Abstract
Background
Brucella melitensis is the most important pathogenic species of Brucella spp. which affects goats and sheep and causes caprine and ovine brucellosis, respectively. Serological tests for diagnosis of brucellosis such as Rose Bengal plate test (RBPT) and enzyme-linked immunosorbent assay (ELISA) usually utilize smooth lipopolysaccharides (S-LPS) as a diagnostic antigen which could give false positive serological reactions. Outer membrane proteins (OMP) of B. melitensis have been used as alternative diagnostic antigens rather than S-LPS for differential serological diagnosis of brucellosis, mainly in ELISA with single recombinant OMP (rOMP) as a diagnostic antigen. Nevertheless, the use of single format mainly showed lack of sensitivity against the desired rOMP. Therefore, this study aimed to determine whether a newly developed rOMPs indirect ELISA (rOMPs I-ELISA), based on combination of rOMP25, rOMP28 and rOMP31of B. melitensis, has a potential benefit for use in the serodiagnosis of brucellosis.
Methods
In this study, omp25, omp28 and omp31 of B. melitensis were cloned and expressed using prokaryotic pET-32 Ek/LIC system and their respective rOMPs were combined as one coating antigen to develop rOMPs I-ELISA. Three groups of BALB/c mice were used to elicit antibody response. Group 1, infected with B. melitensis strain 0331 field strain; group 2, injected with B. melitensis Rev.1 vaccine strain and group 3, infected with Yersinia enterocolitica O:9. Antibody responses in three groups of mice were investigated using Rose Bengal plate test (RBPT) and rOMPs I-ELISA.
Results
The production of rOMP25, rOMP28 and rOMP31 of B. melitensis were achieved and Western immunoblotting analysis demonstrated their reactivity. The RBPT was unable to differentiate the vaccinated mice (group 2) and mice infected with Y. enterocolitica O:9 (group 3) and categorized them wrongly as positive for brucellosis. In contrast, the rOMPs I-ELISA was able to differentiate the mice infected with B. melitensis strain 0331 (group 1) from both of group 2 and group 3, and recorded 100% sensitivity and 100% specificity.
Conclusions
The results of this study suggested that rOMPs of B. melitensis has potential diagnostic ability to differentiate the FPSR in serological diagnosis of brucellosis.
Keywords: Brucella melitensis, rOMPs, FPSR, Mice, ELISA, Recombinant protein
Background
Brucellosis is one of the most important bacterial zoonoses worldwide [1]. Brucella melitensis is the main etiological agent of sheep and goats, and human brucellosis [2]. In control programs of brucellosis, practical solutions for diagnosis of the disease require inexpensive, simple, rapid and specific test to identify the infected animals [3]. Therefore, an indirect diagnosis approach of brucellosis using serological methods mainly Rose Bengal plate test (RBPT), complement fixation test (CFT) and enzyme-linked immunosorbent assay (ELISA) are recommended for large-scale surveillance and/or eradication purposes [4]. These tests usually use S-LPS, part of S-LPS or whole cells as an antigen to detect antibodies to smooth Brucella spp. which could give false positive serological reactions (FPSR) results due to difficulties to differentiate between animals vaccinated with B. melitensis Rev.1 strain and infected animals [5–7]. Another reason which can lead to FPSR is cross-reactivity with other Gram-negative bacteria like Yersinia enterocolitica O:9, Salmonella spp. and Escherichia coli [2, 8, 9].
The outer membrane proteins (OMP) of Brucella spp. were found to be attractive alternative antigens rather than S-LPS for serological diagnosis to minimize the FPSR [10]. Brucella OMPs are grouped according to their apparent molecular weights as group 1 (94 or 88 kDa), group 2 (36–38 kDa), and group 3 (25–27 and 31–34 kDa). Group 1 was identified as minor whereas group 2 and 3 OMPs were identified as major OMPs [11]. Group 3 major OMPs have been approved to be useful for the differentiation of antibody responses between naturally infected animals and Rev.1 vaccinated animals [12, 13]. Two genes were identified for the group 3 proteins of Brucella and were named omp25 and omp31, coding for 25–27 and 31–34 kDa major OMPs, respectively [14, 15]. In addition, OMP28 of B. melitensis has been identified as another member of group 3 OMPs which is coded by omp28 gene [16]. Others reported that OMP28 is a cytosoluble 28 kDa protein (CP28) which is localized in the periplasm [13], or 26 kDa periplasmic protein (BP26) which is coded by bp26 gene [17]. Several individual omp genes have been cloned and their expressed proteins were tested in immunoenzymatic assays for serodiagnosis of brucellosis in animals like recombinant OMP25 [18], recombinant OMP28 [19] and recombinant OMP31 [20]. However, lack of sensitivity to detect antibodies against the desired rOMP was the main obstacle facing these recombinant proteins. For that reason, combination of more than one recombinant protein in a single immunoenzymatic test could increase the sensitivity [21].
Small laboratory animals are frequently employed as models in brucellosis research [22]. Among them, BALB/c mice, has been extensively used in brucellosis research for many years mainly due to economic and practical reasons [22–24]. In addition the well-known biology of this murine species, especially the humoral and cellular immunity, makes it the ideal model for brucellosis research [22].
Accordingly, this study aimed to describe the expression and purification of three recombinant proteins, rOMP25, rOMP28 and rOMP31, of B. melitensis using E. coli expression system. The produced recombinant proteins were combined and used as one coating antigen in an indirect ELISA (I-ELISA) to evaluate its differential serodiagnosis using mouse mode.
