Abstract
Owing to technological progress and a growing body of clinical experience, indication criteria for cochlear implants (CI) are being extended to less severe hearing impairments. It is, therefore, worth reconsidering these indication criteria by introducing novel testing procedures. The diagnostic evidence collected will be evaluated. The investigation includes postlingually deafened adults seeking a CI. Prior to surgery, speech perception tests [Freiburg Speech Test and Oldenburg sentence (OLSA) test] were performed unaided and aided using the Oldenburg Master Hearing Aid (MHA) system. Linguistic skills were assessed with the visual Text Reception Threshold (TRT) test, and general state of health, socio-economic status (SES) and subjective hearing were evaluated through questionnaires. After surgery, the speech tests were repeated aided with a CI. To date, 97 complete data sets are available for evaluation. Statistical analyses showed significant correlations between postsurgical speech reception threshold (SRT) measured with the adaptive OLSA test and pre-surgical data such as the TRT test (r=−0.29), SES (r=−0.22) and (if available) aided SRT (r=0.53). The results suggest that new measures and setups such as the TRT test, SES and speech perception with the MHA provide valuable extra information regarding indication for CI.
Key words: cochlear implant, medical audiology, psychoacoustics/hearing science, speech perception.
Introduction
For more than 25 years now, cochlear implants (CIs) have been used to replace the function of the inner ear and thus restore hearing in people suffering from profound hearing loss or deafness.1,2 Initially, indication criteria were defined such that only individuals with no residual hearing in either ear could receive a CI. The ongoing technical development of these implants and growing clinical experience are also enabling an increasing number of people with less severe hearing loss to be provided with CIs. Before surgery, it is desirable to predict the expected benefit from a CI as accurately as possible in order to decide whether implantation is indicated. If there is significant residual hearing, the expected performance using the CI can be compared with that achieved using a hearing aid, enabling the best hearing device to be chosen for the individual patient. In cases without residual hearing, however, accurate prediction is also desirable as the basis for the patient's decision as to whether or not to undergo surgery. Another useful application of accurate performance prediction is the subsequent evaluation and optimization of individual fitting. If, for example, a patient's performance does not reach the predicted range, this could be a signal that the individual fitting is not optimal and should be re-evaluated. In any case, before a prediction model can be developed, factors influencing performance with a CI have to be identified.
To date, no reliable predictors for outcomes with different hearing devices have been identified so that audiologists rely mainly on their own experience. Some research groups detected correlations between speech perception with CI and demographic data, but the detected relationships vary widely. Age at deafness was detected to be an important factor if the patient is under 18 years old, but the authors do not quantify the relationship.3 Duration of deafness was more broadly supported. For example, Battmer et al.4 classified 245 CI users backwards into three performance groups depending on the speech perception with CI and looked for differences in the demographic group mean data. Duration of deafness was seen to be highly significant (P<0.01) with the best performance group being deaf for 7.3 years on average and the two worse groups being deaf for 17.9 years. They also found significant differences between the groups concerning onset of deafness; the best group was 40.5 years old and the other two groups 28.8 years old at onset of deafness, which was significantly lower (P<0.01). David et al.5 reported that duration of deafness was a significant predictor with 115 adult participants, but did not quantify the relationship. Gantz et al.6 found a significant correlation coefficient of r=−0.45 (shorter duration time is better) (P=0.002) but with only 39 adult participants. Shea et al.7 investigated 20 subjects and determined the influence of the duration of profound hearing loss by performing a stepwise multiple regression to r=0.518 (P<0.05). Van Dijk et al.8 investigated 37 subjects and found duration of deafness (r=−0.47) and residual hearing (r=0.42) to have the highest correlation coefficients to the CI outcomes. Age at implantation was also identified by Gantz et al.6 to be a significant factor (r=−0.28, P=0.044, n=39, lower age is better). The results of speech tests conducted before surgery (monosyllabic word tests) are also used to predict subsequent performance. Friedland et al.9 investigated the data of 58 subjects and found a regression coefficient of r=0.43 (P<0.001) between duration of deafness and speech perception. In the study by Leung et al.,10 the data sets of 749 adults were investigated by separating the subjects into two age groups and performing a multiple regression analysis. In the younger group, duration of deafness was found to be highly significant (P<0.001) and in the older group not (P=0.13). Thus, up to now, no reliable correlations with the CI outcome, such as for example, speech perception have been found, as the predictive power of the data used is apparently too low. Nevertheless, the current indication criteria depend (apart from medical findings) mainly on speech tests in what is termed the best aided condition. However, this condition is not clearly defined as the hearing aids used by the patients are not standardized, the algorithms vary widely and the hardware limited amplification is not always appropriate, even if the individual fitting is optimized. But since a fair grading of the patients requires the same testing conditions for each patient, the way in which patients' hearing aids are compared needs to be improved.
In essence, therefore, the established test procedures (pure tone audiometry, speech perception in quiet, demographical data, therapeutic assessments, electrophysiological measurements, vestibular tests and neuro-imaging) are not reliable predictors of hearing performance. Furthermore, hearing-aid technology is rapidly improving and, for patients with significant residual hearing, considerable benefit from hearing aids can be expected. Also, with increasingly improved performance levels for cochlear implants as well as hearing aids, new, more sophisticated tests such as, for example, speech tests in noise can be applied.11 When it comes to deciding for or against an implantable device, therefore, it is increasingly important to reliably predict outcomes such as, for example, speech perception with CI or subjective hearing improvements. Thus, novel test modalities that offer a more conclusive diagnosis have to be used.
This paper presents some of the tests that have been recently incorporated into our routine clinical practice and the diagnostic evidence they provide is investigated. Prior to surgery, speech tests were performed unaided and with a (theoretically) optimally fitted hearing aid. Linguistic skills were assessed, irrespective of hearing capabilities, by means of the visual Text Reception Threshold (TRT) test, whereas general state of health, socio-economic status (SES) and subjective hearing loss were surveyed using questionnaires. After surgery, the speech perception tests were repeated with the CI four days after initial activation and again after six months of device use. Correlations between the tests performed prior to surgery and the outcomes after surgery were investigated in order to identify factors influencing subsequent success with a CI.
Materials and Methods
Standard tests
The set of standard tests that subjects are required to complete in our routine clinical practice, before a decision about CI candidacy can be made, includes pure-tone audiometry and speech tests in quiet (Freiburg Speech Test consisting of monosyllabic words and polysyllabic numbers)12 with and without the patient's own conventional hearing aid. Prior to testing, the patient's own hearing aid is optimized by our hearing aid experts. This is based on the assumption derived from our clinical experience that the aided performance with an optimal hearing aid at 65 dB should be comparable to the unaided performance at 100 dB. Thus, the fitting of the hearing aid is adapted until this target is reached or until it is confirmed that the target is not reachable. Also electrophysiological measurements are taken and neuro-imaging performed. Demographic data are also obtained, such as duration of normal hearing, duration of hearing impairment (the time between the subject first realizing that his or her hearing is impaired and the onset of deafness), duration of hearing aid use, and duration of deafness. At our center, the onset of deafness is defined as being the moment at which the subject no longer feels able to communicate on the telephone. This standard set of tests is complemented by an additional set including the following test procedures that are new for assessing CI candidacy.
