confirm whether parameters analyzed (y) correlated or not with lifespan (x = mean lifespan) or metabolic rate (x = temperature ). Pearson correlation coefficient (r) was calculated and data are shown as R square (R) and statistical significance (P) in Table S1. The level of statistical significance was established as p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors want to thank Dr Rhoda Stefanatos for editing the manuscript and feedback about the meaning and interpretation of the results.
Author Contributions
FS, ASr, AN, VA, MJ: performed experiments and analyzed data; RP, AS: wrote the manuscript and designed and supervised the project. All authors discussed the data and critically revised the manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This study was supported by the European Research Council (ERC Starting Grant to A.S.), the Academy of Finland (Research Academy Fellowship to A.S), the Spanish Ministry of Economy and Competitiveness (BFU2009-11879/BFI; RD12/0043/0018 and PI1400328 to R.P.), and the Autonomous Government of Catalonia (2014SGR168 to R.P).
References
- 1.Merker K, Stolzing A, Grune T. Proteolysis, caloric restriction and aging. Mech Ageing Dev 2001; 122:595-615; PMID:11322989; http://dx.doi.org/ 10.1016/S0047-6374(01)00219-6 [DOI] [PubMed] [Google Scholar]
- 2.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc 1972; 20:145-7; PMID:5016631; http://dx.doi.org/ 10.1111/j.1532-5415.1972.tb00787.x [DOI] [PubMed] [Google Scholar]
- 3.Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal 2013; 19:1420-45; PMID:23642158; http://dx.doi.org/ 10.1089/ars.2012.5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest 2013; 123:951-7; PMID:23454757; http://dx.doi.org/ 10.1172/JCI64125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scialo F, Mallikarjun V, Stefanatos R, Sanz A. Regulation of lifespan by the mitochondrial electron transport chain: reactive oxygen species-dependent and reactive oxygen species-independent mechanisms. Antioxid Redox Signal 2013; 19:1953-69; PMID:22938137; http://dx.doi.org/ 10.1089/ars.2012.4900 [DOI] [PubMed] [Google Scholar]
- 6.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 2007; 87:1175-213; PMID:17928583; http://dx.doi.org/ 10.1152/physrev.00047.2006 [DOI] [PubMed] [Google Scholar]
- 7.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim Biophys Acta 2008; 1777:1249-62; PMID:18721793; http://dx.doi.org/ 10.1016/j.bbabio.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Shmookler Reis RJ, Xu L, Lee H, Chae M, Thaden JJ, Bharill P, Tazearslan C, Siegel E, Alla R, Zimniak P, et al. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging (Albany NY) 2011; 3:125-47; PMID:21386131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Raamsdonk JM, Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci U S A 2012; 109:5785-90; PMID:22451939; http://dx.doi.org/ 10.1073/pnas.1116158109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 2003; 16:29-37; PMID:14679299; http://dx.doi.org/ 10.1152/physiolgenomics.00122.2003 [DOI] [PubMed] [Google Scholar]
- 11.Gems D, Partridge L. Genetics of Longevity in Model Organisms: Debates and Paradigm Shifts. Annu Rev Physiol 2012; PMID:23190075 [DOI] [PubMed] [Google Scholar]
- 12.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 2006; 5:2087-102; PMID:17012837; http://dx.doi.org/ 10.4161/cc.5.18.3288 [DOI] [PubMed] [Google Scholar]
- 13.Gems D, de la Guardia Y. Alternative Perspectives on Aging in Caenorhabditis elegans: Reactive Oxygen Species or Hyperfunction? Antioxid Redox Signal 2013; 19:321-9; PMID:22870907; http://dx.doi.org/ 10.1089/ars.2012.4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab 2011; 13:668-78; PMID:21641548; http://dx.doi.org/ 10.1016/j.cmet.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spindler SR, Mote PL, Flegal JM. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age (Dordr) 2014; 36:9659; PMID:24816553; http://dx.doi.org/ 10.1007/s11357-014-9659-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 2010; 11:35-46; PMID:20074526; http://dx.doi.org/ 10.1016/j.cmet.