Results
Construction of pET-32 Ek/LIC-omp cloning vector
Using polymerase chain reaction (PCR), the omp25, omp28 and omp31 gene were amplified from the chromosomal DNA of B. melitensis strain 0331 using gene specific primers and produced the expected product sizes of 668, 779 and 749 bp for omp25, omp28 and omp31 respectively (Fig. 1).
Fig. 1.
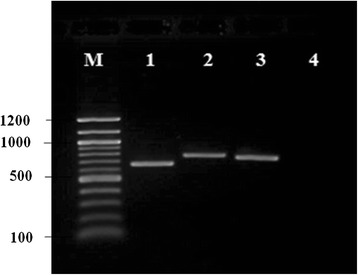
Agarose gel electrophoresis of PCR products of omp genes of B. melitensis strain 0331. Using gene specific primers that include the indicated 5′ LIC extensions, producing expected bands. Lane M, 100 bp DNA ladder (New England Biolabs, USA); lane 1, omp25 with product size 668 bp; lane 2, omp28 with product size 779 bp; lane 3, omp31 with product size 749 bp; lane 4, negative control
Nucleotide sequence of omp25, omp28 and omp31
Nucleotide sequence analysis of pET-32 Ek/LIC-omp25, pET-32 Ek/LIC-omp28 and pET-32 Ek/LIC-omp31 inserts revealed the presence of open reading frame (ORF) of 642, 753 and 723 nucleotides for the three genes respectively. The sequences of omp25, omp28 and omp31 were deposited in the Genbank and assigned the accession numbers [GenBank: JX627633], [GenBank: JX627634] and [GenBank: JX627635] respectively.
SDS-PAGE analysis and immunoreativity of recombinant fusion proteins
The majority of the expressed rOMP25, OMP28 and OMP31 were found in the soluble fraction. Therefore, the expressed proteins were extracted and purified under native conditions. Approximately final concentration of 50 μg/ml purified recombinant protein could be achieved. The SDS-PAGE analysis showed the presence of the expected 42 k, 45 kDa and Da 48 kDa of purified recombinant fusion proteins (Figs. 2, 3 and 4). The results of Western immunoblot analysis revealed the immunoreactivity of purified rOMP25, OMP28 and OMP31 with the three types of antibodies; two types of monoclonal antibodies (His.Tag and S.Tag HRP conjugated monoclonal antibodies), in addition to rabbit polyclonal antibodies against B. melitensis strain 0331 (Figs. 5, 6 and 7).
Fig. 2.
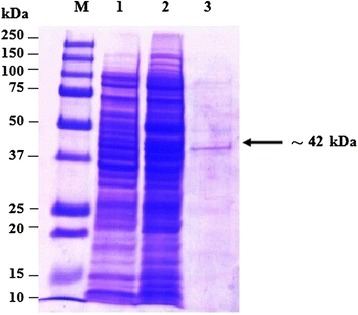
Analysis by SDS–PAGE of rOMP25 fusion protein. Lane M, Protein Marker; lane 1, soluble cell proteins of E. coli BL21(DE3) cells with recombinant omp25 before induction; lane 2, soluble cell proteins of E. coli BL21(DE3) cells with recombinant omp25 after autoinduction; lane 3, rOMP25 fusion protein (arrow) purified by affinity purification
Fig. 3.
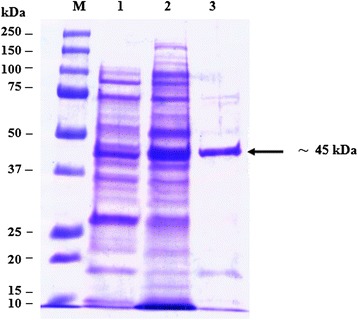
Analysis by SDS–PAGE of rOMP28 fusion protein. Lane M, Protein Marker; lane 1, soluble cell proteins of E. coli BL21(DE3) cells with recombinant omp28 before induction; lane 2, soluble cell proteins of E. coli BL21(DE3) cells with recombinant omp28 after autoinduction; lane 3, rOMP28 fusion protein (arrow) purified by affinity purification
Fig. 4.
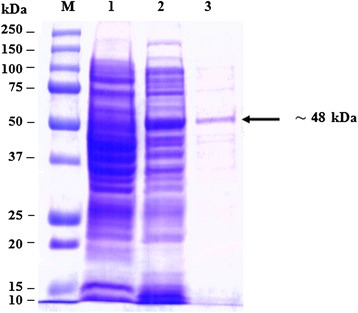
Analysis by SDS–PAGE of rOMP31 fusion protein. Lane M, Protein Marker; lane 1, soluble cell proteins of E. coli BL21(DE3) cells with recombinant omp31 before induction; lane 2, soluble cell proteins of E. coli BL21(DE3) cells with recombinant omp31 after autoinduction; lane 3, rOMP31 fusion protein (arrow) purified by affinity purification
Fig. 5.
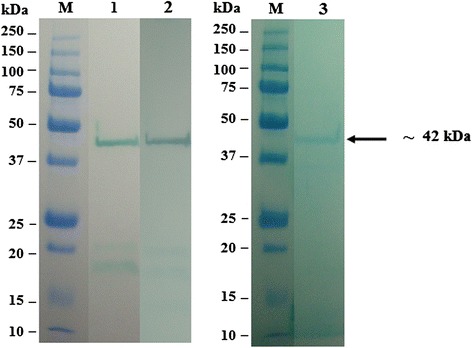
Western immunoblot analysis of purified rOMP25 fusion protein. Lane M, Precision Plus Protein All Blue Standards (Bio-Rad, USA); lane 1, purified rOMP25 detected using His.Tag monoclonal antibody; lane 2, purified rOMP25 detected using S.Tag monoclonal antibody; lane 3, purified rOMP25 detected using rabbit polyclonal antibodies against B. melitensis strain 0331
Fig. 6.