Regarding the expected relationships to the later performance with CI, we hypothesize demographic data such as age, duration of good hearing, impaired hearing, deafness and hearing-aid use to correlate with CI-aided performance with a long period of good hearing (unaided or with a hearing aid) and a short period of partial or total hearing loss resulting in better performance. Also, we expect significant residual hearing to lead to better performance with CI.
Additional set of tests
The novel set of tests before surgery consists of a visual test assessing linguistic competence and speech tests in quiet and in noise which are performed unaided and aided with the PC-based Oldenburg Master Hearing Aid,13 and questionnaires which address SES, subjective hearing abilities and general state of health.
Postoperatively, on Day 4 of the switch-on week the speech tests are repeated. The performance with CI (speech perception, subjective hearing ability) at the switch-on varies widely, as the subjects need different amounts of time to get used to their new way of hearing. Thus, during the first months of CI use, large improvements are usually seen. As this article focuses on the equilibrium performance after getting used to the implant, here the data concerning the switch-on appointment is not included.
After six months of CI use, the speech tests and the questionnaires assessing subjective hearing abilities are repeated.
Text reception threshold test
Background
The linguistic competence of a patient is investigated by means of the purely visually presented TRT test, modified after Zekveld et al.14 Their rationale was to detect relationships to a speech test in noise and to quantify how much this visual test relates to the performance in a speech test in noise. This test was carried out with normal hearing subjects, but good test-retest reliability and promising correlations were described. We chose this test because we thought it to be a useful tool for assessing linguistic skills irrespective of hearing capabilities. Hardware limitations and signal processing of cochlear implants lead to imperfect signal transmissions. Thus, for successful speech perception the patients have to reconstruct speech from degraded information on the audio signal. With additional background noise, this problem becomes even more serious and cognitive skills are required to successfully decode the audio information.14 Thus, the ability to understand the degraded signal, which varies significantly from patient to patient, could possibly be investigated with this visual TRT test. According to our rationale, we made some adaptations. A first evaluation was made with 20 normal hearing subjects. Consistent results with low standard deviations were obtained, so we included this modified test into our additional set of tests for CI candidates.
This test involves the sentences only being displayed visually on a monitor, with the subjects required to read them aloud. The sentences are masked with patterns of dots or bars, and the degree of masking varies depending on the individual performance (Figure 1).
Figure 1.
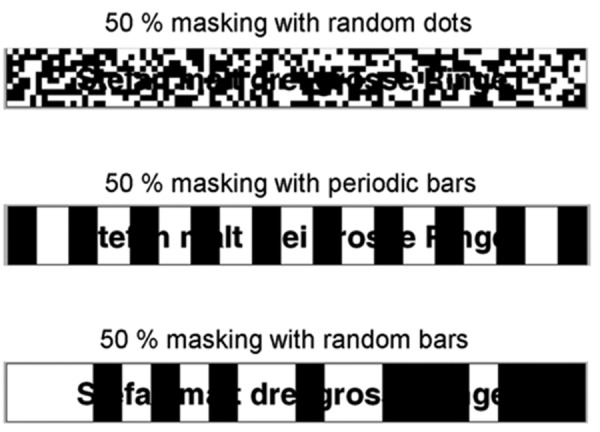
Example of the text reception threshold test: German sentence Stefan malt drei grosse Ringe, 50% masked with three different patterns. Top, random dots; middle, periodic bars; bottom, random bars.
In the same manner, as an adaptive auditory speech test in noise, in an adaptive staircase procedure the masking degree is adapted to the L50, which is the degree of masking at which the patient correctly repeats 50% of the words. Higher values thus correspond to better performance. Zekveld et al.14 determined the proportion of unmasked sentences that are necessary in order to understand 50% of the words. Therefore, with the test conducted in this manner, low values correspond to better performance.
The test material used consists of sentences from the Oldenburg sentence (OLSA) test, developed by Wagener et al.15 Forty-five different test lists are available, each consisting of 20 sentences. The sentences are displayed in black using the Arial typeface, font size 40, on a 17-inch monitor with a resolution of 1024–768 pixels. To ensure similarity with an auditory test, the computer software (MATLAB) limits the display time to 3.5 seconds, which is roughly the same time as that needed for the sentence to be spoken. Zekveld et al.14 use a Dutch test with Arial font, size 27, but do not indicate the size of the monitor or the resolution of the display. In their study, sentences are displayed in red. However, we decided not to adopt this because we did not think it was significant for CI candidates. Here, too, the display time is limited to 3.5 s. In the study by Zekveld et al., sentences are progressively displayed adding one word at a time, whereas we conducted the test such that the whole sentence is displayed immediately.
Three different patterns were used for masking purposes: random dots, periodic bars and random bars. In the random-dot pattern, the degree of masking is varied by changing the number of fixed-sized dots (size 12). The periodic-bar pattern uses 12 bars, the thickness of which is changed. The random-bar pattern involves mimicking a modulated noise, such as the ICRA5-noise.16 Here, both the number and thickness of the bars is varied according to the envelope of this noise. Zekveld et al.14 use only one masking pattern with periodic bars varying in thickness. Instead, we investigated different masking types since also CI subjects have to understand speech in the presence of different types of background noise.
Zekveld et al.14 determined the test-retest reliability of the TRT test to an intraclass correlation coefficient of 0.74, which is stated in their article as being acceptable for group comparisons according to the Scientific Advisory Committee of the Medical Outcomes Trust. Training effects were found in this study in the first list of the TRT test. In the TRT test as conducted in the present study, the characteristics of the OLSA test are important, as these sentences are used. Since the OLSA test uses a pool of 50 different words, the subjects learn these words within the first two lists, so that a strong training effect was described by Wagener and Brand.17 They found the test-retest stability for SRTs in noise to be 0.7 dB, and observed that at least two training lists were needed to remedy training effects. That is why we included two training lists into our TRT test protocol before starting with the test lists.
As for the expected results, we hypothesized a correlation between the performance in the TRT test and the later performance in the speech test in noise, with good TRT test results relating to good results in the speech test.
Experimental setup
The test procedure starts with two training lists in which the sentences are masked with dots or periodic bars, respectively, and the degree of masking is fixed at 15%. As they are included purely for training purposes, the results are not used further. The intrinsic test involves three test lists, one with each masking pattern. The order of the masking patterns and the test lists used are arbitrarily randomized by the examiner who carefully avoids using a list a second time. The lists begin with application of a masking degree of 50%, and this level is adapted to the 50% perception threshold according to the adaptive procedure described by Brand and Kollmeier.18 No repetitions were allowed. Each test takes 3–4 min so the complete test (including the training lists) takes approximately 20 min.
