Abstract
Understanding how the immune response is activated and amplified requires detailed knowledge of the stages in the formation of the immunological synapse (IS) between T lymphocytes and antigen-presenting cells (APCs). We show that tetraspanins CD9 and CD151 congregate at the T-cell side of the IS. Silencing of CD9 or CD151 blunts the IL-2 secretion and expression of the activation marker CD69 by APC-conjugated T lymphocytes, but does not affect the accumulation of CD3 or actin to the IS, or the translocation of the microtubule-organizing center toward the T-B contact area. CD9 or CD151 silencing diminishes the relocalization of α4β1 integrin to the IS and reduces the accumulation of high-affinity β1 integrins at the cell-cell contact. These changes are accompanied by diminished phosphorylation of the integrin downstream targets FAK and ERK1/2. Our results suggest that CD9 and CD151 support integrin-mediated signaling at the IS.
Keywords: CD9, CD151, Immune synapse, Integrins, T-cell activation
INTRODUCTION
Interaction between T lymphocytes and antigen-presenting cells (APCs) is critical for antigen presentation and for the initiation of the immune response. This process is characterized by the formation of a dynamic structure called the immune synapse (IS) at the contact between T cells and APCs [1]. At the T cell side of the IS, T-cell receptors (TCRs) and associated molecules accumulate in the central area (central supramolecular activation cluster; cSMAC), while adhesion receptors and integrins reorganize in a surrounding external ring called peripheral SMAC (pSMAC) [1]. The increased IS stability enhances T-cell sensitivity, stimulates cytoskeletal processes, induces downstream signaling, and drives effective T-cell differentiation [2, 3]. How integrins regulate these processes is not fully understood.
Several membrane receptors and integrins concentrated at the IS — CD3, CD4, ICAM-1, LFA-1 and VLA-4 — are associated with tetraspanins [4, 5], ubiquitously expressed proteins that organize membrane macrocomplexes called tetraspanin-enriched microdomains (TEMs) [6]. Tetraspanins modulate the function of their associated partners and play important roles in immunity, inflammation and other processes [6]. Tetraspanins that accumulate at the IS include CD81, which regulates IS architectural organization and maturation [5], and CD82, which contributes to T cell activation through Rho GTPase-dependent regulation of the actin cytoskeleton [7]. CD82 also increases the adhesion of LFA-1 integrin to its ligand ICAM-1 expressed on APCs [4]. The tetraspanins CD81, CD82, CD53 and CD9 provide T cell costimulatory signals, and CD9 localizes together with TCR signaling molecules in lipid microdomains [4]. Moreover, the T cells of mice deficient for tetraspanins CD151, CD37 and Tssc6 are hyperproliferative [4]. However, it is not known whether CD9 and CD151 play a role during IS formation.
We have investigated the role of tetraspanins CD9 and CD151 in T-cell activation. Our results indicate that CD9 and CD151 contribute to this process by modulating the relocalization to the IS of α4β1 integrin, the local accumulation of high-affinity β1 integrins, the subsequent phosphorylation of focal adhesion kinase (FAK) and extracellular signal-related kinase (ERK1/2), and CD69 expression and IL-2 secretion.
RESULTS AND DISCUSSION
Tetraspanins CD9 and CD151 accumulate at the IS
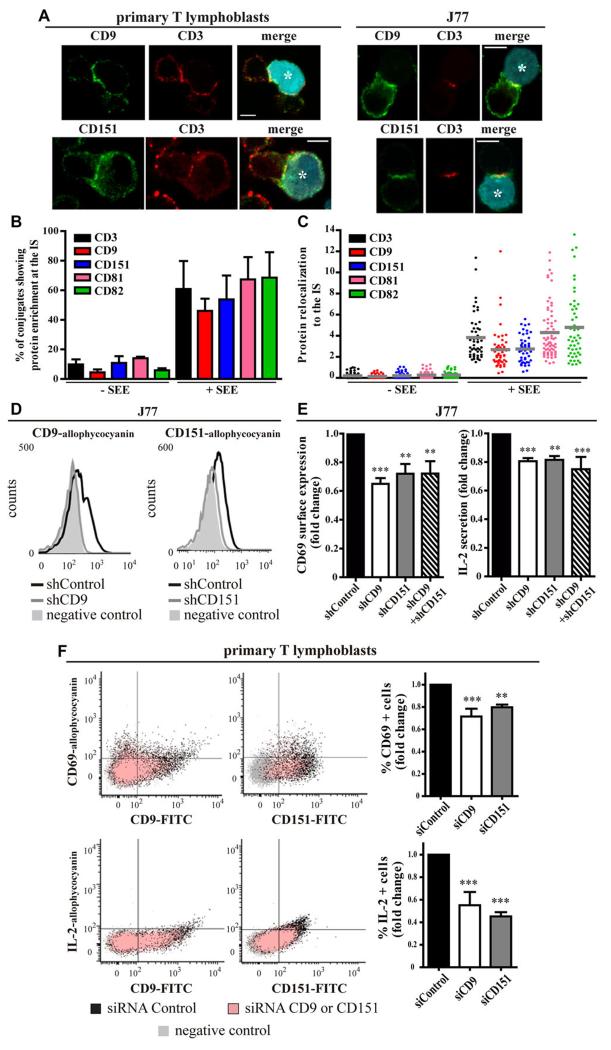