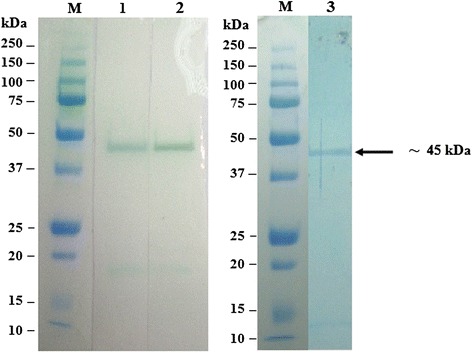
Western immunoblot analysis of purified rOMP28 fusion protein. Lane M, Precision Plus Protein All Blue Standards (Bio-Rad, USA); lane 1, purified rOMP28 detected using His.Tag monoclonal antibody; lane 2, purified rOMP28 detected using S.Tag monoclonal antibody; lane 3, purified rOMP28 detected using rabbit polyclonal antibodies against B. melitensis strain 0331
Fig. 7.
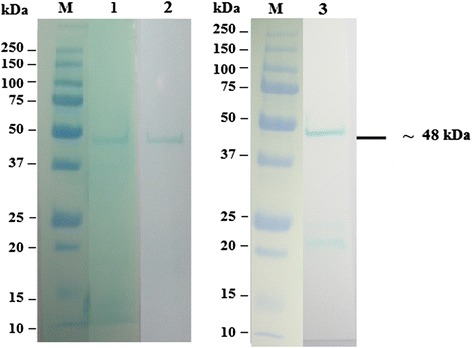
Western immunoblot analysis of purified rOMP31 fusion protein. Lane M, Precision Plus Protein All Blue Standards (Bio-Rad, USA); lane 1, purified rOMP31 detected using His.Tag monoclonal antibody; lane 2, purified rOMP31 detected using S.Tag monoclonal antibody; lane 3, purified rOMP31 detected using rabbit polyclonal antibodies against B. melitensis strain 0331
Rose Bengal plate test
The RBPT gave positive results for the tested sera of the three groups of mice, and it was unable to differentiate the antibody response among these groups. However, the RBPT results showed that the mice in group 1 (n = 45) were able to elicit high titers of anti-Brucella antibodies due to infection with Brucella melitensis strain 0331 and peaked 1100 IU/ml at day 42. While, the mice in group 2 (n = 45) were able to elicit lower titers of antibodies due to vaccination with B. melitensis Rev.1 strain, and peaked 900 IU/ml at day 49. No significant differences (p > 0.05) were found between group 1 and group 2 and this due to inability of RBPT to differentiate between the mice vaccinated with B. melitensis Rev.1 strain and those infected with B. melitensis strain 0331. Although the mice in group 3 (n = 45) developed too low titers of antibodies due to infection with Y. enterocolitica O:9 (peaked 100 IU/ml at day 42), the RBPT was able to detect these cross reacting antibodies (Fig. 8).
Fig. 8.
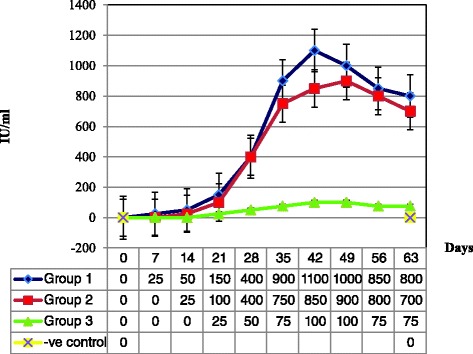
Antibodies titer of mice sera using semi-quantitative RBPT. Group 1, mice infected with B. melitensis strain 0331; group 2, mice vaccinated with B. melitensis Rev. 1 vaccine strain; group 3, mice infected with Y. enterocolitica O:9; (−ve) negative control, mice injected with PBS. ±SE at 95 % CI
Recombinant OMPs I-ELISA
All mice sera in three groups of mice and negative control were tested by I-ELISA using rOMPs as coating antigen. Using the receiver operating characteristics (ROC), a cut-off value of the positivity percent (PP) ≥ 43.30 was considered positive for brucellosis by rOMPs I-ELISA. The rOMPs I-ELISA results showed that antibodies against rOMPs was elicited as early as day 7 (PP = 49.5) and reach the peak at day 56 (PP = 95.5) in group1. While low antibody titers were detected in both group 2 and group 3 and recorded (PP = 25.3 and 13.2) at day 7, and (PP = 33.2 and 29.7) at day 56, respectively (Fig. 9). Tukey’s test analysis of PP values indicated the presence of significant difference (p < 0.05) between group 1 and each of group 2 and group 3. Moreover, by testing sera from mice infected with B. melitensis strain 0331, the results of rOMPs I-ELISA recorded 100 % sensitivity (45/45). When sera from mice vaccinated with B. melitensis Rev.1 vaccine strain was tested by rOMPs I-ELISA, 100 % specificity (45/45) was recorded. Additionally, 100 % specificity (45/45) was recorded when sera from mice infected with Y. enterocolitica O:9 were tested by rOMPs I-ELISA.
Fig. 9.