Master hearing aid
Background
The Oldenburg sentence test15 and the Freiburg Speech Test12 are performed unaided and aided with a conventional hearing aid. Instead of the patient's own hearing aids, the software-based Master Hearing Aid (MHA) developed by Grimm et al.13 was used. Audio signals from the speech tests (see below) are read from the hard disk, processed in real time by the MHA, DA-converted, amplified by a headphone buffer and presented to the subject via Sennheiser HDA200 headphones. The system is implemented on a standard PC system running the Windows operating system and allows a maximum output level of approximately 120 dB SPL. Free-field equivalent calibration was achieved using a B&K type 4153 artificial ear. Signal processing comprises FFT-based multichannel dynamic compression13 that is individually fitted using the CAMFIT fitting rule originally developed by Moore et al.,19 which was modified for severe and profound hearing losses as described below. By using the generic signal processing scheme in combination with the same fitting rule applied to all subjects, any uncontrolled variability introduced by the variety of signal processing schemes and fitting rules used in the patient's own hearing aids is avoided.
Compression was applied in nine overlapping frequency bands using a standard level meter with attack-and-release filter.13 Center frequencies in Hz were 250, 500, 1k, 1.5k, 2k, 3k, 4k 6k, 8k for the different frequency bands. Attack times in ms were 20, 10, 5, 3, 2, 1, 1, 1, 1. Release time was 100 ms in all frequency bands. The number of compression bands was chosen to provide a sufficient frequency resolution to fit sloping losses while avoiding spectral smearing. The center frequencies of the bands were chosen to match the audiological frequencies. The combination of short attack and rather long release times avoids overshoots and distortion, and allows the compressor to follow the short-time maxima of the speech signal. The design rationale of the CAMFIT fitting rule is to achieve normal loudness for a 65 dB speech-shaped signal and to ensure audibility of the minima present in speech.19 The rule was adopted because it provides balanced loudness at a wide range of levels, including high levels,19 and thus should be applicable to the group of subjects with severe and profound hearing loss. However, preliminary experiments with a small group of subjects showed that users complained about distortion. Consequently, we modified the CAMFIT rule to reduce distortion while maintaining audibility of speech information as much as possible. The modification is described in detail below and followed the rationale that the frequency regions with the highest relative hearing loss contribute the least to speech perception and thus can be excluded from amplification. This approach was adapted from the established NAL-NL1 fitting rule20 that also applies a band selection rule. Using the CAMFIT rule with band selection, no complaints about distortion were reported from the experiments and the MHA system using this rule consistently improved speech reception compared with patients' own hearing aids. We, therefore, assume that the CAMFIT rule as modified by us is applicable, even though we cannot currently prove its optimality.
Band selection
The band selection rules was implemented as follows. The frequency bands to be excluded from amplification were determined by applying the following rules: all bands with hearing loss of 110 dB or worse are muted. If the minimum hearing loss across all frequencies is worse than 55 dB, 65 dB, 75 dB or 85 dB, respectively, all bands with a hearing loss above the exclusion threshold of 85 dB, 95 dB, 100 dB or 105 dB are excluded from amplification (i.e. muted). In a second step, the number of muted bands is increased further. This involves further reducing the exclusion threshold value (thus muting more bands) subject to the constraint that three of the four frequency bands in the speech-relevant range from 500 Hz to 2 kHz are not muted. If, however, the frequency of the minimum hearing loss is outside that range, the exclusion threshold value is reduced even further until two of the four frequency bands are muted.
As for the results of the speech perception tests aided with the Master Hearing Aid system, we hypothesize that they correlate positively with CI-aided speech perception.
Experimental setup
The Oldenburg sentence test15 and the Freiburg Speech Test12 were chosen. Before surgery they are performed unaided and aided with Master Hearing Aid (MHA) system, and after surgery aided with CI. Pre-surgically the speech tests are aborted if the patient is not able to understand speech even with the aid of the MHA. The OLSA test and the MHA-aided Freiburg Test were conducted using the Oldenburg Measurement Applications (OMA, www.hoertech.de), whereas the unaided Freiburg Speech Test is carried out with our clinical standard apparatus, as it is already part of our standard set of tests. In both tests, the stimuli are presented monotically via headphones to the ear intended for implantation. The OLSA test begins with a training list in quiet presented at 65 dB SPL that is performed only with the aid of MHA. The basic structure of the sentences and the words that occur are already familiar to patients from the TRT test, so that a second training list is considered unnecessary. The test list used is randomized arbitrarily by the examiner who is careful to avoid using a test list twice. The OLSA test is then administered on an adaptive basis at a fixed noise level of 65 dB SPL. Unmodulated noise (olnoise)15 and modulated noise (ICRA5-250)21 are used. Olnoise is a speech-shaped stationary noise, whereas ICRA5-250 additionally has typical amplitude modulations of a single speaker. The procedure involves determining the SRT, which is the SNR in dB at which the patient understands 50% of the words. This kind of adaptive testing requires that the patient is able to understand at least 50% of the words, but this is usually the case only if he or she has significant residual hearing. Here, the hearing loss of the patient lies in the grey zone between hearing-aid and CI indication. If an individual's performance is not sufficiently good for an adaptive testing procedure, the OLSA test is presented at a fixed SNR of 10 dB for both types of noise mentioned above, with the signal also being fixed at a level of 65 dB SPL.
The Freiburg Speech Test, which consists of one list of monosyllabic words and another list of numbers, is presented in quiet at 65 dB SPL. The results are given as the percentage of correct responses.
After six months, the performance of the overwhelming majority of CI subjects closely approximates the equilibrium performance.2,22,23 This time, the tests are presented via loudspeakers in a sound-treated room. Signal and noise are presented at S0N0 at a distance of 1 m from the patient; again, the OLSA test is presented by means of the OMA and the Freiburg Speech Test using our standard clinical apparatus. If the contralateral ear contributes unaided to the hearing (if the hearing threshold is better than around 95 dB) it is closed with earplugs. The OLSA test starts with a training list in quiet at 65 dB SPL. It is then conducted on an adaptive basis at a fixed noise level of 65 dB SPL. Again, unmodulated (olnoise) and modulated (ICRA5-250) noise are used. If no adaptive measurement was possible before surgery, the fixed-level presentation is repeated with a fixed SNR of 10 dB (noise level 55 dB SPL, signal level 65 dB SPL). In all tests, we are careful to ensure that test lists other than those at the pre-surgery session are used.
The aim of this measurement protocol is two-fold: firstly, so (if possible) adaptive measurements can be taken to grade the performance of the individual CI patient in comparison with all CI patients, and secondly, to obtain data sets in consistent fixed-parameter conditions in order to evaluate each individual's progress.
The Freiburg Speech Test again consists of monosyllabic words and numbers presented in quiet at 65 dB SPL. However, this is already included in our standard set of clinical tests carried out during that particular appointment and is not, therefore, part of the additional set of tests.
Questionnaires
Background
Patients are asked to complete a set of questionnaires. The first of these assesses subjective hearing ability (Oldenburger Inventar).24,25 The estimated variables are subjective hearing in a quiet environment, subjective hearing in a noisy environment, and subjective localization ability, these being added to generate a total score for subjective hearing ability. All variables are estimated both unaided and with the patient's own hearing aid to obtain a score from 0 to 100, with higher values corresponding to better hearing. How the patient's social life is affected by his or her hearing impairment is investigated using the German version of the Gothenburg Profile26 (English version).27 Here, the results are given as inverse scores from 0 to 100, with higher values corresponding to greater severity of impact, so that lower values correspond to better performance. The next questionnaire addresses the general state of health; it is the short version (SF-12) of the Fragebogen zum allgemeinen Gesundheitszustand, SF-36,28 yielding summation scales for mental and physical health, with higher values corresponding to better health. The German normative reference sample generates an overall score of 52.24±8.10 for mental health and a score of 49.03±9.35 for physical health. A final subset of questions (Demographie und Schichtindex)29 investigates patients' SES. This generates a score from 4 to 21, with higher values corresponding to higher SES. Scores between 4 and 8 correspond to lower SES, scores between 9 and 14 to middle SES, and scores between 15 and 21 to upper SES.