To determine the role of CD9 and CD151 in the IS, we first investigated their redistribution to the contact area between T cells (primary T lymphoblasts or J77 T cells) and CMAC-labeled Staphylococcus enterotoxin E (SEE)-loaded Raji B cells (Raji/SEE) as APCs. Confocal microscopy analysis showed that CD9 and CD151 were enriched at the IS, partially colocalizing with CD3, which is enriched at the cSMAC (Fig. 1A). Quantification of the percentage of T–B contacts displaying protein enrichment at the IS over the total number of T–B contacts confirmed the expected high proportion of T–B conjugates showing APC-dependent IS accumulation of CD3, CD81 and CD82, and revealed similar percentages of conjugates with CD9 and CD151 accumulation at the IS (Fig. 1B). Conjugates were further analyzed for the quantitative assessment of the relocalization of proteins to the cell–cell contact area with respect to the fluorescence intensity signal at the rest of the cell membranes. This analysis revealed similarly high levels of antigen-dependent relocalization of tetraspanins and CD3 to the IS (Fig. 1C).
Figure 1. CD9 and CD151 relocalize to the IS and contribute to T-cell activation.
(A) Primary T lymphoblasts or J77 T cells were conjugated for 20 min with CMAC-labeled Raji/SEE B cells (blue, asterisk), plated on poly-L-lysine-coated coverslips, fixed, and stained for with antibodies against CD9 or CD151 (green) and CD3 (red). Maximal projections of confocal stacks, Scale bars = 10 μm. Images are representative of four independent experiments. (B, C) J77 T cells were conjugated (20 min) with Raji/+SEE or Raji/-SEE cells, fixed and stained for with antibodies against the indicated proteins and assessed by confocal microscopy. (B) Percentage of T–B contacts showing protein accumulation at the IS; data show means + SD of at least 300 (+SEE) or 100 (-SEE) contacts, n = 5 independent experiments. (C) Protein relocalization to the IS was determined by analyzing the fluorescence intensity signal at the IS with respect to the fluorescence intensity signal at the rest of cell membranes, using the Synapsemeasure plugin in ImageJ. Each symbol represents an individual conjugate (n = at least 50, pooled from 3 independent experiments) and horizontal bars indicate the means. (D) Flow cytometry analysis of surface expressed CD9 and CD151 in allophycocyanin fluorochrome-labeled T cells. Flow cytometry gating strategy is shown in Supplementary Information Fig. 2A. J77 T cells were transduced with shCD9 or shCD151 (gray lines) or with empty vector (shControl-black). Filled histograms represent control cells labeled with only secondary antibodies. Plots are representative of five independent experiments. (E) J77/shRNA cells were conjugated with Raji/SEE for 16 hours. Cells were analyzed by flow cytometry after staining with CD69-allophycocyanin antibody. Flow cytometry gating strategy is shown in Supplementary Information Fig. 2A. IL-2 content was measured in supernatants by ELISA. Data are presented relative to shControl cells, means + SEM, n = 5 independent experiments performed in triplicate. **p < 0.01, ***p < 0.001. (F) Primary T lymphoblasts treated with siControl, siCD9 (left panels) or siCD151 (right panels) were conjugated with Raji/SEE for 16 hours. Surface CD69 (upper panels) and intracellular IL-2 (bottom panels) were analyzed by flow cytometry after immunolabelling with CD69-allophycocyanin or IL-2-allophycocyanin and CD9-FITC or CD151-FITC antibodies. Flow cytometry gating strategy is shown in Supplementary Information Fig. 2A. Cells labelled with isotype control antibodies are in gray. The graphs show the fold change in percent CD69+ (upper) or IL-2+ (bottom) T lymphoblasts related to control cells (siRNA Control). Means + SEM, n = 2 (CD9), n = 3 (CD151) independent experiments performed in triplicate. **p < 0.01, ***p < 0.001.
We next assessed whether CD9 and CD151 accumulation at the IS changed with IS progression. J77 T cells transfected with CD3ζ-mEGFP and either CD9-mCherry or mRuby-CD151 were conjugated with Raji/SEE and analyzed by time-lapse confocal microscopy. CD9 and CD151 relocalized to the IS within the first minutes of contact, showing a diffuse distribution throughout the contact area. At all times analyzed, colocalization of CD9 or CD151 with CD3 (Supporting Information Fig. 1A-B) was lower than previously observed for CD81 [5], suggesting that, unlike CD81, CD9 and CD151 might not regulate the reorganization of CD3 at the IS.