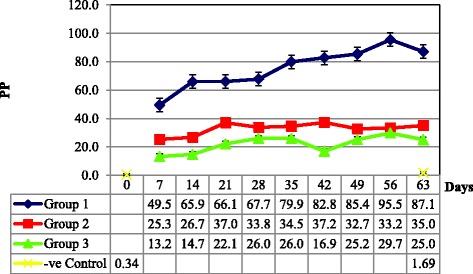
Percentage of positivity (PP) values of the sera from three groups of mice tested using in-house rOMPs I-ELISA. Group 1, mice infected with B. melitensis strain 0331; group 2, mice vaccinated with B. melitensis Rev. 1 vaccine strain; group 3, mice infected with Y. enterocolitica O:9; (−ve) negative control, mice injected with PBS. ±SE at 95 % CI
Discussion
In this study, rOMP25, rOMP28 and rOMP31 have been successfully produced and used together to develop rOMPs I-ELISA in a hope to increase the sensitivity of the developed test for differential serodiagnosis of brucellosis using mouse model. The isolation, cloning and expression of B. melitensis strain 0331 omp25, omp28 and omp31 genes were achieved using prokaryotic system pET-32 Ek/LIC. Using BioEdit sequence alignment editor software, the DNA sequencing results confirm that omp25, omp28 and omp31 genes had correct orientation, and they were in the open reading frame (ORF), which in turn enable full amino acids translation of these genes in the subsequent steps. The results of BLASTN alignment showed that omp25 has 99 % identity with published sequence of B. melitensis 16 M reference strain which is due to the difference in nucleotide number 276, which is C instead of T [18, 25]. In order to show whether this nucleotide difference resulting in differences in the amino acids sequence, the alignment of amino acids was performed. The result showed that the nucleotide difference at number 276 in the omp25 did not cause any differences in the translation of the amino acid glycine (Gly). While, the omp28 and omp31 obtained 100 % identity with published sequence of B. melitensis 16 M reference strain [16, 26] and [15, 20] respectively. Using amino acid composition feature of BioEdit software, the predicted molecular weight of the tagged fused proteins was estimated to be 17 kDa. In addition, the amino acids of omp25, omp28 and omp31 were also analyzed.
The SDS-PAGE analysis reveals the presence of protein bands of purified rOMP25, rOMP28 and rOMP31 with molecular weight of 42, 45 and 48 kDa respectively. These sizes were agreed with the theoretically molecular weights prediction of the expressed rOMP25, rOMP28 and rOMP31 fusion proteins respectively. Moreover, the results of this study agreed with results obtained by Letesson, et al. [21] and de Wergifosse, et al. [25] for the rOMP25, and Gupta, et al. [27] and Seco-Mediavilla, et al. [28] for the rOMP28, and Vizcaino, et al. [15] and Gupta, et al. [20] for the rOMP31. Furthermore, Western immunoblotting analysis revealed that the rOMP25, rOMP28 and rOMP31 fusion proteins were detected by His.Tag and S.Tag monoclonal antibodies. Additional conformation was made using rabbit polyclonal antibodies against Brucella melitensis strain 0331 to assure the immunogenicity of the rOMP25, rOMP28 and rOMP31 fusion proteins, which in turn indicate that these recombinant fusion proteins were expressed in soluble and active form.
The purified rOMP25, rOMP28 and rOMP31 were combined together and named rOMPs. Therefore, mouse model was used to determine the sensitivity and specificity of rOMPs I-ELISA. Accordingly, three groups of mice were set in this study. The first group was infected with B. melitensis strain 0331 to demonstrate the antibody response due to infection with B. melitensis field strain. While the second group was injected with B. melitensis Rev.1 vaccine strain, to demonstrate the antibody response due to vaccination, and the third group was infected with Y. enterocolitica O:9, to demonstrate cross reacting antibodies. Antibodies in sera of all mice in the first and second groups were detected using RBPT as early as 15 days after infection, and peaked at 35–49 days, while the sera from mice in the third group showed a much lower antibody reactivity using RBPT. According to OIE, the RBPT is considered as reference test for serological diagnosis of brucellosis. However, the inability to differentiate between infected and vaccinated animals are the main problem facing RBPT because it is based on detection of antibody to S-LPS that is immunodominant in the serological responses of both infected and vaccinated animals [4, 5]. Although low antibodies titer was elicited in group 3, the RBPT was able to detect cross reacting antibodies because of smooth Brucella strains share common epitopes on the O-PS with cross-reacting bacteria mainly Y. enterocolitica O:9 [2, 9], and in less extent, the natural infection by a number of other Gram negative bacteria can also produce cross reacting antibodies, mainly, E. coli O:157 and Salmonella spp. [2, 8]. The results of rOMPs I-ELISA, testing sera from three groups of mice, clearly indicated the presence of significant differences between group 1 and each of group 2 and group 3, and this due to ability of rOMPs I-ELISA to detect anti-OMP25, anti-OMP28 or antiOMP31 antibodies in mice infected with B. melitensis strain 0331 rather than mice vaccinated with B. melitensis Rev. 1 vaccine strain or infected with Y. enterocolitica O:9. Earlier studies showed greater accessibility of OMP25 OMP28 and OMP31 epitopes to antibodies generated due to infection with B. melitensis [19, 29]. However, previously developed ELISA based on single OMP was challenging due to low sensitivity recorded, like rOMP28 (88.7 %) [19] and rOMP31 (81.8 %) [20]. In our study, the rOMPs I-ELISA recorded 100 % sensitivity (45/45), means that all the mice infected with B. melitensis strain 0331 were recognized as positive by our developed ELISA. Additionally, the rOMPs I-ELISA recorded 100 % specificity (45/45), means that all mice vaccinated with Rev. 1 vaccine strain or infected with Y. enterocolitica O:9 were recognized as negative.
In summary, in this study, the developed rOMPs I-ELISA was able to increase the sensitivity to detect the infected animals. Moreover, it was also able to differentiate the animals with FPSR. These findings suggesting that rOMPs I-ELISA has potential diagnostic properties which could be used as an effective tool in the differential serodiagnosis of brucellosis.