As for the expected results, we hypothesize a higher SES to correlate with better CI performance. Furthermore, we expect the subjective hearing as assessed in all tasks (speech perception in quiet and noise, and subjective localization ability) to improve with CI compared to the situation before surgery, and the perceived level of disability to be reduced.
Experimental setup
Before surgery, the patients are asked to complete the questionnaires addressing subjective hearing ability (Oldenburger Inventar),24,25 the impact of the hearing impairment on a patient's social life (Gothenburg Profile),26,27 general state of health (Fragebogen zum allgemeinen Gesundheitszustand)28 and socio-economic status (SES, Demographie und Schichtindex).29
The questionnaires concerning subjective hearing ability (Oldenburger Inventar)24,25 and the impact of the hearing impairment on a patient's social life (Gothenburg Profile)26,27 are repeated at the 6-month appointment, this time referring to the subjective hearing score for unilateral CI with unaided contralateral ear, and (if applicable) for bimodal or bilateral support. The Gothenburg Profile assesses the level of residual disability despite use of a CI. These repeated questionnaires enable the subjective development of hearing and social life with the CI to be evaluated.
Patients
The additional set of tests is conducted with postlingually deafened adults (≥16 years) who do not have other disabilities and are seeking a CI at our hospital. Data sets of 97 ears for which the 6-month appointment has been completed are available so far. The 97 data sets were obtained from 90 patients. All surgical interventions were performed unilaterally. In 74 cases for which the additional testing as described above was performed, it was the first ear that had been implanted; in 23 cases, it was the second ear. For 7 patients who underwent additional testing, the two ears had been sequentially implanted. In such cases, the two surgical procedures were considered independently. There are 32 males (34 male ears) and 58 females (63 female ears) with an average age of 58.1 years±14.7 years at the time of surgery. On average, these patients had normal hearing for 32.0 years±20.0 years, impaired hearing for 21.6 years±19.0 years, were deaf for 4.4 years±7.0 years, and had 12.7 years±12.0 years' experience of hearing-aid use.
The patients' ears were implanted using different brands of implant: the Nucleus Freedom System from Cochlear (n=34), the Advanced Bionics HiRes90k (n=40), and the Sonata from Med-El (n=23).
The TRT Test was also performed with a normal hearing control group of 20 adults (9 males, 11 females) with an average age of 36.8 years±9.3 years. A first evaluation of our implementation of the TRT Test was then made.
This investigation was conducted in accordance with the ethical standards at our institution and in compliance with the Helsinki Declaration as revised in 1983.
Results
As expected, nearly all subjects experienced considerable improvement in their hearing with the CI compared with the situation before surgery, as was shown in our tests. This work aims to identify variables that explain variances in CI outcome, so in this section, for each test the results are given and then the diagnostic evidence of the obtained variables is analyzed. For this purpose, firstly a correlation analysis between the data obtained before surgery and those obtained at the 6-month appointment, as computed using the MATLAB routine corrcoef, was performed. Negative correlation coefficients can be explained by the fact that in some variables high values correspond to good performance and in other variables high values correspond to bad performance. The diagnostic evidence of the variables was, therefore, investigated by calculating the coefficient of determination as defined by Everitt30 to be the square of the correlation coefficient.
Demographical data and residual hearing
Study subjects' demographical data are given in the patients' details in Materials and Methods. Significant correlations to the results with CI were found between duration of impaired hearing and adaptive OLSA with modulated noise (r=0.22, P=0.043, r2=0.05). Also, significant correlations between duration of deafness and Freiburg Monosyllables were found (r=−0.28, P=0.007, r2=0.08). No other significant correlations between demographic data and CI outcome were detected.
As for the residual hearing, the following significant correlation coefficients, as well as the according coefficients of determination, were found: residual hearing at 250 Hz correlated significantly to the Freiburg Numbers with CI (r=0.28, P=0.017, r2=0.08) and residual hearing at 500 Hz correlated significantly to the fixed OLSA condition with unmodulated noise (r=0.28, P=0.037, r2=0.08) as well as to the subjective localization ability with CI (r=0.29, P=0.017, r2=0.09). Residual hearing at 2 kHz correlated to the subjective hearing ability in noise (r=0.33, P=0.030, r2=0.11). Residual hearing at 8 kHz correlated to the adaptive OLSA conditions in unmodulated noise (r=0.56, P=0.025, r2=0.31) as well as modulated noise (r=0.55, P=0.026, r2=0.31), and to the subjective hearing ability in quiet (r=−0.60, P=0.032, r2=0.35) and the sensed handicap (r=0.59, P=0.009, r2=0.35). No other correlations between residual hearing and CI outcome were found to be significant.
Text reception threshold test
The results of the TRT test, which was performed before surgery, are given as degree of masking where the subject correctly repeats 50% of the words. Higher values represent better understanding. When the sentences were masked with dots, the degree of masking was 54.1%±8.7% (control group 65.7%±6.8%); when periodic bars were used, the figure was 57.8%±5.8% (control group 68.0%±4.2%); with random bars, it was found to be 55.5%±6.7% (control group 64.9%±4.2%). The performance in the periodic bar masking pattern was significantly better than in the random dot pattern (P<0.05) and showed a trend for being better than in the random bar pattern (P<0.1), whereas there was no significant difference in performance between the random dot and the random bar patterns. For the control group, there was no significant difference in the results of the three masking patterns; this was tested for both groups by a one-way ANOVA followed by the Scheffé post hoc test on a significance level of P<0.05. The Mann-Whitney-Test was used and showed very significant differences between the groups in all three conditions (P<0.001) (Figure 2).
Figure 2.

Results of the text reception threshold test expressed as a boxplot. Horizontal line in the box indicates the median of the sample, the lower and upper horizontal lines represent the lower and upper quartile (25% and 75% quartiles, respectively). The whiskers indicate the last data point lying in the 1.5 times range of the interquartile range (the difference between the 25% and the 75% quartiles). The small crosses indicate outliers. (Left panel) Results of the cochlear implants (CI) candidates obtained before surgery. (Right panel) Results of the normal hearing control group. Visual masking was performed with random dots (left), periodic bars (middle) and random bars (right).
Diagnostic evidence
The following significant correlation coefficients and coefficients of determination were found between the results in the TRT test masked by random dots and the CI outcome. This variable correlated to the adaptive OLSA test with unmodulated noise (r=−0.23, P=0.036, r2=0.05) as well as modulated noise (r=−0.29, P=0.007, r2=0.09), to the fixed OLSA test with modulated noise (r=0.26, P=0.026, r2=0.07) and to the subjective hearing ability in noise (r=0.22, P=0.049, r2=0.05). The results of the TRT test masked by periodic bars correlated to the subjective hearing ability in noise (r=0.30, P=0.007, r2=0.09). The TRT test masked with random bars yielded the following significant correlations: this variable correlated to the adaptive OLSA test with unmodulated noise (r=−0.27, P=0.012, r2=0.07) as well as modulated noise (r=−0.28, P=0.009, r2=0.08).