CD9 and CD151 contribute to T-cell activation by modulating integrin signaling
To further investigate the role of CD9 and CD151 during antigen recognition, we studied the effect on T-cell activation of silencing CD9 and CD151 expression (Fig. 1D, flow cytometry gating strategy in Supporting Information Fig. 2A). In non-conjugated J77 T cells, variations of surface expression levels of molecules involved in T-cell activation, such as other tetraspanins (CD81, CD82 and CD53), CD3, CD4 and integrins (α4, αL, β1), were not statistically significant in CD9 or CD151 knocked-down compared with controls (Supporting Information Fig. 2B-D). However, upon Raji/SEE activation, CD9- and CD151-silenced J77 cells showed reduced surface expression of the T-cell activation marker CD69 and lower secretion of IL-2 (Fig. 1E). Simultaneous downregulation of both tetraspanins did not produce a stronger inhibitory effect (Fig. 1E). CD151 knockdown in CH7-C17 cells, which do not express endogenous CD9 (Supporting Information Fig. 2C), blunted the spike in CD69 surface expression and IL-2 secretion in response to Staphylococcus enterotoxin B (SEB, Raji/SEB) or in an antigen-specific model (HA peptide, Hom-2/HA) (Supporting Information Fig. 2E). Similarly, CD9 and CD151 knocked-down primary T lymphoblasts were conjugated with Raji/SEE, and T-cell activation was measured by flow cytometry. In the various experiments performed, only 5-20% of T lymphoblasts were positive for Vβ8 TCR, and thus able to recognize the SEE presented by SEE-loaded Raji B cells (data not shown). Despite the low percentage of responsive T lymphoblasts in each experiment, CD9 and CD151 silencing resulted in reduced expression of surface CD69 and intracellular IL-2 (Fig. 1F). Off-target effects were ruled out by using multiple RNAi sequences in the three conjugation systems (data not shown). Together, these results indicate that CD9 and CD151 play a role in T-cell activation.
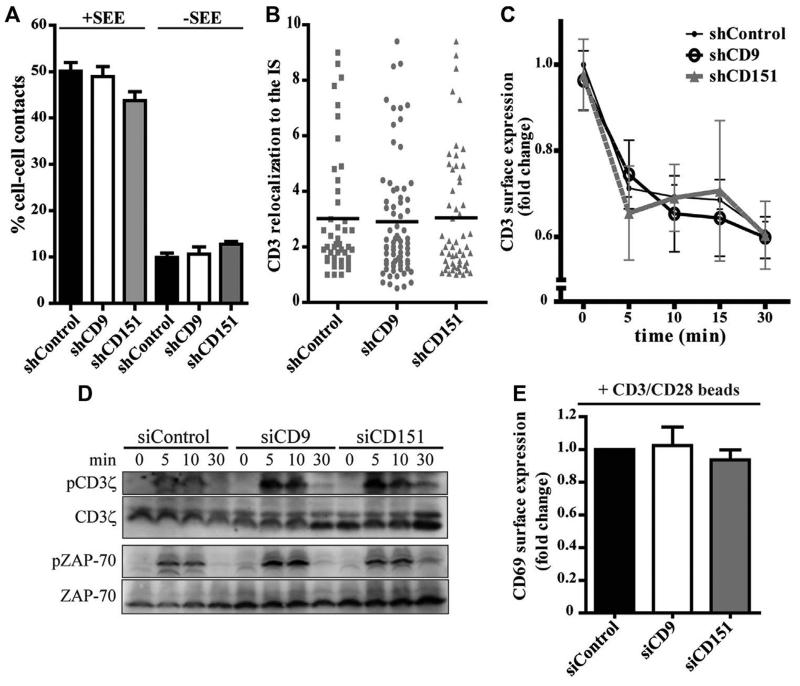
We next examined whether altered IS organization might also account for the impaired T-cell activation observed in the absence of CD9 and CD151. Silencing of CD9 or CD151 did not affect the total number of conjugates (Fig. 2A), the contact duration (Supporting Information Fig. 3A), the translocation of the microtubule-organizing center (MTOC) toward the IS (Supporting Information Fig. 3B), or the relocalization of F-actin (Supporting Information Fig. 3C) or CD3 (Fig. 2B) to the contact area. Tetraspanin knock-down did not affect CD3 surface expression upon Raji/SEE conjugation for different times (Fig. 2C), indicating that CD3 internalization following T-cell activation was not impaired in silenced cells. Moreover, CD3ζ and ZAP-70 phosphorylation in CD9- and CD151-silenced cells was similar to control cells (Fig. 2D). Accordingly, CD9 and CD151 silencing did not modify CD69 induction upon stimulation with anti-CD3/anti-CD28-coated beads (Fig. 2E). Together, these results indicate that CD9 and CD151 do not modulate CD3-mediated T cell signaling. The role of CD9 and CD151 during T-cell activation might be TEM-mediated, contrasting with CD81, which regulates synapse formation and subsequent T-cell activation through TCR signaling events [5], and with CD82, which regulates the T cell actin cytoskeleton [7].