Conclusions
Combination of rOMP25, rOMP28 and rOMP31 together clearly increased the sensitivity of the developed rOMPs I-ELISA. In addition, the results also indicated that the rOMPs I-ELISA has the ability to detect specific OMP antibodies due to infection with B. melitensis strain 0331 rather than vaccinal or cross reacting antibodies, and this means it has the ability to differentiate between infection with B. melitensis and FPSR. However, these result reflect the strict controlled conditions of our study, further evaluations should be performed which involves testing of the developed rOMPs I-ELISA in sheep and goats, as real hosts of B. melitensis, to achieve a definitive conclusion about its usefulness in differentiation of FPSR in the serological diagnosis of brucellosis.
Methods
Bacterial stain and growth conditions
Brucella melitensis strain 0331 Malaysian isolate has been used in this study as a source of the bacterial DNA for cloning and expression of omp25, omp28 and omp31 genes. This isolate has been confirmed as B. melitensis biovar 1 by Veterinary Laboratory Agency (VLA, Weybridge, UK). Fresh colonies of B. melitensis strain 0331 were cultured in Brucella broth (BBL™, BD, USA) for 4 days at 37 °C with continuous shacking; the bacterial cells were harvested by centrifuging.
PCR amplification and cloning
The DNA extraction and purification were performed following the manufacturer instructions (Qiagen, USA). Whereas, cloning was performed using pET-32 Ek/LIC system (Novagen, USA). Based on published sequence of B. melitensis 16 M reference strain [15, 16, 18], specific sets of primers were designed (Table 1) and the omp25, omp28 and omp31 genes were amplified using polymerase chain reaction (PCR). The expected products have extra 26 bp, represent vector cohesive overhangs (bold) for efficient cloning with pET-32 Ek/LIC vector. After amplification the produced insert was treated with LIC-qualified T4 DNA Polymerase, and then, annealed to the pET-32 Ek/LIC vector without the need for restriction digestion or ligation. The resultant nicked, circular plasmids are named pET-32 Ek/LIC-omp25, pET-32 Ek/LIC-omp28 and pET-32 Ek/LIC-omp31 transformed into NovaBlue GigaSingles™ Escherichia coli competent cells as initial cloning host. Positive inserts were screened by direct colony PCR using the designed gene specific primers and vector specific primers which includes T7 promoter and T7 terminator. Purified plasmid was sent to First BASE laboratories, (Malaysia) for sequencing. The obtained sequences were analyzed using The Basic Local Alignment Search Tool (BLAST®) of the National Center for Biotechnology Information (NCBI) database and, BioEdit sequence alignment editor software, version 7.0 [30].
Table 1.
Primers used for amplification of B. melitensis omp25, omp28 and omp31 genes with vector cohesive overhangs (bold)
| No | Primer name | Sequence (5′-3′) | Length | Expected product bp |
|---|---|---|---|---|
| 1. | B. melitensis omp25 F | GACGACGACAAGATGCGCACTCTTAAGTCTCT | 32 | 668 |
| 2. | B. melitensis omp25 R | GAGGAGAAGCCCGGTTAGAACTTGTAGCCGAT | 32 | |
| 3. | B. melitensis omp28 F | GACGACGACAAGATGAACACTCGTGCTAGCAATT | 34 | 779 |
| 4. | B. melitensis omp28 R | GAGGAGAAGCCCGGTTACTTGATTTCAAAAACGA | 34 | |
| 5. | B. melitensis omp31 F | GACGACGACAAGATGAAATCCGTAATTTTGGCGT | 34 | 749 |
| 6. | B. melitensis omp31 R | GAGGAGAAGCCCGGTTAGAACTTGTAGTTCAGAC | 34 |
Induction of expression and purification of recombinant protein
The positive constructed recombinant clones were used for transformation in expression prokaryotic host Escherichia coli BL21(DE3) competent cells (Novagen, USA). Direct colony PCR screening was performed to verify the positive clones using T7 promoter and T7 terminator primer sets. E. coli harboring pET-32 Ek/LIC-omp25, pET-32 Ek/LIC-omp28 and pET-32 Ek/LIC-omp31 plasmids were grown in LB medium and the cells were induced by Overnight Express™ Autoinduction System (Novagen, USA). This system is designed for high-level protein expression with pET expression system without the need to monitor cell growth. To obtain the soluble fraction of the cells, the protein extraction was performed using BugBuster® protein extraction kit with Benzonase® Nuclease (Novagen, USA). Metal chelation chromatography based method was used to purify the rOMP25, rOMP28 and rOMP31 using Ni-NTA His-Bind® Purification Kit (Novagen, USA). The uninduced cell lysate, induced cell lysate and purified rOMPs were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Production of rabbit hyperimmune serum against Brucella melitensis strain 0331
Two six months New Zealand white rabbits were used for raising hyperimmune against B. melitensis strain 0331 according to the method of Cloeckaert, et al. [31]. Ethical approval letter, AUP No: 10R1 17/Feb 11-Jan 12 was obtained from Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Universiti Putra Malaysia, before starting the experiment. The animals were housed under standard conditions at the animal house facility having free access to feed and water ad libitum.
The killed antigen was prepared by growing B. melitensis 0331 in 100 ml of brucella broth (BBL™, UK) for 3 days at 37 °C using incubator shaker. The broth was pelleted down and the cells were washed in phosphate buffer saline (PBS). Afer that the cells were killed by heating and adjusted to concentration of 109 organism/ml in PBS containing 0.5 % phenol by optical density measurment at 600 nm in a spectrophotometer (eppendorf Biophotometer plus).