Master hearing aid
The Master Hearing Aid system is a standardized and (in theory) optimized hearing aid used for pre-surgery testing of speech perception. One drawback of this system is that the subjects do not have sufficient familiarization time. To determine the extent of this disadvantage, the performance of the CI candidates using the Master Hearing Aid system was compared with their performance using their own hearing aids by conducting the Freiburg Monosyllabic Test. Testing with their own hearing aid took place following optimization during the preliminary examinations some weeks prior to CI surgery. This comparison was made with all subjects who were able to understand at least one word using one of the devices (n=35). All other subjects, who had no speech perception with either device, were excluded from this comparison, as in these cases performance differences between the devices cannot be investigated. The distribution of the differences with both devices is given in Figure 3. If a statistical variation of ±10 percentage points is taken into account, 19 subjects performed comparably with both devices and 16 subjects performed better with the Master Hearing Aid system, whereas no individual performed better using his or her own hearing aid. The lack of familiarization time with the Master Hearing Aid system is not, therefore, considered to be a critical factor.
Figure 3.
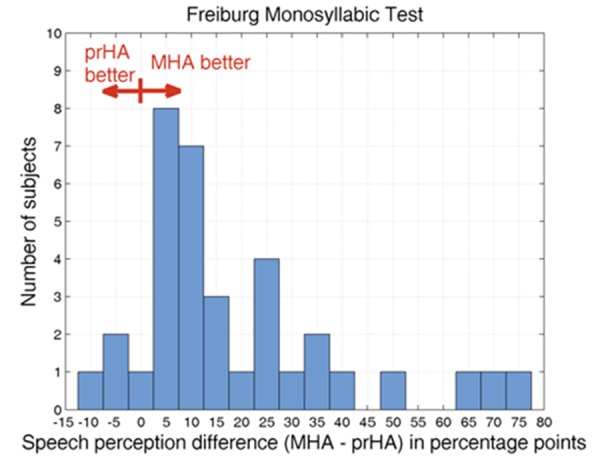
Comparison between performance with own hearing aid (ownHA) and Master Hearing Aid system (MHA) using the Freiburg Monosyllabic Test at 65 dB SPL, given as a histogram plot. On the abscissa, performance differences are given as percentage points; the ordinate gives the numbers of subjects who achieved these differences.
Speech tests
Before surgery, the OLSA test was performed adaptively using the Master Hearing Aid system in 28 subjects with an average SRT of 8.9 dB SNR±6.1 dB SNR in unmodulated noise and 11.2 dB SNR±5.3 dB SNR in modulated noise. Using the Wilcoxon's test, highly significant differences between performances with the two noise types were found (P<0.01); this test was also applied for statistical analysis of the differences found throughout this section. In the other cases, the OLSA test was performed with fixed SNR, yielding 24.5%±16.8% correct in unmodulated and 17.9%±13.3% correct in modulated noise. Here, a trend towards better performance in unmodulated noise was detected (P<0.1). At the 6-month appointment, the CI-aided OLSA test could be performed on an adaptive basis in 87 cases. This means that in 59 cases the adaptive OLSA could be successfully completed after surgery with CI and not before surgery with the MHA system. With CI, the OLSA yielded SRTs of 1.7 dB SNR±4.1 dB SNR in unmodulated noise and 6.6 dB SNR±5.1 dB SNR in modulated noise. Here, the performance in modulated noise was very significantly poorer than in unmodulated noise (P<0.001); for both noises, however, performance with a CI was extremely significantly (P<0.001, unmodulated noise) or highly significantly (P<0.01, modulated noise) better than that assessed before surgery with the Master Hearing Aid. When the fixed SNR was presented, the subjects understood on average 79.6±21.1% in unmodulated noise and 68.2±5.5% in modulated noise, with the differences between the noises being extremely significant (P<0.001) and the differences between the devices being highly significant (P<0.01).
Within the Freiburg Speech Test, when using the MHA prior to surgery, the subjects understood 14.9±18.4% in the monosyllabic test and 51.0 ±31.5% in the numbers test. After six months, the CI-aided results improved extremely significantly (P<0.001) to 62.2±23.2% in the monosyllabic test and to 96.4±12.2% in the numbers test (Figure 4).
Figure 4.
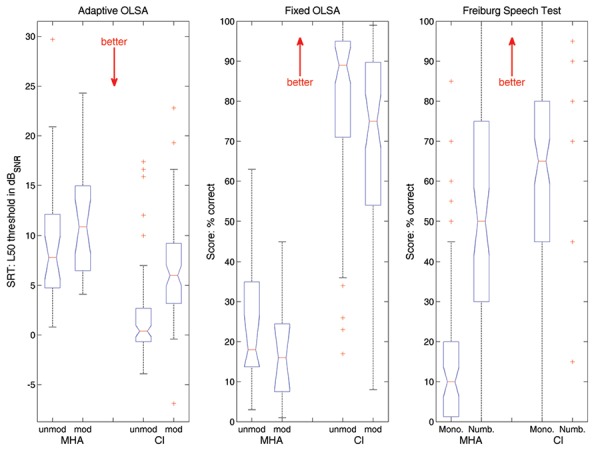
Results of the speech tests. (Left panel) Results of the adaptive Oldenburg sentence (OLSA) test. (Middle panel) Outcome of the fixed-level OLSA test. (Right panel) Results of the Freiburg Speech Test. All measurements were carried out before surgery using the Master Hearing Aid (MHA) and at the 6-month appointment with a cochlear implant (CI). Both OLSA test conditions (adaptive and fixed) are performed in unmodulated and modulated noise (left and right, respectively), and the Freiburg Speech Test consists of the monosyllabic test and the numbers test (left and right, respectively).
Diagnostic evidence
For the adaptive OLSA test in unmodulated noise aided with MHA, the following significant correlations were found: this variable correlated to the adaptive OLSA test with CI in unmodulated noise (r=0.47, P=0.015, r2=0.22) as well as to the subjective localization with CI (r=0.63, P=0.002, r2=0.40).
For the adaptive OLSA test in modulated noise aided with MHA these correlations were found: this variable correlated to the adaptive OLSA test with CI in unmodulated noise (r=0.50, P=0.015, r2=0.25) as well as modulated noise (r=0.53, P=0.013, r2=0.28), as well as to the subjective localization ability with CI (r=0.65, P=0.003, r2=0.42).
For the fixed OLSA test, no significant correlations to the CI outcome were detected.
The Freiburg Monosyllables aided with MHA correlated to the subjective hearing ability in noise (r=−0.29, P=0.033, r2=0.09), to the subjective localization ability (r=−0.44, P=0.001, r2=0.19) and to the sensed handicap (r=0.25, P=0.049, r2=0.06). The Freiburg Numbers aided with MHA were detected to correlate significantly to the subjective hearing in quiet with CI (r=−0.33, P=0.010, r2=0.11) as well as to the subjective hearing in noise with CI (r=−0.45, P=0.000, r2=0.20), to the subjective localization ability with CI (r=−0.43, P=0.001, r2=0.19) and to the total subjective rating of the hearing (r=−0.41, P=0.001, r2=0.17).