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009; 326:140-4; PMID:19797661; http://dx.doi.org/ 10.1126/science.1177221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460:392-5; PMID:19587680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell 2014; 13:616-22; PMID:24655348; http://dx.doi.org/ 10.1111/acel.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med 2007; 43:477-503; PMID:17640558; http://dx.doi.org/ 10.1016/j.freeradbiomed.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 21.Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics 2003; 165:2295-9; PMID:14704205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, Abdueva D, Skvortsov D, Ford D, Luu A, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol 2007; 8:R262; PMID:18067683; http://dx.doi.org/ 10.1186/gb-2007-8-12-r262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanatos R, Sriram A, Kiviranta E, Mohan A, Ayala V, Jacobs HT, Pamplona R, Sanz A. dj-1beta regulates oxidative stress, insulin-like signaling and development in Drosophila melanogaster. Cell Cycle 2012; 11:3876-86; PMID:22983063; http://dx.doi.org/ 10.4161/cc.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz A, Fernandez-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging (Albany NY) 2010; 2:200-23; PMID:20453260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 2013; 155:699-712; PMID:24243023; http://dx.doi.org/ 10.1016/j.cell.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sestini EA, Carlson JC, Allsopp R. The effects of ambient temperature on life span, lipid peroxidation, superoxide dismutase, and phospholipase A2 activity in Drosophila melanogaster. Exp Gerontol 1991; 26:385-95; PMID:1936197; http://dx.doi.org/ 10.1016/0531-5565(91)90050-V [DOI] [PubMed] [Google Scholar]
- 27.Miquel J, Lundgren PR, Bensch KG, Atlan H. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev 1976; 5:347-70; PMID:823384; http://dx.doi.org/ 10.1016/0047-6374(76)90034-8 [DOI] [PubMed] [Google Scholar]
- 28.Pearl R. The Rate of Living, Being an Account of Some Experimental Studies on the Biology of Life Duration. London: University of London Press, 1928. [Google Scholar]
- 29.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 2005; 60:1369-77; PMID:16339321; http://dx.doi.org/ 10.1093/gerona/60.11.1369 [DOI] [PubMed] [Google Scholar]
- 30.de Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci 2007; 62:149-60; PMID:17339640; http://dx.doi.org/ 10.1093/gerona/62.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brys K, Vanfleteren JR, Braeckman BP. Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol 2007; 42:845-51; PMID:17379464; http://dx.doi.org/ 10.1016/j.exger.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Hulbert AJ, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp Gerontol 2004; 39:1137-43; PMID:15288688; http://dx.doi.org/ 10.1016/j.exger.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 33.Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 2004; 3:87-95; PMID:15153176; http://dx.doi.org/ 10.1111/j.1474-9728.2004.00097.x [DOI] [PubMed] [Google Scholar]
- 34.Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res 2007; 10:215-24; PMID:17523876; http://dx.doi.org/ 10.1089/rej.2006.0516 [DOI] [PubMed] [Google Scholar]
- 35.Overgaard J, Sorensen JG, Petersen SO, Loeschcke V, Holmstrup M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J Insect Physiol 2005; 51:1173-82; PMID:16112133; http://dx.doi.org/ 10.1016/j.jinsphys.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 36.Pamplona R, Barja G, Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann N Y Acad Sci 2002; 959:475-90; PMID:11976221; http://dx.doi.org/ 10.1111/j.1749-6632.2002.tb02118.x [DOI] [PubMed] [Google Scholar]
- 37.Pamplona R, Barja G. An evolutionary comparative scan for longevity-related oxidative stress resistance mechanisms in homeotherms. Biogerontology 2011; 12:409-35; PMID:21755337; http://dx.doi.org/ 10.1007/s10522-011-9348-1 [DOI] [PubMed] [Google Scholar]