The rabbites were injected subcutanously (s.c.) with B. melitensis 0331 (killed antigen) 1 × 109 colony forming units (cfu)/ml with Freund’s complete adjuvant (FCA) (Merck, Germany), followed by s.c. injection of two booster doses of B. melitensis (killed antigen) 0.5 × 109 cfu/ml with Freund’s incomplete adjuvant (FIA) (Merck, Germany) at days 21 and 35 respectively and the third booster dose was performed at day 45 by s.c. injection of B. melitensis (killed antigen) 0.5 × 109 cfu/ml with out any adjuvant. Blood samples were collected from ear vein under sedation at day 0 as self control, to reduce the number of animals included in the study, and subsequently every 2 weeks interval to assess the immune response of the rabbits. The hyperimmune serum against B. melitensis was collected by cardiac puncture under anesthesia on day 56. Once the required blood volume was collected, the rabbit was euthanized while still deeply anesthetized. The collected serum was stored at −20 °C until used.
Western Immunoblotting assay
The expressed rOMP25, rOMP28 and rOMP31 were confirmed to be soluble fusion protein by Western immunoblotting using His.Tag and S.Tag HRP conjugated monoclonal antibodies (Novagen, USA). In addition, rabbit polyclonal antibodies against Brucella melitensis strain 0331 was also used to confirm the results. Each purified rOMP was transferred from the unstained polyacrylamide gel onto 0.45 μm nitrocellulose membrane (Bio-Rad, USA) according to the standard procedure by Towbin, et al. [32]. Each blotted membrane which represents one of the rOMP was cut into three membrane parts and blocked using 1 % casein-TBST (Novagen, USA). After washing with TBST, the first membrane part was incubated with a dilution of 1:1500 of His-protein HRP conjugated monoclonal antibody. While the second membrane part was incubated with a dilution of 1:5000 of S-protein HRP conjugated monoclonal antibody. After washing, the colorimetric detection was carried out using TMB peroxidase substrate (Calbiochem, USA) until the color developed. The reaction was stopped by rinsing the membrane in deionized water. The third membrane part was incubated with 1:500 dilution of rabbit hyperimmune serum for 2 h and goat anti rabbit HRP conjugate (KPL, USA) was used as secondary antibody with 1:3000 dilution for 1 h. The remaining washing and colorimetric detection steps were the same as stated for His.Tag and S. Tag.
Mouse model for evaluation of rOMP25, rOMP28 and rOMP31
Total of (165) five-week-old female BALB/c mice were obtained from the animal facility, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM). Ethical approval letter, AUP No: 10R117/Feb 11-Jan 12 was obtained from IACUC, Faculty of Veterinary Medicine, Universiti Putra Malaysia, before starting the experiment. The mice were randomly distributed into three groups of 55 mice and housed in separated plastic cages in controlled animal house facilities for one week to acclimate their environment. The mice were provided with food and water ad libitum.
Following the procedure of Jimenez de Bagues, et al. [33], 45 mice in group 1 were infected intraperitoneally (i.p.) with 5 × 104 cfu of B. melitensis strain 0331 and 45 mice in group 2 were injected s.c. with B. melitensis Rev. 1 vaccine strain 5 × 105 cfu (CZveterinaria, Spain). While in group 3, 45 mice were infected i.p. with Y. enterocolitica O:9 3 × 104 cfu [34]. Additionally, for each of the three groups, five mice were sampled pre-infection and five mice were injected with PBS as negative control. Using cardiac puncture technique under general anesthesia, five serum samples were collected from the mice in each group every seven days interval starting at day seven to day 63. The negative controls were sampled at day 63. Once the required blood volume is collected, the mouse was euthanized while still deeply anesthetized using cervical dislocation to produce rapid euthanasia. Sera samples were stored at −20 °C until used.
Rose Bengal plate test
Sera samples collected from three groups of mice during the experiment period (0–63 days), in addition to negative control samples were tested using semi-quantitative RBPT as described by OIE [35] with some modifications. Briefly, isotonic saline solution was used to prepare serial dilutions of the mice sera (1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128), then equal volume of the diluted seum and RBPT antigen have been mixed and the results was read within 4 min for any positive agglutination. The titer can be calculated by multiplying the dilution factor by the detection limit 25, to give the IU/ml concentration. The RBPT antigen and control sera were obtained from Veterinary Laboratory Agency (VLA, Weybridge, UK).
Recombinant OMPs I-ELISA
Sera obtained from the three groups of mice were analyzed by rOMPs I-ELISA. The rOMPs coating antigen was produced by combination of equal concentrations of rOMP25, rOMP28 and rOMP31 and used as one coating antigen. The wells of polystyrene plates (Maxisorp, Nunc, Denmark) were coated with 100 μl of purified rOMPs at final concentration of 0.39 μg/ml in 0.05 M bicarbonate buffer (pH 9.6) and incubated overnight at 4 °C. This concentration was determined by checkerboard titration to reach optimal working conditions of sensitivity and specificity following the procedure discribed by Crowther [36]. The wells were emptied and washed three times with phosphate buffer saline-Tween20 (PBST) and then blocked with 5 % skim milk (oxoid, UK). Following incubation 100 μl of mice sera samples, positive and negative control sera diluted 1:200 were added into the plates in duplicate wells and incubated at 37 °C for 1 h. After washing with PBST for three times the plates were incubated with 1:5000 goat anti mouse HRPO IgG (H + L conjugate) (KPL, USA) for 1 h at 37 °C. After washing with PBST, the wells were filled with substrate solution containing TMB (3,3′,5,5′-tetramethyl benzidine) (KPL, USA). Finally, color development was stopped by adding 1 N HCL, after 10 min of incubation of the plates in dark at room temperature. The optical density was measured at 450 nm wavelength using an ELISA reader (Bio-Rad, USA).