Questionnaires
The scores obtained in the questionnaires are shown in Figure 5. Patients had a mean SES score of 12.0±4.0 (middle SES) with higher values corresponding to higher SES.
Figure 5.
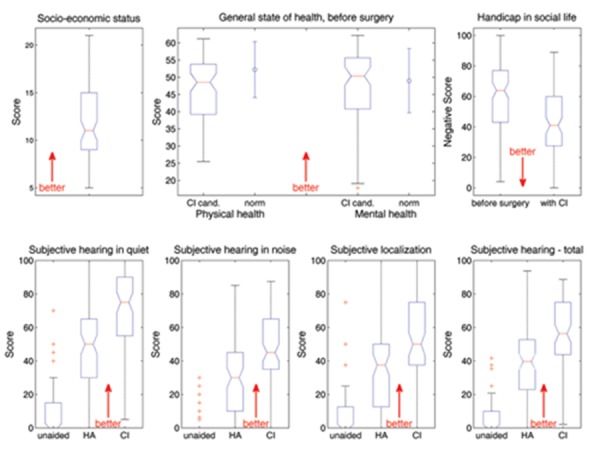
Results of the questionnaires. (Top left) Socio-economic status. (Top center) General state of health as assessed before surgery by the SF-12 is given as a boxplot, subdivided into physical (left) and mental (right) health. The error bars adjacent to the boxplots represent the normative values taken from Bullinger and Kirchberger.28 (Top right) The handicap in social settings as assessed by the Gothenburg Profile is plotted before surgery (left) and after 6-month CI use (right). (Bottom) Results of the Oldenburger Inventar estimating subjective hearing ability in quiet (bottom left), in noise (bottom, second from left), subjective localization ability (bottom, second from right) and totalled to produce an overall score (bottom right). All variables are assessed unaided (on the left of each diagram), with own hearing aid (HA, middle) and with a CI after six months of use (right).
The subjective hearing ability as estimated by the Oldenburger Inventar is broken down into hearing in quiet, hearing in noise and localization ability, yielding a total score with higher values corresponding to better hearing in each case. Values are obtained in all three conditions: unaided, with a hearing aid and with a CI. The score for hearing in quiet improved on average from 8.2±14.7 unaided and 49.0±23.5 with a hearing aid to 67.9±24.4 with a CI. All improvements were found to be extremely significant (P<0.001), tested using a one-way ANOVA and subjected to post hoc correction by means of the Scheffé procedure which was also used to analyze differences in the other three tasks making up the Oldenburger Inventar. The average hearing-in-noise score ranged from 2.9±7.0 unaided and 29.7±20.9 with a hearing aid to 46.0±23.0 with a CI. Furthermore, these improvements were found to be extremely significant (P<0.001). Localization scores were 7.6±14.4 unaided, 36.4±25.9 with a hearing aid and 46.1±26.7 with a CI, again with extremely significant differences (P<0.001) between unaided and both aided conditions, and significant differences (P<0.05) between aided with a hearing aid and with a CI. The total score improved from 5.9±9.9 unaided and 38.9±20.3 with a hearing aid to 55.8±21.4 using a CI, all these improvements being extremely significant (P<0.001).
The experienced handicap in social settings is estimated inversely by the Gothenburg Profile, with lower values corresponding to better performance. For our patients, this handicap was reduced from 59.5±23.1 with a hearing aid before surgery to 42.8±23.0 with a CI. Using the Wilcoxon's test, this reduction was found to be extremely significant (P<0.001).
The SF12 estimates the general state of health before surgery. The scale is subdivided into physical and mental health, yielding a physical health score of 46.4±8.4 (normative range 49.03±9.35)28 and a mental health score of 47.0±10.5 (normative range 52.24±8.10),28 with higher values indicating a better state of health.
Diagnostic evidence
SES correlated to the sensed handicap (r=−0.36, P=0.001, r2=0.13). The unaided subjective hearing ability in quiet correlated to the Freiburg Monosyllables with CI (r=−0.27, P=0.017, r2=0.08). The subjective hearing ability in quiet with hearing aids correlated to the subjective localization ability with CI (r=0.34, P=0.003, r2=0.12) as well as to the sensed handicap with CI (r=−0.32, P=0.003, r2=0.10). The unaided subjective hearing ability in noise correlated to the adaptive OLSA test with CI in modulated noise (r=−0.36, P=0.002, r2=0.13). The subjective hearing ability in noise with hearing aids correlated to the subjective hearing ability in noise with CI (r = 0.28, P=0.018, r2= 0.08) as well as to the subjective localization ability with CI (r=0.53, P=0.000, r2=0.28), to the total subjective rating of the hearing (r=0.33, P=0.004, r2=0.11) and to the sensed handicap (r=−0.38, P=0.000, r2=0.14).
For the unaided subjective localization ability no significant correlations to the CI outcomes were detected. The subjective localization ability with hearing aids correlated significantly to the subjective localization ability with CI (r=0.47, P=0.000, r2=0.22).
The subjective rating of the unaided hearing ability correlated to the results of the Freiburg Monosyllables with CI (r=−0.24, P=0.040, r2=0.06). The subjective rating of the hearing ability with hearing aid correlated significantly to the subjective localization with CI (r=0.49, P=0.000, r2=0.24), to the subjective rating of the hearing ability with CI (r=0.26, P=0.027, r2=0.07) and to the sensed handicap (r=−0.37, P=0.001, r2=0.14).
The sensed handicap before surgery correlated significantly to the subjective hearing ability in noise with CI (r=−0.25, P=0.026, r2=0.06), to the subjective localization ability with CI (r=−0.35, P=0.002, r2=0.12) and to the sensed handicap with CI (r=0.68, P=0.000, r2=0.47).
A significant correlation was detected between the physical health and the sensed handicap with CI outcome (r=−0.29, P=0.014, r2=0.09). The mental health correlated to the subjective localization ability with CI (r=0.39, P=0.002, r2=0.15) and to the sensed handicap with CI (r=−0.38, P=0.001, r2=0.14).
Discussion
Overall, the novel tests which complement our current standard set of tests yield consistent results that clearly reflect the improvements our subjects experienced with their cochlear implant. The analysis of the coefficient of determination enables us to identify some new factors with diagnostic evidence that could help in determining CI candidacy. It must be borne in mind that we could not identify one single factor influencing CI performance that has complete explanatory power. CI performance is still affected by several factors.
Demographic data and residual hearing
In our investigation, we found, as expected, some diagnostic evidence that the duration of impaired hearing as well as the duration of deafness in the speech perception tests with CI with short duration of impaired hearing and deafness correspond to better performance.
The other investigated demographic factors did not contribute to the CI outcome. Also, we found no diagnostic evidence concerning subjective rating of CI.