- 38.Wang PY, Neretti N, Whitaker R, Hosier S, Chang C, Lu D, Rogina B, Helfand SL. Long-lived Indy and calorie restriction interact to extend life span. Proc Natl Acad Sci U S A 2009; 106:9262-7; PMID:19470468; http://dx.doi.org/ 10.1073/pnas.0904115106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11:298-300; PMID:13332224; http://dx.doi.org/ 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 40.Vermeulen CJ, Bijlsma R. Changes in mortality patterns and temperature dependence of lifespan in Drosophila melanogaster caused by inbreeding. Heredity 2004; 92:275-81; PMID:14679396; http://dx.doi.org/ 10.1038/sj.hdy.6800412 [DOI] [PubMed] [Google Scholar]
- 41.Guderley H, St-Pierre J. Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. J Exp Biol 2002; 205:2237-49; PMID:12110658 [DOI] [PubMed] [Google Scholar]
- 42.Geer BW, Perille TJ. Effects of dietary sucrose and environmental temperature on fatty acid synthesis in Drosophila melanogaster. Insect Biochemistry 1977; 7:371-9; http://dx.doi.org/ 10.1016/0020-1790(77)90040-3 [DOI] [Google Scholar]
- 43.Gomez A, Sanchez-Roman I, Gomez J, Cruces J, Mate I, Lopez-Torres M, Naudi A, Portero-Otin M, Pamplona R, De la Fuente M, et al. Lifelong treatment with atenolol decreases membrane fatty acid unsaturation and oxidative stress in heart and skeletal muscle mitochondria and improves immunity and behavior, without changing mice longevity. Aging Cell 2014; 13:551-60; PMID:24612513; http://dx.doi.org/ 10.1111/acel.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valencak TG, Ruf T. Feeding into old age: long-term effects of dietary fatty acid supplementation on tissue composition and life span in mice. J Comp Physiol B 2011; 181:289-98; PMID:20981551; http://dx.doi.org/ 10.1007/s00360-010-0520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Dominguez JA, Ramsey JJ, Tran D, Imai DM, Koehne A, Laing ST, Griffey SM, Kim K, Taylor SL, Hagopian K, et al. The influence of dietary fat source on life Span in calorie restricted mice. J Gerontol A Biol Sci Med Sci 2014; http://dx.doi.org/ 10.1093/gerona/glu177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J 2006; 20:1064-73; PMID:16770005; http://dx.doi.org/ 10.1096/fj.05-5568com [DOI] [PubMed] [Google Scholar]
- 47.Pamplona R, Portero-Otin M, Sanz A, Requena J, Barja G. Modification of the longevity-related degree of fatty acid unsaturation modulates oxidative damage to proteins and mitochondrial DNA in liver and brain. Exp Gerontol 2004; 39:725-33; PMID:15130667; http://dx.doi.org/ 10.1016/j.exger.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 48.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 2007; 6:95-110; PMID:17266679; http://dx.doi.org/ 10.1111/j.1474-9726.2006.00267.x [DOI] [PubMed] [Google Scholar]
- 49.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans 2011; 39:460-5; PMID:21428920; http://dx.doi.org/ 10.1042/BST0390460 [DOI] [PubMed] [Google Scholar]
- 50.Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell reports 2013; 4:913-20; PMID:23994476; http://dx.doi.org/ 10.1016/j.celrep.2013.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012; 4:899-916; PMID:23443503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 2010; 8:e1000556; PMID:21151885; http://dx.doi.org/ 10.1371/journal.pbio.1000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001; 292:104-6; PMID:11292874; http://dx.doi.org/ 10.1126/science.1057991 [DOI] [PubMed] [Google Scholar]
- 54.Hashizume O, Shimizu A, Yokota M, Sugiyama A, Nakada K, Miyoshi H, Itami M, Ohira M, Nagase H, Takenaga K, et al. Specific mitochondrial DNA mutation in mice regulates diabetes and lymphoma development. Proc Natl Acad Sci U S A 2012; 109:10528-33; PMID:22689997; http://dx.doi.org/ 10.1073/pnas.1202367109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanz A, Soikkeli M, Portero-Otin M, Wilson A, Kemppainen E, McIlroy G, Ellila S, Kemppainen KK, Tuomela T, Lakanmaa M, et al. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc Natl Acad Sci U S A 2010; 107:9105-10; PMID:20435911; http://dx.doi.org/ 10.1073/pnas.0911539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