Figure 2. CD9 and CD151 do not modulate CD3-mediated signaling.
(A) J77/shRNA T cells and Raji/+SEE (+SEE) or Raji/-SEE (-SEE) B cells were conjugated for 20 min, fixed, stained for tubulin. The percentage of cell-cell contacts was quantified by confocal microscopy. Data are means + SEM of at least 100 contacts, pooled from 3 independent experiments. (B) J77/shRNA cells conjugated with Raji/SEE for 20 min were fixed, stained for CD3, and protein relocalization to the IS was quantified by confocal microscopy. Each symbol represents an individual conjugate and horizontal lines represent the means of at least 50 conjugates, pooled from 2 independent experiments. (C) J77/shRNA cells were conjugated with Raji/SEE for the indicated times. Surface CD3 was analyzed by flow cytometry. Flow cytometry gating strategy is shown in Supplementary Information Fig. 2A. Data are presented relative to shControl cells incubated with SEE-unloaded Raji B cells (time 0), means ± SEM. Data are pooled from 3 independent experiments performed in triplicate. (D) J77/siRNA cells were incubated at a 10:1 ratio with Raji/SEE for the indicated times, then cells were lysed and immunoblotted for phosphorylated and total CD3ζ, and phosphorylated and total ZAP-70. Total CD3ζ and total ZAP-70 are loading controls. The blots shown are from a representative experiment out of three. (E) J77/siRNA cells were stimulated with anti-CD3/anti-CD28-coated dynabeads for 16 hours, then immunolabelled with CD69-allophycocyanin and analyzed by flow cytometry. Flow cytometry gating strategy is shown in Supplementary Information Fig. 2A. Data are presented relative to siControl cells and show means + SEM. Data are pooled from 2 independent experiments performed in triplicates.
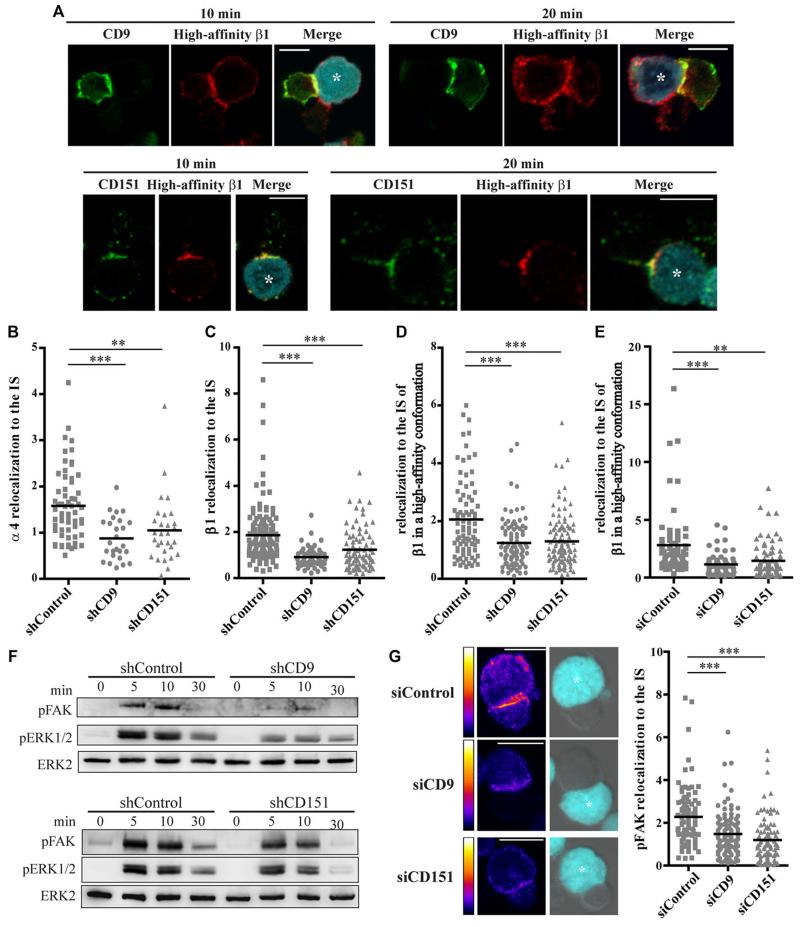
Adhesion of lymphocytes to the extracellular matrix and to other cells is mediated by integrins [8], and well characterized interactions between integrins and tetraspanins are important for the regulation of cell adhesion and migration [4, 9]. B-cell migration is regulated by CD9 through β1 integrin-mediated activation of tyrosine kinases, and also by CD81 in a process dependent on α4β1 integrin [4]. CD151 and α3β1 integrin play a role in neutrophil migration in vivo [4], and association of CD9 with ICAM-1 and of CD151 with VCAM-1 is important for leukocyte transendothelial migration [10]. Tetraspanins modulate the avidity of integrins for their ligands [11] and their diffusion mode at the plasma membrane [12]. To investigate the possible role of tetraspanin-integrin interactions in T-cell activation, we assessed integrin enrichment at the IS. At the IS of primary T lymphoblasts conjugated for 10 or 20 min with Raji/SEE, CD9 and CD151 partially colocalized with β1 integrin in a high-affinity conformation, as immunolabelled with HUTS21 mAb (Fig. 3A). Interestingly, relocalization to the IS of integrins α4 (Fig. 3B) and β1 (Fig. 3C) was significantly impaired in CD9- and CD151-silenced T cells. Moreover, the accumulation at the IS of high-affinity conformation β1 integrins was also reduced in knocked-down cells (Fig. 3D). Likewise, conjugates formed between CD9- and CD151-silenced T lymphoblasts and Raji/SEE displayed reduced IS accumulation of high-affinity β1 integrins (Fig. 