For the residual hearing, especially at high frequencies, we detected a high diagnostic evidence for objective and subjective CI outcome. Significant residual hearing in the high frequency range is only available in those patients whose hearing loss lies in the grey zone between cochlear implant and hearing aid. The need for additional diagnostic information is especially important in this area as these patients have a significant residual hearing to lose. Therefore, we think that the diagnostic evidence of residual hearing is very promising.
Text reception threshold test
In the control group, consistent results were found with low standard deviations. Therefore, we think that our implementation of the test including our adaptations to Zekveld et al.14 is suitable as an initial approach, although test-retest stability and age dependency remain to be evaluated. The masking degrees obtained in the TRT-Test with the CI candidates were lower than the results of the control group, but the average age of the patients was clearly higher than that of the control group. Nevertheless, we detected diagnostic evidence of the TRT test concerning postsurgical OLSA test outcomes with a CI with good results in the TRT test corresponding to good results in the speech test with CI, as also expected. This supports our hypothesis (also stated by Zekveld et al.)14 that certain prerequisites for speech recognition, such as cognitive skills, linguistic skills and concentration, are at least partly independent of intrinsic hearing and can thus be estimated by means of a purely visual task. Therefore, we think that this test clearly adds to the assessment of CI candidacy.
Speech perception aided with the master hearing aid
In our additional set of tests, the speech tests are conducted before surgery using the Master Hearing Aid system, with which a conventional hearing aid is simulated. The main virtue of this approach is the standardized, well-defined hearing aid algorithm and the (theoretically) optimal and standardized fitting, instead of various patients' own conventional hearing aids, ranging from cheap and old-fashioned devices paid for by health insurance providers to up-to-date systems with increased convenience for the individual patient. It was shown that, even without familiarization time, the subjects' performance with a Master Hearing Aid system matched or even exceeded that achieved when using their own hearing aid.
In particular, the adaptive OLSA test provided high diagnostic evidence concerning speech tests with CI, again with good results in the speech tests aided with the MHA system corresponding to good results in the speech tests with CI, as expected. This test relates to residual hearing. Before surgery, this test can be performed only on patients whose hearing loss lies in the grey zone between hearing aid and CI indication. There is a particular need for accurate prediction of CI outcome in these patients because they may also benefit from a good hearing aid, and the best hearing device for a given individual has to be chosen with great care. In any case, if there is no residual hearing or speech understanding, there is no decision to be made between a hearing aid and a CI in any case. Thus, the evidence provided offers additional diagnostic information that is extremely useful for determining CI candidacy, especially in this most challenging group of patients.
The aim in introducing the modulated ICRA5-250 noise is to mimic everyday life situations more closely than with the unmodulated standard noise. Both noises are speech shaped, which means that they consist of the same frequency range as speech. The modulated noise also includes temporal characteristics of speech by mimicking speech modulations. With acoustic hearing, subjects usually perform better in this modulated noise, as they are able to hear in the gaps and thus obtain enough information to reconstruct the original sentences.17 In contrast, our CI subjects performed worse than in the unmodulated noise. A possible explanation for this observation is that because of reduced spectral resolutions, the current CI signal-processing strategies make hardly any allowance for separate speakers operating over the same frequency range. It follows that CI subjects experience difficulties in distinguishing the speaker's voice from the speech-shaped modulated noise. This effect was also previously observed by Arlinger and Gustafsson31 who found that the advantages normal-hearing subjects can experience by hearing into the gaps in modulated noise were reduced with ongoing hearing impairment. Qin and Oxenham32 tested normal-hearing subjects by conducting simulations of CI signal processing. Here, processed and unprocessed speech stimuli are presented and the results compared. Studies like these can possibly also contribute to our understanding of hearing phenomena with CI. In this study, too, the modulated noise was found to be beneficial compared with the unmodulated noise for unprocessed speech stimuli, but this benefit disappeared for the speech stimuli processed with the signal processing of a typical cochlear implant.
Questionnaires
The questionnaires reflect well the development of hearing improvement. Subjective hearing ability in quiet was generally better than ability in noise, and both variables, as well as subjective localization ability and the total score, improved markedly from unaided hearing to hearing-aid use to six months of CI use. Nevertheless, most subjects experienced a floor effect with unaided subjective hearing abilities, thus yielding little diagnostic evidence concerning the aided conditions.
What is striking is the evidences of the SES to the subsequent CI outcome in speech tests and subjective improvement. Furthermore, Niparko et al.33 found in a multicenter study that language development in children is highly dependent on household SES. One reason for this influence could be that people of lower SES do not train their residual hearing ability to its full potential because they are unable to afford a sufficiently good hearing aid and have to rely on a simpler system paid for by their health insurance provider.
Conclusions
These novel tests offer a more in-depth evaluation of our patients than before and results confirm their usefulness. They showed highly significant improvements, including speech perception under more realistic conditions, as well as subjective improvements in hearing and social life. The Master Hearing Aid system can be used for standardized speech testing as all subjects were able to understand speech as well as, or better than, when they used their own hearing aid, even without a long period of familiarization. This makes it possible to carry out more sophisticated speech tests before surgery, such as sentence tests with different noises that were shown to predict subsequent performance with a CI. The innovative TRT test allows the linguistic competence of CI candidates to be assessed before surgery, and this also has high diagnostic potential.
The reason for incorporating these tests into our routine clinical practice is to utilize them for a later statistical model predicting the outcome with a CI, so that the expected benefit from an implant can be compared with that from a hearing aid. In this way, the most suitable hearing device for a given individual can be chosen. Furthermore, an accurate prediction model enables the subsequent fitting of the implant to be evaluated and verified. The diagnostic evidence found between data obtained before surgery and subsequent CI outcome show a promising trend for this model, which is currently under development.
Acknowledgements:
this investigation was carried out under the auspices of the Audiologie-Initiative Niedersachsen, an initiative funded by the Ministry of Science and Culture of Lower Saxony, Germany. We would like to thank Nina Wardenga, Hannover, for assisting with the measurements and the data management, Ralf Meyer, Oldenburg, for implementing the TRT test, Thomas Bisitz, Oldenburg, for supporting the Master Hearing Aid system in our clinic, and Birger Kollmeier, Oldenburg, for his valuable support.