Statistical analysis
IBM SPSS Statistics 19 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Differences in antibody titers expressed in IU/ml among the three groups using semi-quantitative RBPT were estimated by comparing the means using one-way ANOVA, and a P value < 0.05 was considered statistically significant at 95 % confidence interval (CI). The percentage of positivity (PP) value was calculated as described in the following equation.
The PP values were statistically analyzed using on-way ANOVA, Tukey’s multiple comparison test, to detect any significant differences among three groups of mice and a P value < 0.05 was considered statistically significant at 95 % CI. The receiver operating characteristics (ROC) was used in order to determine the cut-off value of the trade off between sensitivity and specificity of rOMPs I-ELISA using mice sera from group 1 and 2 including negative controls and the sensitivity and specificity of rOMPs I-ELISA were estimated accordingly [36].
Acknowledgements
This research was funded by Ministry of Higher Education, Malaysia. Grant 5450544. The authors would like to thank Pascual Rey, product manager of CZveterinaria, Spain for providing B. melitensis Rev.1 vaccine.
Abbreviations
- RBPT
Rose Bengal plate test
- ELISA
Enzyme-linked immunosorbent assay
- S-LPS
Smooth lipopolysaccharides
- FPSR
False positive serological reactions
- OMP
Outer membrane proteins
- rOMPs
Recombinant outer membrane proteins
- rOMPs I-ELISA
rOMPs indirect ELISA
- CFT
Complement fixation test
- PCR
Polymerase chain reaction
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- IACUC
Institutional animal care and use committee
- PP
Percentage of positivity
- CI
Confidence interval
- SE
Standard error
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AI was involved in experimental design, carried out the molecular work, sampling, sample processing, data analysis and drafting of manuscript. KB was involved in supervision, research conception and design, data analysis, interpretation and manuscript revision. LH was involved in data interpretation, statistical analysis and manuscript revision. BA was involved in supervision and manuscript revision. OA was involved in supervision and manuscript revision. All authors read and approved the final manuscript.
Contributor Information
Ihsan Muneer Ahmed, Email: ihsanahmad1@yahoo.com.
Siti Khairani-Bejo, Email: skhairani@upm.edu.my.
Latiffah Hassan, Email: latiffah@upm.edu.my.
Abdul Rani Bahaman, Email: rani@upm.edu.my.
Abdul Rahman Omar, Email: aro@upm.edu.my.
References
- 1.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–86. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Blasco JM, Molina-Flores B. Control and eradication of Brucella melitensis infection in sheep and goats. Vet Clin N Am Food A. 2011;27:95–104. doi: 10.1016/j.cvfa.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Boschiroli ML, Foulongne V, O’Callaghan D. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol. 2001;4:58–64. doi: 10.1016/S1369-5274(00)00165-X. [DOI] [PubMed] [Google Scholar]
- 4.OIE . Manual of diagnostic tests and vaccines for terrestrial animals. Paris: OIE; 2009. Office International des Épizooties, Caprine and ovine brucellosis (excluding Brucella ovis), chapter 2.7.2; pp. 1–10. [Google Scholar]
- 5.Nielsen K, Yu W. Serological diagnosis of brucellosis. Prilozi. 2010;31:65–89. [PubMed] [Google Scholar]
- 6.Godfroid J, Nielsen K, Saegerman C. Diagnosis of brucellosis in livestock and wildlife. Croat Med J. 2010;51:296–305. doi: 10.3325/cmj.2010.51.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poester FP, Nielsen K, Samartino LE, Yu WL. Diagnosis of brucellosis. Open Vet Sci J. 2010;4:46–60. doi: 10.2174/1874318801004010046. [DOI] [Google Scholar]
- 8.Velasco J, Romero C, López-Goñi I, Leiva J, Díaz R, Moriyón I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol. 1998;48:759–68. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 9.Weynants V, Tibor A, Denoel PA, Saegerman C, Godfroid J, Thiange P, et al. Infection of cattle with Yersinia enterocolitica O: 9 a cause of the false positive serological reactions in bovine brucellosis diagnostic tests. Vet Microbiol. 1996;48:101–12. doi: 10.1016/0378-1135(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 10.Garin-Bastuji B, Blasco JM, Marín C, Albert D. The diagnosis of brucellosis in sheep and goats, old and new tools. Small Rumin Res. 2006;62:63–70. doi: 10.1016/j.smallrumres.2005.08.004. [DOI] [Google Scholar]
- 11.Cloeckaert A, Vizcaíno N, Paquet J-Y, Bowden RA, Elzer PH. Major outer membrane proteins of Brucella spp.: past, present and future. Vet Microbiol. 2002;90:229–47. doi: 10.1016/S0378-1135(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 12.Cloeckaert A, Baucheron S, Vizcaino N, Zygmunt MS. Use of recombinant BP26 protein in serological diagnosis of Brucella melitensis infection in sheep. Clin Diagn Lab Immunol. 2001;8:772–5. doi: 10.1128/CDLI.8.4.772-775.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debbarh HSA, Cloeckaert A, Bezard G, Dubray G, Zygmunt MS. Enzyme-linked immunosorbent assay with partially purified cytosoluble 28-kilodalton protein for serological differentiation between Brucella melitensis-infected and B. melitensis Rev. 1-vaccinated sheep. Clin Diagn Lab Immunol. 1996;3:305–8. doi: 10.1128/cdli.3.3.305-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cloeckaert A, Zygmunt M, Bézard G, Dubray G. Purification and antigenic analysis of the major 25-kilodalton outer membrane protein of Brucella abortus. Res Microbiol. 1996;147:225–7. doi: 10.1016/0923-2508(96)81383-0. [DOI] [PubMed] [Google Scholar]