3E). Since integrins have been demonstrated to be enriched at the pSMAC [2], CD9 and CD151 seem to contribute to pSMAC organization by regulating integrin accumulation at the IS, unlike CD81, which contributes to cSMAC formation by regulating CD3 dynamics at the IS [5]. Previous work showed that tetraspanins stabilize integrins in their active conformations [13] and that CD9 promotes β1 activation [14]. Activation of LFA-1 integrin is regulated by the actin linker protein talin [15], which has been implicated in integrin “inside-out” signaling [16]. However, talin relocalization to the IS in CD9- and CD151-silenced T cells remained similar to control cells (Supporting Information Fig. 3D), suggesting that “inside-out” integrin signaling might not be the mechanism driving the reduction in T-cell activation.
Figure 3. CD9 and CD151 promote integrin-mediated signaling.
(A) Primary T lymphoblasts were conjugated with Raji/SEE (blue, asterisk) for 10 or 20 min, fixed, immunolabelled for high-affinity β1 integrin (red), and CD9 or CD151 (green), then analyzed by confocal microscopy. A single confocal plane is shown. Scale bars = 10μm. Images are representative of two independent experiments. (B-D) Protein relocalization to the IS was assessed by confocal microscopy in J77/shRNA cells conjugated with Raji/SEE for 20 min, fixed and immunolabelled for (B) α4 integrin, (C) β1 integrin, (D) or high-affinity β1 integrin. Each symbol represents an individual conjugate (B and C, n = at least 50; D, n = at least 100) and means are indicated with horizontal lines. Data are pooled from (B) 2 and (C and D) 3 independent experiments. Protein relocalization in conjugates of J77/shCD9 or J77/shCD151 cells was compared to J77/shControl cells; **p < 0.01, ***p < 0.001. (E) Protein relocalization to the IS was assessed by confocal microscopy in CD9- and CD151-silenced primary T lymphoblasts conjugated with Raji/SEE for 20 min, fixed and immunolabelled for high-affinity β1 integrin. Each symbol represents an individual conjugate (n = 60) and horizontal lines indicate means. Data are pooled from 2 independent experiments. Protein relocalization in conjugates of J77/shCD9 or J77/shCD151 cells was compared to J77/shControl cells, **p < 0.01, ***p < 0.001. (F) J77/shRNA cells were incubated at a 10:1 ratio with Raji/SEE cells for the times indicated, lysed and immunoblotted for phosphorylated FAK, phosphorylated ERK1/2 and total ERK1/2, as loading control. The blots shown are from a representative experiment out of three. (G) Protein relocalization to the IS was assessed by confocal microscopy in CD9- and CD151-silenced primary T lymphoblasts conjugated with Raji/SEE for 20 min, fixed, and immunolabelled for phosphorylated FAK (pFAK). Example images of pFAK distribution in pseudocolor intensity-coding format (maximum intensity in white), and merged bright-field and blue channels (Raji/SEE, asterisks). Maximal projections of confocal stacks, Scale bar = 10 μm. The right graph shows pFAK relocalization to the IS. Each symbol represents an individual conjugate (n = 90) and horizontal lines indicate means. Data are pooled from 2 independent experiments. Protein relocalization in conjugates of J77/shCD9 or J77/shCD151 cells was compared to J77/shControl cells, ***p < 0.001.