References
- 1.Wilson BS, Dorman MS. Cochlear implants: current designs and future possibilities. J Rehabil Res Dev. 2008;45:695–730. doi: 10.1682/jrrd.2007.10.0173. [DOI] [PubMed] [Google Scholar]
- 2.Krueger B, Joseph G, Rost U, Strauß-Schier A, Lenarz T, et al. Performance groups in adult cochlear implant users: speech perception results from 1984 until today. Otol Neurotol. 2008;29:509–12. doi: 10.1097/MAO.0b013e318171972f. [DOI] [PubMed] [Google Scholar]
- 3.Lehnhard E, Aschendorff A. Prognostic factors in 187 adults provided with the nucleus cochlear mini-system 22. Adv Otorhinolaryngol. 1993;48:146–52. doi: 10.1159/000422575. [DOI] [PubMed] [Google Scholar]
- 4.Battmer RD, Gupta SP, Allum-Mecklenburg DJ, Lenarz T. Factors influencing cochlear implant performance in 132 adults. Ann Otol Rhinol Laryngol Suppl. 1995;166:185–7. [PubMed] [Google Scholar]
- 5.David EE, Ostroff JM, Shipp D, Nedzelski JM, Chen JM, et al. Speech coding strategies and revised cochlear implant candidacy: an analysis of post-implant performance. Otol Neurotol. 2003;24:228–33. doi: 10.1097/00129492-200303000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Gantz BJ, Tylor RS, Knutson JF, Woodworth G, Abbas P, et al. Evaluation of five different cochlear implant designs: Audiologic assessment and predictors of performance. Laryngoscope. 1998;98:1100–6. doi: 10.1288/00005537-198810000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Shea JJJ, III, Domico EH, Orchik DJ. Speech recognition ability as a function of duration of deafness in multichannel cochlear implant patients. Laryngoscope. 1990;100:223–6. doi: 10.1288/00005537-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk JE, van Olphen AF, Langereis MC, Mens LHM, Brokx JPL, et al. Predictors of cochlear implant performance. Audiology. 1999;38:109–16. doi: 10.3109/00206099909073010. [DOI] [PubMed] [Google Scholar]
- 9.Friedland D, Venick H, Niparko J. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24:582–9. doi: 10.1097/00129492-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Leung J, Wang N, Yeagle J, Chinnici J, Bowditch S, et al. Predictive Models for Cochlear Implantation in Elderly Candidates. Arch Otolaryngol Head Neck Surg. 2005;131:1049–54. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]
- 11.Haumann S, Lenarz T, Büchner A. Speech perception with cochlear implants as measured using a roving-level adaptive test method. ORL. 2010;72:312–8. doi: 10.1159/000318872. [DOI] [PubMed] [Google Scholar]
- 12.Hahlbrock KH. Über Sprachaudiometrie und neue Wörterteste. Arch Ohren Nasen Kehlkopfheilkd. 1953;162:394–431. [PubMed] [Google Scholar]
- 13.Grimm G, Herzke T, Berg D, Hohmann V. The Master Hearing Aid: A PC-based platform for algorithm development and evaluation. Acta Acustica united with Acustica. 2006;92:618–28. [Google Scholar]
- 14.Zekveld AA, George EL, Kramer SE, Goverts ST, Houtgast T. The development of the text reception threshold test: a visual analogue of the speech reception threshold test. J Speech Lang Hear Res. 2007;50:576–84. doi: 10.1044/1092-4388(2007/040). [DOI] [PubMed] [Google Scholar]
- 15.Wagener K, Kühnel V, Kollmeier B. Entwicklung und Evaluation eines Satztests für die deutsche Sprache I: Design des Oldenburger Satztests. Zeitschr Audiol. 1999;38:4–15. [Google Scholar]
- 16.Dreschler WA, Verschuure H, Ludvigsen C, Westermann S. ICRA noises: artificial noise signals with speech-like spectral and temporal properties for hearing instrument assessment. Int J Audiol. 2001;40:148–57. [PubMed] [Google Scholar]
- 17.Wagener KC, Brand T. Sentence intelligibility in noise for listeners with normal hearing and hearing impairment: Influence of measurement procedure and masking parameters. Int J Audiol. 2005;44:144–56. doi: 10.1080/14992020500057517. [DOI] [PubMed] [Google Scholar]
- 18.Brand T, Kollmeier B. Efficient adaptive procedures for threshold and concurrent slope estimates for psychophysics and speech intelligibility tests. J Acoust Soc Am. 2002;111:2801–10. doi: 10.1121/1.1479152. [DOI] [PubMed] [Google Scholar]
- 19.Moore BCJ, Alcántara JI, Stone MA, Glasberg BR. Use of a loudness model for hearing aid fitting: II. Hearing aids with multi-channel compression. Br J Audiol. 1999;33:157–70. doi: 10.3109/03005369909090095. [DOI] [PubMed] [Google Scholar]
- 20.Byrne D, Dillon H, Ching T, Katsch R, Keidser G. NAL-NL1 procedure for fitting nonlinear hearing aids: characteristics and comparison with other procedures. J Am Acad Audiol. 2001;12:37–51. [PubMed] [Google Scholar]
- 21.Wagener KC, Brand T, Kollmeier B. The role of silent intervals for sentence intelligibility in fluctuating noise in hearing impaired listeners. Int J Audiol. 2006;45:26–33. doi: 10.1080/14992020500243851. [DOI] [PubMed] [Google Scholar]
- 22.Staller S, Menapace C, Domico E, Mills D, Dowell RC, et al. Speech perception abilities of adult and pediatric nucleus implant recipients using the spectral peak (SPEAK) coding strategy. Otolaryngol Head Neck Surg. 1997;117:236–42. doi: 10.1016/s0194-5998(97)70180-3. [DOI] [PubMed] [Google Scholar]
- 23.Gstoettner W, Adunka O, Hamzavi J, Lautischer M, Baumgartner WD. Speech discrimination in post-lingually deaf patients with cochlear implants. Wien Klin Wochenschr. 2000;112:487–91. [PubMed] [Google Scholar]
- 24.Holube I, Kollmeier B. Ein Fragebogen zur Erfassung des subjektiven Hörvermögens: Erstellung der Fragen und Beziehungen zum Tonschwellenaudiogramm. Audiologische Akustik. 1991;30:48–64. [Google Scholar]
- 25.Holube I, Kollmeier B. Modifikation eines Fragebogens zur Erfassung des subjektiven Hörvermögens und dessen Beziehung zur Sprachverständlichkeit in Ruhe und unter Störgeräuschen. Audiologische Akustik. 1994;33:22–35. [Google Scholar]
- 26.Kießling J, Kollmeier B, Diller G. Stuttgart: Georg Thieme; 1997. Versorgung und Rehabilitation mit Hörgeräten. [Google Scholar]
- 27.Ringdahl A, Eriksson-Mangold M, Andersson G. Psychometric evaluation of the Gothenburg Profile for measurement of experienced hearing disability and handicap: applications with new hearing aid candidates and experienced hearing aid users. Br J Audiol. 1998;32:375–85. doi: 10.3109/03005364000000089. [DOI] [PubMed] [Google Scholar]
- 28.Bullinger M, Kirchberger I. Handanweisung: Hogreve Verlag; 1998. SF-36: Fragebogen zum Gesundheitszustand. [Google Scholar]
- 29.Ahrens W, Bellach BM, Jöckel KH. München: MMV Medizin Verlag; 1998. Messung soziodemographischer Merkmale in der Epidemiologie. RKI-Schriften 1/98. [Google Scholar]
- 30.Everitt BS. 2nd ed. Cambridge: Cambridge University Press; 2002. The Cambridge Dictionary of Statistics. [Google Scholar]
- 31.Arlinger S, Gustafsson HÅ. Masking of speech by amplitude-modulated noise. J Sound Vib. 1991;151:441–5. doi: 10.1121/1.408346. [DOI] [PubMed] [Google Scholar]
- 32.Qin MK, Oxenham AJ. Effects of simulated cochlear-implant processing on speech reception in fluctuating maskers. J Acoust Soc Am. 2003;114:446–54. doi: 10.1121/1.1579009. [DOI] [PubMed] [Google Scholar]
- 33.Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]