- 15.Vizcaino N, Cloeckaert A, Zygmunt MS, Dubray G. Cloning, nucleotide sequence, and expression of the Brucella melitensis omp31 gene coding for an immunogenic major outer membrane protein. Infect Immun. 1996;64:3744–51. doi: 10.1128/iai.64.9.3744-3751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindler LE, Hadfield TL, Tall BD, Snellings NJ, Rubin FA, Van De Verg LL, et al. Cloning of a Brucella melitensis group 3 antigen gene encoding Omp28, a protein recognized by the humoral immune response during human brucellosis. Infect Immun. 1996;64:2490–9. doi: 10.1128/iai.64.7.2490-2499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossetti OL, Arese AI, Boschiroli ML, Cravero SL. Cloning of Brucella abortus gene and characterization of expressed 26-kilodalton periplasmic protein: potential use for diagnosis. J Clin Microbiol. 1996;34:165–9. doi: 10.1128/jcm.34.1.165-169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloeckaert A, Verger JM, Grayon M, Zygmunt MS, Grepinet O. Nucleotide sequence and expression of the gene encoding the major 25-kilodalton outer membrane protein of Brucella ovis: Evidence for antigenic shift, compared with other Brucella species, due to a deletion in the gene. Infect Immun. 1996;64:2047–55. doi: 10.1128/iai.64.6.2047-2055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhuri P, Prasad R, Kumar V, Basavarajappa AG. Recombinant OMP28 antigen-based indirect ELISA for serodiagnosis of bovine brucellosis. Mol Cell Probes. 2010;24:142–5. doi: 10.1016/j.mcp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Gupta VK, Verma DK, Singh SV, Vihan VS. Serological diagnostic potential of recombinant outer membrane protein (Omp31) from Brucella melitensis in goat and sheep brucellosis. Small Rumin Res. 2007;70:260–6. doi: 10.1016/j.smallrumres.2006.01.012. [DOI] [Google Scholar]
- 21.Letesson JJ, Tibor A, Van Eynde G, Wansard V, Weynants V, Denoel P, et al. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997;4:556–64. doi: 10.1128/cdli.4.5.556-564.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva TM, Costa EA, Paixao TA, Tsolis RM, Santos RL. Laboratory animal models for brucellosis research. J Biomed Biotechnol. 2011;2011:1–9. doi: 10.1155/2011/518323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grillo MJ, Blasco JM, Gorvel JP, Moriyon I, Moreno E. What have we learned from brucellosis in the mouse model. Vet Res. 2012;43:1244–50. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plommet M. Brucellosis and immunity: Humoral and cellular components in mice. Res Microbiol. 1987;138:105–9. doi: 10.1016/0769-2609(87)90086-X. [DOI] [PubMed] [Google Scholar]
- 25.de Wergifosse P, Lintermans P, Limet JN, Cloeckaert A. Cloning and nucleotide sequence of the gene coding for the major 25-kilodalton outer membrane protein of Brucella abortus. J Bacteriol. 1995;177:1911–4. doi: 10.1128/jb.177.7.1911-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cloeckaert A, Debbarh HSA, Vizcaíno N, Saman E, Dubray G, Zygmunt MS. Cloning, nucleotide sequence, and expression of the Brucella melitensis bp26 gene coding for a protein immunogenic in infected sheep. FEMS Microbiol Lett. 1996;140:139–44. doi: 10.1111/j.1574-6968.1996.tb08327.x. [DOI] [PubMed] [Google Scholar]
- 27.Gupta VK, Kumari R, Vohra J, Singh SV, Vihan VS. Comparative evaluation of recombinant BP26 protein for serological diagnosis of Brucella melitensis infection in goats. Small Rumin Res. 2010;93:119–25. doi: 10.1016/j.smallrumres.2010.05.009. [DOI] [Google Scholar]
- 28.Seco-Mediavilla P, Verger JM, Grayon M, Cloeckaert A, Marin CM, Zygmunt MS, et al. Epitope Mapping of the Brucella melitensis BP26 Immunogenic Protein: Usefulness for Diagnosis of Sheep Brucellosis. Clin Vaccine Immunol. 2003;10:647–51. doi: 10.1128/CDLI.10.4.647-651.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen-xing L, Sen H, Zu-jian Q, Wei-ye C, Lin-tao L, Fang-kun W, et al. Expression, purification, and improved antigenic specificity of a truncated recombinant bp26 protein of Brucella melitensis M5-90: a potential antigen for differential serodiagnosis of brucellosis in sheep and goats. Biotechnol Appl Biochem. 2011;58:32–8. doi: 10.1002/bab.11. [DOI] [PubMed] [Google Scholar]
- 30.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. 1999. pp. 95–8. [Google Scholar]
- 31.Cloeckaert A, De Wergifosse P, Dubray G, Limet JN. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect Immun. 1990;58:3980–7. doi: 10.1128/iai.58.12.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez de Bagues MP, Elzer PH, Blasco JM, Marin CM, Gamazo C, Winter AJ. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect Immun. 1994;62:632–8. doi: 10.1128/iai.62.2.632-638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins-Browne RM, Prpic JK. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985;47:774–9. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.OIE . Manual of diagnostic tests and vaccines for terrestrial animals. Paris: OIE; 2009. Office International des Épizooties, Bovine brucellosis, chapter 2.4.3; pp. 1–35. [Google Scholar]
- 36.Crowther JR. Methods in molecular biology: The ELISA guidebook. 2. New York: Humana Press; 2009. [DOI] [PubMed] [Google Scholar]