The impaired T-cell activation in the absence of CD9 and CD151 might instead be related to a reduction in direct integrin downstream signaling, as a consequence of reduced integrin accumulation at the contact area. Tetraspanins modulate downstream integrin signaling, promoting the phosphorylation of FAK, Src, p130CAS and paxillin [17]. FAK and Src mediate the activation of small GTPases [18], which are associated to tetraspanins [19, 20]. CD151 specifically supports integrin-dependent activation of small GTPases [19, 21] and their downstream target ERK1/2 [21], which is also increased by CD9 [22]. Our data reveal that silencing of CD9 or CD151 in J77 T cells, although did not affect CD3 downstream early signaling, reduced the phosphorylation of FAK and ERK1/2 over the conjugation period with Raji/SEE (Fig. 3F). Moreover, CD9 and CD151 knock-down in primary T lymphoblasts impaired the accumulation of phosphorylated FAK at the IS (Fig. 3G). Together, these results are consistent with the reductions in CD69 and IL-2 expression, since they both depend on ERK1/2 activation, and CD69 expression has been related to FAK phosphorylation after T-cell integrin engagement [23, 24].
CONCLUDING REMARKS
The results of this study provide evidence that tetraspanins CD9 and CD151 promote T-cell activation through the regulation of integrins within TEMs. CD9 and CD151 promote the relocalization of α4β1 integrins as well as the accumulation of high-affinity β1 integrins at the T-B IS, thus creating the context for focal integrin-mediated activation of FAK and ERK1/2 and downstream T-cell activation and IL-2 secretion.
MATERIAL AND METHODS
Antibodies, reagents and recombinant DNA constructs
Poly-L-lysine (PLL), influenza HA peptide, unconjugated and FITC-conjugated anti-α-tubulin were from Sigma. Staphylococcus enterotoxins B and E were from Toxin Technology. Alexa-488 and 647, Streptavidin-488 and -647, Rhodamine X, phalloidin-488 and 647, anti-α-tubulin-647, and cell tracker CMAC (7-amino-4-chloromethylcoumarin) were from Invitrogen. The antibodies T3b (anti-CD3), TP1/40 (anti-αL integrin), HP2/1 (anti-α4), TS2/16 (anti- β1), HUTS21 (anti- β1 in a high-affinity conformation), TP1/55 (anti-CD69), VJ1/20 (anti-CD9), LIA1/1 (anti-CD151), VJ1/16 (anti-CD151) and HP2/6 (anti-CD4) were produced in our laboratory. CD81 mAb 5A6, CD3ζ 488 pAb, TS82b CD82 mAb and MEM53 CD53 mAb were kindly provided by Dr. S. Levy (Stanford, CA, USA), Dr. B. Alarcón (CBM, Spain), Dr. E. Rubinstein (Institut André Lwoff, Villejuif, France) and Dr. V. Horejsí (Czechoslovak Academy of Sciences, Praha, Czech Republic), respectively. Antibodies against phospho-ZAP-70 (Y493) and phospho-Erk1/2 (T202/Y204) were from Cell Signaling. ZAP-70 Ab, phospho-FAK (Y397), and phospho-CD3ζ (Y83) were from Abcam. Erk1/2 Ab was from Millipore, and CD69- allophycocyanin, IL-2- allophycocyanin and Vβ8-FITC from BD Biosciences.
CD9-mCherry (tag at the C-terminal domain) and mRuby-CD151 (tag at the N-terminal domain) were kindly provided by Dr. Rubinstein (Institut André Lwoff, France) and Dr. M.W. Davidson (Tallahassee, USA), respectively. The constructs pEGFP-N1-CD3 ζ [25], provided by Dr. B. Alarcón (CBM, Spain), CD9-EGFP and ICAM-1-EGFP were as described [10].
Cells, cell transfection and silencing
Cell culture conditions were as described [5] for Vβ8 Jurkat T cells (J77), HA-specific Vβ3 Jurkat T cells (CH7-C17), lymphoblastoid Raji and Hom2 (HLA-DR1 EBV-transformed) B cells, HEK293T cells, and peripheral blood lymphocytes from healthy donors
J77 cells (2×107) were washed twice with HBSS (Lonza) and transiently transfected by electroporation with plasmids (20 μg) or siRNA (1 μM) in OPTIMEM medium (Gibco, Invitrogen) at 240 V and 32 ms (Gene Pulser II, Bio-Rad). Control siRNA, siRNA for CD9 (GAGCAUCUUCGAGCAAGAA) and siRNA for CD151 (CAUGUGGCACCGUUUGCCU) were purchased from Eurogentec. siRNA knockdown was tested by flow cytometry at 48 hours post-transfection. Primary SEE T lymphoblasts were transfected by electroporation with siRNA 4-5 days after isolation using the same protocol. Lentiviral silencing of endogenous CD9 or CD151 was performed as described [5].
Conjugate formation
B cells (Raji or Hom2) were loaded with the CMAC tracker and SEE (0.5-1 μg/mL), SEB (0.5-1 μg/mL) or HA peptide (100-200 μg/mL) as appropriate. T and B cells were mixed (1:1) and incubated at 37°C for the indicated periods. Measurements of IL-2 secretion and immunoblotting were performed as described [5].
Flow cytometry
Conjugates were immunolabelled with specific primary antibodies followed by species-matched secondary antibodies coupled to fluorochromes. B cells were distinguished by CMAC staining and in shRNA experiments T cells were distinguished by Zsgreen fluorescence. Data were acquired with a FACSCantoII flow cytometer (BD), and then analyzed with FlowJo (Inc). For analysis of intracellular IL-2, primary T lymphoblasts were conjugated with Raji/SEE for 16 hours, then incubated 4 hours with 25mg/mL Brefeldin A (GolgiPlug, BD), fixed, permeabilized with PBS 0.3% Saponin for 10 min, and stained for CD9-FITC or CD151-FITC and for IL-2-allophycocyanin in the presence of PBS 0.1% Saponin. For activation of J77 T cells with antibodies, we used anti-CD3/anti-CD28-coated Dynabeads (Human T activator CD3/CD28, Gibco).
Fluorescence confocal microscopy
Conjugates were plated on poly-L-lysine-coated coverslips (40 μg/mL) for 10 min at 37°C. Fluorescence confocal microscopy and time-lapse live-cell fluorescence imaging were performed as described [5]. Confocal images were obtained with a Leica TCS-SP5 confocal scanning laser unit attached to an inverted epifluorescence DMI6000B microscope fitted with an HCX PL APO lambda blue 63X/1.4 NA oil immersion objective, using Las-AF acquisition software (Leica Microsystems), or alternatively with a Zeiss LSM700 confocal scanning laser unit attached to an inverted epifluorescence microscope (Observer.Z1) fitted with a Pan APO Chromat 63X/1.4 NA oil immersion objective, using ZEN 2009 acquisition sorftware (Carl Zeiss Microscopy GmbH). Images were analyzed with Leica LAS-AF, Metamorph or ImageJ (NIH).
Quantitative imaging analyses
Protein relocalization to the IS was quantified using the Synapsemeasure plugin in Image J [26]. Briefly, the quantification takes into account the fluorescence intensity signals measured in selected regions with similar areas at the T–APC contact zone (IS), the APC membrane not in contact with the T cell (B), the T cell membrane not in contact with the APC (T), and the background (Bg). Bg signal was subtracted from all other measurements, and then signal accumulation at the IS relative to the rest of the T cell surface was analyzed according to the formula (IS - B)/T. In the charts, each dot corresponds to a T-B conjugate.
Measurements of Pearson′s coefficient for the co-localization of CD3ζ-mEGFP and CD9-mCherry or mRuby-CD151 were performed with Imaris (Bitplane) from reconstructed 3D time-lapse stack confocal microscopy videos [5]. For each cell-cell conjugate, measurement of GFP-Cherry or GFP-mRuby co-localization was performed considering for all confocal stacks only the 1 μm closest to the Raji B cell membrane.
Statistical analysis
Statistical analyses were performed with GraphPad Prism (GraphPad Software Inc.). p values were calculated by using one-way ANOVA with Bonferroni’s post hoc multiple-comparison test (J77 T cells or primary T lymphoblasts) or two-tailed Student’s t test (CH7-C17 T cells).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank S. Bartlett (CNIC) for manuscript editing. Microscopy analysis was carried out at the CNIC Microscopy & Dynamic Imaging Unit. This study was supported by grants SAF2011-25834 from the Spanish Ministry of Economy and Competitiveness, INDISNET-S2011/BMD-2332 from the Comunidad de Madrid, Cardiovascular Network RD12-0042-0056 from the Instituto Salud Carlos III, and ERC-2011-AdG 294340-GENTRIS.
List of abbreviations
- APC
antigen-presenting cell
- CMAC
7-amino-4-chloromethylcoumarin
- cSMAC
central supramolecular activation cluster
- ERK1/2
extracellular signal-related kinase 1/2
- FAK
focal adhesion kinase
- HA
hemagglutinin
- Hom-2/HA
CMAC-labeled HA-loaded Hom-2 B cells
- ICAM-1
intracellular adhesion molecule 1
- IL-2
interleukin 2
- IS
immune synapse
- LFA-1
lymphocyte function-associated antigen 1
- pSMAC
peripheral supramolecular activation cluster
- Raji/SEB
CMAC-labeled SEB-loaded Raji B cells
- Raji/SEE
CMAC-labeled SEE-loaded Raji B cells
- SEB
Staphylococcus enterotoxin B
- SEE
Staphylococcus enterotoxin E
- TCR
T-cell receptor
- TEMs
tetraspanin-enriched microdomains
- VCAM-1
vascular cell adhesion molecule 1
- VLA-4
very late antigen 4
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- 2.Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, et al. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci U S A. 2004;101:11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24:107–115. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarrant JM, Robb L, van Spriel AB, Wright MD. Tetraspanins: molecular organisers of the leukocyte surface. Trends Immunol. 2003;24:610–617. doi: 10.1016/j.it.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Rocha-Perugini V, Zamai M, Gonzalez-Granado JM, Barreiro O, Tejera E, Yanez-Mo M, Caiolfa VR, Sanchez-Madrid F. CD81 controls sustained T cell activation signaling and defines the maturation stages of cognate immunological synapses. Mol Cell Biol. 2013;33:3644–3658. doi: 10.1128/MCB.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 7.Delaguillaumie A, Lagaudriere-Gesbert C, Popoff MR, Conjeaud H. Rho GTPases link cytoskeletal rearrangements and activation processes induced via the tetraspanin CD82 in T lymphocytes. J Cell Sci. 2002;115:433–443. doi: 10.1242/jcs.115.2.433. [DOI] [PubMed] [Google Scholar]
- 8.Herter J, Zarbock A. Integrin Regulation during Leukocyte Recruitment. J Immunol. 2013;190:4451–4457. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- 9.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 10.Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigelson SW, Grabovsky V, Shamri R, Levy S, Alon R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J Biol Chem. 2003;278:51203–51212. doi: 10.1074/jbc.M303601200. [DOI] [PubMed] [Google Scholar]
- 12.Yang XH, Mirchev R, Deng X, Yacono P, Yang HL, Golan DE, Hemler ME. CD151 restricts the alpha6 integrin diffusion mode. J Cell Sci. 2012;125:1478–1487. doi: 10.1242/jcs.093963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishiuchi R, Sanzen N, Nada S, Sumida Y, Wada Y, Okada M, Takagi J, et al. Potentiation of the ligand-binding activity of integrin alpha3beta1 via association with tetraspanin CD151. Proc Natl Acad Sci U S A. 2005;102:1939–1944. doi: 10.1073/pnas.0409493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez-Lopez MD, Ovalle S, Yanez-Mo M, Sanchez-Sanchez N, Rubinstein E, Olmo N, Lizarbe MA, et al. A functionally relevant conformational epitope on the CD9 tetraspanin depends on the association with activated beta1 integrin. J Biol Chem. 2003;278:208–218. doi: 10.1074/jbc.M207805200. [DOI] [PubMed] [Google Scholar]
- 15.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada M, Sumida Y, Fujibayashi A, Fukaguchi K, Sanzen N, Nishiuchi R, Sekiguchi K. The tetraspanin CD151 regulates cell morphology and intracellular signaling on laminin-511. FEBS J. 2008;275:3335–3351. doi: 10.1111/j.1742-4658.2008.06481.x. [DOI] [PubMed] [Google Scholar]
- 18.Choma DP, Milano V, Pumiglia KM, DiPersio CM. Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J Invest Dermatol. 2007;127:31–40. doi: 10.1038/sj.jid.5700505. [DOI] [PubMed] [Google Scholar]
- 19.Hong IK, Jeoung DI, Ha KS, Kim YM, Lee H. Tetraspanin CD151 stimulates adhesion-dependent activation of Ras, Rac, and Cdc42 by facilitating molecular association between beta1 integrins and small GTPases. J Biol Chem. 2012;287:32027–32039. doi: 10.1074/jbc.M111.314443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tejera E, Rocha-Perugini V, Lopez-Martin S, Perez-Hernandez D, Bachir AI, Horwitz AR, Vazquez J, et al. CD81 regulates cell migration through its association with Rac GTPase. Mol Biol Cell. 2013;24:261–273. doi: 10.1091/mbc.E12-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawada S, Yoshimoto M, Odintsova E, Hotchin NA, Berditchevski F. The tetraspanin CD151 functions as a negative regulator in the adhesion-dependent activation of Ras. J Biol Chem. 2003;278:26323–26326. doi: 10.1074/jbc.C300210200. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki T, Takeda Y, Maruyama K, Yokosaki Y, Tsujino K, Tetsumoto S, Kuhara H, et al. Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro. J Biol Chem. 2013;288:2118–2131. doi: 10.1074/jbc.M112.424291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neto EH, Coelho AL, Sampaio AL, Henriques M, Marcinkiewicz C, De Freitas MS, Barja-Fidalgo C. Activation of human T lymphocytes via integrin signaling induced by RGD-disintegrins. Biochim Biophys Acta. 2007;1773:176–184. doi: 10.1016/j.bbamcr.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Hao J, Metzger DL, Ao Z, Chen L, Ou D, Verchere CB, et al. B7-H4 Treatment of T Cells Inhibits ERK, JNK, p38, and AKT Activation. PloS one. 2012;7:e28232. doi: 10.1371/journal.pone.0028232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Martin N, Fernandez-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabia-Linares C, Robles-Valero J, de la Fuente H, Perez-Martinez M, Martin-Cofreces N, Alfonso-Perez M, Gutierrez-Vazquez C, et al. Endosomal clathrin drives actin accumulation at the immunological synapse. J Cell Sci. 2011;124:820–830. doi: 10.1242/jcs.078832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.